Abstract
In the present work, we report a new class of potent steroid sulphatase (STS) inhibitors based on 6-(1-phenyl-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamate derivatives. Within the set of new STS inhibitors, 6-(1-(1,2,3-trifluorophenyl)-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamate 3L demonstrated the highest activity in the enzymatic assay inhibiting the STS activity to 7.98% at 0.5 µM concentration. Furthermore, to verify whether the obtained STS inhibitors are able to pass through the cellular membrane effectively, cell line experiments have been carried out. We found that the lowest STS activities were measured in the presence of compound 3L (remaining STS activity of 5.22%, 27.48% and 99.0% at 100, 10 and 1 nM concentrations, respectively). The measured STS activities for Irosustat (used as a reference) were 5.72%, 12.93% and 16.83% in the same concentration range. Moreover, a determined IC50 value of 15.97 nM for 3L showed that this compound is a very promising candidate for further preclinical investigations.
Keywords: Steroid sulphatase, hormone-dependent cancer, breast cancer, STS inhibitors, triazoles
Introduction
Cancer is the second leading cause of death worldwide. As reported by International Agency for Research on Cancer, there were more than 18 million new cases diagnosed and 9.5 million tumour-related deaths in 2018 worldwide1. The most common types of cancer are lung, breast, colorectal, prostate, and stomach, which constitute almost 50% of all tumour cases. Moreover, lung, colorectal, stomach, liver, and breast cancers are responsible for nearly 50% of all deaths. National Cancer Institute estimates that more than 270 000 (15.3%) new cases of breast cancer will be diagnosed and more than 42 000 (7.0%) deaths will be reported of this disease in the United States of America in 20202. Approximately 95% of breast cancer cases in the primary stage are hormone-sensitive, and therefore, biological active hormones (including oestrogens and androgens) play a crucial role in the proliferation of tumours cells3. The biosynthesis of active steroids in cancer tissues mainly depends on the following three enzymatic pathways: aromatase (responsible for the transformation of androgens into oestrogens), 17β-hydroxysteroid dehydrogenase (implicated in the reduction of oestrone to oestradiol) and steroid sulphatase (STS). STS acts by hydrolysing steroid sulphates (including oestrone sulphate and dehydroepiandrosterone sulphate) and therefore plays a pivotal role in human steroidogenesis process4–7. Current breast cancer therapies are based on chemotherapeutics that act as selective oestrogen receptor modulators (SERMs) (e.g. Tamoxifen) or inhibitors of the aromatase enzyme complex (e.g. Letrozole or Anastrozole)8–10. Unfortunately, both methods of treatment are unsatisfactory. The main reason for the failure of the therapy based on aromatase inhibitors is the fact that aromatase expression is significantly lower than STS11. Furthermore, there are studies demonstrating a significant increment of STS and 17β-HSD1 following aromatase inhibitor therapy of ER positive postmenopausal breast carcinoma patients due to the compensatory response of breast carcinoma tissues to oestrogen depletion12.
Nowadays, STS has been considered as an attractive molecular target for the development of hormone-dependent cancer therapies, and therefore, the synthesis of new, efficient, selective STS inhibitors is of particular importance for modern medicinal chemistry. Recently, there has been intensive research towards finding novel STS inhibitors. Scientists have developed, both, steroidal and non-steroidal compounds containing various functional groups (e.g. sulphamate and phosphorus moieties)13. For example, one of the most promising drug candidate based on a sulphamoylated coumarin core is Irosustat (also known as 667-COUMATE or STX64). Irosustat is in clinical trials (phase II clinical studies) and exhibits quite good results towards treating hormone-dependent breast cancer (without having in vivo and in vitro oestrogenic properties)14–20. Although Irosustat showed very promising clinical effects in the treatment of hormone-dependent tumours, in relation to endometrium (where occurs high STS activity) Irosustat did not demonstrate activity sufficient for future commercial development21. For this reason, the search for more effective STS inhibitors is still ongoing.
In the present work, we described our recent research on the design, synthesis, and biological evaluation of compounds based on non-steroidal core containing triazole and naphthalene rings in their structure as new and potent STS inhibitors. The presence of the triazole ring in the structure of obtained compounds is justified by the fact that triazoles are a class of compounds showing very attractive properties and are successfully used in the design of new pharmaceuticals22. One of the most important triazoles feature is that they do not undergo hydrolysis under acidic, basic, and redox conditions and they are stable to metabolic degradation. Moreover, these compounds are weakly acidic and weakly basic. Some properties like hydrogen bond formation, π-π stacking interaction, strong dipole moments, and bioisosteric effects make triazole derivatives an attractive type of compounds in the field of medicinal chemistry. Despite the fact that 1,2,3-triazoles do not exist in the nature23 such compounds exhibit many biological activities (e.g. anticancer24,25, antimicrobial26,27, anti-infective28).
Materials and methods
General methods and materials
6-Bromo-2-naphthol, trimethylsilylacetylene, palladium(II) chloride, triphenylphosphine, copper(I) iodide, triethylamine, the appropriate aniline derivatives, tert-butyl nitrite (t-BuONO), azidotrimethylsilane (TMSN3), 1 M solution of tetrabutylammonium fluoride (TBAF) in tetrahydrofuran (THF), sodium ascorbate, copper(II) sulphate pentahydrate, chlorosulphonyl isocyanate, N,N-dimethylacetamide (N,N-DMA), and formic acid were commercially available from Sigma-Aldrich. Radiolabelled [3H] oestrone sulphate for enzyme and cellular assays was purchased from PerkinElmer. Acetonitrile (ACN) and dichloromethane (DCM) were dried and distilled using standard procedures. Melting points (uncorrected) were determined with a Stuart Scientific SMP30 apparatus. Infra-red spectra were measured on a Nicolet 8700 spectrometer. 1H and 13C NMR spectra were recorded on a Bruker Avance III HD 400 MHz spectrometer. Chemical shifts δ are reported in parts per million relative to the residual solvent peak (CDCl3 = 7.26 ppm for 1H, 77.0 ppm for 13C, DMSO-d6 2.49 ppm for 1H, and 39.5 ppm for 13C). Coupling constants are given in Hertz. Mass spectra were recorded on an Agilent 6540 Accurate Mass Q-TOF LC/MS System. Elemental analysis was performed using CHNS-Carlo Erba EA-1108. Preparative thin-layer chromatography (TLC) was carried out with Polygram SIL G/UV254, silica gel (Macherey-Nagel GmbH & Co. KG, Düren, Germany). Column chromatography was performed using silica gel 60 (230-400 mesh, Merck).
General method for the synthesis of 6-((trimethylsilyl)ethynyl)naphthalen-2-ol 1
A solution of 6-bromo-2-naphthol (892 mg, 4 mmol), trimethylsilylacetylene (0.831 ml, 6 mmol), palladium(II) chloride (35.5 mg, 0.20 mmol), triphenylphosphine (105 mg, 0.40 mmol), copper(I) iodide (19 mg, 0.10 mmol) and triethylamine (3.94 ml, 28.2 mmol) in dry ACN (20 ml) was refluxed under an argon atmosphere. After 3 h of heating, the reaction mixture was cooled down and filtered. The filtrate was concentrated under reduced pressure and crude product was purified with column chromatography using AcOEt/hexane (1:4) as eluent to afford the desired compound 1.
6-((Trimethylsilyl)ethynyl)naphthalen-2-ol 1 Yield 68%; mp 92-94 °C; νmax (KBr)/cm−1 3245, 2166, 1602, 1506, 1248, 941, 836, 703; 1H NMR δH (400 MHz, CDCl3) 7.96 (1H, s, Ar-H), 7.73-7.70 (1H, m, Ar-H), 7.61 (1H, d, J = 8.5 Hz, Ar-H), 7.49 (1H, dd, J = 8.5, 1.6 Hz, Ar-H), 7.14-7.11 (2H, m, Ar-H), 5.55-4.53 (1H, brs, OH) 0.31 (9H, s, CH3); 13C NMR δC (101 MHz, CDCl3) 154.1, 134.2, 132.0, 129.9, 129.3, 128.3, 126.3, 118.4, 118.1, 109.6, 105.7, 93.8, 0.1; Anal. calcd for: C15H16OSi, C, 74.95; H, 6.71; O, 6.66; Si, 11.68. Found: C, 74.90; H, 6.75; O, 6.70; Si, 11.68%. HRMS (m/z) [M—H]– calcd 239.1155, found 239.0897.
General method for the synthesis of 6–(1-phenyl-1H-1,2,3-triazol-4-yl)naphthalen-2-ol derivatives 2 A-L
To an ice-cooled solution of corresponding amine (2.63 mmol) in ACN (6.1 ml), t-BuONO (325 mg, 3.16 mmol) was added dropwise, followed by TMSN3 (333 mg, 2.89 mmol). Solution was stirred at room temperature for 4 h and in the next step, 1 (632 mg, 2.63 mmol) and 1 M solution of TBAF in THF (2.89 ml) were added. The reaction mixture was stirred at 0 °C for 30 min. Subsequently, CuSO4 · 5H2O (65.7 mg, 0.263 mmol) and a freshly prepared aqueous solution (0.526 ml) of sodium ascorbate (104 mg, 0.526 mmol) were added, and the obtained solution was stirred for 24 h under an argon atmosphere at room temperature. Afterwards, the reaction mixture was concentrated under vacuum and the crude product was dissolved in AcOEt (30 ml). Obtained solution was washed with 0.1 M hydrochloric acid. After separation organic phase was dried using MgSO4, solvent was evaporated and the resulting residue was crystallised from ACN to give the desired products 2 A-L.
6-(1-Phenyl-1H-1,2,3-triazol-4-yl)naphthalen-2-ol 2A Yield 64%; mp (with decomposition) 228-230 °C; νmax (KBr)/cm−1 3293, 3137, 1598, 1504, 1228, 1036, 871, 683; 1H NMR δH (400 MHz, DMSO-d6) 9.87 (1H, s, OH), 9.35 (1H, s, CH), 8.39 (1H, s, Ar-H), 8.02-7.96 (3H, m, Ar-H), 7.86 (1H, d, J = 8.8 Hz, Ar-H), 7.82 (1H, d, J = 8.7 Hz, Ar-H), 7.65 (2H, t, J = 7.9 Hz, Ar-H), 7.53 (1H, t, J = 7.4 Hz, Ar-H), 7.18 (1H, d, J = 2.3 Hz, Ar-H), 7.15 (1H, dd, J = 8.7, 2.4 Hz, Ar-H); 13C NMR δC (101 MHz, DMSO-d6) 156.2, 148.2, 137.2, 134.9, 130.4, 130.2, 129.1, 128.2, 127.3, 125.0, 124.4, 124.3, 120.4, 119.8, 119.7, 109.3; Anal. calcd for: C18H13N3O, C, 75.25; H, 4.56; N, 14.63; O, 5.57. Found: C, 75.31; H, 4.49; N, 14.71; O, 5.49%. HRMS (m/z) [M + H]+ calcd 288.1132, found 288.3504.
6-(1-(3-Fluorophenyl)-1H-1,2,3-triazol-4-yl)naphthalen-2-ol 2B Yield 43%; mp (with decomposition) 219-220 °C; νmax (KBr)/cm−1 3251, 3125, 2352, 1600, 1498, 1231, 1033, 870, 677; 1H NMR δH (400 MHz, DMSO-d6) 9.88 (1H, s, OH), 9.40 (1H, s, CH), 8.37 (1H, s, Ar-H), 7.95 (1H, dd, J = 8.5, 1.7 Hz, Ar-H), 7.93-7.80 (4H, m, Ar-H), 7.70 (1H, td, J = 8.3, 6.5 Hz, Ar-H), 7.38 (1H, tdd, J = 8.5, 2.5, 0.7 Hz, Ar-H), 7.18 (1H, d, J = 2.1 Hz, Ar-H), 7.15 (1H, dd, J = 8.7, 2.4 Hz, Ar-H); 13C NMR δC (101 MHz, DMSO-d6) 162.9 (d, 1JC-F = 245.1 Hz), 156.3, 148.3, 138.4 (d, 3JC-F = 10.5 Hz), 134.9, 132.4 (d, 3JC-F = 9.2 Hz), 130.2, 128.1, 127.3, 124.8, 124.3, 119.9, 119.8, 116.3 (d, 4JC-F = 3.0 Hz), 115.8 (d, 2JC-F = 21.0 Hz), 109.3, 107.9 (d, 2JC-F = 26.5 Hz); Anal. calcd for: C18H12FN3O, C, 70.81; H, 3.96; F, 6.22; N, 13.76; O, 5.24. Found: C, 70.79; H, 3.99; F, 6.24; N, 13.69; O, 5.29%. HRMS (m/z) [M + H]+ calcd 306.1038, found 306.3540.
6-(1-(3-Chlorophenyl)-1H-1,2,3-triazol-4-yl)naphthalen-2-ol 2C Yield 45%; mp (with decomposition) 229-231 °C; νmax (KBr)/cm−1 3254, 3129, 2345, 1598, 1221, 1035, 869, 679; 1H NMR δH (400 MHz, DMSO-d6) 9.88 (1H, s, OH), 9.43 (1H, s, CH), 8.37 (1H, s, Ar-H), 8.12 (1H, t, J = 2.0 Hz, Ar-H), 8.01 (1H, ddd, J = 8.1, 2.0, 0.9 Hz, Ar-H), 7.95 (1H, dd, J = 8.5, 1.7 Hz, Ar-H), 7.86 (1H, d, J = 8.8 Hz, Ar-H), 7.82 (1H, d, J = 8.6 Hz, Ar-H) 7.68 (1H, t, J = 8.1 Hz, Ar-H), 7.59 (1H, ddd, J = 8.1, 1.9, 0.9 Hz, Ar-H), 7.19-7.17 (1H, m, Ar-H), 7.15 (1H, dd, J = 8.7, 2.4 Hz, Ar-H); 13C NMR δC (101 MHz, DMSO-d6) 156.3, 148.3, 138.2, 134.9, 134.7, 132.2, 130.2, 128.9, 128.1, 127.3, 124.8, 124.3, 120.2, 119.9, 119.8, 118.9, 109.3; Anal. calcd for: C18H12ClN3O, C, 67.19; H, 3.76; Cl, 11.02; N, 13.06; O, 4.97. Found: C, 67.09; H, 3.84; Cl, 11.09; N, 12.99; O, 4.99%. HRMS (m/z) [M + H]+ calcd 321.0669, found 322.3397.
6-(1-(3-Bromophenyl)-1H-1,2,3-triazol-4-yl)naphthalen-2-ol 2D Yield 42%; mp (with decomposition) 226-228 °C; νmax (KBr)/cm−1 3271, 3130, 2922, 2350, 1583, 1489, 1034, 782; 1H NMR δH (400 MHz, DMSO-d6) 9.88 (1H, s, OH), 9.43 (1H, s, CH), 8.37 (1H, s, Ar-H), 8.24 (1H, s, Ar-H), 8.04 (1H, d, J = 8.1 Hz, Ar-H), 7.95 (1H, d, J = 8.2 Hz, Ar-H), 7.86 (1H, d, J = 8.8 Hz, Ar-H), 7.82 (1H, d, J = 8.6 Hz, Ar-H), 7.72 (1H, d, J = 7.8 Hz, Ar-H), 7.61 (1H, t, J = 8.0 Hz, Ar-H), 7.18 (1H, s, Ar-H), 7.15 (1H, d, J = 8.9 Hz, Ar-H); 13C NMR δC (101 MHz, DMSO-d6) 156.3, 148.3, 138.3, 134.9, 132.4, 131.8, 130.2, 128.1, 127.3, 124.8, 124.3, 123.0, 122.9, 119.9, 119.8, 119.3, 109.3; Anal. calcd for: C18H12BrN3O, C, 59.03; H, 3.30; Br, 21.82; N, 11.47; O, 4.37. Found: C, 59.11; H, 3.28; Br, 21.87; N, 11.50; O, 4.24%. HRMS (m/z) [M + H]+ calcd 366.0237, found 366.3458.
6-(1-(4-Fluorophenyl)-1H-1,2,3-triazol-4-yl)naphthalen-2-ol 2E Yield 74%; mp (with decomposition) 237-239 °C; νmax (KBr)/cm−1 3651, 3199, 3124, 2920, 2347, 1614, 1507, 1039, 802; 1H NMR δH (400 MHz, DMSO-d6) 9.86 (1H, s, OH), 9.33 (1H, s, CH), 8.37 (1H, s, Ar-H), 8.06-8.00 (2H, m, Ar-H), 7.96 (1H, dd, J = 8.5, 1.5 Hz, Ar-H), 7.86 (1H, d, J = 8.8 Hz, Ar-H), 7.82 (1H, d, J = 8.6 Hz, Ar-H), 7.52 (2H, t, J = 8.8 Hz, Ar-H), 7.17 (1H, s, Ar-H), 7.15 (1H, dd, J = 8.8, 2.3 Hz, Ar-H); 13C NMR δC (101 MHz, DMSO-d6) 162.1 (d, 1JC-F = 245.8 Hz), 156.2, 148.2, 134.9, 133.8 (d, 4JC-F = 2.8 Hz), 130.1, 128.2, 127.3, 125.0, 124.4, 124.2, 122.8 (d, 3JC-F = 8.8 Hz), 120.0, 119.8, 117.3 (d, 2JC-F = 23.3 Hz), 109.2; Anal. calcd for: C18H12FN3O, C, 70.81; H, 3.96; F, 6.22; N, 13.76; O, 5.24. Found: C, 70.75; H, 3.99; F, 6.27; N, 13.81; O, 5.18%. HRMS (m/z) [M + H]+ calcd 306.1038, found 306.3524.
6-(1-(4-Chlorophenyl)-1H-1,2,3-triazol-4-yl)naphthalen-2-ol 2F Yield 74%; mp (with decomposition) 256-257 °C; νmax (KBr)/cm−1 3286, 3119, 2294, 1611, 1550, 1032, 831; 1H NMR δH (400 MHz, DMSO-d6) 9.86 (1H, s, OH), 9.38 (1H, s, CH), 8.37 (1H, s, Ar-H) 8.03 (2H, d, J = 8.9 Hz, Ar-H), 7.95 (1H, dd, J = 8.5, 1.6 Hz, Ar-H), 7.86 (1H, d, J = 8.8 Hz, Ar-H), 7.82 (1H, d, J = 8.6 Hz, Ar-H), 7.73 (2H, d, J = 8.9 Hz, Ar-H), 7.17 (1H, s, Ar-H), 7.15 (1H, dd, J = 8.7, 2.3 Hz, Ar-H); 13C NMR δC (101 MHz, DMSO-d6) 156.3, 148.3, 136.0, 134.9, 133.4, 130.4, 130.2, 128.2, 127.3, 124.9, 124.4, 124.3, 122.1, 119.8, 109.3; Anal. calcd for: C18H12ClN3O, C, 67.19; H, 3.76; Cl, 11.02; N, 13.06; O, 4.97. Found: C, 67.24; H, 3.69; Cl, 11.09; N, 13.04; O, 4.49%. HRMS (m/z) [M + H]+ calcd 322.0742, found 322.3345.
6-(1-(4-Bromophenyl)-1H-1,2,3-triazol-4-yl)naphthalen-2-ol 2G Yield 62%; mp (with decomposition) 254-256 °C; νmax (KBr)/cm−1 3470, 3119, 1612, 1495, 1200, 1030, 867, 810; 1H NMR δH (400 MHz, DMSO-d6) 9.86 (1H, s, OH), 9.38 (1H, s, CH), 8.37 (1H, s, Ar-H), 7.99-7.93 (3H, m, Ar-H), 7.88-7.80 (4H, m, Ar-H), 7.17 (1H, s, Ar-H), 7.15 (1H, dd, J = 8.7, 2.4 Hz, Ar-H); 13C NMR δC (101 MHz, DMSO-d6) 156.3, 148.3, 136.4, 134.9, 133.3, 130.2, 128.1, 127.3, 124.9, 124.4, 124.3, 122.3, 121.7, 119.8, 119.7, 109.3; Anal. calcd for: C18H12BrN3O, C, 59.03; H, 3.30; Br, 21.82; N, 11.47; O, 4.37. Found: C, 59.07; H, 3.32; Br, 21.78; N, 11.54; O, 4.29%. HRMS (m/z) [M + H]+ calcd 366.0237, found 366.3154.
6-(1-(2-Fluorophenyl)-1H-1,2,3-triazol-4-yl)naphthalen-2-ol 2H Yield 47%; mp (with decomposition) 207-208 °C; νmax (KBr)/cm−1 3177, 1614, 1511, 1048, 864, 819; 1H NMR δH (400 MHz, DMSO-d6) 9.86 (1H, s, OH), 9.12 (1H, d, J = 1.9 Hz, CH), 8.41 (1H, s, Ar-H), 8.02-7.91 (2H, m, Ar-H), 7.83 (2H, dd, J = 20.5, 8.7 Hz, Ar-H), 7.68-7.58 (2H, m, Ar-H), 7.54-7.45 (1H, m, Ar-H), 7.19-7.09 (2H, m, Ar-H); 13C NMR δC (101 MHz, DMSO-d6) 156.3, 154.3 (d, 1JC-F = 250.8 Hz), 147.8, 134.8, 131.8 (d,
2JC-F = 7.8 Hz), 130.2, 128.2, 127.3, 126.5, 126.1 (d, 3JC-F = 3.7 Hz), 125.3 (d, 2JC-F = 11.0 Hz), 124.8, 124.4, 122.9 (d, 3JC-F = 4.1 Hz), 119.8, 117.8, 117.6, 108.9; Anal. calcd for: C18H12FN3O, C, 70.81; H, 3.96; F, 6.22; N, 13.76; O, 5.24. Found: C, 70.78; H, 3.99; F, 6.28; N, 13.70; O, 5.25%. HRMS (m/z) [M + H]+ calcd 306.1038, found 306.3529.
6-(1-(2-Chlorophenyl)-1H-1,2,3-triazol-4-yl)naphthalen-2-ol 2I Yield 62%; mp 104-106 °C; νmax (KBr)/cm−1 3133, 1616, 1493, 1493, 1196, 1050, 862, 804; 1H NMR δH (400 MHz, DMSO-d6) 9.86 (1H, s, OH), 9.08 (1H, s, CH), 8.39 (1H, s, Ar-H), 7.96 (1H, dd, J = 8.5, 1.6 Hz, Ar-H), 7.90-7.75 (4H, m, Ar-H), 7.73–7.59 (2H, m, Ar-H), 7.21-7.11 (2H, m, Ar-H); 13C NMR δC (101 MHz, DMSO-d6) 156.3, 147.4, 135.1, 134.9, 132.2, 131.1, 130.2, 129.0, 128.9, 128.8, 128.2, 127.3, 124.9, 124.4, 124.3, 123.7, 119.8, 109.3; Anal. calcd for: C18H12ClN3O, C, 67.19; H, 3.76; Cl, 11.02; N, 13.06; O, 4.97. Found: C, 67.22; H, 3.70; Cl, 11.08; N, 13.11; O, 4.89%. HRMS (m/z) [M + H]+ calcd 322.0742, found 322.3377.
6-(1-(2-Bromophenyl)-1H-1,2,3-triazol-4-yl)naphthalen-2-ol 2J Yield 68%; mp 105-107 °C; νmax (KBr)/cm−1 3135, 1611, 1493, 1283, 1048, 909; 1H NMR δH (400 MHz, DMSO-d6) 9.86 (1H, s, OH), 9.05 (1H, s, CH), 8.39 (1H, s, Ar-H), 7.97-7.95 (2H, m, Ar-H), 7.83 (2H, dd, J = 18.6, 8.7 Hz, Ar-H), 7.77 (1H, dd, J = 7.8, 1.6 Hz), 7.67 (1H, td, J = 7.7, 1.4 Hz, Ar-H), 7.60 (1H, td, J = 7.7, 1.4 Hz, Ar-H), 7.21-7.18 (1H, m, Ar-H), 7.14 (1H, dd, J = 8.8, 2.4 Hz, Ar-H); 13C NMR δC (101 MHz, DMSO-d6) 156.2, 147.3, 136.8, 134.9, 134.2, 132.5, 130.2, 129.5, 129.2, 128.2, 127.3, 125.0, 124.4, 124.3, 123.7, 119.8, 119.4, 109.3; Anal. calcd for: C18H12BrN3O, C, 59.03; H, 3.30; Br, 21.82; N, 11.47; O, 4.37. Found: C, 59.10; H, 3.26; Br, 21.86; N, 11.36; O, 4,42%. HRMS (m/z) [M + H]+ calcd 366.0237, found 366.3130.
6-(1-(3,5-Difluorophenyl)-1H-1,2,3-triazol-4-yl)naphthalen-2-ol 2K Yield 39%; mp (with decomposition) 210-212 °C; νmax (KBr)/cm−1 3128, 1607, 1482, 1303, 1050, 911; 1H NMR δH (400 MHz, DMSO-d6) 9.88 (1H, s, OH), 9.43 (1H, s, CH), 8.35 (1H, s, Ar-H), 7.95-7.80 (4H, m, Ar-H), 7.46 (1H, tt, J = 9.2, 2.2 Hz, Ar-H), 7.20-7.17 (2H, m, Ar-H), 7.15 (1H, dd, J = 8.7, 2.4 Hz, Ar-H); 13C NMR δC (101 MHz, DMSO-d6) 163.2 (dd, 1JC-F = 246.9, 3JC-F = 14.6 Hz), 156.4, 148.4, 138.9 (t, 3JC-F = 13.3 Hz), 135.0, 130.2, 128.1, 127.4, 124.6, 124.4, 124.2, 120.0, 119.9, 109.3, 104.4, 104; Anal. calcd for: C18H11F2N3O, C, 66.87; H, 3.43; F, 11.75; N, 13.00; O, 4.95. Found: C, 66.90; H, 3.39; F, 11.83; N, 12.96; O, 4.92%. HRMS (m/z) [M + H]+ calcd 324.0943, found. 324.3554.
6-(1-(2,3,4-Trifluorophenyl)-1H-1,2,3-triazol-4-yl)naphthalen-2-ol 2L Yield 13%; mp (with decomposition) 212-214 °C; νmax (KBr)/cm−1 3175, 1630, 1509, 1305, 1043, 912; 1H NMR δH (400 MHz, DMSO-d6) 9.88 (1H, s, OH), 9.14 (1H, d, J = 1.7 Hz, CH), 8.40 (1H, s, Ar-H), 7.97 (1H, dd, J = 8.5, 1.6 Hz, Ar-H), 7.89-7.77 (3H, m, Ar-H), 7.65 (1H, qd, J = 8.5, 1.6 Hz, Ar-H), 7.18-7.16 (1H, m, Ar-H), 7.14 (1H, dd, J = 8.7, 2.3 Hz, Ar-H); 13C NMR δC (101 MHz, DMSO-d6) 156.3, 152.4-152.0 (m), 149.8-149.5 (m), 147.9, 145.8-145.4 (m), 143.2-142.9 (m), 141.6-141.2 (m), 139.2-138.7 (m), 135.0, 130.2, 128.1, 127.3, 124.5, 124.5, 124.4, 123.1 (dd, 3JC-F = 8.3, 4JC-F = 3.7 Hz), 122.9 (d, 4JC-F = 3.3 Hz), 121.0 (dd, 3JC-F = 8.5, 4JC-F = 3.9 Hz), 119.8, 113.8 (dd, 2JC-F = 18.6, 3JC-F = 3.8 Hz), 109.; Anal. calcd for: C18H10F3N3O, C, 63.35; H, 2.95; F, 16.70; N, 12.31; O, 4.69. Found: C, 63.41; H, 2.89; F, 16.78; N, 12.39; O, 4.53%. HRMS (m/z) [M + H]+ calcd 342.0849, found. 342.3574.
General method for the synthesis of 6-(1-phenyl-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamate derivatives 3A-L
A solution of chlorosulphonyl isocyanate (212.0 mg, 1.50 mmol) in anhydrous DCM (0.5 ml) was prepared. In the next step, a solution of formic acid (70.9 mg, 1.54 mmol) and N,N-DMA (1.4 mg, 0.016 mmol) was added, and the reaction mixture was stirred at 40 °C for 3.5 h. Then, a solution of the corresponding derivative 2A-L (1.00 mmol) in N,N-DMA (3.4 ml) was added, and the obtained solution was stirred at room temperature overnight. Subsequently, reaction mixture was poured into water (50 ml). The resulting precipitate was filtered and washed with water. The crude product was recrystallized from ACN to give the desired products 3A-L.
6-(1-Phenyl-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamate 3A Yield 70%; mp (with decomposition) 210-211 °C; νmax (KBr)/cm−1 3119, 2915, 2358, 1595, 1352, 1168, 759, 672; 1H NMR δH (400 MHz, DMSO-d6) 9.47 (1H, s, CH), 8.59 (1H, s, Ar-H), 8.16 (1H, dd, J = 8.6, 1.5 Hz, Ar-H), 8.15-8.10 (4H, m, Ar-H, NH2), 8.00 (2H, d, J = 7.6 Hz, Ar-H), 7.88 (1H, d, J = 2.2 Hz, Ar-H), 7.67 (2H, t, J = 7.9 Hz, Ar-H), 7.54 (1H, t, J = 7.4 Hz, Ar-H), 7.51 (1H, dd, J = 8.9, 2.4 Hz, Ar-H); 13C NMR δC (101 MHz, DMSO-d6) 148.5, 147.6, 137.1, 133.4, 131.9, 130.5, 130.4, 129.3, 129.1, 128.4, 125.0, 124.0, 122.9, 120.6, 120.5, 119.8; Anal. calcd for: C18H14N4O3S, C, 59.01; H, 3.85; N, 15.29; O, 13.10; S, 8.75. Found: C, 58.98; H, 3.88; N, 15.34; O, 13.12; S, 8.68%. HRMS (m/z) [M + H]+ calcd 367.0860, found 367.3750.
6-(1-(3-Fluorophenyl)-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamate 3B Yield 43%; mp (with decomposition) 210-212 °C; νmax (KBr)/cm−1 3120, 2560, 1600, 1358, 1173, 782, 673; 1H NMR δH (400 MHz, DMSO-d6) 9.52 (1H, s, CH), 8.58 (1H, s, Ar-H), 8.18-8.06 (5H, m, Ar-H, NH2), 7.96-7.86 (3H, m, Ar-H), 7.72 (1H, q, J = 7.4 Hz, Ar-H), 7.50 (1H, dd, J = 8.9, 2.2 Hz, Ar-H), 7.41 (1H, td, J = 8.7, 2.3 Hz, Ar-H); 13C NMR δC (101 MHz, DMSO-d6) 162.9 (d, 1JC-F = 245.2 Hz), 148.6, 147.7, 138.3, (d, 3JC-F = 10.5 Hz), 133.5, 132.4 (d, 3JC-F = 9.1 Hz), 131.9, 130.5, 129.2, 128.2, 125.0, 124.1, 122.9, 120.8, 119.8, 116.4 (d, 4JC-F = 3.0 Hz), 116.0 (d, 2JC-F = 21.0 Hz), 108.0 (d, 2JC-F = 26.5 Hz); Anal. calcd for: C18H13FN4O3S, C, 56.24; H, 3.41; F, 4.94; N, 14.58; O, 12.49; S, 8.34. Found: C, 56.30; H, 3.45; F, 4.90; N, 14.60; O, 12.41; S, 8,43%. HRMS (m/z) [M + H]+ calcd 385.0766, found 385.3810.
6-(1-(3-Chlorophenyl)-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamate 3C Yield 67%; mp (with decomposition) 214-216 °C; νmax (KBr)/cm−1 3132, 2915, 2376, 1591, 1381, 1177, 784, 676; 1H NMR δH (400 MHz, DMSO-d6) 9.53 (1H, s, CH), 8.57 (1H, s, Ar-H), 8.16-8.07 (6H, m, Ar-H, NH2), 8.01 (1H, dd, J = 7.9, 1.2 Hz, Ar-H), 7.88 (1H, d, J = 2.1 Hz, Ar-H), 7.69 (1H, t, J = 8.1 Hz, Ar-H), 7.61 (1H, dd, J = 8.0, 0.8 Hz, Ar-H), 7.51 (1H, dd, J = 8.9, 2.3 Hz, Ar-H); 13C NMR δC (101 MHz, DMSO-d6) 148.5, 147.7, 138.1, 134.7, 133.5, 132.2, 131.9, 130.5, 129.2, 129.0, 128.2, 124.9, 124.1, 122.9, 120.8, 120.3, 119.8, 119.0; Anal. calcd for: C18H13ClN4O3S, C, 53.94; H, 3.27; Cl, 8.84; N, 13.98; O, 11.97; S, 8.00. Found: C, 53.98; H, 3.29; Cl, 8.80; N, 13.94; O, 11.92; S, 8.07%. HRMS (m/z) [M + H]+ calcd 401.0470, found 401.3646.
6-(1-(3-Bromophenyl)-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamate 3D Yield 34%; mp (with decomposition) 219-221 °C; νmax (KBr)/cm−1 3298, 3171, 2363, 1484, 1368, 1182, 800; 1H NMR δH (400 MHz, DMSO-d6) 9.55 (1H, s, CH), 8.58 (1H, s, Ar-H), 8.26 (1H, t, J = 1.8 Hz, Ar-H), 8.16-8.09 (5H, m, Ar-H, NH2), 8.06 (1H, dd, J = 8.1, 1.2 Hz, Ar-H), 7.88 (1H, d, J = 2.1 Hz, Ar-H), 7.75 (1H, d, J = 8.0 Hz, Ar-H), 7.63 (1H, t, J = 8.1 Hz, Ar-H), 7.50 (1H, dd, J = 8.9, 2.3 Hz, Ar-H); 13C NMR δC (101 MHz, DMSO-d6) 148.6, 147.7, 138.2, 133.5, 132.4, 132.0, 131.9, 130.5, 129.2, 128.2, 124.9, 124.1, 123.0, 122.99, 122.90, 120.9, 119.8, 119.4; Anal. calcd for: C18H13BrN4O3S, C, 48.55; H, 2.94; Br, 17.94; N, 12.58; O, 10.78; S, 7.20. Found: C, 48.59; H, 2.96; Br, 17.90; N, 12.55; O, 10.74; S, 7.26%. HRMS (m/z) [M + H]+ calcd 444.9965, found 445.3408.
6-(1-(4-Fluorophenyl)-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamate 3E Yield 36%; mp (with decomposition) 226-227 °C; νmax (KBr)/cm−1 3314, 3159, 1515, 1357, 1180, 838; 1H NMR δH (400 MHz, DMSO-d6) 9.44 (1H, s, CH), 8.57 (1H, s, Ar-H), 8.17-8.09 (5H, m, Ar-H, NH2), 8.07-8.01 (2H, m, Ar-H), 7.88 (1H, dd, J = 2.0 Hz, Ar-H), 7.58-7.46 (3H, m, Ar-H); 13C NMR δC (101 MHz, DMSO-d6) 162.2 (d, 1JC-F = 245.9 Hz), 148.5, 147.6, 133.7 (d, 4JC-F = 2.8 Hz), 133.4, 131.9, 130.5, 129.1, 128.4, 125.0, 124.0, 122.9 (d, 3JC-F = 5.0 Hz), 122.8, 120.9, 119.8, 117.3 (d, 2JC-F = 23.3 Hz); Anal. calcd for: C18H13FN4O3S, C, 56.24; H, 3.41; F, 4.94; N, 14.58; O, 12.49; S, 8.34. Found: C, 56.27; H, 3.37; F, 4.93; N, 14.62; O, 12.53; S, 8.28%. HRMS (m/z) [M + H]+ calcd 385.0766, found 385.3823.
6-(1-(4-Chlorophenyl)-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamate 3F Yield 40%; mp (with decomposition) 226-229 °C; νmax (KBr)/cm−1 3372, 3269, 1502, 1352, 1173, 813; 1H NMR δH (400 MHz, DMSO-d6) 9.49 (1H, s, CH), 8.58 (1H, s, Ar-H), 8.15-8.09 (5H, m, Ar-H, NH2), 8.04 (2H, d, J = 8.9 Hz, Ar-H), 7.88 (1H, d, J = 2.2 Hz, Ar-H), 7.75 (2H, d, J = 8.8 Hz, Ar-H), 7.50 (1H, dd, J = 8.9, 2.4 Hz, Ar-H); 13C NMR δC (101 MHz, DMSO-d6) 148.5, 147.7, 135.9, 133.52, 133.48, 131.9, 130.5, 130.4, 129.2, 128.3, 125.0, 124.1, 122.9, 122.2, 120.7, 119.7; Anal. calcd for: C18H13ClN4O3S, C, 53.94; H, 3.27; Cl, 8.84; N, 13.98; O, 11.97; S, 8.00. Found: C, 53.90; H, 3.31; Cl, 8.91; N, 14.01; O, 11.94; S, 7.93%. HRMS (m/z) [M + H]+ calcd 401.0470, found 401.3596.
6-(1-(4-Bromophenyl)-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamate 3G Yield 45%; mp (with decomposition) 234-235 °C; νmax (KBr)/cm−1 3378, 3273, 3143, 1498, 1144, 710; 1H NMR δH (400 MHz, DMSO-d6) 9.50 (1H, s, CH), 8.58 (1H, s, Ar-H), 8.16-8.10 (5H, m, Ar-H, NH2), 7.97 (2H, d, J = 8.9 Hz, Ar-H), 7.89-7.86 (3H, m, Ar-H), 7.50 (1H, dd, J = 8.9, 2.3 Hz, Ar-H); 13C NMR δC (101 MHz, DMSO-d6) 148.5, 147.7, 136.3, 133.5, 133.4, 131.9, 130.5, 129.2, 128.2, 125.0, 124.1, 122.9, 122.4, 121.9, 120.7, 119.7; Anal. calcd for: C18H13BrN4O3S, C, 48.55; H, 2.94; Br, 17.94; N, 12.58; O, 10.78; S, 7.20. Found: C, 48.59; H, 2.90; Br, 17.97; N, 12.61; O, 10.81; S, 7.12%. HRMS (m/z) [M + H]+ calcd 445.3490, found 446.9945.
6-(1-(2-Fluorophenyl)-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamate 3H Yield 34%; mp (with decomposition) 206-207 °C; νmax (KBr)/cm−1 3398, 3277, 3148, 1502, 1111, 728; 1H NMR δH (400 MHz, DMSO-d6) 9.50 (1H, d, J = 1.8 Hz, CH), 8.61 (1H, s, Ar-H), 8.21-8.06 (5H, m, Ar-H, NH2), 7.95 (1H, td, J = 7.9, 1.1 Hz, Ar-H), 7.88 (1H, d, J = 2.0 Hz, Ar-H), 7.70-7.60 (2H, m, Ar-H), 7.55-7.45 (2H, m, Ar-H); 13C NMR δC (101 MHz, DMSO-d6) 154.5 (d, 1JC-F = 250.9 Hz), 148.5, 147.2, 133.5, 131.9, 130.5, 129.1, 128.2, 126.5, 126.1 (d, 3JC-F = 3.8 Hz), 125.3 (d, 2JC-F = 10.5 Hz), 125.1, 124.1, 123.8 (d, 3JC-F = 4.1 Hz), 122.9, 119.7, 117.8, 117.6; Anal. calcd for: C18H13FN4O3S, C, 56.24; H, 3.41; F, 4.94; N, 14.58; O, 12.49; S, 8.34. Found: C, 56.31; H, 3.39; F, 4.90; N,14.56; O, 12.53; S, 8.31%. HRMS (m/z) [M + H]+ calcd 385.0766, found 385.3785.
6-(1-(2-Bromophenyl)-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamate 3J Yield 52%; mp (with decomposition) 188-189 °C; νmax (KBr)/cm−1 3307, 3146, 3052, 1605, 1491, 1370, 929; 1H NMR δH (400 MHz, DMSO-d6) 9.19 (1H, s, CH), 8.60 (1H, s, Ar-H), 8.20-8.06 (5H, m, Ar-H, NH2), 7.98 (1H, dd, J = 8.0, 1.3 Hz, Ar-H), 7.88 (1H, d, J = 2.2 Hz, Ar-H), 7.79 (1H, dd, J = 7.8, 1.6 Hz, Ar-H), 7.68 (1H, td, J = 7.6, 1.4 Hz, Ar-H), 7.61 (1H, td, J = 7.7, 1.7 Hz, Ar-H), 7.50 (1H, dd, J = 8.9, 2.4 Hz, Ar-H); 13C NMR δC (101 MHz, DMSO-d6) 153.7, 150.4, 137.9, 133.1, 132.2, 132.1, 131.4, 129.0, 128.9, 128.5, 126.2, 125.8, 124.7, 122.1, 120.7, 118.7, 115.5, 114.9; Anal. calcd for: C18H13BrN4O3S, C, 48.55; H, 2.94; Br, 17.94; N, 12.58; O, 10.78; S, 7.20. Found: C, 48.59, H, 2.96; Br, 17.93; N, 12.57; O, 10.72; S, 7.23%. HRMS (m/z) [M + H]+ calcd 444.9965, found. 445.3399
6-(1-(3,5-Difluorophenyl)-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamate 3K Yield 37%; mp (with decomposition) 214-215 °C; νmax (KBr)/cm−1 3296, 3153, 3052, 1627, 1473, 1357, 916; 1H NMR δH (400 MHz, DMSO-d6) 9.55 (1H, s, CH), 8.56 (1H, s, Ar-H), 8.19-8.05 (5H, m, Ar-H, NH2), 7.88 (1H, d, J = 2.1 Hz, Ar-H), 7.85 (2H, dd, J = 7.8, 2.0 Hz, Ar-H), 7.51 (1H, dd, J = 8.8, 2.3 Hz, Ar-H), 7.46 (1H, dt, J = 9.2, 2.2 Hz, Ar-H); 13C NMR δC (101 MHz, DMSO-d6) 163.3 (dd, 1JC-F = 247.0 Hz, 3JC-F = 14.6 Hz), 148.6, 147.7, 138.8 (t, 3JC-F = 13.2 Hz), 133.6, 131.9, 130.5, 129.3, 128.0, 124.9, 124.2, 122.9, 121.0, 119.8, 104.8, 104.5, 104.; Anal. calcd for: C18H12F2N4O3S, C, 53.73; H, 3.01; F, 9.44; N, 13.92; O, 11.93; S, 7.97. Found: C, 53.76; H, 3.06; F, 9.39; N, 13.87; O, 11.96; S, 7.96%. HRMS (m/z) [M + H]+ calcd 403.0671, found. 403.3818.
6-(1-(2,3,4-Trifluorophenyl)-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamate 3L Yield 26%; mp (with decomposition) 198-200 °C; νmax (KBr)/cm−1 3262, 3175, 3021, 1618, 1509, 1384, 915; 1H NMR δH (400 MHz, DMSO-d6) 9.27 (1H, d, J = 1.6 Hz, CH), 8.61 (1H, s, Ar-H), 8.20-8.07 (5H, m, Ar-H, NH2), 7.91-7.83 (2H, m, Ar-H), 7.67 (1H, qd, J = 9.8, 2.2 Hz, Ar-H), 7.50 (1H, dd, J = 8.9, 2.3 Hz, Ar-H); 13C NMR δC (101 MHz, DMSO-d6) 152.4–152.1 (m), 149.9-149.6 (m), 148.6, 147.3, 145.9-145.6 (m), 143.3-143.0 (m), 141.7-141.2 (m), 139.2–138.7 (m), 133.5, 131.9, 130.5, 129.2, 127.9, 125.0, 124.3, 123.8 (d, 4JC-F = 3.3 Hz), 123.0 (dd, 3JC-F = 8.3, 4JC-F = 3.7 Hz), 122.9, 121.1 (dd, 3JC-F = 8.5, 4JC-F = 4.0 Hz), 119.7, 113.8 (dd, 2JC-F = 18.6, 3JC-F = 3.8 Hz); Anal. calcd for: C18H11F3N4O3S, C, 51.43; H, 2.64; F, 13.56; N, 13.33; O, 11.42; S, 7.63. Found: C, 51.39; H, 2.71; F, 13.51; N, 13.29; O, 11.48; S, 7.62%. HRMS (m/z) [M + H]+ calcd 421.0577, found. 421.3945.
Molecular docking
Ligands and molecular target preparation
The 3 D structures of the potential steroid sulphatase inhibitors (ligands) were prepared using Portable HyperChem 8.0.7 Release (Hypercube, Inc., Gainesville, FL, USA)29. Before docking calculations, the structures of all ligands were optimised with an MM + force field30 and the Polak-Ribière conjugate gradient algorithm (terminating at a gradient of 0.05 kcal·mol−1 Å−1). The X-ray structure of human STS was obtained from Protein Data Bank (accession code 1P49) and was prepared using standard procedure. Initially, the NAG, BOG, PO43– and water molecules from crystallisation were removed from the structure, and the catalytic amino acid fGly75 was converted to gem-diol form using the Maestro Protein Preparation Wizard module (Schrödinger, LLC, New York, NY, USA)31. Then, hydrogen atoms were introduced into the structure, and prepared model of the enzyme was optimised using the OPLS-AA force field32.
Molecular docking
Docking calculations of the optimised ligands to the prepared structure of human STS were carried out with AutoDock Vina 1.1.2 software (The Molecular Graphic Laboratory, The Scripps Research Institute, La Jolla, CA, USA)33. For all of docking studies, a grid box was centred on the Cβ atom of amino acid 75 of the prepared STS structure, and the size of the grid box was 24 Å × 24 Å × 24 Å. Then, the best binding modes for a particular ligand were inspected visually. Illustrations of the 3D models were prepared using VMD 1.9 (University of Illinois at Urbana-Champaign, Urbana, IL, USA)34. Identification of the ligand-protein interactions was performed using Discovery Studio Visualiser v20. 1. 0. 19295 (BIOVIA, Dassault Systémes, San Diego, CA, USA)35.
Biological assays
In vitro STS assay
The STS enzyme was extracted from the human placenta, and purified using the 3-step chromatographic procedure according to the described method36. The reaction mixtures, at a final volume of 100 µL, containing 20 mM Tris-HCl pH = 7.4, 3 nM radiolabelled [3H] oestrone sulphate, various concentrations of inhibitor and 5 U of purified enzyme (1 U is the amount of enzyme that hydrolyses 100 nM radiolabelled [3H] oestrone sulphate in 1 h at 37 °C). The reactions were performed at 37 °C for 30 min. Afterwards, the reaction mixtures (90 µL) were collected from each well, and the product formed by STS-mediated hydrolysis of [3H]E1 was extracted with toluene (0.5 ml). Next 0.3 ml of toluene was combined with 0.3 ml of scintillation liquid. STS activity was measured using the radioluminometer MicroBeta (Perkin Elmer). Assays were performed in triplicate.
In vitro cellular assay using MCF-7 breast cancer cell line
Inhibition of STS activity in the MCF-7 breast cancer cell line was measured according to the method described by Purohit et al. 37. MCF-7 cells were maintained in Dulbecco’s Modified Eagle Medium supplemented with 10% of foetal bovine serum and cultured until 80% of confluence was received. Cells were seeded in 24-well microplates (Nest Biotechnology) at a density of 1·105 cells/well. In order to assure an equal amount of cells in each reaction sample the number of cells was determined using a Bürker Counting Chamber. The STS activity was evaluated in living MCF-7 cells. Incubation of cells was conducted for 20 h at 37 °C in a 5% CO2 humidified incubator in a serum-free medium (0.5 ml) with radiolabelled oestrone sulphate [3H]E1S (4·105 cpm, 3 nM) with or without an inhibitor. After incubation, a medium containing STS-mediated reaction product was collected (0.45 ml) from each well and the product was isolated from the mixture by extraction with toluene (4 ml). The STS activity expressed as the level of product radioactivity was measured using the Radioluminometer MicroBeta (Perkin Elmer). Cellular assay was carried out in triplicate. IC50 values were calculated using GraphPad Prism software.
Results and discussion
Molecular docking
Initially, to verify that the 6–(1-phenyl-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamate derivatives are able to effectively bind to the STS active site, molecular docking studies were performed. The X-ray structure of STS was retrieved from the Protein Data Bank (Protein Data Bank accession code 1P49) and properly prepared for docking calculations. The docking procedure of the optimised ligands was performed using AutoDock Vina 1.1.2 software (Molecular Graphics Laboratory, The Scripps Research Institute, LaJolla, CA). The calculated results for the proposed structures of the inhibitors 3A-L were at a satisfactory, comparable level in the range of −6.0 to −8.3 kcal·mol−1 (the measurement error for the AutoDock Vina software is 2.85 kcal·mol−1) (Table 1) and lower than the free energy of binding value of the reference inhibitor Irosustat (–5.4 kcal·mol−1). The most favourable binding energy was determined for compound 3L (–8.3 kcal·mol−1) suggesting that this compound could theoretically create the most stable inhibitor-enzyme complex in the STS active site, leading to effective inhibition.
Table 1.
Free energies of binding calculated for compounds 3 A-L and Irosustat.
 | ||||||
|---|---|---|---|---|---|---|
| No. | R1 | R2 | R3 | R4 | R5 | Free energies of binding [kcal·mol-1] |
| 3A | H | H | H | H | H | –7.4 |
| 3B | H | F | H | H | H | –7.7 |
| 3C | H | Cl | H | H | H | –6.4 |
| 3D | H | Br | H | H | H | –6.3 |
| 3E | H | H | F | H | H | –7.6 |
| 3F | H | H | Cl | H | H | –7.6 |
| 3G | H | H | Br | H | H | –6.0 |
| 3H | F | H | H | H | H | –8.0 |
| 3I | Cl | H | H | H | H | –7.9 |
| 3J | Br | H | H | H | H | –8.0 |
| 3K | H | F | H | F | H | –7.9 |
| 3L | F | F | F | H | H | –8.3 |
| Irosustat | – | – | – | – | – | –5.4 |
In order to analyse the ligand-protein interactions that could be responsible for the stabilisation of the inhibitor-enzyme complexes, studies using BIOVIA, Dassault Systémes, Discovery Studio Visualiser software have been carried out. Our research has shown that the newly designed potential STS inhibitors based on 6–(1-phenyl-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamate derivatives were able to create the complexes with the STS protein stabilised by a number of interactions including π-alkyl, alkyl, π-sulphur, conventional hydrogen bond, carbon hydrogen bond, π-cation, or π-sigma (listed in Table 2). The largest number of interactions was detected for compounds 3E, 3F, 3G. However, apart from the above-mentioned numerous interactions, an extremely important aspect (influencing the inhibitory properties of STS inhibitors based on aryl-sulphamate derivatives) is their ability to undergo the nucleophilic substitution reactions on the sulphur atom. Although the mechanism of action is not confirmed and remains a topic of discussion, a sulphamate functional group (sulphate mimic) might be transferred to fGly75 residue leading to irreversible inhibition of the STS enzyme21. The visualisation of the putative binding mode for compounds 3L using VMD 1.9 (University of Illinois at Urbana-Champaign, Urbana, IL, USA) is shown in Figure 1. The sulphamate functional group, which is directly responsible for the inactivation of the enzyme, is located in the catalytic region of STS close to the fGly75 residue coordinated to Ca2+ and stabilised by π-sulphur interaction with His290 (the distance between the sulphur atom of 3L and OH group of fGly75 is 2.90 Å). For this reason, we suppose that the compound 3L (despite a smaller number of electrostatic interactions indicated by the docking program) may prove to be the most effective due to the very close distance of the sulphamate group to fGly75 residue. Furthermore, the tetracyclic core of compound 3L is well accommodated in the STS active site and is surrounded by some hydrophobic amino acid residues (e.g. Leu103, Leu167, Phe178, Phe182, Phe237, Val486, Phe488, and Phe553). Interestingly, the fluorine atoms of compound 3L are within a short distance to the nitrogen atoms of the Arg98 residue (4.12 and 4.29 Å), indicating the possibility of electrostatic interactions (undetected by the Discovery Studio Visualiser). On the other hand, the presence of the fluorine atoms may be crucial for its potentially increased ability to undergo the enzymatic reaction. Highly electronegative fluorine atoms may reduce the pKa value of the molecule, making it a good leaving group in the nucleophilic substitution reaction on the sulphur atom. In addition, the molecular modelling studies indicated (Figure 1) that the triazole ring of compound 3L is located close to the Thr484 residue, suggesting an additional interaction including hydrogen bond between OH group of Thr484 and ring-nitrogen atom (5.81 Å). These detected interaction points may be responsible for an enhancement of inhibitory potency by stabilisation of the potential STS inhibitor in the enzyme’s active site.
Table 2.
The ligand-protein interactions (and distances [Å]) identified using BIOVIA, Dassault Systémes, Discovery Studio Visualiser.
 | |||||||
|---|---|---|---|---|---|---|---|
| No. | π–Alkyl | Alkyl | π–Sulphur | Conventional Hydrogen Bond | Carbon Hydrogen Bond | π–Cation | π–Sigma |
| 3A | LEU103 (6.16, 6.50); VAL486 (5.86); VAL101 (4.93, 6.33); VAL177 (6.12) | – | HIS290 (5.76); HIS136 (6.93) | THR165 (4.11) | – | – | – |
| 3B | LEU103 (6.13, 6.47); VAL486 (5.50, 6.07), VAL101 (5.31), VAL177 (5.50, 5.57) | – | HIS290 (6.00) | FGLY75 (4.18), LYS368 (5.02) | HIS136 (5.74, 7.12) | – | – |
| 3C | LEU103 (5.91); VAL486 (6.16); VAL101 (5.43); VAL177 (5.43, 5.63) | ARG98 (2.99), TRP550 (6.97) | HIS290 (5.75); HIS136 (7.13) |
– | HIS136 (6.04) | ARG98 (4.40) | – |
| 3D | LEU103 (5.51); VAL101 (4.85); VAL177 (4.85, 5.55) | ARG98 (3.08); TRP550 (6.86) | HIS290 (5.79); HIS136 (7.11) |
– | – | ARG98 (4.83) | |
| 3E | ARG98 (4.57, 6.35); VAL177 (6.26); VAL486 (5.36), VAL101 (6.07) |
– | HIS290 (5.43); HIS136 (6.78) | LYS134 (6.65); ASP36 (4.96); GLN343 (5.61) | THR165 (5.41) | – | VAL101 (4.93) |
| 3F | ARG98 (4.74); VAL177 (6.49); VAL101 (5.81), VAL486 (5.37) |
– | HIS290 (5.29); HIS136 (6.73) | LYS134 (5.37), THR165 (4.20) | – | – | VAL101 (4.78) |
| 3G | ARG98 (4.95); VAL177 (5.24, 5.96), VAL486 (5.30), VAL101 (6.42) | LEU185 (5.88) | HIS290 (4.87, 5.32); HIS136 (6.72) |
LYS368 (5.58), LYS134 (6.45), GLN343 (5.45), ASP36 (4.95), ASP35 (4.95) | HIS136 (5.58) | – | VAL101 (5.29) |
| 3H | LEU103 (6.23, 6.49); VAL486 (6.21); VAL101 (5.38); VAL177 (5.49, 5.69) | – | HIS290 (5.96); HIS136 (7.09) | FGLY75 (4.25, 5.14); LYS368 (3.69) |
HIS136 (5.74) | – | – |
| 3I | PHE178 (4.53); LEU103 (6.27, 6.56); VAL177 (5.46, 5.79); VAL486 (5.96), VAL101 (5.42) | – | HIS290 (6.01); HIS136 (7.08) | LYS368 (5.15) | HIS136 (5.73) | – | – |
| 3J | PHE178 (4.48); LEU103 (6.15, 6.43), VAL177 (5.50, 5.67); VAL486 (6.08); VAL101 (5.34) |
– | HIS290 (5.92); HIS136 (7.05) |
LYS368 (5.18); FGLY75 (3.04, 4.27) |
HIS136 (5.69) | ARG98 (4.90) | – |
| 3K | LEU103 (6.18, 6.60); VAL486 (5.95); VAL101 (5.40); VAL177 (5.26, 5.68) |
– | HIS290 (5.91); HIS136 (7.05) |
ARG98 (5.14) | HIS136 (5.67) | – | – |
| 3L | VAL177 (4.32); VAL486 (5.09, 5.61); LEU74 (6.53) | – | HIS290 (5.57) | – | – | – | VAL101 (5.12) |
| Irosustat | VAL177 (6.51); VAL486 (4.87, 5.08) VAL101 (5.09) |
– | HIS290 (5.73); HIS136 (6.84) |
LYS368 (5.49); FGLY75 (3.04) |
HIS136 (6.03) | – | VAL486 (4.67) |
Figure 1.
Docked binding modes and distance to fGly75, Arg98 and Thr484 for compound 3 L (CPK coloured) and Irosustat (pink).
Chemistry
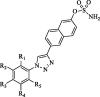
The newly designed compounds 3A-L based on 6–(1-phenyl-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamate derivatives were synthesised according to the route shown in (Scheme 1). In the first step, we synthesised 6-((trimethylsilyl)ethynyl)naphthalen-2-ol 1 by the Sonogashira coupling reaction between 6-bromo-2-naphthol and trimethylsilylacetylene. Next step included the preparation of 6–(1-phenyl-1H-1,2,3-triazol-4-yl)naphthalen-2-ol derivatives 2 A-L by a 1,3-dipolar cycloaddition reaction. In this case the corresponding azide derivatives (obtained from commercially available anilines by the reaction with t-BuONO and TMSN3) were treated with 1 in the presence of CuSO4 · 5H2O (0.1 equiv.), sodium ascorbate (0.2 equiv.), and TBAF. Finally, 6–(1-phenyl-1H-1,2,3-triazol-4-yl)naphthalen-2-ol derivatives 2 A-L were sulphamoylated using sulphamoyl chloride previously generated in the reaction of chlorosulphonyl isocyanate and formic acid in the presence of a catalytic amount of N,N-DMA. After standard isolation, the desired derivatives 3A-L were obtained.
Scheme 1.
Synthesis of 6-((trimethylsilyl)ethynyl)naphthalen-2-ol 1 and 6–(1-phenyl-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamate derivatives (R1, R2, R3, R4, R5 = H, F, Cl, or Br) 3 A-L.
Biological evaluation
Enzymatic assay using the STS enzyme isolated from the human placenta
In the next step of our investigation, the synthesised new STS inhibitors based on 6–(1-phenyl-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamate derivatives 3A-L were tested in the enzymatic assay (at 0.5 µM of inhibitor concentration) in order to verify their theoretical potential to inhibit of the STS activity. Screening tests were performed using the STS enzyme isolated from the human placenta and purified by a 3-step chromatography procedure. After purification, the obtained fraction was used directly as the enzyme source. In this activity assay, a radiolabelled [3H] oestrone sulphate has been used as a substrate to provide higher selectivity and more reliable results. The summarised results of the enzymatic assay for newly synthesised 6–(1-phenyl-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamate derivatives 3A-L are presented in Table 3. The received data showed that all compounds effectively inhibited the activity of the steroid sulphatase to a level from 7.98 to 27.17%. In the course of the research, we found that the highest inhibitory activity was exhibited by the compound 3L containing three fluorine atoms in its structure (remaining STS activity of 7.98%). This result was in agreement with the data from the molecular modelling studies. It was observed that the substitution of the terminal aromatic ring with a less halogen atoms resulted in a decrease in the activity of the tested compounds towards STS. This observation may confirm our assumptions that the higher number of electronegative heteroatoms may reduce the pKa value of the inhibitor cores, making them more susceptible to nucleophilic substitution reaction on the sulphur atom. The obtained results may also suggest that the position of the halogen substitution in the above-mentioned aryl ring may be crucial for the potency of evaluated compounds. As it turns out, the presence of halogen atoms at the R1 and R2 positions may have the greatest influence on the increase of inhibitory potency. On the other hand, the type of substituent is slightly less significant.
Table 3.
The STS inhibitory effect of the newly synthesised compounds 3 A-L at 0.5 µM inhibitor concentration.
 | ||||||
|---|---|---|---|---|---|---|
| No. | R1 | R2 | R3 | R4 | R5 | Remaining STS activity [%] |
| 3A | H | H | H | H | H | 24.50 ± 1.36 |
| 3B | H | F | H | H | H | 17.40 ± 0.87 |
| 3C | H | Cl | H | H | H | 9.66 ± 0.48 |
| 3D | H | Br | H | H | H | 9.62 ± 0.48 |
| 3E | H | H | F | H | H | 23.75 ± 1.22 |
| 3F | H | H | Cl | H | H | 18.71 ± 0.94 |
| 3G | H | H | Br | H | H | 23.07 ± 1.15 |
| 3H | F | H | H | H | H | 27.17 ± 1.36 |
| 3I | Cl | H | H | H | H | 16.93 ± 0.83 |
| 3J | Br | H | H | H | H | 15.93 ± 0.80 |
| 3K | H | F | H | F | H | 14.03 ± 0.70 |
| 3L | F | F | F | H | H | 7.98 ± 0.40 |
Evaluation of STS inhibition in the MCF-7 cell line
The next step of the biological evaluation was to verify whether the obtained new STS inhibitors based on 6–(1-phenyl-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamates 3A-L are able to inhibit STS activity in the MCF-7 cancer cell line. In the course of our study, we determined the level of STS inhibition in the MCF-7 cells after incubation in the presence of inhibitors at 100, 10, and 1 nM concentrations. The summarised results are presented in Table 4. In the course of our research, we found that in the presence of inhibitor at 100 nM concentration, the lowest STS activities were measured for derivatives 3K (5.43% of STS activity) and 3L (5.29% of STS activity) substituted at R1, R2 or R3 position with fluorine atoms. These results were comparable with those obtained for the reference compound – Irosustat (remaining STS activity of 5.72%). In the next step, six of the most promising derivatives 3G-L were selected and tested at an inhibitor concentration of 10 nM. Among these compounds, the highest STS inhibitory activities were observed for the derivatives 3I, 3K, and 3L (remaining STS activity of 42.09%, 42.01% and 27.48%, respectively). As the research showed, the tested compounds showed slightly weaker activity in comparison with Irosustat (remaining STS activity of 12.93% at an inhibitor concentration of 10 nM). In order to further verify the potential of the new selected compounds, the STS activity was measured in the presence of 3I, 3K, and 3L at the inhibitor concentration of 1 nM. For these compounds, an IC50 parameter was determined as well. The studies showed that the tested compounds based on 6–(1-phenyl-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamates 3I, 3K, and 3L found to be very potent STS inhibitors characterised by low IC50 values of 30.14, 17.02, 15.97 nM, respectively (IC50 parameter determined for Irosustat was 1.14 nM). The obtained results indicated that 6–(1-phenyl-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamates may be very promising in further in vivo studies. Overall, among all the newly prepared compounds, the highest activity was shown by those with fluorine atoms in their structures. We assume that their effectiveness may be influenced by the possibility of electrostatic interactions between fluorine atoms and the Arg98 residue in the STS active site (as suggested in molecular modelling studies) as well as a higher susceptibility of these structures to nucleophilic substitution reaction on the sulphur atom. Moreover, the presence of C-F bonds in the structure of biologically active compounds often affects a number of favourable properties, including metabolic stability, leading to higher therapeutic effectiveness.
Table 4.
Remaining STS activity in MCF-7 cells after incubation with compounds 3 A-L and Irosustat at 100, 10 and 1 nM inhibitor concentrations.
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | R1 | R2 | R3 | R4 | R5 | Remaining STS activity [%] |
|||
| 100 [nM] | 10 [nM] | 1 [nM] | IC50 [nM] | ||||||
| 3A | H | H | H | H | H | 15.52 ± 0.78 | – | – | – |
| 3B | H | F | H | H | H | 12.22 ± 0.61 | – | – | – |
| 3C | H | Cl | H | H | H | 22.05 ± 1.10 | – | – | – |
| 3D | H | Br | H | H | H | 20.59 ± 1.03 | – | – | – |
| 3E | H | H | F | H | H | 11.32 ± 0.57 | – | – | – |
| 3F | H | H | Cl | H | H | 13.25 ± 0.66 | – | – | – |
| 3G | H | H | Br | H | H | 8.68 ± 0.43 | 59.86 ± 2.99 | – | – |
| 3H | F | H | H | H | H | 8.80 ± 0.44 | 55.84 ± 2.79 | – | – |
| 3I | Cl | H | H | H | H | 7.44 ± 0.37 | 42.09 ± 2.10 | 84.32 ± 4.22 | 30.14 ± 1.51 |
| 3J | Br | H | H | H | H | 7.30 ± 0.37 | 59.45 ± 2.97 | – | – |
| 3K | H | F | H | F | H | 5.43 ± 0.27 | 42.01 ± 2.10 | 79.30 ± 3.96 | 17.02 ± 0.85 |
| 3L | F | F | F | H | H | 5.29 ± 0.26 | 27.48 ± 1.37 | 99.00 ± 4.95 | 15.97 ± 0.80 |
| Irosustat | – | – | – | – | – | 5.72 ± 0.29 | 12.93 ± 0.65 | 16.83 ± 0.84 | 1.14 ± 0.06 |
Conclusions
In the present work, we described our research on molecular modelling, synthesis, and biological evaluation of 6–(1-phenyl-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamate derivatives 3A-L as new very potent STS inhibitors. Screening enzymatic assay, performed using the STS enzyme isolated from the human placenta indicated that all of the newly synthesised inhibitors 3A-L were able to effectively inhibit the action of STS. Among them, the highest inhibitory activity was exhibited by compound 3L containing three fluorine atoms in its structure (remaining STS activity of 7.98%). In the course of the cell line experiment, we observed the highest inhibition of STS in the presence of 6–(1-phenyl-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamates 3I, 3K, and 3L characterised by low IC50 values of 30.14, 17.02, 15.97 nM, respectively (IC50 value determined for Irosustat was 1.14 nM). The presented results showed that 6–(1-phenyl-1H-1,2,3-triazol-4-yl)naphthalen-2-yl sulphamate derivatives may be very promising anticancer agents and their therapeutic potential should be confirmed in further in vivo studies. Furthermore, the data of enzymatic and cell line experiments suggested that the possibility of creating electrostatic interactions between the fluorine atoms of compounds and the Arg98 residue in the active site of STS as well as a higher susceptibility to nucleophilic substitution reaction on the sulphur atom could be critical for their inhibitory effects.
Funding Statement
Financial support for this study was provided by the National Centre for Research and Development (Poland) for: TANGO program - grant agreement no. TANGO-IV-A/0004/2019-00.
Disclosure statement
The authors report no conflict of interest.
References
- 1.IARC: CANCER FACT SHEETS [Internet] . Lyon (France): International Agency for Research on Cancer. Available from: https://gco.iarc.fr/today/factsheets-cancers [last accessed 10 May 2020].
- 2.NCI [Internet] . Maryland (U.S.): National Cancer Institiute. Available from: https://seer.cancer.gov/statfacts/html/breast.html [last accessed on 10 May 2020].
- 3.Pasqualini JR. The selective estrogen enzyme modulators in breast cancer: a review. Biochim Biophys Acta Rev 2004;1654:123–43. [DOI] [PubMed] [Google Scholar]
- 4.Purohit A, Woo LWL, Potter BVL.. Steroid sulfatase: a pivotal player in estrogen synthesis and metabolism. Mol Cell Endocrinol 2011;340:154–60. [DOI] [PubMed] [Google Scholar]
- 5.Shah R, Singh J, Singh D, et al. Sulfatase inhibitors for recidivist breast cancer treatment: a chemical review. Eur J Med Chem 2016;114:170–90. [DOI] [PubMed] [Google Scholar]
- 6.Reed MJ, Purohit A, Woo LWL, et al. Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr Rev 2005;26:171–202. [DOI] [PubMed] [Google Scholar]
- 7.Zaraei S-O, Abduelkarem AR, Anbar HS, et al. Sulfamates in drug design and discovery: pre-clinical and clinical investigations. Eur J Med Chem 2019;179:257–71. [DOI] [PubMed] [Google Scholar]
- 8.Ring A, Dowsett M.. Mechanisms of tamoxifen resistance. Endocr Relat Cancer 2004;11:643–58. [DOI] [PubMed] [Google Scholar]
- 9.Foster PA, Chander SK, Newman SP, et al. A new therapeutic strategy against hormone-dependent breast cancer: the preclinical development of a dual aromatase and sulfatase inhibitor. Clin Cancer Res 2008;14:6469–77. [DOI] [PubMed] [Google Scholar]
- 10.Dixon JM. Endocrine resistance in breast cancer. New J Sci 2014;2014:1–7. [Google Scholar]
- 11.Foster A, Reed PA, Purohit MJ.. Recent developments of steroid sulfatase inhibitors as anti-cancer agents. Anticancer Agents Med Chem 2008;8:732–8. [DOI] [PubMed] [Google Scholar]
- 12.Chanplakorn N, Chanplakorn P, Suzuki T, et al. Increased estrogen sulfatase (STS) and 17β-hydroxysteroid dehydrogenase type 1(17β-HSD1) following neoadjuvant aromatase inhibitor therapy in breast cancer patients. Breast Cancer Res Treat 2010;120:639–48. [DOI] [PubMed] [Google Scholar]
- 13.Daśko M, Demkowicz S, Biernacki K, et al. Recent progress in the development of steroid sulphatase inhibitors - examples of the novel and most promising compounds from the last decade. J Enzyme Inhib Med Chem 2020;35:1163–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malini B, Purohit A, Ganeshapillai D, et al. Inhibition of steroid sulphatase activity by tricyclic coumarin sulphamates. J Steroid Biochem Mol Biol 2000;75:253–8. [DOI] [PubMed] [Google Scholar]
- 15.Woo LWL, Purohit A, Malini B, et al. Potent active site-directed inhibition of steroid sulphatase by tricyclic coumarin-based sulphamates. Chem Biol 2000;7:773–91. [DOI] [PubMed] [Google Scholar]
- 16.Stanway SJ, Purohit A, Woo LWL, et al. Phase I study of STX 64 (667 Coumate) in breast cancer patients: the first study of a steroid sulfatase inhibitor. Clin Cancer Res 2006;12:1585–92. [DOI] [PubMed] [Google Scholar]
- 17.Palmieri C, Januszewski A, Stanway S, Coombes RC.. Irosustat: a first-generation steroid sulfatase inhibitor in breast cancer. Expert Rev Anticancer Ther 2011;11:179–83. [DOI] [PubMed] [Google Scholar]
- 18.Palmieri C, Stein RC, Liu X, et al. IRIS study: a phase II study of the steroid sulfatase inhibitor Irosustat when added to an aromatase inhibitor in ER-positive breast cancer patients. Breast Cancer Res Treat 2017;165:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmieri C, Stein RC, Liu X, et al. A Phase II study to assess the safety and efficacy of the steroid sulfatase inhibitor Irosustat when added to an aromatase inhibitor in ER positive locally advanced or metastatic breast cancer patients (IRIS) – trial results. J Clin Oncol 2016;34:549. [Google Scholar]
- 20.Palmieri C, Szydlo R, Miller M, et al. IPET study: an FLT-PET window study to assess the activity of the steroid sulfatase inhibitor irosustat in early breast cancer. Breast Cancer Res Treat 2017;166:527–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potter BVL. Sulfation pathways: steroid sulphatase inhibition via aryl sulphamates: clinical progress, mechanism and future prospects. J Mol Endocrinol 2018;61:T233–T252. [DOI] [PubMed] [Google Scholar]
- 22.Daśko M, Demkowicz S, Rachon J, et al. New potent STS inhibitors based on fluorinated 4-(1-phenyl-1H-[1,2,3]triazol-4-yl)-phenyl sulfamates. J Asian Nat Prod Res 2020;22:1037–44. [DOI] [PubMed] [Google Scholar]
- 23.Bozorov K, Zhao J, Aisa HA.. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: a recent overview. Bioorg Med Chem 2019;27:3511–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kommidi H, Guo H, Nurili F, et al. 18F-positron emitting/trimethine cyanine-fluorescent contrast for image-guided prostate cancer management. J Med Chem 2018;61:4256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang S, Han Y, Chen M, et al. Radiosynthesis and biological evaluation of 18F-labeled 4-anilinoquinazoline derivative (18F-FEA-Erlotinib) as a potential EGFR PET agent. Bioorganic Med Chem Lett 2018;28:1143–8. [DOI] [PubMed] [Google Scholar]
- 26.Zhou J, Stapleton P, Haider S, Healy J.. Boronic acid inhibitors of the class A β-lactamase KPC-2. Bioorg Med Chem 2018;26:2921–7. [DOI] [PubMed] [Google Scholar]
- 27.Lopes SMM, Novais JS, Costa DCS, et al. Hetero-Diels-Alder reactions of novel 3-triazolyl-nitrosoalkenes as an approach to functionalized 1,2,3-triazoles with antibacterial profile. Eur J Med Chem 2018;143:1010–20. [DOI] [PubMed] [Google Scholar]
- 28.Batra N, Rajendran V, Agarwal D, et al. Synthesis and antimalarial evaluation of [1,2,3]-Triazole-Tethered Sulfonamide-Berberine Hybrids. ChemistrySelect 2018;3:9790–3. [Google Scholar]
- 29.HyperChem(TM) Professional 7.51, Hypercube, Inc., 1115 NW 4th Street , Gainesville, Florida, USA. Available from: www.hyper.com
- 30.Hocquet A, Langgård M.. An evaluation of the MM + force field. J Mol Model 1998;4:94–122. [Google Scholar]
- 31.Schrödinger Release 2020-3: Maestro , Schrödinger , LLC, New York, NY, 2020. [Google Scholar]
- 32.Kaminski G, Duffy E, Matsui T, Jorgensen W.. Free energies of hydration and pure liquid properties of hydrocarbons from the OPLS All-Atom Model. J Phys Chem 1994;98:13077–82. [Google Scholar]
- 33.Trott O, Olson AJ.. Autodock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 2010;31:455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humphrey W, Dalke A, Schulten K.. VMD: visual molecular dynamics. J Mol Graph 1996;14:33–8. [DOI] [PubMed] [Google Scholar]
- 35.BIOVIA , Dassault Systèmes. Discovery Studio Visualizer, v20. 1. 0. 19295. San Diego: Dassault Systèmes; 2020 [Google Scholar]
- 36.Hernandez-Guzman FG, Higashiyama T, Osawa Y, Ghosh D.. Purification, characterization and crystallization of human placental estrone/dehydroepiandrosterone sulfatase, a membrane-bound enzyme of the endoplasmic reticulum. J Steroid Biochem Mol Biol 2001;78:441–50. [DOI] [PubMed] [Google Scholar]
- 37.Purohit A, Williams GJ, Howarth NM, et al. Inactivation of steroid sulfatase by an active site-directed inhibitor, estrone-3-O-sulfamate. Biochemistry 1995;34:11508–14. [DOI] [PubMed] [Google Scholar]




