Abstract
Motivated behaviors are controlled by the mesocorticolimbic dopamine (DA) system, consisting of projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) and prefrontal cortex (PFC), with input from structures including the medial preoptic area (mPOA). Sex differences are present in this circuit, and gonadal hormones (e.g., estradiol and testosterone) are important for regulating DA transmission. Early life stress (ELS) also regulates the mesocorticolimbic DA system. ELS modifies motivated behaviors and the underlying DA circuitry, increasing risk for disorders such as substance use disorder, major depression, and schizophrenia. ELS has been shown to change gonadal hormone signaling in both sexes. Thus, one way that ELS could impact mesocorticolimbic DA is by altering the efficacy of gonadal hormones. This review provides evidence for this idea by integrating the gonadal hormone, motivation, and ELS literature to argue that ELS alters gonadal hormone signaling to impact motivated behavior. We also discuss the importance of these effects in the context of understanding risk and treatments for psychiatric disorders in men and women.
Keywords: early life stress, estradiol, testosterone, sex differences, dopamine, mesocorticolimbic, substance use disorder, depression, schizophrenia, motivated behaviors
Introduction
Motivation is crucial for survival. It encompasses the processes that allow an individual to regulate its interactions with stimuli in the environment; for example, directing organisms toward stimuli that are rewarding and away from those that are aversive, as well as activating behaviors to seek or avoid different stimuli (Salamone et al. 2016). Eating, reproducing, and providing parental care can all be considered motivated behaviors as they direct an individual toward a specific stimulus (e.g., food or a conspecific) and animals will demonstrate a high level of speed, vigor, or persistence to initiate or maintain these behaviors (Salamone et al. 2016). This can be contrasted with reflexive behaviors, for example, which do not require a high level of behavioral activation. Expression of these behaviors depends on the appropriate function of the neural reward system, including the mesocorticolimbic pathway, which consists primarily of dopaminergic neurons that project from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) and prefrontal cortex (PFC) (Kelley and Berridge 2002, Salamone et al. 2016). The mesocorticolimbic pathway does not work in isolation and is highly influenced by hypothalamic and limbic regions involved in social processing (O’Connell and Hofmann 2011). In addition to dopamine (DA), other signaling molecules, such as endogenous opioids, are important for motivated behavior (Spanagel, Herz and Shippenberg 1992, Devine et al. 1993). Dysregulation of any of these interacting networks and the signaling molecules involved can therefore disrupt motivation for natural rewards and contribute to pathology. Either an excess or a lack of motivation in different contexts can impair an individual’s quality of life and ability to survive and is therefore thought to be maladaptive. As an example, continued seeking of a certain stimulus, such as a drug of abuse, despite negative consequences (e.g., overdose, loss of job, loss of relationships) is often characterized as maladaptive. On the other end of the spectrum, a lack of motivation for life-sustaining behaviors, such as eating or socializing, is also considered maladaptive. These types of dysregulated motivated behavior are linked to the etiology and symptomatology of psychiatric disorders, such as substance use disorder (SUD), major depressive disorder (MDD), and schizophrenia (Dailly et al. 2004, Nestler 1993, Laruelle et al. 1999, Seeman and Seeman 2014).
The link between maladaptive motivated behaviors and psychiatric disorders has prompted much research into factors that contribute to disruptions in reward circuitry. One such factor is early life stress (ELS). For the purposes of this review, ELS is broadly defined as any adverse experiences spanning from prenatal development through the onset of puberty and ranging from mild to severe. In humans, this can take a wide variety of forms including poverty, abuse, neglect, and more. Clinical studies have repeatedly shown that ELS increases risk for motivation-related disorders, including SUD, MDD, and schizophrenia (Enoch 2011, Jumper 1995, Molnar, Buka and Kessler 2001). Much research has focused on how this type of stress directly or indirectly via epigenetic processes affects brain development and there is evidence that it can alter the maturation of brain regions, including those involved in motivated behavior (Bath, Manzano-Nieves and Goodwill 2016, Manzano Nieves et al. 2019, Goodwill et al. 2018, Reincke and Hanganu-Opatz 2017, Honeycutt et al. 2019). However, ELS can also affect gonadal hormones that regulate brain function. This review will argue that an important mechanism by which ELS can alter motivated behavior is via regulating gonadal hormones. Support for this idea will come from integrating data demonstrating that gonadal hormones regulate motivated behavior, with data revealing how ELS affects motivated behavior. Given that gonadal hormones occur at different concentrations in males and females, this review will also highlight sex differences in hormonal mechanisms by which ELS can impact motivated behavior.
Gonadal Hormones and Motivated Behavior
Sexual differentiation of the brain
Sexual differentiation of both the male and female brain is dependent on gonadal hormone signaling, as well as chromosomal sex and environmental factors (McCarthy and Arnold 2011). While emerging data is highlighting the important role of sex chromosome effects in contributing to sex differences in behavior (Arnold, Chen and Itoh 2012, Arnold and Chen 2009), here we focus on gonadal hormonals, as this review argues that these hormones mediate some effects of ELS on motivated behavior. There are several periods in development where gonadal hormones are particularly influential on the brain. The perinatal period represents a sensitive window during which testosterone (through its conversion to estradiol in rodents) triggers masculinization and defeminization processes in the male-typical brain (Amateau and McCarthy 2004, Wright, Burks and McCarthy 2008). Early estradiol is also critical for feminization processes in the female-typical brain (Bakker et al. 2002, Bakker and Brock 2010, Brock, Baum and Bakker 2011). These processes lead to permanent, or organizational, effects on brain development and function. The organization by gonadal hormones, in turn, determines the activational effects of sex hormones on sex-specific behaviors later in life (Lenz et al. 2011). For example, in adult female mammals, circulating ovarian hormones can enact transient effects on the female-organized brain to activate sex-specific adult behavioral responses, such as lordosis in rodents (Kow and Pfaff 1975). Most adult males, on the other hand, will not display lordosis behavior even when primed with the appropriate ovarian hormones (Yamanouchi and Arai 1976). Thus, although both male and female adults have detectable levels of both estrogens and androgens, these hormones do not have the same activational effects in males and females because of the organizational sex differences already present in the brain by adulthood (Sisk and Zehr 2005). The levels of androgens and estrogens available both during development to exert organizational effects, as well as during adulthood to exert activational effects are critical components of male-typical and female-typical brain development and behavior.
Most studies investigating the effects of gonadal hormones on regions involved in motivated behavior have explored the effect of circulating levels of these hormones in adult animals on the mesolimbic portion of the mesocorticolimbic dopaminergic pathway, which involves the release of DA in the NAc (Steinberg et al. 2014). There is also evidence for organizational and activational effects of testosterone and estradiol in other brain regions involved in motivational behaviors and reward processing, including the PFC and the medial preoptic area (mPOA). An in-depth review of the roles of estradiol and testosterone in mediating motivational brain circuitry has been covered elsewhere (Becker and Chartoff 2019, Becker 1999, Becker 2016, Becker and Hu 2008), so here we overview key findings.
Clinical data on estrogens and disorders characterized by changes in motivated behavior
SUD, MDD, and schizophrenia are all characterized by changes in motivated behavior and regulated by gonadal hormones. In general, high levels of estrogens are linked to the dysregulated increase in motivated behavior found in SUD (Bobzean, DeNobrega and Perrotti 2014). In contrast, low levels or dropping estrogen levels are associated with the amotivation that characterizes some patients with depression and schizophrenia (Young et al. 2000, Seeman 1996). Understanding the nuances of how estrogens affect these disorders is critical to elucidating their etiology and finding better treatments.
Although historically men have suffered from SUD at higher rates than women, this sex difference is likely cultural and as more women have the opportunity to access drugs of abuse this disparity is getting smaller (Van Etten and Anthony 2001, Etten, Neumark and Anthony 1999, Becker and Hu 2008). In fact, when women do access some drugs, including MDMA and nicotine, they are more likely to report subjective drugs effects and they escalate their intake more quickly than men (Liechti, Gamma and Vollenweider 2001, Sofuoglu and Mooney 2009), although men have reported greater subjective effects of cocaine than women (Lukas et al. 1996, Sofuoglu et al. 1999). Women also take drugs for different reasons than men, and stress triggers more use in women (Hudson and Stamp 2011). Some of these sex differences may be driven by the higher levels of estrogens in women. For example, estradiol increases the subjective drug effects of amphetamine. Women in the luteal phase of the menstrual cycle, which is characterized by relatively high levels of estrogens, report “feeling” amphetamine more than women in the follicular phase of the menstrual cycle, which is characterized by relatively low levels of estrogens (Justice and de Wit 1999). The same researchers also reported that subjective amphetamine effects were greater during the late follicular phase, when estrogen levels are rising, than the early follicular phase, when estrogen levels are low (Justice and De Wit 2000). To our knowledge, no studies have been conducted to directly investigate the role of estrogens in the escalation of drug taking in humans, which is an important gap in the literature. However, women compared to men exhibit a shorter latency from the onset of drug taking to seeking treatment for abuse of alcohol (Randall et al. 1999) and heroin (Hser, Anglin and Booth 1987). Given that women have higher levels of ovarian hormones, this finding suggests that these hormones potentiate escalation to problematic drug use.
In contrast to SUD, decreased motivation is observed in MDD and schizophrenia. Reduced estrogens may contribute to this amotivation. The sex difference in MDD emerges during puberty (Angold and Worthman 1993, Cyranowski et al. 2000), which could suggest that higher levels of estrogens are a risk factor for women with MDD. However, it seems instead that a portion of women after puberty become sensitive to dropping estradiol levels and this sensitivity contributes to conditions, such as premenstrual dysphoric disorder, postpartum depression, and depressive symptoms in some perimenopausal women (Payne 2003). For example, depressive symptoms increase during the perimenopausal window, when estrogen levels begin to drop (Freeman et al. 2004), and estrogen treatment seems to improve mood in the peri- and postmenopausal periods (Grigoriadis 2002, Schmidt et al. 2015). Similarly, estradiol treatment alleviates depressive symptoms in postpartum women (Ahokas et al. 2001, Gregoire et al. 1996), however its widespread use as a treatment is limited because it antagonizes lactation (Kelsey 1996).
While in MDD dropping levels of estrogens precipitate symptoms in some women, in schizophrenia the higher levels of estrogens in women relative to men may confer resilience. Women tend to have a later schizophrenia onset than men and clinical symptoms worsen during phases of the menstrual cycle when estrogen levels are low (Grigoriadis and Seeman 2002). The idea of estrogens having protective effects against schizophrenia is further supported by a study showing that co-administration of antipsychotics and estrogen treatment reduced symptoms more than antipsychotic treatment alone in female patients (Akhondzadeh et al. 2003). Variations in the ESR1 gene, which codes for ERα, have also been associated with schizophrenia risk in women (Miranda-Angulo et al. 2008).
In sum, MDD and schizophrenia are very complex disorders and there are many contributing factors. Collectively though, these studies suggest that low or dropping estrogens may increase symptoms. One possibility is that they do so by decreasing motivation. More studies are needed to directly test this hypothesis. Also, lacking are neuroimaging studies linking the symptom-reducing effect of estradiol treatment to changes in the mesocorticolimbic system. Given the inadequate treatments for MDD and schizophrenia, more studies in patient populations exploring whether estradiol alleviates amotivation and alters reward circuitry are warranted. That said, as reviewed below, much preclinical work supports the idea that estrogens alter the function of the mesocorticolimbic system.
Clinical data on androgens and disorders characterized by changes in motivated behavior
Another gonadal hormone associated with symptoms of SUD, MDD, and schizophrenia is testosterone. In fact, high levels of testosterone are linked to SUD. For example, young men with high testosterone consume more alcohol, engage in more binge drinking, are more frequently intoxicated, and have a greater incidence of alcohol dependence than men with low testosterone levels (Eriksson et al. 2005, La Grange et al. 1995). Anabolic androgenic steroids (AAS) are synthetic derivatives of testosterone and their use is associated with SUD. Specifically, AAS users are at higher risk for opioid and alcohol abuse than non-users (Arvary and Pope 2000, DuRant et al. 1993, Wines Jr et al. 1999). The role of testosterone in potentiating SUD is consistent with testosterone-increased responsitivy to reward in healthy subjects. Interestingly, women admistered testosterone increase risk taking (van Honk et al. 2004) and show activation of the ventral striatium during reward anticipation (Hermans et al. 2010) compared to controls. Together, these data suggest that testosterone can enhance reward process, which may contribute to SUD.
Lower levels of testosterone have also been linked to MDD. A meta-analysis of studies of testosterone therapy showed that testosterone administration improved Hamilton ratings of depression in men across seven studies (Zarrouf et al. 2009). Testosterone may be especially effective in treating depressive symptoms in aging men (Carnahan and Perry 2004), as there is evidence that lower levels of testosterone is associated with increased risk for depression in older men in particular (Almeida et al. 2008, Seidman and Walsh 1999). Interestingly, the protective effect of testosterone on depression may only be true for men, because depressed women have elevated testosterone levels compared to controls (Baischer et al. 1995). More research is needed to elucidate the role of testosterone and sex differences therein in MDD.
Reduced testosterone levels have also been linked to schizophrenia symptoms. Free and plasma testosterone levels are lower in male schizophrenia patients than in controls (Akhondzadeh et al. 2006). Several lines of evidence suggest that lower testosterone may drive negative symptoms (e.g., flattened affect) rather than positive symptoms (e.g., hallucinations). For example, testosterone levels are significantly lower in male patients with predominantly negative symptoms compared to healthy controls, but no such effect was found for patients with predominantly positive symptoms (Goyal et al. 2004). There is also a negative correlation between testosterone levels and the severity of negative symptoms (Ko et al. 2007). Additionally, testosterone levels also predict cognitive performance in men with schizophrenia, but not in healthy men (Moore et al. 2013).
In sum, alterations in testosterone are associated with psychiatric disorders. High testosterone contributes to SUD, while low testosterone is linked to MDD and schizophrenia symptoms. It is noteworthy that these effects parallel the effects of estrogens in these disorders, and testosterone can be converted to estradiol via aromatase. Given the challenges of dissociating the effects of testosterone from estradiol in humans, it is possible that mechanistically changes in testosterone may affect brain circuits via their conversion to estradiol. Future studies are needed to assess this possibility.
Preclinical data on estrogens and the mesocorticolimbic DA system
Preclinically, the role of estrogens in motivated behavior has been studied most in animal models of SUD. Interest in estrogenic effects was initially sparked by studies revealing sex differences in drug taking behavior. Recall that clinically, women more rapidly progress from the onset of drug taking to seeking treatment (Randall et al. 1999, Hser, Anglin and Booth 1987), which suggests they escalate drug taking more quickly than men. Preclinical studies in rodents are consistent with this finding and reveal that females acquire drug self-administration training procedures faster than males (Hu et al. 2003, Lynch 2006, Roth and Carroll 2004). Compared to male rats, female rats also show more rapid escalation of drug taking when given prolonged access to drugs (Reichel et al. 2012), greater motivation to seek drugs (Cummings et al. 2011, Westenbroek, Perry and Becker 2013), and more relapse-like behaviors (Hudson and Stamp 2011). There is evidence that some of these sex differences are driven by circulating estrogen levels, because animals exhibit faster acquisition of drug self-administration procedures following estradiol administration (Hu et al. 2003, Jackson, Robinson and Becker 2005, Lynch et al. 2001, Perry, Westenbroek and Becker 2013, Roth, Casimir and Carroll 2002). Additionally, female mice exhibit greater conditioned place preference to cocaine during proestrus/estrus, when circulating estradiol levels are high, suggesting that estradiol enhances cocaine reward in females (Calipari et al. 2017).
The link between ovarian hormones and drug self-administration suggests a role for estrogenic regulation of reward circuits. Such regulation is possible because estrogen receptors (ERs) are present throughout the mesocorticolimbic DA system (Creutz and Kritzer 2002, Creutz and Kritzer 2004). For example, the VTA, which is the major source of DA mesocorticolimbic system, is regulated by estradiol. Ovariectomy (the surgical removal of the ovaries) in mice and rats reduces the number of neurons in the VTA that are immunopositive for tyrosine hydroxylase (TH), the rate-limiting enzyme of DA synthesis (Johnson et al. 2010). Hormone replacement in ovariectomized females with 17β-estradiol, the selective ERα agonist propyl-pyrazole-triol (PPT), or the selective ERβ agonist diarylpropionitrile (DPN) attenuates the effects of ovariectomy (Johnson et al. 2010), suggesting that both types of receptors play a role in controlling the population of dopaminergic neurons in the VTA. It is worth noting that ER agonists such as DPN have relatively low selectivity and can bind to both ERα and ERβ at high doses (Carroll et al. 2012). However, complementary work in ER knockout mouse models further support the roles that ERs play in modulating VTA DA. ERα knockout mice that have lacked the receptor throughout development show diminished numbers of TH-positive neurons in the VTA, while ERβ knockout mice show cell counts comparable to control animals (Johnson et al. 2010), suggesting that ERα is more crucial for this effect than ERβ. Collectively, these data indicate that estradiol via ERα activation increases TH, an effect that would enhance the capacity of VTA neurons to synthesize DA.
In addition to increasing the number of TH positive neurons in the VTA, estradiol also alters their physiology. Although basal DA firing in the VTA is highest during estrus (relatively low estrogens) and lowest during proestrus (relatively high estrogens) (Zhang et al. 2008), rats with high estradiol levels show increased sensitivity of VTA DA neurons to both the excitatory effects of ethanol and the inhibitory effects of administered DA acting locally on autoreceptors in the VTA (Vandegrift et al. 2017). These findings suggest that estradiol can enhance both excitatory and inhibitory effects on DA transmission. Ovariectomized rats, on the other hand, show no VTA fMRI response to amphetamine, an effect that was rescued by administration of either selective ERα (16α-lactone-estradiol) or ERβ (DPN) agonists (Sárvári et al. 2014). Overall, these findings point to a key role for estradiol in modulating the effects of drugs on the physiological action of VTA DA neurons.
One target of VTA DA release is the NAc. Therefore, regulation of the VTA by estradiol can impact DA release in this brain area. Consistent with this idea, circulating estradiol in adulthood triggers DA release in the NAc (Alderson and Baum 1981, Becker 1999, Di Paolo, Rouillard and Bédard 1985, Hernandez et al. 1994, Lammers et al. 1999, Landry, Lévesque and Di Paolo 2002). In fact, DA release in the NAc fluctuates throughout the estrous cycle, with stimulated DA release increasing during phases characterized by high levels of estrogens (Thompson and Moss 1997). Extracellular DA in the dorsolateral striatum is higher in female rats in cycle stages with high estradiol than ovariectomized females and males (Xiao and Becker 1994). Increased DA in the NAc can increase reward sensitivity (Drevets et al. 2001, Volkow et al. 1996, Volkow et al. 1999), so it may not be surprising that estradiol alone in the NAc produces conditioned place preference (Walf et al. 2006). There is evidence that the reward-enhancing effects of estrogens in the NAc are exerted particularly through ERβ rather than ERα signaling. For instance, in female mice, estradiol and the ERβ agonist DPN both enhanced cocaine-induced place preference and induced cFOS expression in the NAc, whereas the ERα agonist PPT had neither effect (Satta et al. 2018). Similarly, estradiol and DPN, but not PPT, increase cocaine-induced DA release in the NAc (Yoest, Cummings and Becker 2019). Furthermore, RNA interference-mediated knockdown of ERβ, but not ERα, impaired cocaine-induced place preference in female mice (Satta et al. 2018). These studies point to a specific role for ERβ in mediating the effects of estradiol on DA action in the NAc of female rodents.
As noted, estradiol can affect DA release in the NAc via direct regulation of DA neurons in the VTA. However, estradiol can also regulate DA transmission in the NAc via the modulation of opioid function. Endogenous opioids and their receptors (μ, δ, and κ) are present throughout the mesolimbic DA system (Dilts and Kalivas 1989, Unterwald et al. 1989, Dilts and Kalivas 1990, Mansour et al. 1987). Activation of μ and δ opioid receptors in this circuit can increase DA release, whereas activation of κ opioid receptors reduces DA release (Chartoff et al. 2016, Spanagel, Herz and Shippenberg 1990, Spanagel, Herz and Shippenberg 1992, Ebner et al. 2010). There is not much research on the role of estradiol in regulating opioid receptors in the VTA. However, a recent paper found that a supraphysiological dose of estradiol valerate, known to produce a β-endorphin neuronal deficit, reduced δ opioid receptors in the VTA (Molina-Martínez and Juárez 2020). Yet, how physiological levels of estrogens or the estrous cycle impact opioid receptor expression or function in the VTA is unknown. More research has been conducted in the NAc. Ovariectomy decreases mRNA levels of the endogenous opioid preproenkephalin in the core and shell of the NAc, an effect that was prevented by treatment with estradiol, the ERα agonist PPT, and the ERβ agonist DPN (Le Saux and Di Paolo 2005). Estradiol treatment also increases μ opioid receptors in the NAc (Di Chiara and Imperato 1988, Le Saux and Di Paolo 2005). In contrast, estradiol blunts κ opioid receptor signaling in the NAc (Abraham et al. 2018). A combined estradiol-induced increase in μ opioid receptor signaling and suppression of κ opioid receptor signaling would increase DA transmission in the NAc, providing another mechanism by which estrogens can potentiate DA function.
Motivated behavior is also regulated through DA reuptake, the process by which DA is taken via DA transporters (DAT) into the presynaptic neuron for degradation and resynthesis. Blocking DATs pharmacologically causes DA to stay longer in the synapse and is linked to increased motivation. For example, administration of the DAT inhibitor GBR12909 heightened food reward seeking in a progressive ratio breakpoint task in mice (Young and Geyer 2010, Milienne-Petiot et al. 2017b), as well as increased motivation for rewarding intracranial self-stimulation (Esumi et al. 2013). Similarly, genetic knockdown of DAT in mice results in a heightened progressive ratio breakpoint for seeking a food reward (Cagniard et al. 2006, Milienne-Petiot et al. 2017a). There is evidence that estradiol can regulate DA reuptake, but the direction of the effects on reuptake vary. One study found that 48 hours of estradiol treatment in ovariectomized rats decreased the rate of DA uptake and increased DA clearance time following an injection of DA into the NAc, an effect that would allow for more DA action in the synapse for a longer period (Thompson 1999). In contrast, DA reuptake in the broadly dissected striatum was shown to peak during proestrus in naturally cycling rats, when estradiol levels are highest (Morissette and Di Paolo 1993, Morissette and Paolo 1993), which could be a mechanism designed to compensate for excess DA release, as DA levels in the striatum also peaked during proestrus (Morissette and Di Paolo 1993). Similarly, other studies have demonstrated that estradiol treatment of ovariectomized rats increases binding density at DA uptake sites in the striatum, which would suggest a faster uptake of DA from the synapse (Morissette, Biron and Di Paolo 1990), however, this appears to be true only for nigrostriatal sites, such as the substantia nigra pars compacta, and not true for the NAc (Morissette and Paolo 1993). While the exact mechanism of estradiol effects on DA reuptake in the NAc remains unclear, one possibility is that estrogens directly impact DAT expression. Treatment of ovariectomized females with the selective ERα agonist, 16α-lactone-estradiol, increased DAT mRNA in VTA neurons (Sárvári et al. 2014). However, this effect is not observed following estradiol or DPN treatment. Collectively, these studies suggest that changing levels of estradiol do impact the rate of DA uptake and the density of DA binding sites in certain dopaminergic nuclei. Further research on the effects of estradiol on DA reuptake in specific nuclei of the striatum and the mechanisms that regulate uptake are warranted.
DA signals through two families of receptors: D1-like (which includes D1 and D5 receptors) and D2-like (which includes D2, D3, and D4 receptors). These receptors generally have opposite effects at the cellular level, with D1-like activation stimulating and D2-like activation inhibiting postsynaptic adenylyl cyclase activity (Sibley and Monsma 1992, Hopf et al. 2003, Gingrich and Caron 1993). Additionally, D2-like receptor activation has been linked to increased K+ channel activity and decreased Ca2+ channel activity, effects that can contribute to postsynaptic inhibition, and these types of receptors can also be located presynaptically as inhibitory autoreceptors (Dominguez and Hull 2005, Sibley and Monsma 1992, Gingrich and Caron 1993). Estradiol treatment can increase striatal D1 receptor density in both male (Hruska and Nowak 1988) and ovariectomized female rats (Lévesque and Di Paolo 1989). Similalry, estradiol treatment increases striatal D2 receptor density in male (Hruska, Ludmer and Silbergeld 1980) and female rats (Di Paolo, Poyet and Labrie 1982), including specifically the NAc (Di Paolo et al. 1979) in females. In female rats, estradiol and ERβ agonist (DPN) treatment, but not ERα agonist (PPT) treatment, also prevented an ovariectomy-induced decrease in D2 binding in the NAc core (Le Saux, Morissette and Di Paolo 2006). In many cases, a simultaneous increase in D1 and D2 receptors may not alter the net effect of DA on NAc function as these receptors generally oppose each other in their effects. However, the two receptors have been shown to work together for certain outcomes, in a model known as D1/D2 receptor synergism (Gershanik, Heikkila and Duvoisin 1983, Dziedzicka-Wasylewska 2004). Concurrent activation of D1 and D2 receptors is necessary for DA-mediated stereotyped behaviors (White et al. 1988) and simultaneous D1 and D2 receptor activation in the NAc has rewarding effects in rats (Ikemoto et al. 1997). This synergism between D1 and D2 receptors in the NAc suggests that estradiol-induced increases in D1 and D2 receptor expression may be able to enhance reward processing, despite their apparent opposite actions. On the other hand, ovariectomized female rats show increased D2 receptor expression in the NAc (Gordon and Fields 1989). This may lead to an imbalance in D1 and D2 receptors which could cause hyper- or hypo-activity of the mesolimbic reward system. Furthermore, estradiol treatment of ovariectomized rats has been shown to increase both D1 and D2 receptor density in striatum in response to irreversible peripheral DA receptor blockade (Lévesque and Di Paolo 1991). These studies point to an important role for estradiol not only in potentiating DA signaling in the mesolimbic system, but also in regulation and maintenance of the appropriate balance of D1 to D2 receptor levels.
A focus of the field has been on estrogenic regulation of DA in the NAc, however, DA release into other brain regions is also regulated by estrogens. Specifically, dopaminergic projections from the VTA also regulate the PFC to mediate cognitive control (Floresco and Magyar 2006, Cools 2008) and estrogens can also increase mesocortical DA release (Aubele and Kritzer 2010). Female rats have a higher proportion of PFC-projecting DA neurons in the VTA than males at baseline (Kritzer and Creutz 2008). Estradiol administration increased spinophilin immunoreactivity (suggesting greater spine density) in PFC of nonhuman primates and rats (Khan et al. 2013, Lasley et al. 2004) and increased dendritic spine density in the PFC of aged ovariectomized females (Bailey et al. 2011, Khan et al. 2013, Velázquez-Zamora, Garcia-Segura and González-Burgos 2012). When synapses are specifically assessed, estradiol treatment increases their number in the PFC of rats (Chisholm and Juraska 2012) and restores multisynaptic bouton levels in the aged female primate PFC (Hara et al. 2016). Collectively, this estradiol-induced plasticity could make the PFC more responsive to DA afferents. Moreover, estradiol alters DA function in the PFC. Specifically, estradiol-treated female rats show higher levels of DA and its metabolites in the PFC compared to ovariectomized controls (Sárvári et al. 2014). Additionally, estradiol-treated animals showed an upregulation of DA receptors in the PFC and a more robust blood-oxygen-level dependent signal (BOLD) response to amphetamine in the PFC (Sárvári et al. 2014). Correspondingly, ovariectomy significantly lowers spine density in the PFC (Wallace et al. 2006) and increases the density of DA β-hydroxylase axons in the dorsolateral PFC, suggesting higher rates of DA conversion to norepinephrine (Kritzer and Kohama 1999). Together, these findings demonstrate an enhancing effect of estradiol on DA transmission in the PFC at a variety of endpoints.
The effects of estrogens on the mesocorticolimbic system are summarized in Table 1. When considered together, there is evidence for estrogenic regulation of the mesocorticolimbic system at every part of the circuit. Thus, environmental insults, such as ELS, that can alter estrogen levels would be expected to alter motivated behavior.
Table 1.
Effects of estrogens in the mesocorticolimbic dopamine system.
| Brain Region | Effects of Estrogens | Species | Sex | Citat |
|---|---|---|---|---|
| VTA | increased number of TH- positive cells following ovariectomy | rat | female | Johnson et al. 2010 |
| enhanced fMRI response to amphetamine | rat | female | Sarvari et al. 2014 | |
| increased DAT | rat | female | Sarvari et al. 2014 | |
| NAc | restored DA levels after castration | rat | male | Alderson and Baum 1981 |
| increased DA turnover | rat | female | Di Paolo, Rouillard and Bédard 1985 | |
| induced c-fos expression | mouse | female | Satta et al. 2018 | |
| enhanced cocaine-induced place preference | mouse | female | Satta et al. 2018 | |
| decreased DA uptake rate | rat | female | Thompson 1999 | |
| increased DA clearance time | rat | female | Thompson 1999 | |
| increased D1 receptor density | rat | male | Hruska and Nowak 1988 | |
| increased D1 receptor density | rat | female | Levesque and Di Paolo 1989 | |
| increased D2 receptor density | rat | male | Hruska, Ludmer and Silbergeld 1980 | |
| increased D2 receptor density | rat | male and female | Di Paolo, Poyet and Labrie 1982 | |
| increased D2 receptor density | rat | female | Di Paolo et al. 1979 | |
| increased preproenkephalin expression | rat | female | Le Saux and Di Paolo 2005 | |
| PFC | increased spinophilin expression and number of dendritic spines | rat | female | Khan et al. 2013 |
| increased number of spines | rhesus monkey | female | Lasley et al. 2004 | |
| increased spine density | rhesus monkey | female | Bailey et al. 2011 | |
| increased number of synaptophysin-positive boutons | rat | female | Chisholm and Juraska 2012 | |
| increased multisynaptic bouton levels | rhesus monkey | female | Hara et al. 2016 | |
| increased levels of DA and its metabolites | rat | female | Sarvari et al 2014 | |
| upregulated DA receptors | rat | female | Sarvari et al. 2014 | |
Preclinical data on androgens and the mesocorticolimbic DA system
As noted, testosterone can be converted to estradiol and estrogens can regulate the mesocorticolimbic system. However, testosterone is also poised to directly regulate structures in the mesocorticolimbic system because there are androgen receptors (AR) present in the VTA (Creutz and Kritzer 2002, Creutz and Kritzer 2004). Testosterone has organizational effects of dopaminergic neurons in the VTA. Specifically, male sheep have more TH-positive cells in the VTA than females, and prenatal testosterone exposure increased TH-positive cell numbers in females (Brown et al. 2015). Furthermore, TH-positive cells in males and testosterone-treated females showed greater density of staining, suggesting increased levels of TH within the neurons (Brown et al. 2015). Testosterone can also have activational effects on the VTA. Central administration of testosterone increased Fos expression in the VTA of the male Syrian hamster without altering AR or ER expression in the brain (DiMeo and Wood 2006). There is one study that reported that DA levels in the VTA of male rats did not change after castration (Mitchell and Stewart 1989). However, there is evidence that testosterone is synthesized locally within the corticolimbic system, as gonadectomy does not eliminate testosterone from the mesocorticolimbic system (Tobiansky 2018), suggesting testosterone plays a crucial role in the normal regulation of this system.
As male rats age and testosterone levels drop (Coquelin and Desjardins 1982), DA metabolism in the VTA also decreases (Goudsmit, Feenstra and Swaab 1990). The AR antagonist flutamide administered prenatally during late gestation decreased production of MAP2, a protein found primarily in dendrites, in the VTA and PFC at both prepubertal and adult timepoints, suggesting a reduction in dendritic arborization (Pallarés et al. 2014). Animals treated with flutamide also exhibited a reduction in the number of TH-positive cells in the VTA at a prepubertal time point, but reached normal cell numbers by adulthood (Pallarés et al. 2014), suggesting testosterone is especially important in mesocorticolimbic transmission during adolescence. Blocking conversion of testosterone into dihydrotestosterone (DHT), a non-aromatizable androgen, by finasteride reduced TH expression in the VTA in adolescent male rats (Li et al. 2018). Together, these findings show that testosterone plays an important role in the modulation of DA transmission at the level of the VTA.
Androgens can also regulate opioids in the mesocorticolimbic system. β-endorphin binds to μ opioid receptors in the VTA to trigger DA release in the NAc (Spanagel et al. 1991). VTA levels of β endorphins are increased after exposure to drugs of abuse, including alcohol (Jarjour, Bai and Gianoulakis 2009) and cannabis (Solinas et al. 2004). Similarly, AAS can increase β-endorphin levels in the male rat VTA (Johansson et al. 1997). This effect may explain some of the rewarding effects of AAS and points to another route by which androgen signaling can enhance mesolimbic DA transmission. More work is needed to assess how variations in physiological levels of testosterone affect the opioid system to impact DA transmission.
Testosterone has similar DA-enhancing effects in the NAc. For example, intranasal testosterone was found to increase DA in the NAc of male rats (de Souza Silva et al. 2009) and there is also evidence that intra-NAc testosterone infusion itself has rewarding properties, explaining the addictive properties of AAS (Packard, Cornell and Alexander 1997). This rewarding effect can be blocked by administration of the DA receptor antagonist α-flupenthixol (Packard, Schroeder and Alexander 1998), demonstrating that testosterone induces its rewarding effects in the NAc by leading to activation of mesolimbic DA receptors. Similarly, castration of male rats reduced DA levels in the NAc, while testosterone, estradiol, and DHT all reversed this effect (Alderson and Baum 1981), showing further support for the idea that both androgens and estrogens can enhance NAc DA transmission. In addition to these activational effects of testosterone in the NAc, there are organizational effects of testosterone. Specifically, brain masculinization by neonatal administration of testosterone in female rats decreased NAc DAT expression compared to both controls and estradiol-treated females (Dib et al. 2018). A decrease in DAT expression in the NAc would lead to a longer period of action for DA in the synapse, suggesting that testosterone exposure early in development causes organizational alterations to the brain that enhance the capacity of DA in the NAc to exert its synaptic effects. Together, these studies point to a role for testosterone in potentiating dopaminergic transmission in the mesolimbic reward system.
Another key region in the mesocorticolimbic circuit, the PFC, is regulated by testosterone. Gonadectomy of adult male rats decreased extracellular DA levels in the mPFC compared to intact controls four days after surgery but increased extracellular DA in the long-term (Aubele and Kritzer 2010). Both effects were rescued by testosterone replacement (Aubele and Kritzer 2010). Aged male rats show a reduction in TH fibers in the PFC, an effect that is not mirrored in aged females (Chisholm, Kim and Juraska 2013). Because testosterone can be converted into estrogens via aromatization, it is not always clear whether these effects on mesolimbic DA functioning are due to testosterone signaling or estrogen signaling. However, some studies have demonstrated that DHT can impact DA transmission. For instance, one study showed that DHT treatment of castrated males restored DA turnover in the NAc to normal levels (Yang and Shieh 2007). The effects of androgens on the mesocorticolimbic system are summarized in Table 2. In summary, while both estrogens and testosterone enhance DA function in the mesocorticolimbic system, there is evidence that some effects directly result from testosterone rather than indirectly from its conversion to estrogens.
Table 2.
Effects of androgens in the mesocorticolimbic dopamine system.
| Brain Region | Effects of Androgens | Species | Sex | Citation |
|---|---|---|---|---|
| VTA | organizational increase in TH-positive neurons | sheep | male an female | Brown et al. 2015 |
| greater density of TH staining | sheep | male an female | Brown et al. 2015 | |
| increased FOS expression | Syrian hamster | male | DiMeo and Wood 2006 | |
| enhanced dendritic arborization | rat | male | Pallares et al. 2014 | |
| increased beta endorphin levels | rat | male | Johansson et al. 1997 | |
| NAc | increased DA release | rat | male | de Souza Silva et al. 2009 |
| organizational decrease in DAT expression | rat | female | Dib et al. 2018 | |
| PFC | increased extracellular DA | rat | male | Aubele and Kritzer 2010 |
Preclinical data on gonadal hormone regulation of DA in the mPOA
In addition to acting directly on the mesocorticolimbic pathway, estrogens and androgens can also modulate reward processing for reproductive behaviors by acting on brain areas in the social behavior network that have outputs to the mesocorticolimbic system. The mPOA is critical for male sexual behavior, as it integrates relevant sensory input and, in turn, projects to motor regions critical for the copulation and the mesocorticolimbic system to mediate reward (Simerly and Swanson 1986, Simerly and Swanson 1988). Given its integratory role, the mPOA is important for both the consummatory aspects (i.e., execution) of male sexual behavior, as well as some appetitive (i.e., motivational) aspects of male sexual behavior (Will, Hull and Dominguez 2014, Paredes, Highland and Karam 1993, Dominguez and Hull 2005). Early lesion studies found that ablation of the mPOA reduced or eliminated male sexual behavior in rats (Heimer and Larsson 1967, Paredes and Agmo 1992, Paredes et al. 1993, Paredes, Tzschentke and Nakach 1998, Lupo et al. 1983, Hansen et al. 1982, Paredes and Baum 1995), as well as decreased several measures of sexual motivation, including preference for a receptive female over a non-receptive female (Edwards and Einhorn 1986) and precopulatory pursuit behavior (Paredes et al. 1993). The role of the mPOA in male sexual behavior and motivation is modulated by gonadal hormone action. The sexually dimorphic nucleus of the mPOA is larger in males than in females, and this sex difference is organized estradiol converted from perinatal testosterone surge (Bloch and Gorski 1988, Döhler et al. 1984). However, it is not this specific nucleus, but rather the larger mPOA that is necessary for the full expression of male sexual behavior (Arendash and Gorski 1983). When considering hormone effects on the mPOA more broadly, activational effects of gonadal hormones can regulate reproductive behavior. Implants of either testosterone propionate (Davidson 1966) or estradiol (Christensen and Clemens 1975, Davis and Barfield 1979) into the mPOA of male rats castrated in adulthood restored sexual behavior. Furthermore, blockade of aromatization of testosterone into estradiol in the mPOA prevented testosterone from restoring sexual behavior in castrated male rats, revealing that estradiol (converted from testosterone) drives this behavior (Christensen and Clemens 1975, Clancy, Zumpe and Michael 1995, Vagell and McGinnis 1997). Collectively, these studies demonstrate a critical effect of estradiol in controlling male reproductive behaviors.
DA mediates both the appetitive and consummatory behaviors required for male reproduction, in part via regulation of the mPOA (Will et al. 2014, Dominguez and Hull 2005). DA levels in the mPOA increase in response to a sexual stimulus (e.g., female rat in estrus) and during copulation (Hull et al. 1995, Hull et al. 1993). An increase in DA in the mPOA facilitates motivation, genital reflexes, and motor patterns required for copulation (Dominguez and Hull 2005, Hull et al. 1992, Markowski et al. 1994, Hull et al. 1995). Gonadal hormones regulate DA in the mPOA, because gonadectomy reduces DA release in this region (Du, Lorrain and Hull 1998). Testosterone treatment restores DA in the mPOA and normal copulatory behavior (Putnam et al. 2001). Further studies determined the role of specific testosterone metabolites on mPOA DA release: estradiol maintains basal levels of DA in the mPOA, while DHT is required for DA release caused by a sexual stimulus (Putnam, Sato and Hull 2003). One way by which testosterone can facilitate mPOA DA release is via the upregulation of nitric oxide synthesis (Putnam et al. 2005). However, there is much we do not know about how gonadal hormones regulate DA in the mPOA, because few studies have investigated how gonadal hormones regulate the incertohypothalamic DA system. The incertohypothalamic DA system consists of DA-producing cells in the periventricular hypothalamus (A14 group) and the rostral zona incerta (A13), which release DA into the mPOA (Moore 1995, Björklund, Lindvall and Nobin 1975, Lookingland and Moore 1984). Gonadectomy in male and female rats reduces TH immunoreactivity in the incertohypothalamic DA system, an effect reversed by hormone replacement (Sanghera et al. 1991). The similarities between the effect of gonadal hormones on TH in incertohypothalamic system and mesocorticolimbic system suggest that these hormones affect dopaminergic systems similarly throughout the brain. However, TH is just one aspect of DA function and much more research is needed on the effects of estradiol and testosterone on the hypothalamic DA system.
The source of DA for the mPOA is hypothalamic, but the mPOA projects to the mesocorticolimbic dopaminergic system to further regulate motivated behavior (Hull and Dominguez 2007). Specifically, there is an important afferent from the mPOA to the VTA (Tobiansky et al. 2016, Simerly and Swanson 1988), and there is emerging evidence that this projection can be regulated by gonadal hormones. There is an organizational impact of gonadal hormone signaling on this projection, as neonatal overexpression of ERα in mPOA increases the number of TH-positive neurons in the VTA (Peña and Champagne 2015). In adulthood, projections from the mPOA to the VTA continue to modulate DA signaling, with estrogen-sensitive neurons in the mPOA synapsing onto both GABAergic and dopaminergic neurons in the VTA. Indeed, microinjections of estradiol in the mPOA of adult rats enhances DA release in the NAc in response to cocaine administration (Tobiansky et al. 2016), demonstrating that hormonal signaling can also impact this circuit indirectly via the mPOA.
Collectively these studies reveal gonadal hormone regulation of the DA in the mPOA. A focus of this work is on male reproductive behavior. However, given the role of the mPOA in regulating appetitive aspects of natural reward and its ability to modulate the mesocorticolimbic dopaminergic system, it is possible the gonadal hormone regulation of the mPOA also contributes to aspects of SUD, or other disorders where reward circuitry is dysregulated.
Summary: Gonadal hormones and motivated behavior
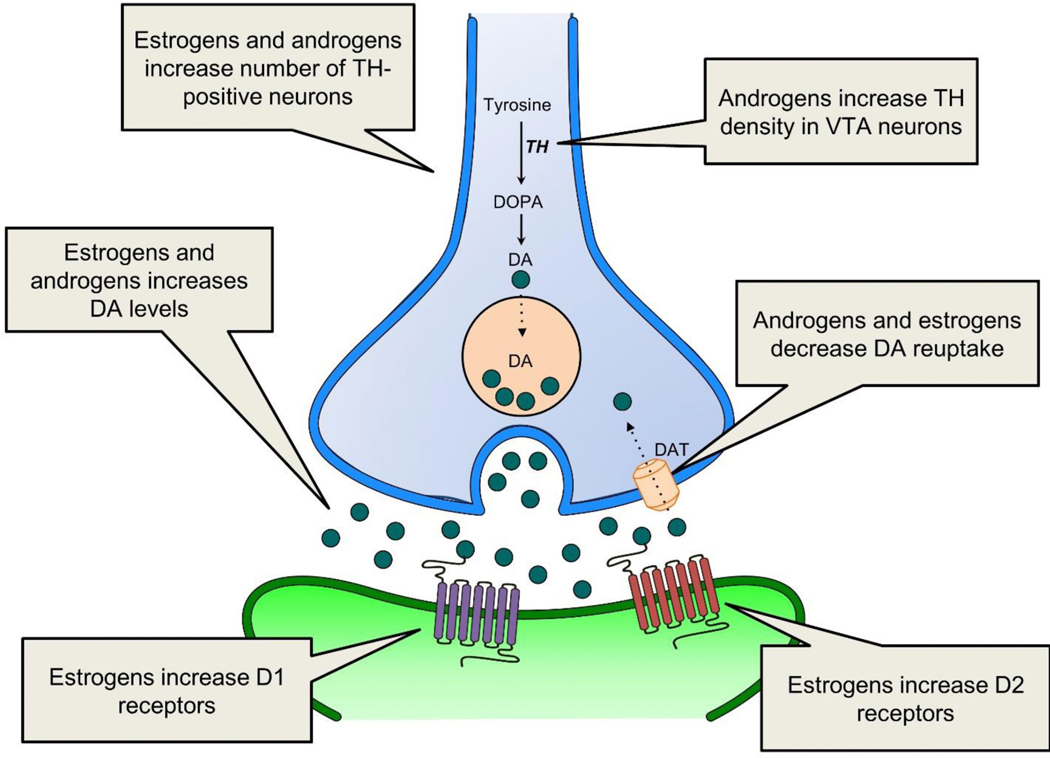
In summary, estradiol and testosterone facilitate DA synthesis and release. Estradiol can also induce structural changes in the mesocorticolimbic system. A schematic depicting the effects of estrogens and androgens at the VTA-NAc synapse is shown in Figure 1. The consequence of these hormonal effects is altered motivated behavior. As noted, ELS can also alter motivated behavior. Next, we will discuss specific ways by which this can happen, with a focus on ELS-induced changes in gonadal hormones as an important mediating factor. Given that ELS is, by definition, early in life, we will discuss evidence suggesting this stress can induce organizational changes. We will also review data indicating that ELS causes lasting alterations in plasma gonadal hormone levels, which could induce activational effects on motivated behavior.
Figure 1.
This schematic shows how estrogens and androgens regulate the mesolimbic DA system at the VTA-NAc synapse. Estrogens enhance DA activity by increasing the number of VTA DA neurons (blue), increasing DA release, decreasing the rate of DA reuptake, and increasing D1 and D2 receptor density at the postsynaptic neuron (green). Androgens can also enhance DA activity by increasing TH expression, increasing DA release and decreasing the expression of DAT, however, some of the effects of androgens may be mediated by aromatization to estrogens. Different forms of ELS can increase or decrease estrogen and androgen levels in males and females, altering gonadal hormone regulation of the mesolimbic DA system. DA, dopamine; TH, tyrosine hydroxylase; DOPA, L-dihydroxyphenylalanine; DAT, dopamine transporter; VTA, ventral tegmental area.
ELS and Motivated Behavior
Typical methods for modeling ELS in rodents
Much of the research exploring the neurobiological mechanisms by which ELS affects reward circuitry comes from rodent studies using rats and mice. In these species, several animal models of ELS, including both prenatal and postnatal stressors, have been developed (Liu et al. 1997, Pryce and Feldon 2003, Surakul, Weerachatyanukul and Chutabhakdikul 2011, Liu et al. 2000, Ivy et al. 2008, Rice et al. 2008). Some studies employ prenatal stressors aimed at stressing pregnant dams before parturition. The most common approach is restraint stress from days 14 through 21 of pregnancy (Ward and Weisz 1984), but other gestational timepoints and stressors (e.g., chronic variable stress, swim stress, corticosterone administration) are also used (Mueller and Bale 2008, Leuner et al. 2014, Brummelte and Galea 2010). A common postnatal stressor is the maternal separation model. In this manipulation, pre-weaning rat or mouse pups are separated from the dam and removed from the home cage for a period of time ranging from 15 min to 24 h per day (Schmidt, Wang and Meijer 2011). Variations of this model that utilize a shorter period of separation result in increased levels of maternal care behaviors, which is associated with higher stress resiliency and lower anxiety in offspring, whereas variations that utilize a longer period of separation induce impaired maternal care behaviors and are used as models of ELS (Nishi et al. 2013). Another common ELS model is the limited bedding and nesting manipulation, which is typically implemented from postnatal days 2 through 9. Dams in the limited resource environment lack accessing bedding and proper nesting materials, which results in fragmented maternal care that stresses the offspring (Ivy et al. 2008, Rice et al. 2008). Given the variability in ELS manipulations and implementation, it is not surprising that differing ELS protocols can result in different outcomes for the offspring. However, there are many similarities in the way ELS manipulations affect motivated behavior and gonadal hormones.
A role for estradiol in mediating the effects of ELS on motivated behavior
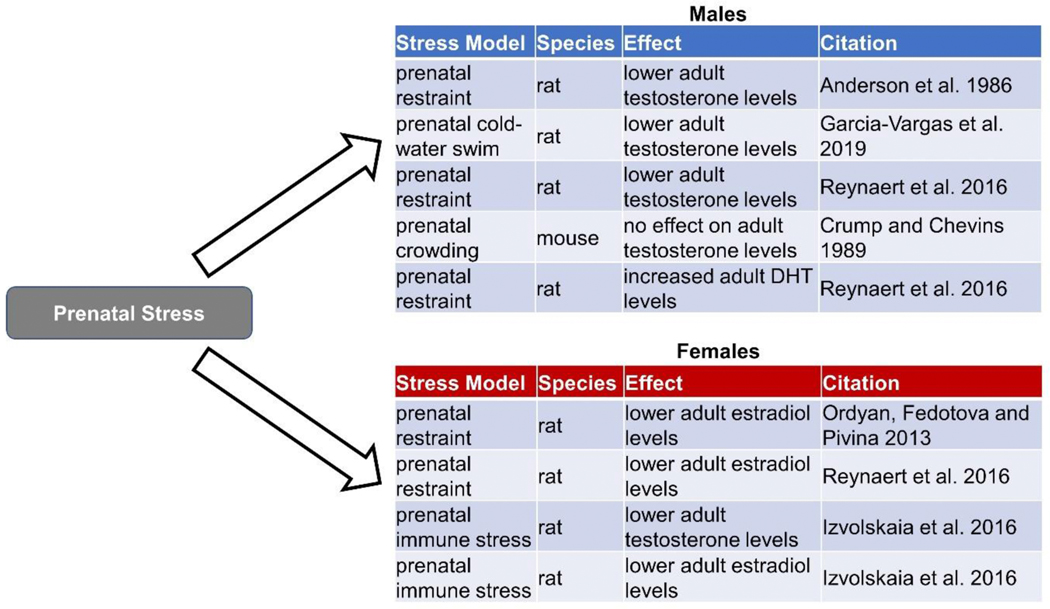
Studies on the effects of ELS on mesocorticolimbic signaling reveal a fairly consistent pattern of downregulation of dopaminergic activity in females. For instance, in the NAc specifically, prenatal restraint stress decreases DA levels in adult female rats (Reynaert et al. 2016) and maternal separation decreased D1 receptor expression in adult female mice (Sasagawa et al. 2017). Further, maternal separation stress also decreased acute stress-induced DA release in the NAc of juvenile (post-weaning but pre-puberty; postnatal day 26–30) male and female rats (McCormick et al. 2002), suggesting DA suppression following ELS. There are several theories as to why this as occurs. One theory is that ELS disturbs the development of the HPA axis, leading to changes in circulating glucocorticoid levels which can impact sex-specific organization of DA circuitry in the early postnatal period (Chocyk et al. 2015, Majcher-Maślanka et al. 2017, Gillies and McArthur 2010, de Kloet et al. 2005). ELS has also been proposed to interfere with the normal maturation of DA circuitry by altering the processes of overproduction and subsequent pruning of dopaminergic synapses (Majcher-Maślanka et al. 2017, Manzano Nieves et al. 2019). Still another theory suggests that ELS may enact its changes in brain function by affecting the timing normal maturational processes in females, with evidence for delayed sexual development (Manzano Nieves et al. 2019), as well as altered maturation of parvalbumin systems in the orbitofrontal cortex (Goodwill et al. 2018). However, one idea that has received less attention is that ELS induces changes in estradiol, which in turn alters DA signaling in the NAc. A summary of the effects of ELS on estradiol levels in males and females can be found in Figures 2 and 3. Recall that estradiol enhances DA signaling in the NAc by multiple mechanisms, including increased DA release via ERβ activation, increased density of D1 and D2 receptors, and increased number of TH-positive neurons in the VTA that project to the NAc (Satta et al. 2018, Le Saux et al. 2006, Lévesque and Di Paolo 1989, Di Paolo et al. 1979, Johnson et al. 2010). Interestingly, both prenatal restraint stress (Ordyan, Fedotova and Pivina 2013, Reynaert et al. 2016) and prenatal exposure to lipopolysaccharide-induced immunological stress (Izvolskaia et al. 2016) lower adult estradiol levels in females relative to their unstressed counterparts (Reynaert et al. 2016). Although more studies are needed, this result suggests that reduced DA signaling in reward circuity in prenatally stressed females may result from changes in estrogens.
Figure 2.
The effects of prenatal stress on plasma levels of androgens and estrogens in male and female rodents.
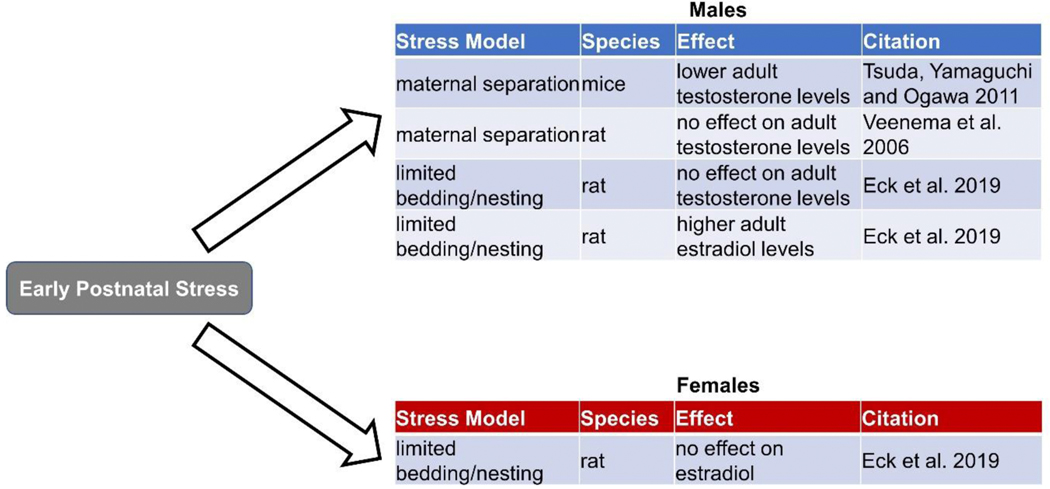
Figure 3.
The effects of early postnatal stress on plasma levels of androgens and estrogens in male and female rodents.
In unstressed females, estradiol potentiates reward, which could contribute to aspects of SUD (Justice and de Wit 1999, Justice and De Wit 2000). However, abnormally low levels of DA signaling are also linked to addiction risk (Melis, Spiga and Diana 2005), and as noted earlier, low estradiol is associated with MDD and schizophrenia (Young et al. 2000, Seeman 1996). One shared aspect of these disorders, which may be driven by low estradiol is anhedonia. Lower baseline levels of mesolimbic DA can lead to anhedonia in response to natural rewards like food (Melis et al. 2005). This anhedonia can increase risk for substance abuse because drugs of abuse become the only stimulus potent enough to induce a rewarding effect (Garfield, Lubman and Yücel 2013, Hatzigiakoumis et al. 2011). Anhedonia is also reported in patients with MDD and schizophrenia (Gard et al. 2007, Pelizza and Ferrari 2009). There is evidence from rodent studies that prenatal stress increases anhedonia in adult females (Reynaert et al. 2016). This effect was reversed by estradiol treatment (Reynaert et al. 2016). Taken together these studies suggest that anhedonia resulting from prenatal stress in females is mediated by low estradiol. This anhedonic state can contribute to disorders characterized by maladaptive motivated behaviors.
In contrast to the effect of prenatal stress, postnatal stress seems to impact estradiol differently and can do so in a sex-specific manner. Exposure to the limited bedding and nesting manipulation from postnatal day 2–9 significantly increases plasma estradiol in adult male rats, but not in females (Eck et al. 2019). Consistent with the data revealing that high estradiol increases DA function in the NAc, studies have demonstrated enhanced mesolimbic DA signaling following ELS in males. For instance, in the NAc, parental separation in the biparental mandarin vole has been linked to an increase in DA levels and D1 receptor expression in adult males and females, as well as an increase in D2 receptor expression in males only (Yu et al. 2013). In the biparental Octodon degus, parental separation actually increases DAT density in the NAc in both male and female adults (Kunzler et al. 2015). This increased DAT would result in faster DA clearance from the synapse, but perhaps this helps compensate for a stress-induced increase in DA release. Notably, estradiol and ELS causes the same changes in DA release, receptors, and uptake, suggesting that effects of postnatal ELS on NAc DA function in males may be mediated by high estradiol. This elevated estradiol and subsequent enhancement of DA transmission in the mesolimbic system of stressed males could increase the risk of developing later substance abuse issues, as elevated DA transmission in the NAc has been linked to increased reward sensitivity to drugs of abuse, including amphetamine (Bradberry et al. 1991, Piazza et al. 1991) and cocaine (Stacy Hooks et al. 1992, Hooks et al. 1991).
Rodent models of ELS typically alter maternal care, such as increasing time apart from pups in the maternal separation model or causing fragmented maternal care in the limited bedding and nesting model (Walker et al. 2017). However, there is variability in maternal care, even in unstressed dams. There is a large literature on how individual differences in maternal care affect estradiol signaling in hypothalamic brain regions that regulate reward circuitry. The paraventricular nucleus (PVN) of the hypothalamus and mPOA also interact with the mesolimbic pathway to increase DA release in the NAc (Numan and Stolzenberg 2009, Melis et al. 2007, Succu et al. 2007). Adult female offspring of rat dams that engage in low levels of licking and grooming (LG) behaviors exhibit increased ERα expression in the PVN (Cameron et al. 2008). In contrast, ERα expression in the mPOA is increased in high LG adult female offspring compared to low LG adult offspring (Champagne et al. 2003). It is unclear exactly how the altered maternal care in postnatal stress models would regulate ERs in these regions, but, based on these data, their regulation is likely. Future studies investigating estrogenic mediation of ELS effects on motivated behavior will also need to consider changes in ERs in afferents to mesocorticolimbic regions.
A role for androgens in mediating the effects of ELS on motivated behavior
As noted, testosterone has organizational and activational effects that masculinize the brain. ELS-induced changes in either of these surges could alter motivated behaviors. Recall that testosterone increases DA release in the NAc and PFC, likely through its ability to increase TH, and thus DA synthesis, in the VTA (Brown et al. 2015, Pallarés et al. 2014, Li et al. 2018). Moreover, DA release in the NAc can further be potentiated by androgen-induced increases in β-endorphin (Johansson et al. 1997). Therefore, increases in testosterone facilitate DA function in the mesocorticolimbic circuit.
There is some evidence that ELS causes lasting changes in testosterone that persist into adulthood, although the degree and consistency of these changes seems to depend on time of exposure and type of stressor used. A summary of the effects of different types of ELS on androgen levels in males and females can be found in Figures 2 and 3. Prenatal stress reliably lowers adult plasma testosterone in male rats (Anderson et al. 1986, García-Vargas et al. 2019, Reynaert et al. 2016), although this effect was not observed in one mouse study, perhaps indicating a species difference (Crump and Chevins 1989). Lower testosterone would be expected to reduce DA function in reward circuits, an effect that could contribute to symptoms of amotivation that can occur in MDD and schizophrenia. Interestingly, one study by Reynaert and colleagues tested the effect of prenatal restraint stress on levels of both testosterone and DHT in adult male rats. The authors found the expected decrease in testosterone, but also an increase in DHT (Reynaert et al. 2016). Because testosterone is synthesized into DHT via the enzyme 5α-reductase, this result is consistent with increased 5α-reductase activity observed in 10-day old rats following prenatal stress (Reznikov and Tarasenko 2007). Reynaert and colleagues (Reynaert et al. 2016) also found that prenatal restraint stress increased preference for natural rewards in adult male rats, an effect that was mimicked by DHT in unstressed males and abolished by castration in stressed males. Taken together, these studies suggest that typically prenatal stress reduces T, but that change in testosterone can be accompanied by an increase in DHT. Dissociating the effects of these changes in testosterone and DHT on reward circuitry will be critical to understand how gonadal hormones mediate effects of ELS on motivated behavior.
Stress manipulations that occur postnatally do not always result in changes in testosterone, but when testosterone is altered, it is also decreased. One study found that maternal separation in male mice led to a reduction in testosterone levels in adulthood (Tsuda, Yamaguchi and Ogawa 2011). Yet, another study that used maternal separation in rats demonstrated a more nuanced effect on testosterone: there were no differences in plasma testosterone levels in adult male rats, but swim stress caused a smaller in increase in testosterone levels in adult male rats exposed to maternal separation vs. the control housing condition (Veenema et al. 2006). In contrast, the limited bedding and nesting model did not alter adult plasma testosterone levels in males (Eck et al. 2019), indicating different postnatal stressors have different effects on adult testosterone. Collectively, these studies indicate that prenatal stress is a more potent regulator of adult testosterone levels than postnatal stress, and thus prenatal stress is more likely to influence motivated behavior via changes in testosterone levels.
Not only can ELS change adult testosterone levels, but there is evidence that ELS alters the early androgen exposure that masculinizes the brain. There are sex differences in dopaminergic mesocorticolimbic circuits that are observed throughout development and may be critical for driving aspects of sex-specific reproductive behavior (Gillies et al. 2014). Although some these early sex differences are driven by sex chromosome complement (Sibug et al. 1996, Seney et al. 2013, Arnold 2014), testosterone treatment also contributes to sexual differentiation of this system. In fact, prenatal testosterone exposure increases TH-positive neurons in the VTA (Brown et al. 2015) and catecholamine activity in the frontal cortex of rats (Stewart and Rajabi 1994). Similarly, a sex difference in impulsivity, which is mediated by the mesocortical system, is organized by perinatal testosterone exposure (Bayless, Darling and Daniel 2013). Many studies have linked prenatal stress to altered androgen exposure and subsequent demasculinization in males, and in some cases, masculinization in females (Ward 1972, Dahlöf, Hård and Larsson 1978, Sachser, Hennessy and Kaiser 2011). One proxy for androgen exposure is anogenital distance (AGD), such that a reduction in AGD is associated with lower prenatal androgen exposure in both sexes (Clemens, Gladue and Coniglio 1978, van den Driesche et al. 2011, Hotchkiss et al. 2007). Prenatal stress in rodents and humans is typically associated with shorter AGDs in males and longer AGDs in females (Barrett et al. 2013, Morgan and Bale 2011, Desaulniers, Lamberson and Safranski 2016, Vom Saal et al. 1990). Interestingly, postnatal stress decreased AGD in both male and female juvenile rats, suggesting that stress during the postnatal critical period when androgens can still affect the brain may also alter masculinization (Eck et al. 2019). These changes in early androgen exposure have been linked to reduced reproductive behaviors, including decreased preferences for sexual odors, which can be influenced by reward circuitry (Ward 1972, Vom Saal and Bronson 1978, Zehr, Gans and McClintock 2001, Hotchkiss et al. 2002, Rhees et al. 1997, Freeman, Sheehan and Ophir 2019). However, how stress-induced changes in prenatal androgen exposure regulate behaviors more directly related to those observed in pathological conditions (e.g., self-administration, anhedonia) is unclear, and much more research is needed.
Conclusions and Implications
It is well established that ELS contributes to disorders characterized by changes in motivation. There are multiple mechanisms by which this can occur. However, given the link between gonadal hormones and reward circuitry, as well as the evidence that ELS can alter gonadal hormone levels, we propose that ELS-induced changes in gonadal hormones are one important way by which stress can increase risk for certain psychiatric disorders. More research is needed to fully characterize hormonal changes induced by ELS in rodents and humans, particularly in patient populations. Such research is worthy of investment because manipulating gonadal hormones is relatively easy to accomplish. However, it is important to note that enthusiasm for hormone replacement therapies dwindled after initial findings from the Women’s Health Initiative reported increased risk of invasive breast cancer and coronary heart disease after the treatment of postmenopausal women with estrogen plus progestin (Rossouw et al. 2002). These surprisingly negative outcomes have been attributed to the timing of replacement, and contrast to the beneficial effects observed if replacement is started within 10 years of menopause (Kling and Manson 2017, Rocca, Grossardt and Shuster 2010). The Women’s Health Initiative did not focus on psychiatric disorders, but limited evidence suggests a beneficial effect of estrogen therapy. As noted, estradiol replacement reduces the incidence depression in vulnerable women as they go through menopause (Schmidt et al. 2015), and perhaps this occurs via estrogenic regulation of reward circuitry. Moreover, estrogen administration as an adjunctive therapy to antipsychotic medication is more effective at reducing schizophrenia symptoms than antipsychotic medication alone (Akhondzadeh et al. 2003). Much more research is needed. However, it is possible that in the future, such hormone treatments can be extended to other patient populations who have experienced ELS-induced endocrine changes to better alleviate symptoms and improve patient outcomes.
Highlights.
Mesocorticolimbic dopamine signaling underlies motivated behaviors
Mesocorticolimbic dopamine is modulated by gonadal hormones
Gonadal hormones may mediate some early life stress-induced changes in dopamine
Gonadal hormones are a potential therapeutic target for motivation-related deficits
Acknowledgments:
This work was supported by T32 DA007237 (SRE), NSF CAREER grant IOS-1552416 (DAB), and NSF IOS-1929829 (DAB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham AD, Schattauer SS, Reichard KL, Cohen JH, Fontaine HM, Song AJ, Johnson SD, Land BB & Chavkin C. (2018) Estrogen Regulation of GRK2 Inactivates Kappa Opioid Receptor Signaling Mediating Analgesia, But Not Aversion. The Journal of Neuroscience, 38, 8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahokas A, Kaukoranta J, Wahlbeck K & Aito M. (2001) Estrogen deficiency in severe postpartum depression: successful treatment with sublingual physiologic 17B-estradiol: a preliminary study. J Clin Psychiatry, 62, 332–6. [DOI] [PubMed] [Google Scholar]
- Akhondzadeh S, Nejatisafa AA, Amini H, Mohammadi MR, Larijani B, Kashani L, Raisi F & Kamalipour A. (2003) Adjunctive estrogen treatment in women with chronic schizophrenia: a double-blind, randomized, and placebo-controlled trial. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 27, 1007–1012. [DOI] [PubMed] [Google Scholar]
- Akhondzadeh S, Rezaei F, Larijani B, Nejatisafa A-A, Kashani L & Abbasi SH (2006) Correlation between testosterone, gonadotropins and prolactin and severity of negative symptoms in male patients with chronic schizophrenia. Schizophrenia Research, 84, 405–410. [DOI] [PubMed] [Google Scholar]
- Alderson LM & Baum MJ (1981) Differential effects of gonadal steroids on dopamine metabolism in mesolimbic and nigro-striatal pathways of male rat brain. Brain Research, 218, 189–206. [DOI] [PubMed] [Google Scholar]
- Allen E. (1922) The oestrous cycle in the mouse. American Journal of Anatomy, 30, 297–371. [Google Scholar]
- Almeida OP, Yeap BB, Hankey GJ, Jamrozik K & Flicker L. (2008) Low Free Testosterone Concentration as a Potentially Treatable Cause of Depressive Symptoms in Older Men. Archives of General Psychiatry, 65, 283–289. [DOI] [PubMed] [Google Scholar]
- Amateau SK & McCarthy MM (2004) Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nature Neuroscience, 7, 643–650. [DOI] [PubMed] [Google Scholar]
- Anderson RH, Fleming DE, Rhees RW & Kinghorn E. (1986) Relationships between sexual activity, plasma testosterone, and the volume of the sexually dimorphic nucleus of the preoptic area in prenatally stressed and non-stressed rats. Brain Research, 370, 1–10. [DOI] [PubMed] [Google Scholar]
- Angold A & Worthman CW (1993) Puberty onset of gender differences in rates of depression: a developmental, epidemiologic and neuroendocrine perspective. Journal of Affective Disorders, 29, 145–158. [DOI] [PubMed] [Google Scholar]
- Arendash GW & Gorski RA (1983) Effects of discrete lesions of the sexually dimorphic nucleus of the preoptic area or other medial preoptic regions on the sexual behavior of male rats. Brain Research Bulletin, 10, 147–154. [DOI] [PubMed] [Google Scholar]
- Arnold AP & Chen X. (2009) What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Frontiers in neuroendocrinology, 30, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Chen X & Itoh Y. (2012) What a difference an X or Y makes: sex chromosomes, gene dose, and epigenetics in sexual differentiation. Handbook of experimental pharmacology, 67–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP (2014) Conceptual frameworks and mouse models for studying sex differences in physiology and disease: why compensation changes the game. Experimental neurology, 259, 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvary D & Pope HG (2000) Anabolic–Androgenic Steroids as a Gateway to Opioid Dependence. New England Journal of Medicine, 342, 1532–1532. [DOI] [PubMed] [Google Scholar]
- Aubele T & Kritzer MF (2010) Gonadectomy and Hormone Replacement Affects In Vivo Basal Extracellular Dopamine Levels in the Prefrontal Cortex but Not Motor Cortex of Adult Male Rats. Cerebral Cortex, 21, 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey ME, Wang ACJ, Hao J, Janssen WGM, Hara Y, Dumitriu D, Hof PR & Morrison JH (2011) Interactive effects of age and estrogen on cortical neurons: implications for cognitive aging. Neuroscience, 191, 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baischer W, Koinig G, Hartmann B, Huber J & Langer G. (1995) Hypothalamic-pituitary-gonadal axis in depressed premenopausal women: Elevated blood testosterone concentrations compared to normal controls. Psychoneuroendocrinology, 20, 553–559. [DOI] [PubMed] [Google Scholar]
- Bakker J & Brock O. (2010) Early Oestrogens in Shaping Reproductive Networks: Evidence for a Potential Organisational Role of Oestradiol in Female Brain Development. Journal of Neuroendocrinology, 22, 728–735. [DOI] [PubMed] [Google Scholar]
- Bakker J, Honda S-I, Harada N & Balthazart J. (2002) The aromatase knock-out mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. The Journal of neuroscience : the official journal of the Society for Neuroscience, 22, 9104–9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett ES, Parlett LE, Sathyanarayana S, Liu F, Redmon JB, Wang C & Swan SH (2013) Prenatal exposure to stressful life events is associated with masculinized anogenital distance (AGD) in female infants. Physiology & Behavior, 114–115, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath K, Manzano-Nieves G & Goodwill H. (2016) Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Hormones and behavior, 82, 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayless DW, Darling JS & Daniel JM (2013) Mechanisms by which neonatal testosterone exposure mediates sex differences in impulsivity in prepubertal rats. Hormones and Behavior, 64, 764–769. [DOI] [PubMed] [Google Scholar]
- Becker JB (1999) Gender Differences in Dopaminergic Function in Striatum and Nucleus Accumbens. Pharmacology Biochemistry and Behavior, 64, 803–812. [DOI] [PubMed] [Google Scholar]
- Becker JB & Hu M. (2008) Sex differences in drug abuse. Frontiers in Neuroendocrinology, 29, 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB (2016) Sex differences in addiction. Dialogues in Clinical Neuroscience, 18, 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB & Chartoff E. (2019) Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 44, 166–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund A, Lindvall O & Nobin A. (1975) Evidence of an incerto-hypothalamic dopamine neurone system in the rat. Brain Research, 89, 29–42. [DOI] [PubMed] [Google Scholar]
- Bloch GJ & Gorski RA (1988) Cytoarchitectonic analysis of the SDN-POA of the intact and gonadectomized rat. Journal of Comparative Neurology, 275, 604–612. [DOI] [PubMed] [Google Scholar]
- Bobzean SAM, DeNobrega AK & Perrotti LI (2014) Sex differences in the neurobiology of drug addiction. Experimental Neurology, 259, 64–74. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Gruen RJ, Berridge CW & Roth RH (1991) Individual differences in behavioral measures: Correlations with nucleus accumbens dopamine measured by microdialysis. Pharmacology Biochemistry and Behavior, 39, 877–882. [DOI] [PubMed] [Google Scholar]
- Brock O, Baum MJ & Bakker J. (2011) The development of female sexual behavior requires prepubertal estradiol. The Journal of neuroscience : the official journal of the Society for Neuroscience, 31, 5574–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ECZ, Steadman CJ, Lee TM, Padmanabhan V, Lehman MN & Coolen LM (2015) Sex differences and effects of prenatal exposure to excess testosterone on ventral tegmental area dopamine neurons in adult sheep. European Journal of Neuroscience, 41, 1157–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelte S & Galea LAM (2010) Chronic corticosterone during pregnancy and postpartum affects maternal care, cell proliferation and depressive-like behavior in the dam. Hormones and Behavior, 58, 769–779. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Collins WE & Fugo NW (1974) Plasma Concentration of LH, FSH, Prolactin, Progesterone and Estradiol-17β Throughout the 4-Day Estrous Cycle of the Rat. Endocrinology, 94, 1704–1708. [DOI] [PubMed] [Google Scholar]
- Cagniard B, Balsam PD, Brunner D & Zhuang X. (2006) Mice with Chronically Elevated Dopamine Exhibit Enhanced Motivation, but not Learning, for a Food Reward. Neuropsychopharmacology, 31, 1362–1370. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Ribeiro E, Roman-Ortiz C, Ramakrishnan C, Deisseroth K, Han M-H & Nestler EJ (2017) Dopaminergic dynamics underlying sex-specific cocaine reward. Nature Communications, 8, 13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron NM, Shahrokh D, Del Corpo A, Dhir SK, Szyf M, Champagne FA & Meaney MJ (2008) Epigenetic Programming of Phenotypic Variations in Reproductive Strategies in the Rat Through Maternal Care. Journal of Neuroendocrinology, 20, 795–801. [DOI] [PubMed] [Google Scholar]
- Carnahan RM & Perry PJ (2004) Depression in Aging Men. Drugs & Aging, 21, 361–376. [DOI] [PubMed] [Google Scholar]
- Carroll VM, Jeyakumar M, Carlson KE & Katzenellenbogen JA (2012) Diarylpropionitrile (DPN) enantiomers: synthesis and evaluation of estrogen receptor β-selective ligands. Journal of medicinal chemistry, 55, 528–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Weaver ICG, Diorio J, Sharma S & Meaney MJ (2003) Natural Variations in Maternal Care Are Associated with Estrogen Receptor α Expression and Estrogen Sensitivity in the Medial Preoptic Area. Endocrinology, 144, 4720–4724. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Ebner SR, Sparrow A, Potter D, Baker PM, Ragozzino ME & Roitman MF (2016) Relative Timing Between Kappa Opioid Receptor Activation and Cocaine Determines the Impact on Reward and Dopamine Release. Neuropsychopharmacology, 41, 989–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm NC & Juraska JM (2012) Effects of long-term treatment with estrogen and medroxyprogesterone acetate on synapse number in the medial prefrontal cortex of aged female rats. Menopause (New York, N.Y.), 19, 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm NC, Kim T & Juraska JM (2013) Males, but not females, lose tyrosine hydroxylase fibers in the medial prefrontal cortex and are impaired on a delayed alternation task during aging. Behavioural Brain Research, 243, 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chocyk A, Majcher-Maślanka I, Przyborowska A, Maćkowiak M & Wędzony K. (2015) Early-life stress increases the survival of midbrain neurons during postnatal development and enhances reward-related and anxiolytic-like behaviors in a sex-dependent fashion. International Journal of Developmental Neuroscience, 44, 33–47. [DOI] [PubMed] [Google Scholar]
- Christensen LW & Clemens LG (1975) BLOCKADE OF TESTOSTERONE-INDUCED MOUNTING BEHAVIOR IN MALE RAT WITH INTRACRANIAL APPLICATION OF AROMATIZATION INHIBITOR, ANDROST-1,4,6-TRIENE-3,17-DIONE. Endocrinology, 97, 1545–1551. [DOI] [PubMed] [Google Scholar]
- Clancy AN, Zumpe D & Michael RP (1995) INTRACEREBRAL INFUSION OF AN AROMATASE INHIBITOR, SEXUAL-BEHAVIOR AND BRAIN ESTROGEN RECEPTOR-LIKE IMMUNOREACTIVITY IN INTACT MALE-RATS. Neuroendocrinology, 61, 98–111. [DOI] [PubMed] [Google Scholar]
- Clemens LG, Gladue BA & Coniglio LP (1978) Prenatal endogenous androgenic influences on masculine sexual behavior and genital morphology in male and female rats. Hormones and Behavior, 10, 40–53. [DOI] [PubMed] [Google Scholar]
- Cools R. (2008) Role of Dopamine in the Motivational and Cognitive Control of Behavior. The Neuroscientist, 14, 381–395. [DOI] [PubMed] [Google Scholar]
- Coquelin A & Desjardins C. (1982) Luteinizing hormone and testosterone secretion in young and old male mice. American Journal of Physiology-Endocrinology and Metabolism, 243, E257–E263. [DOI] [PubMed] [Google Scholar]
- Creutz LM & Kritzer MF (2002) Estrogen receptor-β immunoreactivity in the midbrain of adult rats: Regional, subregional, and cellular localization in the A10, A9, and A8 dopamine cell groups. Journal of Comparative Neurology, 446, 288–300. [DOI] [PubMed] [Google Scholar]
- Creutz LM & Kritzer MF (2004) Mesostriatal and mesolimbic projections of midbrain neurons immunoreactive for estrogen receptor beta or androgen receptors in rats. Journal of Comparative Neurology, 476, 348–362. [DOI] [PubMed] [Google Scholar]
- Crump CJ & Chevins PFD (1989) Prenatal stress reduces fertility of male offspring in mice, without affecting their adult testosterone levels. Hormones and Behavior, 23, 333–343. [DOI] [PubMed] [Google Scholar]
- Cummings JA, Gowl BA, Westenbroek C, Clinton SM, Akil H & Becker JB (2011) Effects of a selectively bred novelty-seeking phenotype on the motivation to take cocaine in male and female rats. Biology of Sex Differences, 2, 3–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranowski JM, Frank E, Young E & Shear MK (2000) Adolescent Onset of the Gender Difference in Lifetime Rates of Major Depression: A Theoretical Model. Archives of General Psychiatry, 57, 21–27. [DOI] [PubMed] [Google Scholar]
- Dahlöf L-G, Hård E & Larsson K. (1978) Influence of maternal stress on the development of the fetal genital system. Physiology & Behavior, 20, 193–195. [DOI] [PubMed] [Google Scholar]
- Dailly E, Chenu F, Renard CE & Bourin M. (2004) Dopamine, depression and antidepressants. Fundamental & Clinical Pharmacology, 18, 601–607. [DOI] [PubMed] [Google Scholar]
- Davidson JM (1966) Activation of the Male Rat’s Sexual Behavior by Intracerebral Implantation of Androgen. Endocrinology, 79, 783–794. [DOI] [PubMed] [Google Scholar]
- Davis PG & Barfield RJ (1979) Activation of Masculine Sexual Behavior by Intracranial Estradiol Benzoate Implants in Male Rats. Neuroendocrinology, 28, 217–227. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Sibug RM, Helmerhorst FM & Schmidt M. (2005) Stress, genes and the mechanism of programming the brain for later life. Neuroscience & Biobehavioral Reviews, 29, 271–281. [DOI] [PubMed] [Google Scholar]
- de Souza Silva MA, Mattern C, Topic B, Buddenberg TE & Huston JP (2009) Dopaminergic and serotonergic activity in neostriatum and nucleus accumbens enhanced by intranasal administration of testosterone. European Neuropsychopharmacology, 19, 53–63. [DOI] [PubMed] [Google Scholar]
- Desaulniers AT, Lamberson WR & Safranski TJ (2016) Prenatal heat stress reduces male anogenital distance at birth and adult testis size, which are rescued by concurrent maternal Artemisia absinthium consumption. Journal of Thermal Biology, 57, 84–91. [DOI] [PubMed] [Google Scholar]
- Devine DP, Leone P, Pocock D & Wise RA (1993) Differential involvement of ventral tegmental mu, delta and kappa opioid receptors in modulation of basal mesolimbic dopamine release: in vivo microdialysis studies. Journal of Pharmacology and Experimental Therapeutics, 266, 1236. [PubMed] [Google Scholar]
- Di Chiara G & Imperato A. (1988) Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. Journal of Pharmacology and Experimental Therapeutics, 244, 1067. [PubMed] [Google Scholar]
- Di Paolo T, Carmichael R, Labrie F & Raynaud J-P (1979) Effects of estrogens on the characteristics of [3H]spiroperidol and [3H]RU24213 binding in rat anterior pituitary gland and brain. Molecular and Cellular Endocrinology, 16, 99–112. [DOI] [PubMed] [Google Scholar]
- Di Paolo T, Poyet P & Labrie F. (1982) Effect of prolactin and estradiol on rat striatal dopamine receptors. Life Sciences, 31, 2921–2929. [DOI] [PubMed] [Google Scholar]
- Di Paolo T, Rouillard C & Bédard P. (1985) 17β-estradiol at a physiological dose acutely increases dopamine turnover in rat brain. European Journal of Pharmacology, 117, 197–203. [DOI] [PubMed] [Google Scholar]
- Dib T, Martínez-Pinto J, Reyes-Parada M, Torres GE & Sotomayor-Zárate R. (2018) Neonatal programming with testosterone propionate reduces dopamine transporter expression in nucleus accumbens and methylphenidate-induced locomotor activity in adult female rats. Behavioural Brain Research, 346, 80–85. [DOI] [PubMed] [Google Scholar]
- Dilts RP & Kalivas PW (1989) Autoradiographic localization of μ-opioid and neurotensin receptors within the mesolimbic dopamine system. Brain Research, 488, 311–327. [DOI] [PubMed] [Google Scholar]
- Dilts RP & Kalivas PW (1990) Autoradiographic localization of delta opioid receptors within the mesocorticolimbic dopamine system using radioiodinated [2-d-penicillamine, 5-d-penicillamine]enkephalin (125I-DPDPE). Synapse, 6, 121–132. [DOI] [PubMed] [Google Scholar]
- DiMeo AN & Wood RI (2006) ICV testosterone induces Fos in male Syrian hamster brain. Psychoneuroendocrinology, 31, 237–249. [DOI] [PubMed] [Google Scholar]
- Döhler KD, Srivastava SS, Shryne JE, Jarzab B, Sipos A & Gorski RA (1984) Differentiation of the Sexually Dimorphic Nucleus in the Preoptic Area of the Rat Brain Is Inhibited by Postnatal Treatment with an Estrogen Antagonist. Neuroendocrinology, 38, 297–301. [DOI] [PubMed] [Google Scholar]
- Dominguez JM & Hull EM (2005) Dopamine, the medial preoptic area, and male sexual behavior. Physiology & Behavior, 86, 356–368. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL & Mathis CA (2001) Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biological Psychiatry, 49, 81–96. [DOI] [PubMed] [Google Scholar]
- Du J, Lorrain DS & Hull EM (1998) Castration decreases extracellular, but increases intracellular, dopamine in medial preoptic area of male rats. Brain Research, 782, 11–17. [DOI] [PubMed] [Google Scholar]
- DuRant RH, Rickert VI, Ashworth CS, Newman C & Slavens G. (1993) Use of Multiple Drugs among Adolescents Who Use Anabolic Steroids. New England Journal of Medicine, 328, 922–926. [DOI] [PubMed] [Google Scholar]
- Dziedzicka-Wasylewska M. (2004) Brain dopamine receptors--research perspectives and potential sites of regulation. Polish Journal of Pharmacology, 56, 659–71. [PubMed] [Google Scholar]
- Ebner SR, Roitman MF, Potter DN, Rachlin AB & Chartoff EH (2010) Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology, 210, 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eck SR, Ardekani CS, Salvatore M, Luz S, Kim ED, Rogers CM, Hall A, Lee DE, Famularo ST, Bhatnagar S & Bangasser DA (2019) The effects of early life adversity on growth, maturation, and steroid hormones in male and female rats. European Journal of Neuroscience, n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DA & Einhorn LC (1986) Preoptic and midbrain control of sexual motivation. Physiology & Behavior, 37, 329–335. [DOI] [PubMed] [Google Scholar]
- Enoch M-A (2011) The Role of Early Life Stress as a Predictor for Alcohol and Drug Dependence. Psychopharmacology, 214, 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson CJP, Kaprio J, Pulkkinen L & Rose RJ (2005) Testosterone and Alcohol Use among Adolescent Male Twins: Testing Between-Family Associations in Within-Family Comparisons. Behavior Genetics, 35, 359–68. [DOI] [PubMed] [Google Scholar]
- Esumi S, Sagara H, Nakamoto A, Kawasaki Y, Gomita Y & Sendo T. (2013) Effect of GBR12909 on affective behavior: Distinguishing motivational behavior from antidepressant-like and addiction-like behavior using the runway model of intracranial self-stimulation. Behavioural Brain Research, 243, 313–321. [DOI] [PubMed] [Google Scholar]
- Etten MLV, Neumark YD & Anthony JC (1999) Male-female differences in the earliest stages of drug involvement. Addiction, 94, 1413–1419. [DOI] [PubMed] [Google Scholar]
- Floresco SB & Magyar O. (2006) Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology, 188, 567–585. [DOI] [PubMed] [Google Scholar]
- Freeman AR, Sheehan MJ & Ophir AG (2019) Anogenital distance predicts sexual odour preference in African giant pouched rats. Animal Behaviour, 148, 123–132. [Google Scholar]
- Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB & Hollander L. (2004) Hormones and Menopausal Status as Predictors of Depression in Womenin Transition to Menopause. Archives of General Psychiatry, 61, 62–70. [DOI] [PubMed] [Google Scholar]
- García-Vargas D, Juárez-Rojas L, Rojas Maya S & Retana-Márquez S. (2019) Prenatal stress decreases sperm quality, mature follicles and fertility in rats. Systems Biology in Reproductive Medicine, 65, 223–235. [DOI] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP & Green MF (2007) Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schizophrenia Research, 93, 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield JBB, Lubman DI & Yücel M. (2013) Anhedonia in substance use disorders: A systematic review of its nature, course and clinical correlates. Australian & New Zealand Journal of Psychiatry, 48, 36–51. [DOI] [PubMed] [Google Scholar]
- Gershanik O, Heikkila RE & Duvoisin RC (1983) Behavioral correlations of dopamine receptor activation. Neurology, 33, 1489. [DOI] [PubMed] [Google Scholar]
- Gillies GE & McArthur S. (2010) Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacological reviews, 62, 155–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies GE, Virdee K, McArthur S & Dalley JW (2014) Sex-dependent diversity in ventral tegmental dopaminergic neurons and developmental programing: A molecular, cellular and behavioral analysis. Neuroscience, 282, 69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich J & Caron M. (1993) Recent advances in the molecular biology of dopamine receptors. Annual review of neuroscience, 16, 299–321. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS & Cooper RL (2007) The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Research Part B: Developmental and Reproductive Toxicology, 80, 84–97. [DOI] [PubMed] [Google Scholar]
- Goodwill HL, Manzano-Nieves G, LaChance P, Teramoto S, Lin S, Lopez C, Stevenson RJ, Theyel BB, Moore CI, Connors BW & Bath KG (2018) Early Life Stress Drives Sex-Selective Impairment in Reversal Learning by Affecting Parvalbumin Interneurons in Orbitofrontal Cortex of Mice. Cell Reports, 25, 2299–2307.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JH & Fields JZ (1989) A permanent dopamine receptor up-regulation in the ovariectomized rat. Pharmacology Biochemistry and Behavior, 33, 123–125. [DOI] [PubMed] [Google Scholar]
- Goudsmit E, Feenstra MGP & Swaab DF (1990) Central monoamine metabolism in the male brown-Norway rat in relation to aging and testosterone. Brain Research Bulletin, 25, 755–763. [DOI] [PubMed] [Google Scholar]
- Goyal RO, Sagar R, Ammini AC, Khurana ML & Alias AG (2004) Negative Correlation between Negative Symptoms of Schizophrenia and Testosterone Levels. Annals of the New York Academy of Sciences, 1032, 291–294. [DOI] [PubMed] [Google Scholar]
- Gregoire A, Kumar R, Everitt B, Henderson A & Studd J. (1996) Transdermal oestrogen for treatment of severe postnatal depression. Lancet, 347, 930–3. [DOI] [PubMed] [Google Scholar]
- Grigoriadis (2002) Role of Estrogen in the Treatment of Depression. American journal of therapeutics., 9, 503. [DOI] [PubMed] [Google Scholar]
- Grigoriadis S & Seeman MV (2002) The Role of Estrogen in Schizophrenia: Implications for Schizophrenia Practice Guidelines for Women. The Canadian Journal of Psychiatry, 47, 437–442. [DOI] [PubMed] [Google Scholar]
- Hansen S, Köhler C, Goldstein M & Steinbusch HVM (1982) Effects of ibotenic acid-induced neuronal degeneration in the medial preoptic area and the lateral hypothalamic area on sexual behavior in the male rat. Brain Research, 239, 213–232. [DOI] [PubMed] [Google Scholar]
- Hara Y, Yuk F, Puri R, Janssen WGM, Rapp PR & Morrison JH (2016) Estrogen Restores Multisynaptic Boutons in the Dorsolateral Prefrontal Cortex while Promoting Working Memory in Aged Rhesus Monkeys. The Journal of Neuroscience, 36, 901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzigiakoumis DS, Martinotti G, Di Giannantonio M & Janiri L. (2011) Anhedonia and Substance Dependence: Clinical Correlates and Treatment Options. Frontiers in Psychiatry, 2, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L & Larsson K. (1967) Impairment of mating behavior in male rats following lesions in the preoptic-anterior hypothalamic continuum. Brain Research, 3, 248–263. [Google Scholar]
- Hermans EJ, Bos PA, Ossewaarde L, Ramsey NF, Fernández G & van Honk J. (2010) Effects of exogenous testosterone on the ventral striatal BOLD response during reward anticipation in healthy women. NeuroImage, 52, 277–283. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Gonzalez L, Murzi E, Páez X, Gottberg E & Baptista T. (1994) Testosterone modulates mesolimbic dopaminergic activity in male rats. Neuroscience Letters, 171, 172–174. [DOI] [PubMed] [Google Scholar]
- Honda S. i., Harada N, Ito S, Takagi Y & Maeda S. (1998) Disruption of Sexual Behavior in Male Aromatase-Deficient Mice Lacking Exons 1 and 2 of thecyp19Gene. Biochemical and Biophysical Research Communications, 252, 445–449. [DOI] [PubMed] [Google Scholar]
- Honeycutt JA, Demaestri C, Peterzell S, Silveri MM, Cai X, Kulkarni P, Cunningham MG, Ferris CF & Brenhouse HC (2019) Too much, too young? Altered corticolimbic axonal innervation and resting state functional connectivity suggests sex-dependent outcomes in a rat model of early life adversity. bioRxiv, 700666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB & Justice JB Jr (1991) Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse, 9, 121–128. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Cascini MG, Gordon AS, Diamond I & Bonci A. (2003) Cooperative Activation of Dopamine D1 and D2 Receptors Increases Spike Firing of Nucleus Accumbens Neurons via G-Protein βγ Subunits. The Journal of Neuroscience, 23, 5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss AK, Lambright CS, Ostby JS, Parks-Saldutti L, Vandenbergh JG & Gray LE Jr. (2007) Prenatal Testosterone Exposure Permanently Masculinizes Anogenital Distance, Nipple Development, and Reproductive Tract Morphology in Female Sprague-Dawley Rats. Toxicological Sciences, 96, 335–345. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Ostby JS, Vandenburgh JG & Gray LE (2002) Androgens and environmental antiandrogens affect reproductive development and play behavior in the Sprague-Dawley rat. Environmental Health Perspectives, 110, 435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska RE, Ludmer LM & Silbergeld EK (1980) Characterization of the striatal dopamine receptor supersensitivity produced by estrogen treatment of male rats. Neuropharmacology, 19, 923–926. [DOI] [PubMed] [Google Scholar]
- Hruska RE & Nowak MW (1988) Estrogen treatment increases the density of D1 dopamine receptors in the rat striatum. Brain Research, 442, 349–350. [DOI] [PubMed] [Google Scholar]
- Hser Y-I, Anglin MD & Booth MW (1987) Sex Differences in Addict Careers. 3. Addiction. The American Journal of Drug and Alcohol Abuse, 13, 231–251. [DOI] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE & Becker JB (2003) Biological Basis of Sex Differences in the Propensity to Self-administer Cocaine. Neuropsychopharmacology, 29, 81. [DOI] [PubMed] [Google Scholar]
- Hudson A & Stamp JA (2011) Ovarian hormones and propensity to drug relapse: A review. Neuroscience & Biobehavioral Reviews, 35, 427–436. [DOI] [PubMed] [Google Scholar]
- Hull EM & Dominguez JM (2007) Sexual behavior in male rodents. Hormones and behavior, 52, 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Du J, Lorrain DS & Matuszewich L. (1995) Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. The Journal of neuroscience : the official journal of the Society for Neuroscience, 15, 7465–7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Eaton RC, Markowski VP, Moses J, Lumley LA & Loucks JA (1992) Opposite influence of medial preoptic D1 and D2 receptors on genital reflexes: Implications for copulation. Life Sciences, 51, 1705–1713. [DOI] [PubMed] [Google Scholar]
- Hull EM, Eaton RC, Moses J & Lorrain D. (1993) Copulation increases dopamine activity in the medial preoptic area of male rats. Life Sciences, 52, 935–940. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Glazier BS, Murphy JM & McBride WJ (1997) Role of Dopamine D1 and D2 Receptors in the Nucleus Accumbens in Mediating Reward. The Journal of Neuroscience, 17, 8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C & Baram TZ (2008) Dysfunctional nurturing behavior in rat dams with limited access to nesting material: A clinically relevant model for early-life stress. Neuroscience, 154, 1132–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izvolskaia MS, Tillet Y, Sharova VS, Voronova SN & Zakharova LA (2016) Disruptions in the hypothalamic–pituitary–gonadal axis in rat offspring following prenatal maternal exposure to lipopolysaccharide. Stress, 19, 198–205. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE & Becker JB (2005) Sex Differences and Hormonal Influences on Acquisition of Cocaine Self-Administration in Rats. Neuropsychopharmacology, 31, 129. [DOI] [PubMed] [Google Scholar]
- Jarjour S, Bai L & Gianoulakis C. (2009) Effect of Acute Ethanol Administration on the Release of Opioid Peptides From the Midbrain Including the Ventral Tegmental Area. Alcoholism: Clinical and Experimental Research, 33, 1033–1043. [DOI] [PubMed] [Google Scholar]
- Johansson P, Ray A, Zhou Q, Huang W, Karlsson K & Nyberg F. (1997) Anabolic androgenic steroids increase β-endorphin levels in the ventral tegmental area in the male rat brain. Neuroscience Research, 27, 185–189. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Ho CC, Day AE, Walker QD, Francis R & Kuhn CM (2010) Oestrogen Receptors Enhance Dopamine Neurone Survival in Rat Midbrain. Journal of Neuroendocrinology, 22, 226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper SA (1995) A meta-analysis of the relationship of child sexual abuse to adult psychological adjustment. Child Abuse & Neglect, 19, 715–728. [DOI] [PubMed] [Google Scholar]
- Justice AJH & de Wit H. (1999) Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology, 145, 67–75. [DOI] [PubMed] [Google Scholar]
- Justice AJH & De Wit H. (2000) Acute Effects of d-Amphetamine During the Early and Late Follicular Phases of the Menstrual Cycle in Women. Pharmacology Biochemistry and Behavior, 66, 509–515. [DOI] [PubMed] [Google Scholar]
- Kelley AE & Berridge KC (2002) The Neuroscience of Natural Rewards: Relevance to Addictive Drugs. The Journal of Neuroscience, 22, 3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey JJ (1996) Hormonal contraception and lactation. Journal of human lactation : official journal of International Lactation Consultant Association, 12, 315–318. [DOI] [PubMed] [Google Scholar]
- Khan MM, Dhandapani KM, Zhang Q.-g & Brann DW (2013) Estrogen regulation of spine density and excitatory synapses in rat prefrontal and somatosensory cerebral cortex. Steroids, 78, 614–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling JM & Manson JE (2017) The Women’s Health Initiative: evolving insights over 15 years. Menopause, 24. [DOI] [PubMed] [Google Scholar]
- Ko Y-H, Jung S-W, Joe S-H, Lee C-H, Jung H-G, Jung I-K, Kim S-H & Lee M-S (2007) Association between serum testosterone levels and the severity of negative symptoms in male patients with chronic schizophrenia. Psychoneuroendocrinology, 32, 385–391. [DOI] [PubMed] [Google Scholar]
- Kow L-M & Pfaff DW (1975) Induction of lordosis in female rats: Two modes of estrogen action and the effect of adrenalectomy. Hormones and Behavior, 6, 259–276. [DOI] [PubMed] [Google Scholar]
- Kritzer MF & Creutz LM (2008) Region and Sex Differences in Constituent Dopamine Neurons and Immunoreactivity for Intracellular Estrogen and Androgen Receptors in Mesocortical Projections in Rats. The Journal of Neuroscience, 28, 9525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF & Kohama SG (1999) Ovarian hormones differentially influence immunoreactivity for dopamine β- hydroxylase, choline acetyltransferase, and serotonin in the dorsolateral prefrontal cortex of adult rhesus monkeys. Journal of Comparative Neurology, 409, 438–451. [DOI] [PubMed] [Google Scholar]
- Kunzler J, Braun K & Bock J. (2015) Early life stress and sex-specific sensitivity of the catecholaminergic systems in prefrontal and limbic regions of Octodon degus. Brain Structure and Function, 220, 861–868. [DOI] [PubMed] [Google Scholar]
- La Grange L, Jones TD, Erb L & Reyes E. (1995) Alcohol consumption: Biochemical and personality correlates in a college student population. Addictive Behaviors, 20, 93–103. [DOI] [PubMed] [Google Scholar]
- Lammers C-H, D’Souza U, Qin Z-H, Lee S-H, Yajima S & Mouradian MM (1999) Regulation of striatal dopamine receptors by estrogen. Synapse, 34, 222–227. [DOI] [PubMed] [Google Scholar]
- Landry M, Lévesque D & Di Paolo T. (2002) Estrogenic Properties of Raloxifene, but Not Tamoxifen, on D2 and D3 Dopamine Receptors in the Rat Forebrain. Neuroendocrinology, 76, 214–222. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, Gil R, Kegeles L & Innis R. (1999) Increased dopamine transmission in schizophrenia: relationship to illness phases. Biological Psychiatry, 46, 56–72. [DOI] [PubMed] [Google Scholar]
- Lasley B, McKay H, Roberts JA, Kordower JH, Hao J, Morrison JH, Allen PB, Hof PR, Greengard P, Rapp PR, Janssen WGM & Tang Y. (2004) Estrogen Replacement Increases Spinophilin-immunoreactive Spine Number in the Prefrontal Cortex of Female Rhesus Monkeys. Cerebral Cortex, 14, 215–223. [DOI] [PubMed] [Google Scholar]
- Le Saux M & Di Paolo T. (2005) Chronic estrogenic drug treatment increases preproenkephalin mRNA levels in the rat striatum and nucleus accumbens. Psychoneuroendocrinology, 30, 251–260. [DOI] [PubMed] [Google Scholar]
- Le Saux M, Morissette M & Di Paolo T. (2006) ERβ mediates the estradiol increase of D2 receptors in rat striatum and nucleus accumbens. Neuropharmacology, 50, 451–457. [DOI] [PubMed] [Google Scholar]
- Lenz KM, Wright CL, Martin RC & McCarthy MM (2011) Prostaglandin E2 Regulates AMPA Receptor Phosphorylation and Promotes Membrane Insertion in Preoptic Area Neurons and Glia during Sexual Differentiation. PLOS ONE, 6, e18500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Fredericks PJ, Nealer C & Albin-Brooks C. (2014) Chronic gestational stress leads to depressive-like behavior and compromises medial prefrontal cortex structure and function during the postpartum period. PloS one, 9, e89912-e89912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque D & Di Paolo T. (1989) Chronic estradiol treatment increases ovariectomized rat striatal D-1 dopamine receptors. Life Sciences, 45, 1813–1820. [DOI] [PubMed] [Google Scholar]
- Lévesque D & Di Paolo T. (1991) Dopamine receptor reappearance after irreversible receptor blockade: effect of chronic estradiol treatment of ovariectomized rats. Molecular Pharmacology, 39, 659. [PubMed] [Google Scholar]
- Li L, Kang Y-X, Ji X-M, Li Y-K, Li S-C, Zhang X-J, Cui H-X & Shi G-M (2018) Finasteride inhibited brain dopaminergic system and open-field behaviors in adolescent male rats. CNS Neuroscience & Therapeutics, 24, 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Gamma A & Vollenweider FX (2001) Gender differences in the subjective effects of MDMA. Psychopharmacology, 154, 161–168. [DOI] [PubMed] [Google Scholar]
- Liu D, Caldji C, Sharma S, Plotsky PM & Meaney MJ (2000) Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinepherine release in the hypothalamic paraventricular nucleus. Journal of neuroendocrinology., 12, 5–12. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM & Meaney MJ (1997) Maternal Care, Hippocampal Glucocorticoid Receptors, and Hypothalamic-Pituitary-Adrenal Responses to Stress. Science, 277, 1659. [DOI] [PubMed] [Google Scholar]
- Long JA & Evans HM. 1922. The oestrous cycle in the rat and its associated phenomena. University of California Press. [Google Scholar]
- Lookingland KJ & Moore KE (1984) Dopamine receptor-mediated regulation of incertohypothalamic dopaminergic neurons in the male rat. Brain Research, 304, 329–338. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Sholar M, Lundahl LH, Lamas X, Kouri E, Wines JD, Kragie L & Mendelson JH (1996) Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology, 125, 346–354. [DOI] [PubMed] [Google Scholar]
- Lupo C, Dessi-Fulgheri F, Musi B & Larsson K. (1983) The effect of medial preoptic-anterior hypothalamic lesions on testosterone plasma levels and testosterone conversion in the hypothalamus of male rats. Neuroscience Letters, 39, 261–265. [DOI] [PubMed] [Google Scholar]
- Lynch WJ (2006) Sex differences in vulnerability to drug self-administration. Experimental and Clinical Psychopharmacology, 14, 34–41. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Mickelberg JL & Carroll ME (2001) Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacology Biochemistry and Behavior, 68, 641–646. [DOI] [PubMed] [Google Scholar]
- Majcher-Maślanka I, Solarz A, Wędzony K & Chocyk A. (2017) The effects of early-life stress on dopamine system function in adolescent female rats. International Journal of Developmental Neuroscience, 57, 24–33. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, & Watson SJ (1987) Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. Journal of Neuroscience, 7 (8), 2445–2464. [PMC free article] [PubMed] [Google Scholar]
- Manzano Nieves G, Schilit Nitenson A, Lee H-I, Gallo M, Aguilar Z, Johnsen A, Bravo M & Bath KG (2019) Early Life Stress Delays Sexual Maturation in Female Mice. Frontiers in molecular neuroscience, 12, 27–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowski VP, Eaton RC, Lumley LA, Moses J & Hull EM (1994) A D1 agonist in the MPOA facilitates copulation in male rats. Pharmacology Biochemistry and Behavior, 47, 483–486. [DOI] [PubMed] [Google Scholar]
- McCarthy MM & Arnold AP (2011) Reframing sexual differentiation of the brain. Nature Neuroscience, 14, 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Kehoe P, Mallinson K, Cecchi L & Frye CA (2002) Neonatal isolation alters stress hormone and mesolimbic dopamine release in juvenile rats. Pharmacology Biochemistry and Behavior, 73, 77–85. [DOI] [PubMed] [Google Scholar]
- Melis M, Spiga S & Diana M. 2005. The Dopamine Hypothesis of Drug Addiction: Hypodopaminergic State In International Review of Neurobiology, 101–154. Academic Press. [DOI] [PubMed] [Google Scholar]
- Melis MR, Melis T, Cocco C, Succu S, Sanna F, Pillolla G, Boi A, Ferri G-L & Argiolas A. (2007) Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. European Journal of Neuroscience, 26, 1026–1035. [DOI] [PubMed] [Google Scholar]
- Milienne-Petiot M, Groenink L, Minassian A & Young JW (2017a) Blockade of dopamine D1-family receptors attenuates the mania-like hyperactive, risk-preferring, and high motivation behavioral profile of mice with low dopamine transporter levels. Journal of Psychopharmacology, 31, 1334–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milienne-Petiot M, Kesby JP, Graves M, van Enkhuizen J, Semenova S, Minassian A, Markou A, Geyer MA & Young JW (2017b) The effects of reduced dopamine transporter function and chronic lithium on motivation, probabilistic learning, and neurochemistry in mice: Modeling bipolar mania. Neuropharmacology, 113, 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Angulo AL, Ward SE, Perlman WR, Weickert CS, Kleinman JE, Weinberger DR, Straub RE, Radhakrishna V & Wong J. (2008) Variants in the estrogen receptor α gene and its mRNA contribute to risk for schizophrenia. Human Molecular Genetics, 17, 2293–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JB & Stewart J. (1989) Effects of castration, steroid replacement, and sexual experience on mesolimbic dopamine and sexual behaviors in the male rat. Brain Research, 491, 116–127. [DOI] [PubMed] [Google Scholar]
- Molina-Martínez LM & Juárez J. (2020) Differential expression of μ-opioid receptors in the nucleus accumbens, amygdala and VTA depends on liking for alcohol, chronic alcohol intake and estradiol treatment. Behavioural Brain Research, 378, 112255. [DOI] [PubMed] [Google Scholar]
- Molnar BE, Buka SL & Kessler RC (2001) Child sexual abuse and subsequent psychopathology: results from the National Comorbidity Survey. American Journal of Public Health, 91, 753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KE (1995) Dopaminergic neuronal systems in tha hypothalamus. Psychopharmacology, 245–256. [Google Scholar]
- Moore L, Kyaw M, Vercammen A, Lenroot R, Kulkarni J, Curtis J, O’Donnell M, Carr VJ, Shannon Weickert C & Weickert TW (2013) Serum testosterone levels are related to cognitive function in men with schizophrenia. Psychoneuroendocrinology, 38, 1717–1728. [DOI] [PubMed] [Google Scholar]
- Morgan CP & Bale TL (2011) Early Prenatal Stress Epigenetically Programs Dysmasculinization in Second-Generation Offspring via the Paternal Lineage. The Journal of Neuroscience, 31, 11748–11755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette M, Biron D & Di Paolo T. (1990) Effect of estradiol and progesterone on rat striatal dopamine uptake sites. Brain Research Bulletin, 25, 419–422. [DOI] [PubMed] [Google Scholar]
- Morissette M & Di Paolo T. (1993) Sex and Estrous Cycle Variations of Rat Striatal Dopamine Uptake Sites. Neuroendocrinology, 58, 16–22. [DOI] [PubMed] [Google Scholar]
- Morissette M & Paolo TD (1993) Effect of Chronic Estradiol and Progesterone Treatments of Ovariectomized Rats on Brain Dopamine Uptake Sites. Journal of Neurochemistry, 60, 1876–1883. [DOI] [PubMed] [Google Scholar]
- Mueller B & Bale T. (2008) Sex-Specific Programming of Offspring Emotionality after Stress Early in Pregnancy. The Journal of neuroscience : the official journal of the Society for Neuroscience, 28, 9055–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JF, Felicio LS, Osterburg HH & Finch CE (1981) Altered Profiles of Estradiol and Progesterone Associated with Prolonged Estrous Cycles and Persistent Vaginal Cornification in Aging C578L/6J Mice1. Biology of Reproduction, 24, 784–794. [DOI] [PubMed] [Google Scholar]
- Nestler EJ (1993) Molecular mechanisms of drug addiction in the mesolimbic dopamine pathway. Seminars in Neuroscience, 5, 369–376. [Google Scholar]
- Nishi M, Horii-Hayashi N, Sasagawa T & Matsunaga W. (2013) Effects of early life stress on brain activity: Implications from maternal separation model in rodents. General and Comparative Endocrinology, 181, 306–309. [DOI] [PubMed] [Google Scholar]
- Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, Russo SJ, Devine SE & McCarthy MM (2015) Brain feminization requires active repression of masculinization via DNA methylation. Nature Neuroscience, 18, 690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M & Stolzenberg DS (2009) Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Frontiers in Neuroendocrinology, 30, 46–64. [DOI] [PubMed] [Google Scholar]
- O’Connell LA & Hofmann HA (2011) The Vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. Journal of Comparative Neurology, 519, 3599–3639. [DOI] [PubMed] [Google Scholar]
- Ordyan NE, Fedotova YO & Pivina SG (2013) Effects of Prenatal Stress on the Activity of the Pituitary-Ovarian System in Female Rats. Bulletin of Experimental Biology and Medicine, 155, 433–435. [DOI] [PubMed] [Google Scholar]
- Packard MG, Cornell AH & Alexander GM (1997) Rewarding affective properties of intra-nucleus accumbens injections of testosterone. Behavioral Neuroscience, 111, 219–224. [DOI] [PubMed] [Google Scholar]
- Packard MG, Schroeder JP & Alexander GM (1998) Expression of Testosterone Conditioned Place Preference Is Blocked by Peripheral or Intra-accumbens Injection of α-Flupenthixol. Hormones and Behavior, 34, 39–47. [DOI] [PubMed] [Google Scholar]
- Pallarés ME, Adrover E, Imsen M, González D, Fabre B, Mesch V, Baier CJ & Antonelli MC (2014) Maternal administration of flutamide during late gestation affects the brain and reproductive organs development in the rat male offspring. Neuroscience, 278, 122–135. [DOI] [PubMed] [Google Scholar]
- Paredes RG & Agmo A. (1992) Facilitation of sexual behavior shortly after electrolytic lesion of the medial preoptic area: what does it mean? Brain Research Bulletin, 29, 125–128. [DOI] [PubMed] [Google Scholar]
- Paredes RG & Baum MJ (1995) Altered sexual partner preference in male ferrets given excitotoxic lesions of the preoptic area/anterior hypothalamus. Journal of Neuroscience, 15, 6619–6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes RG, Highland L & Karam P. (1993) Socio-sexual behavior in male rats after lesions of the medial preoptic area: evidence for reduced sexual motivation. Brain Research, 618, 271–276. [DOI] [PubMed] [Google Scholar]
- Paredes RG, Tzschentke T & Nakach N. (1998) Lesions of the medial preoptic area/anterior hypothalamus (MPOA/AH) modify partner preference in male rats. Brain Research, 813, 1–8. [DOI] [PubMed] [Google Scholar]
- Payne JL (2003) The role of estrogen in mood disorders in women. International Review of Psychiatry, 15, 280–290. [DOI] [PubMed] [Google Scholar]
- Pelizza L & Ferrari A. (2009) Anhedonia in schizophrenia and major depression: state or trait? Annals of General Psychiatry, 8, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña CJ & Champagne FA (2015) Neonatal overexpression of estrogen receptor-α alters midbrain dopamine neuron development and reverses the effects of low maternal care in female offspring. Developmental Neurobiology, 75, 1114–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C & Becker JB (2013) Impact of pubertal and adult estradiol treatments on cocaine self-administration. Hormones and behavior, 64, 10.1016/j.yhbeh.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA & Young WC (1959) ORGANIZING ACTION OF PRENATALLY ADMINISTERED TESTOSTERONE PROPIONATE ON THE TISSUES MEDIATING MATING BEHAVIOR IN THE FEMALE GUINEA PIG1. Endocrinology, 65, 369–382. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Rougé-Pont F, Deminière JM, Kharoubi M, Le Moal M & Simon H. (1991) Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Research, 567, 169–174. [DOI] [PubMed] [Google Scholar]
- Pryce CR & Feldon J. (2003) Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. Neuroscience & Biobehavioral Reviews, 27, 57–71. [DOI] [PubMed] [Google Scholar]
- Putnam SK, Du J, Sato S & Hull EM (2001) Testosterone Restoration of Copulatory Behavior Correlates with Medial Preoptic Dopamine Release in Castrated Male Rats. Hormones and Behavior, 39, 216–224. [DOI] [PubMed] [Google Scholar]
- Putnam SK, Sato S & Hull EM (2003) Effects of testosterone metabolites on copulation and medial preoptic dopamine release in castrated male rats. Hormones and Behavior, 44, 419–426. [DOI] [PubMed] [Google Scholar]
- Putnam SK, Sato S, Riolo JV & Hull EM (2005) Effects of testosterone metabolites on copulation, medial preoptic dopamine, and NOS-immunoreactivity in castrated male rats. Hormones and Behavior, 47, 513–522. [DOI] [PubMed] [Google Scholar]
- Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ & Mattson ME (1999) Telescoping of landmark events associated with drinking: a gender comparison. Journal of Studies on Alcohol, 60, 252–260. [DOI] [PubMed] [Google Scholar]
- Redei E & Freeman EW (1995) Daily plasma estradiol and progesterone levels over the menstrual cycle and their relation to premenstrual symptoms. Psychoneuroendocrinology, 20, 259–267. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Chan C, Ghee SM & See RE (2012) Sex differences in escalation of methamphetamine self-administration: Cognitive and motivational consequences. Psychopharmacology, 223, 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reincke SAJ & Hanganu-Opatz IL (2017) Early-life stress impairs recognition memory and perturbs the functional maturation of prefrontal-hippocampal-perirhinal networks. Scientific Reports, 7, 42042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaert M-L, Marrocco J, Mairesse J, Lionetto L, Simmaco M, Deruyter L, Allorge D, Moles A, Pittaluga A, Maccari S, Morley-Fletcher S, Van Camp G & Nicoletti F. (2016) Hedonic sensitivity to natural rewards is affected by prenatal stress in a sex-dependent manner. Addiction Biology, 21, 1072–1085. [DOI] [PubMed] [Google Scholar]
- Reznikov AG & Tarasenko LV (2007) Hormonal protection of gender-related peculiarities of testosterone metabolism in the brain of prenatally stressed rats. Neuro endocrinology letters., 28, 671. [PubMed] [Google Scholar]
- Rhees RW, Kirk BA, Sephton S & Lephart ED (1997) Effects of Prenatal Testosterone on Sexual Behavior, Reproductive Morphology and LH Secretion in the Female Rat. Developmental Neuroscience, 19, 430–437. [DOI] [PubMed] [Google Scholar]
- Rice CJ, Sandman CA, Lenjavi MR & Baram TZ (2008) A Novel Mouse Model for Acute and Long-Lasting Consequences of Early Life Stress. Endocrinology, 149, 4892–4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR & Shuster LT (2010) Oophorectomy, Menopause, Estrogen, and Cognitive Aging: The Timing Hypothesis. Neurodegenerative Diseases, 7, 163–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J, & Writing Group for the Women’s Health Initiative Investigators (2002). Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA, 288(3), 321–333. 10.1001/jama.288.3.321 [DOI] [PubMed] [Google Scholar]
- Roth ME & Carroll ME (2004) Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology, 172, 443–449. [DOI] [PubMed] [Google Scholar]
- Roth ME, Casimir AG & Carroll ME (2002) Influence of estrogen in the acquisition of intravenously self-administered heroin in female rats. Pharmacology Biochemistry and Behavior, 72, 313–318. [DOI] [PubMed] [Google Scholar]
- Sachser N, Hennessy MB & Kaiser S. (2011) Adaptive modulation of behavioural profiles by social stress during early phases of life and adolescence. Neuroscience & Biobehavioral Reviews, 35, 1518–1533. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Pardo M, Yohn SE, López-Cruz L, SanMiguel N & Correa M. 2016. Mesolimbic Dopamine and the Regulation of Motivated Behavior In Behavioral Neuroscience of Motivation, eds. Simpson EH & Balsam PD, 231–257. Cham: Springer International Publishing. [DOI] [PubMed] [Google Scholar]
- Sanghera MK, Grady S, Smith W, Woodward DJ & Porter JC (1991) Incertohypothalamic A13 Dopamine Neurons: Effect of Gonadal Steroids on Tyrosine Hydroxylase. Neuroendocrinology, 53, 268–275. [DOI] [PubMed] [Google Scholar]
- Sárvári M, Deli L, Kocsis P, Márk L, Maász G, Hrabovszky E, Kalló I, Gajári D, Vastagh C, Sümegi B, Tihanyi K & Liposits Z. (2014) Estradiol and isotype-selective estrogen receptor agonists modulate the mesocortical dopaminergic system in gonadectomized female rats. Brain Research, 1583, 1–11. [DOI] [PubMed] [Google Scholar]
- Sasagawa T, Horii-Hayashi N, Okuda A, Hashimoto T, Azuma C & Nishi M. (2017) Long-term effects of maternal separation coupled with social isolation on reward seeking and changes in dopamine D1 receptor expression in the nucleus accumbens via DNA methylation in mice. Neuroscience Letters, 641, 33–39. [DOI] [PubMed] [Google Scholar]
- Satta R, Certa B, He D & Lasek AW (2018) Estrogen Receptor β in the Nucleus Accumbens Regulates the Rewarding Properties of Cocaine in Female Mice. International Journal of Neuropsychopharmacology, 21, 382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MV, Wang X-D & Meijer OC (2011) Early life stress paradigms in rodents: potential animal models of depression? Psychopharmacology, 214, 131–140. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Ben Dor R, Martinez PE, Guerrieri GM, Harsh VL, Thompson K, Koziol DE, Nieman LK & Rubinow DR (2015) Effects of Estradiol Withdrawal on Mood in Women With Past Perimenopausal Depression: A Randomized Clinical Trial. JAMA Psychiatry, 72, 714–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Menard TA, Smith DA, Albers HE & Sisk CL (2006) Testicular hormone exposure during adolescence organizes flank-marking behavior and vasopressin receptor binding in the lateral septum. Hormones and Behavior, 50, 477–483. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA & Sisk CL (2009) Back to the future: The organizational–activational hypothesis adapted to puberty and adolescence. Hormones and Behavior, 55, 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA & Sisk CL (2004) Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Hormones and Behavior, 45, 242–249. [DOI] [PubMed] [Google Scholar]
- Schulz KM & Sisk CL (2006) Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Molecular and Cellular Endocrinology, 254–255, 120–126. [DOI] [PubMed] [Google Scholar]
- Seeman MV (1996) The role of estrogen in schizophrenia. Journal of psychiatry & neuroscience : JPN, 21, 123–127. [PMC free article] [PubMed] [Google Scholar]
- Seeman MV & Seeman P. (2014) Is schizophrenia a dopamine supersensitivity psychotic reaction? Progress in Neuro-Psychopharmacology and Biological Psychiatry, 48, 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman SN & Walsh BT (1999) Testosterone and Depression in Aging Men. The American Journal of Geriatric Psychiatry, 7, 18–33. [PubMed] [Google Scholar]
- Seney ML, Ekong KI, Ding Y, Tseng GC & Sibille E. (2013) Sex chromosome complement regulates expression of mood-related genes. Biology of Sex Differences, 4, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley DR & Monsma FJ (1992) Molecular biology of dopamine receptors. Trends in Pharmacological Sciences, 13, 61–69. [DOI] [PubMed] [Google Scholar]
- Sibug R, Küppers E, Beyer C, Maxson SC, Pilgrim C & Reisert I. (1996) Genotype-dependent sex differentiation of dopaminergic neurons in primary cultures of embryonic mouse brain. Developmental Brain Research, 93, 136–142. [DOI] [PubMed] [Google Scholar]
- Simerly RB & Swanson LW (1986) The organization of neural inputs to the medial preoptic nucleus of the rat. Journal of Comparative Neurology, 246, 312–342. [DOI] [PubMed] [Google Scholar]
- Simerly RB & Swanson LW (1988) Projections of the medial preoptic nucleus: A Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. Journal of Comparative Neurology, 270, 209–242. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Schulz KM & Zehr JL (2003) Puberty: A Finishing School for Male Social Behavior. Annals of the New York Academy of Sciences, 1007, 189–198. [DOI] [PubMed] [Google Scholar]
- Sisk CL & Zehr JL (2005) Pubertal hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrinology, 26, 163–174. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR & Hatsukami DK (1999) Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Experimental and Clinical Psychopharmacology, 7, 274–283. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M & Mooney M. (2009) Subjective responses to intravenous nicotine: Greater sensitivity in women than in men. Experimental and Clinical Psychopharmacology, 17, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Zangen A, Thiriet N & Goldberg SR (2004) β-Endorphin elevations in the ventral tegmental area regulate the discriminative effects of Δ−9-tetrahydrocannabinol. European Journal of Neuroscience, 19, 3183–3192. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Bals-Kubik R & Shippenberg TS (1991) β-Endorphin-induced locomotor stimulation and reinforcement are associated with an increase in dopamine release in the nucleus accumbens. Psychopharmacology, 104, 51–56. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A & Shippenberg TS (1990) The Effects of Opioid Peptides on Dopamine Release in the Nucleus Accumbens: An In Vivo Microdialysis Study. Journal of Neurochemistry, 55, 1734–1740. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A & Shippenberg TS (1992) Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proceedings of the National Academy of Sciences, 89, 2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy Hooks M, Colvin AC, Juncos JL & Justice JB (1992) Individual differences in basal and cocaine-stimulated extracellular dopamine in the nucleus accumbens using quantitative microdialysis. Brain Research, 587, 306–312. [DOI] [PubMed] [Google Scholar]
- Steinberg EE, Boivin JR, Saunders BT, Witten IB, Deisseroth K & Janak PH (2014) Positive reinforcement mediated by midbrain dopamine neurons requires D1 and D2 receptor activation in the nucleus accumbens. PLoS ONE, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J & Rajabi H. (1994) Estradiol derived from testosterone in prenatal life affects the development of catecholamine systems in the frontal cortex in the male rat. Brain Research, 646, 157–160. [DOI] [PubMed] [Google Scholar]
- Succu S, Sanna F, Melis T, Boi A, Argiolas A & Melis MR (2007) Stimulation of dopamine receptors in the paraventricular nucleus of the hypothalamus of male rats induces penile erection and increases extra-cellular dopamine in the nucleus accumbens: Involvement of central oxytocin. Neuropharmacology, 52, 1034–1043. [DOI] [PubMed] [Google Scholar]
- Surakul P, Weerachatyanukul W & Chutabhakdikul N. (2011) Repeated carbenoxolone injections during late pregnancy alter Snk-SPAR and PSD-95 expression in the hippocampus of rat pups. Neuroscience Letters, 494, 75–79. [DOI] [PubMed] [Google Scholar]
- Thompson TL (1999) Attenuation of dopamine uptake in vivo following priming with estradiol benzoate. Brain Research, 834, 164–167. [DOI] [PubMed] [Google Scholar]
- Thompson TL & Moss RL (1997) Modulation of mesolimbic dopaminergic activity over the rat estrous cycle. Neuroscience Letters, 229, 145–148. [DOI] [PubMed] [Google Scholar]
- Tobiansky DJ (2018) Understanding how Age-Related Decline in Testosterone Affects Male Sexual Behavior: Neurosteroids as the Missing Piece. Current Sexual Health Reports, 10, 305–314. [Google Scholar]
- Tobiansky DJ, Will RG, Lominac KD, Turner JM, Hattori T, Krishnan K, Martz JR, Nutsch VL & Dominguez JM (2016) Estradiol in the Preoptic Area Regulates the Dopaminergic Response to Cocaine in the Nucleus Accumbens. Neuropsychopharmacology, 41, 1897–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda MC, Yamaguchi N & Ogawa S. (2011) Early life stress disrupts peripubertal development of aggression in male mice. Neuroreport., 22, 259–263. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Tempel A, Koob GF & Zukin RS (1989) Characterization of opioid receptors in rat nucleus accumbens following mesolimbic dopaminergic lesions. Brain Research, 505, 111–118. [DOI] [PubMed] [Google Scholar]
- Vagell ME & McGinnis MY (1997) The role of aromatization in the restoration of male rat reproductive behavior. Journal of Neuroendocrinology, 9, 415–421. [DOI] [PubMed] [Google Scholar]
- van den Driesche S, Scott HM, MacLeod DJ, Fisken M, Walker M & Sharpe RM (2011) Relative importance of prenatal and postnatal androgen action in determining growth of the penis and anogenital distance in the rat before, during and after puberty. International Journal of Andrology, 34, e578–e586. [DOI] [PubMed] [Google Scholar]
- Van Etten ML & Anthony JC (2001) Male-Female Differences in Transitions from First Drug Opportunity to First Use: Searching for Subgroup Variation by Age, Race, Region, and Urban Status. Journal of Women’s Health & Gender-Based Medicine, 10, 797–804. [DOI] [PubMed] [Google Scholar]
- van Honk J, Schutter DJLG, Hermans EJ, Putman P, Tuiten A & Koppeschaar H. (2004) Testosterone shifts the balance between sensitivity for punishment and reward in healthy young women. Psychoneuroendocrinology, 29, 937–943. [DOI] [PubMed] [Google Scholar]
- Vandegrift BJ, You C, Satta R, Brodie MS & Lasek AW (2017) Estradiol increases the sensitivity of ventral tegmental area dopamine neurons to dopamine and ethanol. PLoS ONE, 12, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Blume A, Niederle D, Buwalda B & Neumann ID (2006) Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. European Journal of Neuroscience, 24, 1711–1720. [DOI] [PubMed] [Google Scholar]
- Velázquez-Zamora DA, Garcia-Segura LM & González-Burgos I. (2012) Effects of selective estrogen receptor modulators on allocentric working memory performance and on dendritic spines in medial prefrontal cortex pyramidal neurons of ovariectomized rats. Hormones and Behavior, 61, 512–517. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Logan J, Gatley SJ, Wong C, Hitzemann R & Pappas NR (1999) Reinforcing Effects of Psychostimulants in Humans Are Associated with Increases in Brain Dopamine and Occupancy of D2Receptors. Journal of Pharmacology and Experimental Therapeutics, 291, 409. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Ding YS, Logan J, Dewey SL, Hitzemann R & Lieberman J. (1996) Relationship between psychostimulant-induced “high” and dopamine transporter occupancy. Proceedings of the National Academy of Sciences of the United States of America, 93, 10388–10392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vom Saal FS & Bronson FH (1978) In Utero Proximity of Female Mouse Fetuses to Males: Effect on Reproductive Performance during Later Life. Biology of Reproduction, 19, 842–853. [DOI] [PubMed] [Google Scholar]
- Vom Saal FS, Quadagno DM, Even MD, Keisler LW, Keisler DH & Khan S. (1990) Paradoxical Effects of Maternal Stress on Fetal Steroids and Postnatal Reproductive Traits in Female Mice from Different Intrauterine Positions1. Biology of Reproduction, 43, 751–761. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Meade JR, Harney JP & Frye CA (2006) Estradiol-Induced Conditioned Place Preference may Require Actions at Estrogen Receptors in the Nucleus Accumbens. Neuropsychopharmacology, 32, 522. [DOI] [PubMed] [Google Scholar]
- Walker C-D, Bath KG, Joels M, Korosi A, Larauche M, Lucassen PJ, Morris MJ, Raineki C, Roth TL, Sullivan RM, Taché Y & Baram TZ (2017) Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential. Stress, 20, 421–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M, Luine V, Arellanos A & Frankfurt M. (2006) Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Research, 1126, 176–182. [DOI] [PubMed] [Google Scholar]
- Ward IL (1972) Prenatal Stress Feminizes and Demasculinizes the Behavior of Males. Science, 175, 82. [DOI] [PubMed] [Google Scholar]
- Ward IL & Weisz J. (1984) Differential Effects of Maternal Stress on Circulating Levels of Corticosterone, Progesterone, and Testosterone in Male and Female Rat Fetuses and Their Mothers*. Endocrinology, 114, 1635–1644. [DOI] [PubMed] [Google Scholar]
- Wersinger SR & Rissman EF (2000) Dopamine Activates Masculine Sexual Behavior Independent of the Estrogen Receptor α. The Journal of Neuroscience, 20, 4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek C, Perry AN & Becker JB (2013) Pair housing differentially affects motivation to self-administer cocaine in male and female rats. Behavioural brain research, 252, 68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FJ, Bednarz LM, Wachtel SR, Hjorth S & Brooderson RJ (1988) Is stimulation of both D1 and D2 receptors necessary for the expression of dopamine-mediated behaviors? Pharmacology Biochemistry and Behavior, 30, 189–193. [DOI] [PubMed] [Google Scholar]
- Will RG, Hull EM & Dominguez JM (2014) Influences of dopamine and glutamate in the medial preoptic area on male sexual behavior. Pharmacology Biochemistry and Behavior, 121, 115–123. [DOI] [PubMed] [Google Scholar]
- Wines JD Jr, Gruber AJ, Pope HG Jr & Lukas SE (1999) Nalbuphine Hydrochloride Dependence in Anabolic Steroid Users. The American Journal on Addictions, 8, 161–164. [DOI] [PubMed] [Google Scholar]
- Wright CL, Burks SR & McCarthy MM (2008) Identification of prostaglandin E2 receptors mediating perinatal masculinization of adult sex behavior and neuroanatomical correlates. Developmental Neurobiology, 68, 1406–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L & Becker JB (1994) Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: effects of estrous cycle and gonadectomy. Neuroscience Letters, 180, 155–158. [DOI] [PubMed] [Google Scholar]
- Yamanouchi K & Arai Y. (1976) Heterotypical Sexual Behavior in Male Rats: Individual Difference in Lordosis Response. Endocrinologia Japonica, 23, 179–182. [DOI] [PubMed] [Google Scholar]
- Yang S-C & Shieh K-R (2007) Gonadal hormones-mediated effects on the stimulation of dopamine turnover in mesolimbic and nigrostriatal systems by cocaine- and amphetamine-regulated transcript (CART) peptide in male rats. Neuropharmacology, 53, 801–809. [DOI] [PubMed] [Google Scholar]
- Yoest KE, Cummings JA & Becker JB (2019) Oestradiol influences on dopamine release from the nucleus accumbens shell: sex differences and the role of selective oestradiol receptor subtypes. British Journal of Pharmacology, 176, 4136–4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Midgley AR, Carlson NE & Brown MB (2000) Alteration in the Hypothalamic-Pituitary-Ovarian Axis in Depressed Women. Archives of General Psychiatry, 57, 1157–1162. [DOI] [PubMed] [Google Scholar]
- Young JW & Geyer MA (2010) Action of Modafinil—Increased Motivation Via the Dopamine Transporter Inhibition and D1 Receptors? Biological Psychiatry, 67, 784–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, An S, Tai F, Wang J, Wu R & Wang B. (2013) Early social deprivation impairs pair bonding and alters serum corticosterone and the NAcc dopamine system in mandarin voles. Psychoneuroendocrinology, 38, 3128–3138. [DOI] [PubMed] [Google Scholar]
- Zarrouf FA, Artz S, Griffith J, Sirbu C & Kommor M. (2009) Testosterone and Depression. Journal of psychiatric practice., 15, 289–305. [DOI] [PubMed] [Google Scholar]
- Zehr JL, Gans SE & McClintock MK (2001) Variation in reproductive traits is associated with short anogenital distance in female rats. Developmental Psychobiology, 38, 229–238. [DOI] [PubMed] [Google Scholar]
- Zhang D, Yang S, Yang C, Jin G & Zhen X. (2008) Estrogen regulates responses of dopamine neurons in the ventral tegmental area to cocaine. Psychopharmacology, 199, 625–635. [DOI] [PubMed] [Google Scholar]