Abstract
Background
The CNS manifestations of COVID-19 in children have primarily been described in case reports, which limit the ability to appreciate the full spectrum of the disease in paediatric patients. We aimed to identify enough cases that could be evaluated in aggregate to better understand the neuroimaging manifestations of COVID-19 in the paediatric population.
Methods
An international call for cases of children with encephalopathy related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and abnormal neuroimaging findings was made. Clinical history and associated plasma and cerebrospinal fluid data were requested. These data were reviewed by a central neuroradiology panel, a child neurologist, and a paediatric infectious diseases expert. The children were categorised on the basis of their time of probable exposure to SARS-CoV-2. In addition, cases were excluded when a direct link to SARS-CoV-2 infection could not be established or an established alternate diagnostic cause could be hypothesised. The accepted referral centre imaging data, from ten countries, were remotely reviewed by a central panel of five paediatric neuroradiologists and a consensus opinion obtained on the imaging findings.
Findings
38 children with neurological disease related to SARS-CoV-2 infection were identified from France (n=13), the UK (n=8), the USA (n=5), Brazil (n=4), Argentina (n=4), India (n=2), Peru (n=1), and Saudi Arabia (n=1). Recurring patterns of disease were identified, with neuroimaging abnormalities ranging from mild to severe. The most common imaging patterns were postinfectious immune-mediated acute disseminated encephalomyelitis-like changes of the brain (16 patients), myelitis (eight patients), and neural enhancement (13 patients). Cranial nerve enhancement could occur in the absence of corresponding neurological symptoms. Splenial lesions (seven patients) and myositis (four patients) were predominantly observed in children with multisystem inflammatory syndrome. Cerebrovascular complications in children were less common than in adults. Significant pre-existing conditions were absent and most children had favourable outcomes. However, fatal atypical CNS co-infections developed in four previously healthy children infected with SARS-CoV-2.
Interpretation
Acute-phase and delayed-phase SARS-CoV-2-related CNS abnormalities are seen in children. Recurring patterns of disease and atypical neuroimaging manifestations can be found and should be recognised being as potentially due to SARS-CoV-2 infection as an underlying aetiological factor. Studies of paediatric specific cohorts are needed to better understand the effects of SARS-CoV-2 infection on the CNS at presentation and on long-term follow-up in children.
Funding
American Society of Pediatric Neuroradiology, University of Manchester (Manchester, UK).
Video Abstract
Neuroimaging manifestations in children with SARS-CoV-2 infection
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first identified as a cluster of pneumonia cases in China in December, 2019.1 Early in the pandemic, the most severely affected individuals with the highest mortality were older adults with significant comorbidities. By contrast, children accounted for a minority of cases, among which 80% were mildly affected or entirely asymptomatic.2 The number of children who developed severe disease was reported to be low.3
As the pandemic progressed, it became clear that, in addition to respiratory involvement, other systemic symptoms could develop, including neurological symptoms.4 In places where the pandemic was widespread, such as northern Italy and New York City (NY, USA), clinicians encountered numerous adult patients with severe neurological complications from acute COVID-19 infection.5 In adults, cytokine storm and thrombogenic reactions to SARS-CoV-2 infection led to a high incidence of ischaemic stroke and intracerebral haemorrhage.6 By contrast, severe CNS injury in children with COVID-19 was rarely reported.
In children, the temporal evolution of COVID-19 can involve an inflammatory process during the latent period of the disease, termed multisystem inflammatory syndrome in children (MIS-C), also known as paediatric inflammatory multisystem syndrome-temporally associated with SARS-CoV-2 (PIMS-TS).7 It was within this subcohort of COVID-19 cases that neurological manifestations of SARS-CoV-2 infection were first identified in children.8 A review of multiple studies identified an unexpectedly high incidence of neurological symptoms (34%) in children with MIS-C.9
Research in context.
Evidence before this study
We searched PubMed on May 4, 2020, and again on the Oct 1, 2020, with no restriction on language or dates of publication. The search terms used included “COVID-19” and “SARS-CoV-2” combined with “paediatric” and “neurology” or “encephalopathy”. This search yielded 151 publications on May 4 and an additional 49 publications on Oct 1. We focused on publications that yielded imaging manifestations in these cohorts, which were mostly case reports or small case series that limited the ability of the full spectrum of disease to be appreciated in this patient population.
Added value of this study
This study has allowed the systematic evaluation of neuroimaging manifestations in the largest reported cohort of children infected with SARS-CoV-2 to date, and includes children with acute COVID-19 and those in the postinfectious period. Recurring patterns of disease were identified, along with more unusual imaging manifestations that we were able to recognise due to the large number of cases evaluated simultaneously. The neuroimaging manifestations of COVID-19 infection in children can range from mild to fatal, and pre-existing conditions were usually absent. Cerebrovascular complications seem to be less common in children than in adults. The most common imaging findings observed in children resembled immune-mediated parainfectious patterns of disease involving the brain, spine, and nerves. An important observation was that cranial nerve enhancement did not always correlate with cranial nerve deficits. The dominant findings in multisystem inflammatory syndrome in children were splenial lesions and myositis of the face and neck. This study also describes the development of fatal atypical CNS infections in previously healthy children infected with SARS-CoV-2.
Implications of all the available evidence
Acute-phase and delayed-phase SARS-CoV-2-related changes in the CNS in children were identified in this large case study. Different recognisable patterns of brain, cranial nerve, and spinal cord involvement were identified, including multifocal T2 bright lesions in brain white matter, vasculitic patterns with ischaemic lesions, enhancing neuritis or polyradiculitis, venous thrombosis, splenial lesions of the corpus callosum, longitudinally extensive myelitis, and myositis. These findings should be recognised and investigated for possible SARS-CoV-2 infection as the underlying aetiological factor. COVID-19 CNS disease in children, although less frequent than in adults, can occur, with severe CNS involvement in previously healthy, typically developing children. Studies of paediatric-specific cohorts are needed to better understand the effects of SARS-CoV-2 infection on the CNS at presentation and on long-term follow-up in children.
Reports began to emerge of abnormal neuroimaging findings in children with MIS-C.10 These isolated reports made it difficult to appreciate what the prevalence and patterns of CNS manifestations of COVID-19 were in the paediatric population. These unknowns, and the limited conclusions that could be reached from the existing literature, led the American Society of Pediatric Neuroradiology (ASPNR) to initiate an international call for cases. This study presents a review of cases submitted from ten countries, the purpose of which was to identify enough cases that could be evaluated in aggregate to better understand the neuroimaging manifestations of COVID-19 in the paediatric population.
A video abstract of this Article is available online.
Methods
Overview and case collection
The ASPNR distributed a survey and broadcast by email to all ASPNR members an international call for cases of COVID-19 in children and adolescents aged 0–18 years with abnormal neuroimaging. Additionally, calls were put out via the ASPNR Twitter feed and during the International Pediatric Neuroradiology Training Network meetings. The ASPNR survey was created with the online survey development tool SurveyMonkey. The UK paediatric cases were identified via communication through the UK Brain Imaging in COVID-19 Registry (UK-BICoRe), an initiative established in April, 2020, and supported by the British Society of Neuroradiologists to define the spectrum of acute neurological imaging abnormalities that arise from SARS-CoV-2 infection.
Physicians who submitted cases for review were considered part of a multinational, multicentre collaborative called the Pediatric COVID Brain Imaging Group (PECOBIG), formed for the purposes of this study. Collaborating physicians provided the neuroimaging studies, clinical data, results of PCR testing of the upper respiratory tract, serology for SARS-CoV-2 antibodies, cerebrospinal fluid (CSF) analysis (when available), and clinical outcomes.
Institutional review board or ethical approval was obtained by each collaborator for their case(s) according to the respective jurisdictions from where the case(s) originated, with written consent obtained in instances in which the respective jurisdiction mandated such. A list of institutions providing review board approval is given in the appendix (p 3)).
Case selection criteria
Cases were included if patients were aged 0–18 years and had clinical or laboratory evidence of SARS-CoV-2 infection and abnormal neuroimaging findings on MRI or CT that, given the exclusion criteria below, were hypothesised to be attributable to SARS-CoV-2 infection. Six cases in this study (cases 5, 8, 23, 27, 28 and 30) have been submitted separately as case reports to clinical journals, but are included in this Article because their neuroimaging findings were either not presented or were incompletely reported or not identified in other reports. The central review committee deemed that said findings, in these cases, were essential to include and discuss in aggregate within this imaging series. None of the images presented in this study have been previously published.
Initial reviews assessed the quality of available imaging and appropriate imaging sequences, and cases were excluded if the imaging dataset was suboptimal for accurate case characterisation. Cases were also excluded if the link to possible SARS-CoV-2 infection was based on contact exposure alone and if a patient did not have either a positive result on PCR or serology testing for SARS-CoV-2, if the exposure to SARS-CoV-2 was considered too remote in time to presentation to be causal (>12 weeks from symptom onset in non-MIS-C cases), or if imaging abnormalities could be attributed to a pre-existing comorbidity or an alternative diagnosis. This rigorous exclusion process was done to distil a subset of patients in whom a direct clinicoradiological link to SARS-CoV-2 could be established. The risk of reducing the cohort size of reported cases through exclusion of some cases that might have been due to SARS-CoV-2 was accepted as reasonable, with the aim of maximising homogeneity and reducing selection bias within the study cohort.
Case review
Image review was done jointly with the submitting radiologist or clinical team and the central review panel of five practicing paediatric neuroradiologists (CEL, KM, VMS, SMS, and SP) with 100 years of combined experience in tertiary-level paediatric neuroradiology. We used the virtual platform Zoom for reviewing the original anonymised DICOM dataset. A consensus opinion on imaging findings was obtained with agreement from a minimum of four reviewers required for an imaging finding to be documented.
Descriptive statistics were used to assess the incidence and prevalence of imaging abnormalities of the CNS.
Case categorisation
To appropriately understand the neuroimaging findings that can be encountered throughout the clinical course of COVID-19, a clinical categorisation scheme was created by four of the authors (CEL, DR, LKK, and SMS; appendix p 1). Cases were allocated into one of four categories, by agreement in all cases, by physicians with expertise in child neurology, a paediatric infectious disease physician, and a paediatric neuroradiologist (CEL, DR, LKK, and SMS), based on accepted clinical and laboratory data parameters for SARS-CoV-2 infection and the US Centers for Disease Control and Prevention (CDC) guidelines. The categorisation was done according to the temporal and clinical relationship of symptoms to the patient's suspected SARS-CoV-2 exposure, based on understanding of the clinical spectrum of COVID-19 in children and interpretation of molecular and serological assay results. The data form used to report case data for the purposes of the study is presented in the appendix (p 1)).
Role of the funding source
The funders of the study had no role in study design, data collection (beyond funding from the ASPNR for the initial SurveyMonkey call), data analysis, data interpretation, or writing of the report. All authors had access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between the survey dates of April 30 and Sept 8, 2020, we received 429 initial responses from 32 countries. The numbers of survey responses, case submissions, reviews, inclusions, and exclusions are presented in the appendix (p 2)). 60 children with neurological symptoms underwent formal review by the central review panel. Following an analysis of clinical data and image reviews, 17 children were excluded on the basis of clinical or radiological exclusion criteria, and five cases were withdrawn. 38 children were included and formed the basis of this study. The cases represented contributions from France (n=13), the UK (n=8), the USA (n=5), Brazil (n=4), Argentina (n=4), India (n=2), Peru (n=1), and Saudi Arabia (n=1). Patient demographics are shown in the appendix (p 3)).
Cases were grouped into four categories: acute COVID-19 (category 1; 12 [32%] children); asymptomatic acute or subacute COVID-19 (category 2; eight [21%] children); MIS-C (category 3; 11 [29%] children); or indeterminate (category 4; seven [18%] children).
Category 1 comprised 12 children with clinical and laboratory findings in keeping with acute COVID-19, 11 (92%) of whom had positive upper respiratory PCR testing and one of whom had acute clinical symptoms and positive IgM serology compatible with recent SARS-CoV-2 infection. The clinicoradiological findings including comorbidity data and clinical follow-up are summarised in the appendix (pp 4–5).
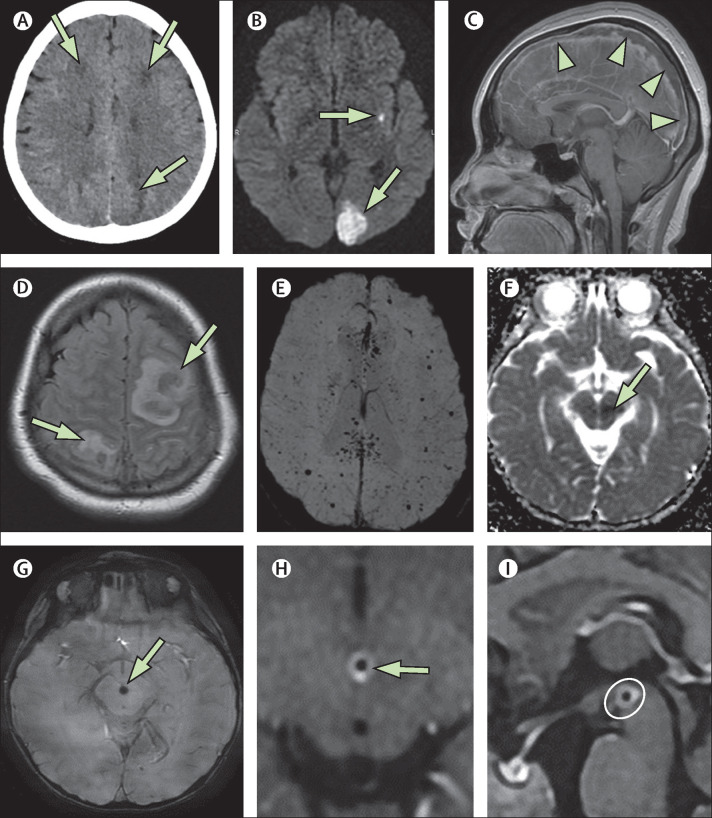
The most frequent imaging findings in category 1 were compatible with autoimmune manifestations, observed in six (50%) of 12 patients, as follows. Patchy T2 hyperintensity involving grey and white matter with or without abnormal enhancement and diffusion restriction was seen in four (33%) patients (cases 1–4; figure 1A–D ). This imaging pattern is referred to herein as acute disseminated encephalomyelitis (ADEM)-like; this descriptive terminology is used to refer to an imaging phenotype analogous to an ADEM-like pattern, but does not imply that the patients met the clinical definition for ADEM per se.11 Two children (cases 2 and 3) with the ADEM-like imaging pattern had T2 signal changes in the splenium of the corpus callosum which, given that these patients had seizures at presentation, was thought to be compatible with this clinical presentation. One patient (case 4) also developed long-segment myelitis with predominant central cord T2 hyperintensity (appendix p 6). In the two other patients (cases 6 and 7) with autoimmune manifestations, we observed enhancement of the cranial nerves or cauda equina, or both, referred to under the general term neuritis (figure 2A–F ).
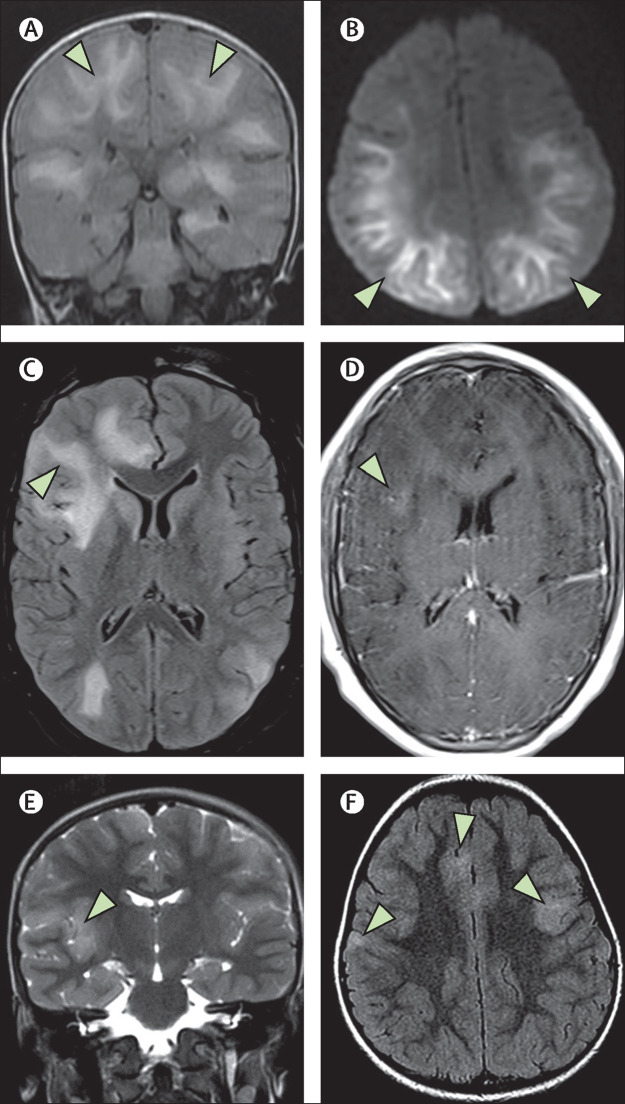
Figure 1.
ADEM-like brain changes
(A, B) A 1-year-old boy (case 2) with acute COVID-19 showed confluent areas of high signal in the subcortical white matter on coronal FLAIR imaging (A; arrows), and reduced diffusion on DWI trace (B; arrows). (C, D) A 13-year-old boy (case 4) showed similar changes on FLAIR imaging with associated mass effect in the right frontal lobe (C; arrow). This area showed some subtle enhancement on postcontrast T1-weighted imaging (D; arrow).(E, F) In a 4-year-old boy (case 38) with an indeterminate timepoint of exposure to SARS-CoV-2, ADEM-like changes were seen on coronal T2-weighted images (E; arrow) and axial FLAIR images (F; arrows). This child was positive for antibodies to myelin oligodendrocyte glycoprotein. ADEM=acute disseminated encephalomyelitis. DWI=diffusion-weighted imaging. FLAIR=fluid-attenuated inversion recovery.
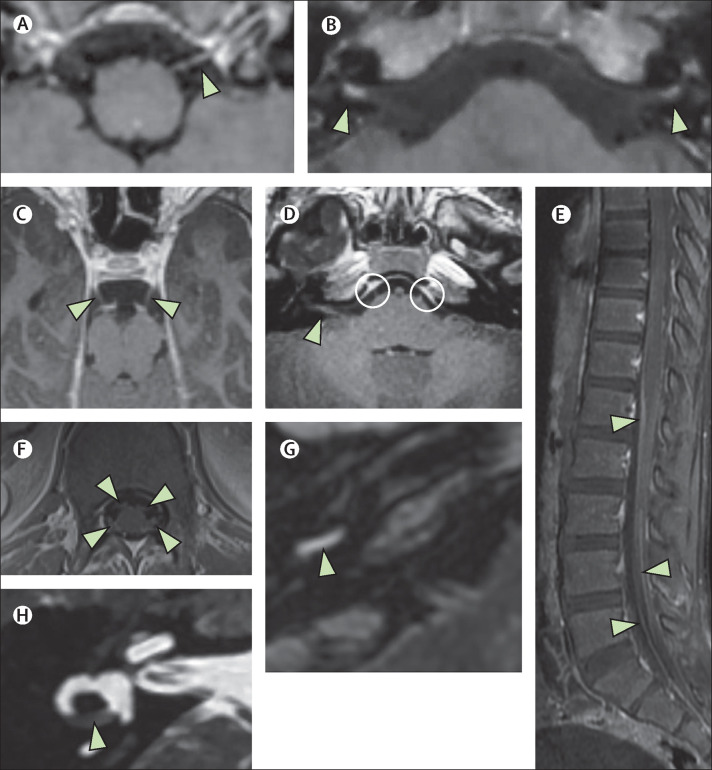
Figure 2.
Neuritis
(A, B) A 5-year-old boy (case 6) with acute COVID-19 presented with acute facial paralysis in conjunction with respiratory failure. He had marked enhancement and thickening of multiple cranial nerves, for example the 12th nerve on the left (A; arrowhead) and the seventh nerves bilaterally (B; arrowheads). (C–F) A 9-year-old boy (case 7), also with acute COVID-19, showed similar cranial nerve enhancement of his third nerves (C; arrowheads) as well as his seventh and eighth nerves (D; arrowhead [shown on patient's right side]) and his sixth nerves bilaterally (D; circles). This child also had enhancement of the cauda equina (E; arrowheads) as well as his cervical spine nerve roots (F; arrowheads). (G, H) A 13-year-old boy (case 33) with labyrinthitis with enhancement of the basal turn of the cochlea (G; arrowhead) and partial obliteration of his horizontal semicircular canal (H; arrowhead). All panels show T1 postcontrast images except for panel H (fast-spin echo T2 image).
Case 5 had an unusually aggressive myelitis. Initially subtle T2-signal prolongation within the medulla and the cervical and thoracic spinal cord on MRI at the time of presentation rapidly progressed within 4 days to marked cord oedema with enhancement, reduced diffusivity, and haemorrhage. MRI 3 weeks later showed severe cord atrophy with unusual, persistent, and prominent restricted diffusion of the spinal cord (figure 3A–H ).
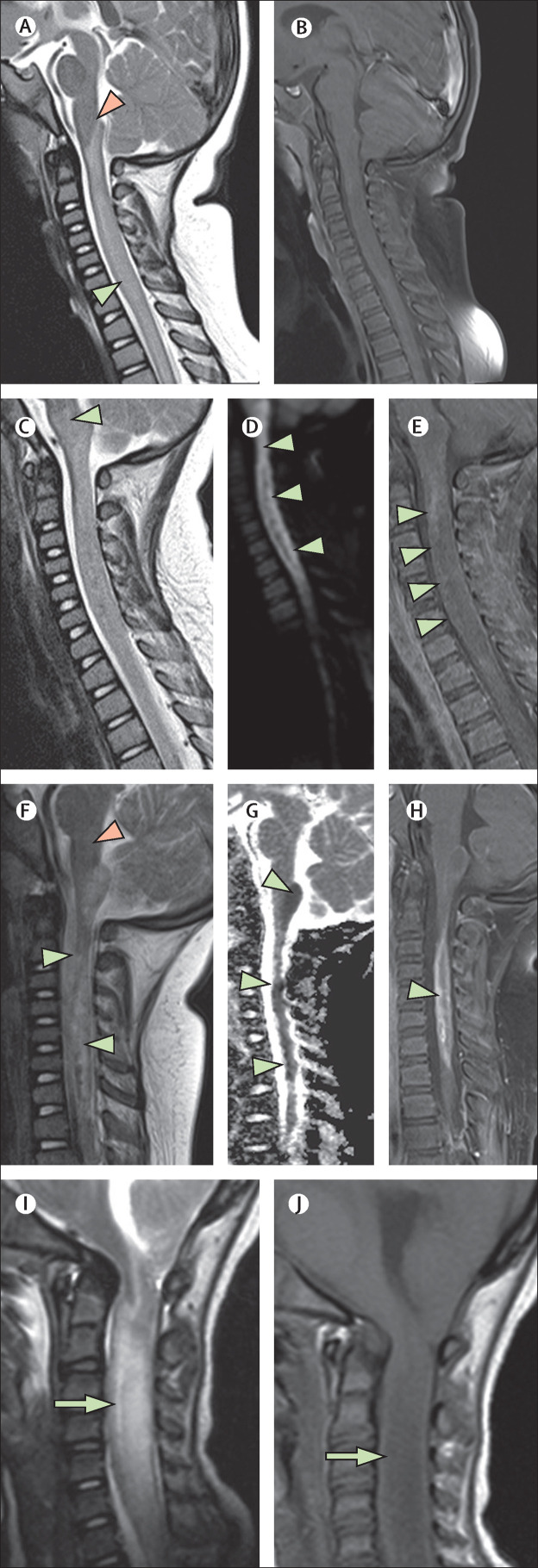
Figure 3.
Acute necrotising myelitis
(A–H) A 3-year-old girl (case 5), who was living in a household with multiple family members who had COVID-19, presented with acute SARS-CoV-2 infection with positive PCR result. Symptoms included acute respiratory failure, confusion, limb weakness, and vomiting. Initial T2-weighted imaging (A) showed central cervical cord signal abnormality (green arrowhead) extending up to the obex (pink arrowhead) but sparing the medulla. No enhancement was seen in the cervical and thoracic cord on the initial T1-weighted postcontrast imaging (B). 4 days later, more extensive myelitis was seen with new involvement of the medulla on T2 imaging (C; arrowhead), new reduced diffusion seen on diffusion trace images (D; arrowheads), and progressive enhancement seen on T1 postcontrast imaging (E; arrowheads). 3·5 weeks later, marked cord atrophy and necrosis were seen on sagittal T2 imaging (F; green arrowheads) with resolution of the medullary signal change (pink arrowhead). Persistent and varied areas of reduced diffusion were seen on sagittal apparent diffusion coefficient maps (G; arrowheads) in addition to enhancement on T1 postcontrast sagittal imaging (H; arrowhead), suggesting ongoing active disease. (I, J) For comparison, a second case of severe myelitis in a 5-year-old girl (case 8) with acute COVID-19 is shown. Sagittal T2-weighted (I) and sagittal T1-weighted (J) images show profound cord swelling (arrowheads). This child (case 8) died with biopsy-proven tuberculous granulomata and electron microscopic evidence of SARS-CoV-2 viral inclusions in the brain. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
In four (33%) previously healthy children (cases 8–11) in category 1, fulminant co-infections ended in rapid demise despite aggressive treatment. Case 8 first developed a small left frontal infarct.12 Rapid clinical deterioration with meningitis and hydrocephalus occurred within 3 days, progressing to fatal cerebral oedema and herniation 3·5 weeks after presentation (figure 4A–C ). Cerebellar biopsy showed SARS-CoV-2 viral inclusions on electron microscopy, and tuberculosis granulomata. Images of the cervical spine showed a fulminant myelitis (figure 3I, J). Case 9 presented 5 days after varicella zoster virus (VZV) infection with acute encephalopathy and fever. Pulmonary infiltrates typical of COVID-19 were present on CT, and sepsis and neurological decline ensued. MRI showed an atypical pattern of cerebral microhaemorrhages and multiple foci of infarction (figure 4D–F). Meticillin-resistant Staphylococcus aureus (MRSA) and VZV were positive in blood and CSF cultures. Case 10 was a child who developed an unusual choroid plexitis and fulminant CNS tuberculosis with ventriculitis, hydrocephalus, and focal cerebral abscesses (figure 4G–J). Case 11 presented with complicated sinusitis and developed meningitis, inflammatory vasculitis, and multiple infarcts in the setting of sepsis (figure 4K–O), and Fusobacterium necrophorum and Streptococcus constellatus were cultured from blood and CSF.
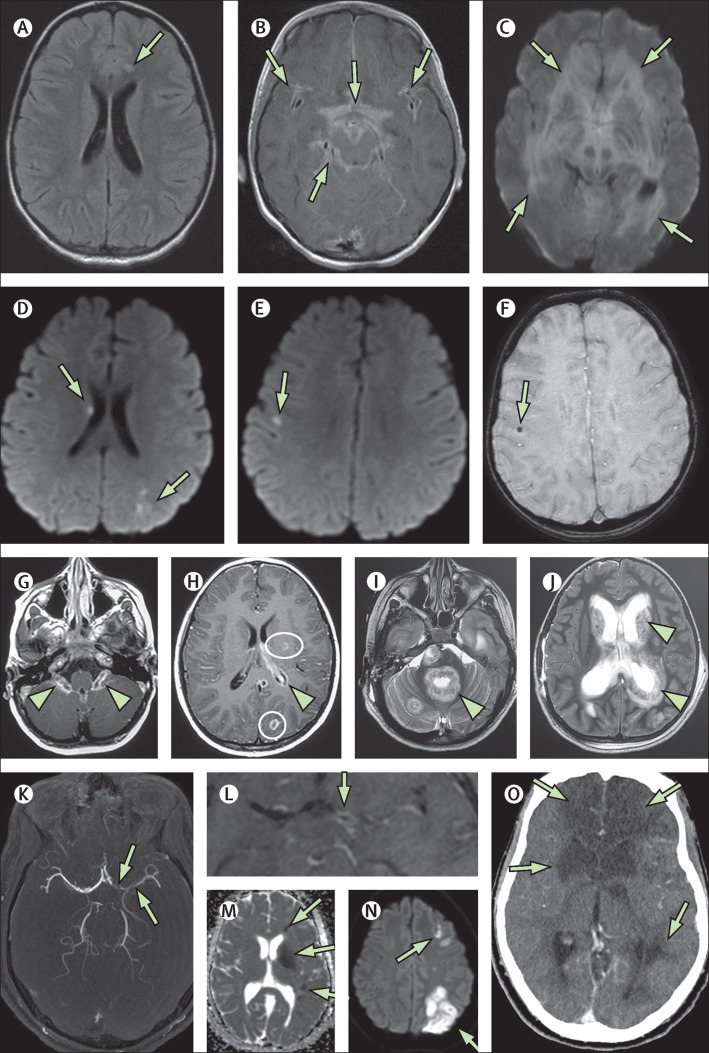
Figure 4.
Fatal co-infections
(A–C) 5-year-old girl (case 8) presented with fever, headache, and seizures. Initial MRI showed an acute small left frontal infarct on axial FLAIR imaging (A; arrow). 5 days later she had extensive leptomeningeal enhancement in the basilar cisterns and perisylvian regions on postcontrast T1-weighted imaging (B; arrows). Findings progressed and, 3 weeks after presentation, markedly reduced diffusion on diffusion trace imaging (C; arrows) and oedema of the brain parenchyma were observed. The patient's brain biopsy was positive for SARS-CoV-2 viral inclusions on electron microscopy and positive for tuberculosis granulomata despite no tuberculosis contact. (D–F) A 5-year-old girl (case 9) presented with encephalopathy and acute respiratory distress and became septic with meticillin-resistant Staphylococcus aureus and varicella zoster virus infections, both of which were culture positive in the blood and CSF. She had multiple small foci of reduced diffusion on axial diffusion trace imaging, in keeping with microinfarcts (D, E; arrows), some of which had associated microhaemorrhage (F; arrow). This patient died 15 days after symptom onset. (G–J) A 6-year-old boy (case 10) with no previous comorbidities presented with scattered T2 hyperintensities in the supratentorial white matter. He also had marked choroid plexitis (G; arrowheads) and foci of ring enhancement on T1 postcontrast imaging, in keeping with small abscesses (H; circles). There was minimal ependymal enhancement (H; arrowhead). Reduced diffusion was noted in the affected choroid and abscesses (not shown). 2 weeks later, axial T2-weighted imaging showed extensive and rapid progression involving multiple supratentorial and infratentorial compartments with ependymal invasion around the fourth ventricle (I; arrowhead), the basal ganglia, and the lateral ventricles (J; arrowhead). These areas also showed enhancement and reduced diffusion (not shown). Mycobacterium tuberculosis infection was confirmed at open biopsy. (K–O) A 16-year-old boy (case 11) presented with encephalopathy, fever, sinusitis, and meningismus, and Fusobacterium necrophorum and Streptococcus constellatus co-infections in the blood and CSF. At presentation he had multivessel stenoses on his magnetic resonance angiogram, most markedly involving the anterior and middle cerebral arteries (K; arrows), and there was associated vessel wall enhancement on postcontrast arterial wall imaging (L; arrow) and multiple vascular territory infarcts on the apparent diffusion coefficient map and diffusion-weighted trace image (M, N; arrows), features all in keeping with multivessel vasculitis. This condition progressed to multiple regional infarctions as shown on CT at day 5 (O; arrows). FLAIR=fluid-attenuated inversion recovery.
Four children in category 1 had thromboischaemic disease, of whom three (cases 8, 9, and 11) had co-infections. The other patient (case 12), in whom co-infection was absent, was 27 weeks pregnant at presentation and developed an occipital infarction in the setting of unusually severe posterior reversible encephalopathy syndrome (figure 5A, B ).
Figure 5.
Vasculitic and thrombotic findings
(A, B) A 15-year-old girl (case 12) presented 27 weeks pregnant with fever, seizures, and hypertension, and COVID-19 pneumonia. Her CT at presentation (A) showed low-density areas in multiple locations (arrows). MRI 7 days later (B) showed small focal infarcts and a larger left occipital infarct (arrows) on diffusion trace imaging, findings compatible with unusually severe posterior reversible encephalopathy syndrome. (C, D) A 15-year-old girl (case 20) with subacute COVID-19 and no classical respiratory symptoms presented with fever, confusion, and headache. Complete occlusive thrombosis of the superior sagittal sinus was shown by the large filling defect in the postcontrast sagittal T1-weighted image (C; arrowheads), with resultant bilateral haemorrhagic venous infarcts on axial FLAIR images (D; arrows). (E) A 15-year-old girl (case 27) with multisystem inflammatory syndrome in children who also developed multiple microthrombi, as shown on SWI. The microthrombi were relatively clinically silent and showed partial resolution at 3 weeks with full clinical resolution of symptoms at 3 months after presentation. (F–I) A 2-year-old girl (case 32, indeterminate category) presented with fever and pharyngeal pain with an acute left midbrain infarction (arrow) shown on the apparent diffusion coefficient map (F; arrow). She had a thrombus in the feeding anterior perforator vessel on SWI (G; arrow) and marked associated vessel wall enhancement on postcontrast T1 arterial wall imaging (H, arrow; I, circle). FLAIR=fluid-attenuated inversion recovery. SWI=susceptibility-weighted imaging.
Among the 12 cases in category 1, one (8%) child with necrotising myelitis is permanently quadriplegic (case 5), all four (33%) patients with co-infections died, and seven (58%) were clinically normal at discharge. The child born to the patient in case 12 was clinically normal for age and has not been tested for SARS-CoV-2 infection.
Category 2 (asymptomatic acute or subacute COVID-19) was formed of eight patients who did not present with clinically acute COVID-19 symptoms (based on CDC criteria) but had upper respiratory PCR tests that showed SARS-CoV-2 infection (cases 13–20). Five (63%) children in this category were found to be serologically positive for COVID-19 exposure. One child tested negative and two were not tested for serology. Clinicoradiological findings, including comorbidity data and clinical follow-up, are presented in the appendix (p 7)). Imaging abnormalities were as follows: two (25%) patients (cases 13 and 14) had ADEM-like changes in the brain and long-segment central cord myelitis; one (13%) patient (case 15) had long-segment central cord myelitis and no brain imaging; one (13%) patient (case 16) had ADEM-like changes in the brain and was diagnosed with anti-N-methyl-D-aspartate receptor (anti-NMDAR) autoimmune encephalitis; four (50%) patients (cases 14 and 17–19) had neuritis manifesting as cauda equina enhancement with variable enhancement of cranial or spinal nerves (one of whom [case 14] also had myelitis and ADEM-like changes); and one (13%) patient (case 20) had extensive superior sagittal sinus thrombosis with parasagittal venous infarcts (figure 5C, D). Regarding outcomes, the child with COVID-19 and anti-NMDAR encephalitis did poorly and remained intubated in the PICU 6 months following presentation. The other seven (88%) had favourable outcomes.
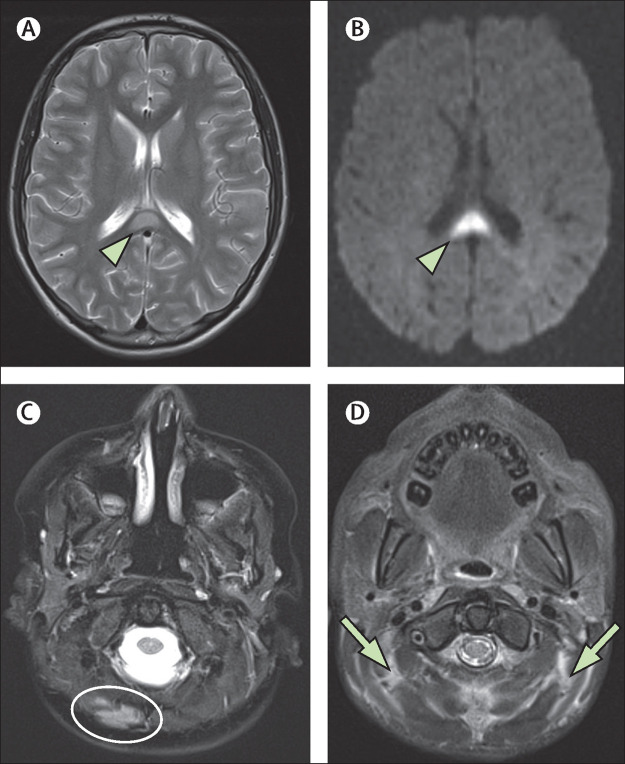
Category 3 comprised 11 patients who met the criteria for MIS-C (cases 21–31). Their clinicoradiological findings, including comorbidity data and clinical follow-up, are summarised in the appendix (p 8)). The imaging findings of these 11 patients were as follows: seven (64%) patients (cases 21, 23, and 26–30) had splenial lesions of the corpus callosum in isolation or in combination with other brain abnormalities (figure 6A, B ); seven (64%; cases 21–27) had ADEM-like brain findings; two (18%; cases 24 and 29) had cranial nerve enhancement; one (9%; case 31) had cauda equina enhancement, one (9%; case 25) had myelitis; one (9%; case 27) had multiple punctate foci of susceptibility-induced signal drop-out in the brain, consistent with microthrombi, that improved on follow-up MRI done 3 weeks after the initial study (figure 5E); and four (36%; cases 22, 23, 28, and 30) had enhancing myositis of the facial or neck musculature (figure 6C, D). Follow-up was favourable in all cases, with five (45%) clinically normal and six (55%) clinically improved at discharge, most with minor residual symptoms.
Figure 6.
Myositis and splenial lesions
(A) Axial T2-weighted image in a 14-year-old boy (case 26) with the classic appearance of a splenial lesion (arrowhead) in post-COVID-19 MIS-C. This lesion showed reduced diffusion at presentation (arrowhead), as shown by the diffusion trace image in the same patient (B). Four patients with MIS-C were also noted to have myositis, which could be focal (C; oval), as seen in an 8-year-old boy (case 28), or diffuse (D; arrows), as seen in a 9-year-old boy (case 23) on axial T2-weighted fat-saturated images. MIS-C=multisystem inflammatory syndrome in children.
Category 4 (indeterminate cases) included seven patients who had had a positive SARS-CoV-2 serology test and positive neuroimaging findings (cases 32–38). They presented at varying times during the course of the global pandemic, and may or may not have had PCR testing initially. Early on in the pandemic PCR testing was not always available. After extensive discussion with the case contributors, alternative causes for their presentation have been excluded to the best of our ability. Clinicoradiological findings, PCR testing availability, and clinical follow-up data are presented in the appendix (p 9)). Imaging abnormalities were as follows: four (71%) of the seven patients had neuritis (cases 33–36); two (29%) had ADEM-like brain manifestations, of whom one also had myelitis (case 37) and the other (case 38) developed anti-myelin oligodendrocyte glycoprotein (MOG) antibodies (figure 1E, F); one (14%) patient (case 36) had cerebellitis in addition to cranial neuritis; and one (14%; case 32) had vasculitis and a midbrain infarct unrelated to a co-infection (figure 5F–J). Outcomes at follow-up were favourable, with three (43%) children clinically normal and four (57%) having improved with residual neurological symptoms.
Discussion
Early in the COVID-19 pandemic, children seemed to be largely unaffected by the disease relative to adults, typically being asymptomatic or having mild symptoms.13 However, as the pandemic progressed, cases of children more severely affected emerged, some manifesting with a Kawasaki-like syndrome labelled MIS-C.14 However, neurological complications in children were rarely reported—a stark difference from what was observed in adults.15 We suspect that children who were neurologically impaired through COVID-19 earlier in the pandemic might not have been identified because of an absence of available PCR testing or because the atypical or delayed symptoms shown by children were not immediately identified as COVID-19 related. To understand the neuroimaging manifestations encountered in the context of the temporal nature of symptoms in children and the variations in available testing, we divided cases into four categories. We identified several consistent neuroimaging patterns in children infected by SARS-CoV-2 in the acute, subacute, and postinfectious phases.
Throughout all phases and presentations of COVID-19, the most prevalent neuroimaging manifestations observed in children resembled an immune-mediated parainfectious pattern of disease involving the brain, spine, cranial nerves, and nerve roots. These manifestations were observed in 13 (65%) of 20 patients in categories 1 and 2. Throughout all four categories, this neuroimaging manifestation was found in 28 (74%) of 38 children. Brain manifestations were most commonly ADEM-like in appearance, with patchy or confluent areas of T2 hyperintensity in the grey and white matter, with or without reduced diffusion or enhancement, the exact pathophysiology of which remains to be clarified.
Of the 16 patients with ADEM-like appearances, anti-MOG antibody testing was available in 11, with negative results in ten. Two patients with ADEM-like neuroimaging had CNS-directed antibodies: one was positive for anti-NMDAR (case 16) and one for anti-MOG antibodies (case 38). Case 16, in category 2, was a 14-year-old girl who presented with fever, seizures, and encephalopathy, and developed anti-NMDAR autoimmune encephalitis. Immune-mediated encephalitis is known to occur in the setting of viral illness, and case reports in adults have noted an association between anti-NMDAR encephalitis and COVID-19.16 Case 38 was a 4-year-old boy in category 4 who presented with seizures and limb dysfunction, and was found to have anti-MOG antibodies. Given that neurological illnesses related to anti-MOG and anti-NMDAR antibodies can appear in post-viral illness, these two cases raise the likelihood that COVID-19 also has an association with immune-mediated CNS pathology. Except for the one child who developed anti-NMDAR encephalitis, all patients with ADEM-like imaging had favourable outcomes.
Myelitis in isolation or in combination with brain abnormalities was seen in eight (21%) cases. It most commonly appeared as T2 hyperintense cord signal abnormality predominantly within the central cord grey matter, similar in appearance to other demyelinating myelopathies such as ADEM, neuromyelitis optica, anti-MOG-associated encephalomyelitis, and idiopathic transverse myelitis (appendix p 6). This pattern of spinal cord pathology has previously been reported in case reports of children and adults with COVID-19.17, 18
There were two atypical cases of severe myelitis, both seen in the acute phase of the disease (category 1). Case 5 is of a child with no pre-existing conditions or other comorbidities who developed a clinically catastrophic myelitis, resulting in permanent quadriplegia. This unusual case was recently published in a single series clinical case report;17 however, the neuroimaging findings were not expounded upon and are worthy of review here. The initial MRI revealed only subtle T2 hyperintensity in the medulla and the cervical and thoracic cord. These changes evolved rapidly over a 4-day period with the development of marked cord oedema, intense enhancement, diffusion restriction, and haemorrhage. On imaging follow-up several weeks later, severe cord atrophy with persistent and striking reduced diffusion was noted, suggesting a progressive acute necrotising myelitis manifesting over an extended period and a distinctly different pathological process from the typical pattern of myelitis seen in the majority of our cases. The second case of severe myelitis was in case 8, a child who succumbed secondary to SARS-CoV-2-related encephalitis and fulminant tuberculosis. To our knowledge, only one other case of acute necrotising myelitis in a patient with COVID-19 (a 69-year-old woman) has been reported in the literature,19 and none have been reported in children so far. The underlying pathological mechanism remains to be clarified; cytokine storm secondary to SARS-CoV-2 has been postulated.
Neuritis, consisting of enhancement of cranial and spinal nerves or the cauda equina, was found in 12 (32%) cases in this study. Similar cranial nerve abnormalities have been reported in adults with acute COVID-19.20 Notably, in cases of cranial nerve involvement by SARS-CoV-2 infection, imaging findings are not always congruous with clinical symptoms. Of the nine cases of cranial neuritis noted across all four categories, seven (78%) had involvement of at least one specific cranial nerve without corresponding nerve palsy. The finding of cranial nerve enhancement on MRI should alert the clinician to possible SARS-CoV-2 infection as a consideration. Taste and smell dysfunction in adults with COVID-19 is well documented and there have been similar case reports in children.21 Anosmia was reported in only one patient in our cohort (case 34) and we did not observe signal abnormalities or enhancement of the olfactory nerves or surrounding tissues during imaging review. Labyrinthitis was present in one child in the indeterminate category (case 33). Given the frequency of cranial nerve involvement within our cohort, such involvement would not be an unexpected finding in the setting of COVID-19 infection. Labyrinthitis has not yet been reported in children with COVID-19. Cauda equina enhancement was observed in eight (21%) of 38 children. Most of these cases presented with neurological correlates supportive of Guillain-Barré syndrome. Cauda equina enhancement has also been reported in adults with SARS-CoV-2 infection.22
Children with COVID-19 and co-infections were the most severely ill patients in our series and all died. All co-infections occurred in the acute phase of COVID-19 and none of the children had pre-existing conditions. Two of these children (cases 8 and 10) developed fulminant M tuberculosis infections of the CNS and had no known tuberculosis exposure. Case 10 also showed striking plexitis. SARS-CoV-2 infection causes a breakdown of the blood–CSF barrier in the choroid plexus, and this mechanism might facilitate entry of tuberculosis into the CSF in children with SARS-CoV-2 infection, potentiating the CNS infection.23 The third case (case 9) was co-infected with MRSA and VZV and had scattered microbleeds or microthrombi and small foci of ischaemic injury. These findings are atypical for VZV-related CNS infection, which usually manifests with a focal, medium-sized vessel vasculopathy and basal ganglia infarcts. The fourth case (case 11) was co-infected with F necrophorum and S constellatus in the blood and CSF, developed meningitis, vasculitis, and died from multifocal cerebral infarctions, despite aggressive treatment. These four cases emphasise the potential for SARS-CoV-2 to impede the host's normal immune responses such that co-infections can work synergistically, contributing to a more severe clinical evolution of infection.24, 25
CNS complications of stroke and cerebral haemorrhage are well documented in adults.26 We found seven (18%) cases in our cohort that had findings that could be characterised as thromboembolic or vasculitic, occurring across all four categories and often manifesting in the setting of confounding comorbid conditions such as co-infections. Case 12 was an adolescent who was 27 weeks pregnant and developed hypertension and encephalopathy. Imaging showed bilateral deep white matter punctate foci of parasagittal diffusion restriction and an occipital lobe infarct, which were atypically severe manifestations of posterior reversible encephalopathy syndrome. Similar COVID-19-related atypical manifestations of posterior reversible encephalopathy syndrome have been reported in critically ill patients.27 Case 20 in category 2 is the only instance of venous thrombosis in our paediatric series. Neuroimaging revealed extensive thrombosis of the superior sagittal sinus and bilateral parasagittal haemorrhagic venous infarcts. Venous thrombosis, including of dural sinuses, has been reported in adults, and the mechanism through which SARS-CoV-2 infection promotes a thrombophilic state has not been completely elucidated, whether related to the cytokine storm inducing a procoagulable state or due to direct procoagulant effects of the virus itself.28 A pattern of scattered punctate foci of susceptibility-induced signal drop throughout the brain was seen in a child with MIS-C (case 27). Similar findings have been reported in adults with acute SARS-CoV-2 infection and are associated with acute critical illness, increased mortality, and poor functional outcome.29, 30 Microhaemorrhages have also been described in adults with COVID-19-related neuroinflammatory syndromes.31 It is also reported that thrombotic microangiopathy related to direct or indirect damage by SARS-CoV-2 to the vascular endothelium can occur.32 In our case, the punctate signal abnormalities largely resolved within 3 weeks, suggesting microthrombi as the most likely aetiology. The child was clinically normal at the 3-month follow-up. The only case of arterial infarction unrelated to co-infection arose in case 32. Vessel wall enhancement compatible with vasculopathy was seen around a thrombosed vessel, resulting in a small midbrain infarct. Case reports of COVID-19-associated cerebral vasculopathy can be found in the paediatric literature.33
Regarding the 11 children with MIS-C, the most frequent brain abnormalities were lesions in the splenium of the corpus callosum. These abnormalities were unrelated to seizure activity and identified in seven (64%) of 11 children in this category. They appear as discrete, ovoid, T2 hyperintense foci in the splenium, sometimes extending into the adjacent white matter, with variable associated restricted diffusion. Splenial lesions have been reported in other cases of MIS-C in the literature10, 34, 35 as well as in other infections.36, 37 It has been postulated that these lesions represent intramyelinic oedema as a result of cytokine-mediated glutamate release.38 Another frequent finding, observed in four (36%) patients with MIS-C, was myositis of the visualised musculature of the neck or face. Myositis has been observed in adults with COVID-19.39 Interpreting neuroradiologists should be aware of the presence of myositis as the cause of reported neck swelling in children with MIS-C.
Regarding outcomes within our entire cohort, most children did well with COVID-19 and were either normal or had some mild residual neurological deficits at last follow-up. However, all children with co-infections died, and two children (one with anti-NMDAR autoimmune encephalitis and one with acute necrotising cord myelitis) were severely impaired at last follow-up.
The limitations of this study include its retrospective nature, the selection bias of cases identified through voluntary submissions (which were likely to be more on the severe end of the spectrum of disease), and the variability in diagnostic, treatment, and imaging approaches at different international institutions. This variability also includes that associated with the non-standardised clinical evaluation in terms of timing and disability measures or neurocognitive testing. Limitations of assay availability and performance should also be considered. In addition, cases might have been erroneously excluded on the basis of the patient having imaging abnormalities that could be ascribed to a pre-existing morbidity but that were in fact related to SARS-CoV-2 infection. Long-term follow-up was absent in some cases, preventing us from fully elucidating the neurological sequelae of SARS-CoV-2 infection.
This is the largest study to date of CNS imaging manifestations of SARS-CoV-2 infection in children. Mild to severe COVID-19 occurred in healthy children without pre-existing conditions. Outcomes were usually favourable but fatalities and severe residua were also observed. Consistent disease patterns emerged, of which ADEM-like abnormalities of the brain and spinal cord and neuritis were the most common. Cranial nerve enhancement was found to occur without correlative cranial nerve deficits. Cerebrovascular manifestations were less frequently encountered in children compared with SARS-CoV-2 CNS complications reported in adults. Vascular complications were seen most often in the setting of comorbid conditions and consisted of regional and small infarcts, arteritis, and thrombosis. Fulminant atypical co-infections occurred in previously healthy children with acute symptomatic SARS-CoV-2 infections and were fatal in all instances. Finally, in children with MIS-C, the most common findings were splenial lesions and myositis of the neck and face.
Despite its limitations, this international collaboration has revealed that children can present with positive neuroimaging findings with or without the classic symptoms of COVID-19. Knowledge of the neuroimaging patterns of COVID-19 is important, as these patterns could be the first indication of SARS-CoV-2 infection in children with neurological abnormalities.
Data sharing
The authors and PECOBIG collaboration are committed to open science. The broader imaging review data and associated clinical data from this study will be made available, wherever possible, within the terms of participant consent and when not otherwise restricted by intellectual property rights or ongoing collaborative research. To preserve individual case anonymity, detailed data are not given in this Article or the appendix, but will be made available on appropriate request to the corresponding author.
Acknowledgments
Acknowledgments
We thank Claudia Gomez (GrupoCTScanner, Mexico City, Mexico) for collaborator outreach; Esmeralda Garcia for help in data curation; the American Society of Pediatric Neuroradiology and the British Society of Neuroradiology and their members; and all members of the PECOBIG Collaborative Group, without whom this work could not have been done.
Contributors
All authors have contributed to writing and manuscript editing. CEL, DR, LKK, SMS, and SP contributed to the literature search. CEL and SMS are responsible for the figures. KM led on the international research network and outreach. All authors are responsible for study design, data collection, analysis, and interpretation. CEL and SMS have accessed and verified the data underlying this study.
Declaration of interests
LKK reports grants from Merck outside of the submitted work. All other authors declare no competing interests.
Contributor Information
ASPNR PECOBIG Collaborator Group:
Sameen Akhtar, Douglas Alden, Suraj Amonkar, Pascale Aouad, Mélodie Aubart, Jose Alejandro Bacalla, Alcino A Barbosa, Romain Basmaci, Laureline Berteloot, Thomas Blauwblomme, Gilles Brun, Olivia Carney, Judith Chareyre, Gérard Chéron, Pablo Picasso De Araujo Coimbra, Volodia Dangouloff-Ros, Felice D'Arco, Rob Dineen, Loic De-Pontual, Isabelle Desguerre, Wissam Elfallal, D. Gareth Evans, Suely Fazio Ferraciolli, Nadine Girard, Fabrício Guimarães Gonçalves, Ivan Gonzalez, P. Ellen Grant, David Grévent, Carolina Valduga de Alencastro Guimaraes, Jane Hassell, Fabiana C.C. Hirata, Ian Kamaly-Asl, Jeffrey Jacob, Kandise Jackson, Blaise V. Jones, Robin Joseph, Ah Young Jung, Amna Kashgari, John-Paul Kilday, Alyssa Kirsch, Manoelle Kossorotoff, Anant Krishnan, Shilpa Kulkarni, Marianne Leruez-Vill, Fabrice Lesage, Raphaël Levy, Yi Li, Carol Cavalcante de Vasconcelos Lima, Lokesh Lingappa, Ulrike Löbel, Roberto Lopez-Alberola, Leandro Tavares Lucato, Daniela Duarte Moreira, Jonathan G. Murnick, Sarah Nahmani, Shubra Pagariya, Julija Pavaine, Bryan Philbrook, Ana Cláudia Piovesan, Kelsey E. Poisson, Nihaal Reddy, Phil Riley, Andrea Romsauerova, Charlies-Joris Roux, Carlos Rugilo, Gaurav Saigal, Gabriel Lucca de Oliveira Salvador, David Seidenwurm, Isabelle Sermet-Gaudelus, Jai Sidpra, Sniya Valsa Sudhakar, María Sol Toronchik, and Gilbert Vézina
Supplementary Material
References
- 1.Zhu N, Zhang D, Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parri N, Lenge M, Buonsenso D. Children with COVID-19 in pediatric emergency departments in Italy. N Engl J Med. 2020;383:187–190. doi: 10.1056/NEJMc2007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Götzinger F, Santiago-García B, Noguera-Julián A. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653–661. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niazkar HR, Zibaee B, Nasimi A, Bahri N. The neurological manifestations of COVID-19: a review article. Neurol Sci. 2020;41:1667–1671. doi: 10.1007/s10072-020-04486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yaghi S, Ishida K, Torres J. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51:2002–2011. doi: 10.1161/STROKEAHA.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaur S, Bansal R, Kollimuttathuillam S. The looming storm: blood and cytokines in COVID-19. Blood Rev. 2020 doi: 10.1016/j.blre.2020.100743. published online Aug 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pouletty M, Borocco C, Ouldali N. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020;79:999–1006. doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiotos K, Bassiri H, Behrens EM. Multisystem inflammatory syndrome in children during the coronavirus 2019 pandemic: a case series. J Pediatric Infect Dis Soc. 2020;9:393–398. doi: 10.1093/jpids/piaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen TH. Neurological involvement associated with COVID-19 infection in children. J Neurol Sci. 2020;418 doi: 10.1016/j.jns.2020.117096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdel-Mannan O, Eyre M, Löbel U. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. 2020;77 doi: 10.1001/jamaneurol.2020.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krupp LB, Tardieu M, Amato MP. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19:1261–1267. doi: 10.1177/1352458513484547. [DOI] [PubMed] [Google Scholar]
- 12.Freij BJ, Gebara BM, Tariq R. Fatal central nervous system co-infection with SARS-CoV-2 and tuberculosis in a healthy child. BMC Pediatr. 2020;20:429. doi: 10.1186/s12887-020-02308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He J, Guo Y, Mao R, Zhang J. Proportion of asymptomatic coronavirus disease 2019: a systematic review and meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.26326. published online July 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim L, Whitaker M, O'Halloran A. Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19—COVID-NET, 14 States, March 1–July 25, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1081–1088. doi: 10.15585/mmwr.mm6932e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahammedi A, Saba L, Vagal A. Imaging in neurological disease of hospitalized COVID-19 patients: an Italian multicenter retrospective observational study. Radiology. 2020;297:E270–E273. doi: 10.1148/radiol.2020201933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monti G, Giovannini G, Marudi A. Anti-NMDA receptor encephalitis presenting as new onset refractory status epilepticus in COVID-19. Seizure. 2020;81:18–20. doi: 10.1016/j.seizure.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaur H, Mason JA, Bajracharya M. Transverse myelitis in a child with COVID-19. Pediatr Neurol. 2020;112:5–6. doi: 10.1016/j.pediatrneurol.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munz M, Wessendorf S, Koretsis G. Acute transverse myelitis after COVID-19 pneumonia. J Neurol. 2020;267:2196–2197. doi: 10.1007/s00415-020-09934-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sotoca J, Rodriguez-Alvarez Y. COVID-19-associated acute necrotizing myelitis. Neurol Neuroimmunol Neuroinflamm. 2020;7:e803. doi: 10.1212/NXI.0000000000000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95:e601–e605. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 21.Erdede O, Sarı E, Uygur Külcü N, Uyur Yalçın E, Sezer Yamanel RG. An overview of smell and taste problems in paediatric COVID-19 patients. Acta Paediatr. 2020;109:2184–2186. doi: 10.1111/apa.15515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abu-Rumeileh S, Abdelhak A, Foschi M, Tumani H, Otto M. Guillain-Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol. 2020 doi: 10.1007/s00415-020-10124-x. published online Aug 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pellegrini L, Albecka A, Mallery DL. SARS-CoV-2 infects brain choroid plexus and disrupts the blood-CSF barrier. bioRxiv. 2020 doi: 10.1101/2020.08.20.259937. published online Aug 21. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Liao B, Cheng L. The microbial coinfection in COVID-19. Appl Microbiol Biotechnol. 2020;104:7777–7785. doi: 10.1007/s00253-020-10814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crisan-Dabija R, Grigorescu C, Pavel CA. Tuberculosis and COVID-19: lessons from the past viral outbreaks and possible future outcomes. Can Respir J. 2020;2020 doi: 10.1155/2020/1401053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Li M, Wang M. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5:279–284. doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parauda SC, Gao V, Gewirtz AN. Posterior reversible encephalopathy syndrome in patients with COVID-19. J Neurol Sci. 2020;416 doi: 10.1016/j.jns.2020.117019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolaji P, Kukoyi B, Ahmad N, Wharton C. Extensive cerebral venous sinus thrombosis: a potential complication in a patient with COVID-19 disease. BMJ Case Rep. 2020;13:8. doi: 10.1136/bcr-2020-236820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitsiori A, Pugin D, Thieffry C, Lalive P, Vargas MI. COVID-19 is associated with an unusual pattern of brain microbleeds in critically ill patients. J Neuroimaging. 2020;30:593–597. doi: 10.1111/jon.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agarwal S, Jain R, Dogra S. Cerebral microbleeds and leukoencephalopathy in critically ill patients with COVID-19. Stroke. 2020;51:2649–2655. doi: 10.1161/STROKEAHA.120.030940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paterson RW, Brown RL, Benjamin L. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iba T, Connors JM, Levy JH. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm Res. 2020;69:1181–1189. doi: 10.1007/s00011-020-01401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirzaee SMM, Gonçalves FG, Mohammadifard M, Tavakoli SM, Vossough A. Focal cerebral arteriopathy in a pediatric patient with COVID-19. Radiology. 2020;297:E274–E275. doi: 10.1148/radiol.2020202197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schupper AJ, Yaeger KA, Morgenstern PF. Neurological manifestations of pediatric multi-system inflammatory syndrome potentially associated with COVID-19. Childs Nerv Syst. 2020;36:1579–1580. doi: 10.1007/s00381-020-04755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abel D, Shen MY, Abid Z. Encephalopathy and bilateral thalamic lesions in a child with MIS-C associated with COVID-19. Neurology. 2020;95:745–748. doi: 10.1212/WNL.0000000000010652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanderschueren G, Schotsmans K, Maréchal E, Crols R. Mild encephalitis with reversible splenial (MERS) lesion syndrome due to influenza B virus. Pract Neurol. 2018;18:391–392. doi: 10.1136/practneurol-2018-001880. [DOI] [PubMed] [Google Scholar]
- 37.Feraco P, Porretti G, Marchiò G, Bellizzi M, Recla M. Mild encephalitis/encephalopathy with reversible splenial lesion (MERS) due to cytomegalovirus: case report and review of the literature. Neuropediatrics. 2018;49:68–71. doi: 10.1055/s-0037-1608779. [DOI] [PubMed] [Google Scholar]
- 38.Starkey J, Kobayashi N, Numaguchi Y, Moritani T. Cytotoxic lesions of the corpus callosum that show restricted diffusion: mechanisms, causes, and manifestations. Radiographics. 2017;37:562–576. doi: 10.1148/rg.2017160085. [DOI] [PubMed] [Google Scholar]
- 39.Mehan WA, Yoon BC, Lang M, Li MD, Rincon S, Buch K. Paraspinal myositis in patients with COVID-19 Infection. AJNR Am J Neuroradiol. 2020;41:1949–1952. doi: 10.3174/ajnr.A6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Neuroimaging manifestations in children with SARS-CoV-2 infection
Data Availability Statement
The authors and PECOBIG collaboration are committed to open science. The broader imaging review data and associated clinical data from this study will be made available, wherever possible, within the terms of participant consent and when not otherwise restricted by intellectual property rights or ongoing collaborative research. To preserve individual case anonymity, detailed data are not given in this Article or the appendix, but will be made available on appropriate request to the corresponding author.