Abstract
STUDY QUESTION
Can the priorities for future research in infertility be identified?
SUMMARY ANSWER
The top 10 research priorities for the four areas of male infertility, female and unexplained infertility, medically assisted reproduction and ethics, access and organization of care for people with fertility problems were identified.
WHAT IS KNOWN ALREADY
Many fundamental questions regarding the prevention, management and consequences of infertility remain unanswered. This is a barrier to improving the care received by those people with fertility problems.
STUDY DESIGN, SIZE, DURATION
Potential research questions were collated from an initial international survey, a systematic review of clinical practice guidelines and Cochrane systematic reviews. A rationalized list of confirmed research uncertainties was prioritized in an interim international survey. Prioritized research uncertainties were discussed during a consensus development meeting. Using a formal consensus development method, the modified nominal group technique, diverse stakeholders identified the top 10 research priorities for each of the categories male infertility, female and unexplained infertility, medically assisted reproduction and ethics, access and organization of care.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Healthcare professionals, people with fertility problems and others (healthcare funders, healthcare providers, healthcare regulators, research funding bodies and researchers) were brought together in an open and transparent process using formal consensus methods advocated by the James Lind Alliance.
MAIN RESULTS AND THE ROLE OF CHANCE
The initial survey was completed by 388 participants from 40 countries, and 423 potential research questions were submitted. Fourteen clinical practice guidelines and 162 Cochrane systematic reviews identified a further 236 potential research questions. A rationalized list of 231 confirmed research uncertainties was entered into an interim prioritization survey completed by 317 respondents from 43 countries. The top 10 research priorities for each of the four categories male infertility, female and unexplained infertility (including age-related infertility, ovarian cysts, uterine cavity abnormalities and tubal factor infertility), medically assisted reproduction (including ovarian stimulation, IUI and IVF) and ethics, access and organization of care were identified during a consensus development meeting involving 41 participants from 11 countries. These research priorities were diverse and seek answers to questions regarding prevention, treatment and the longer-term impact of infertility. They highlight the importance of pursuing research which has often been overlooked, including addressing the emotional and psychological impact of infertility, improving access to fertility treatment, particularly in lower resource settings and securing appropriate regulation. Addressing these priorities will require diverse research methodologies, including laboratory-based science, qualitative and quantitative research and population science.
LIMITATIONS, REASONS FOR CAUTION
We used consensus development methods, which have inherent limitations, including the representativeness of the participant sample, methodological decisions informed by professional judgment and arbitrary consensus definitions.
WIDER IMPLICATIONS OF THE FINDINGS
We anticipate that identified research priorities, developed to specifically highlight the most pressing clinical needs as perceived by healthcare professionals, people with fertility problems and others, will help research funding organizations and researchers to develop their future research agenda.
STUDY FUNDING/COMPETING INTEREST(S)
The study was funded by the Auckland Medical Research Foundation, Catalyst Fund, Royal Society of New Zealand and Maurice and Phyllis Paykel Trust. G.D.A. reports research sponsorship from Abbott, personal fees from Abbott and LabCorp, a financial interest in Advanced Reproductive Care, committee membership of the FIGO Committee on Reproductive Medicine, International Committee for Monitoring Assisted Reproductive Technologies, International Federation of Fertility Societies and World Endometriosis Research Foundation, and research sponsorship of the International Committee for Monitoring Assisted Reproductive Technologies from Abbott and Ferring. Siladitya Bhattacharya reports being the Editor-in-Chief of Human Reproduction Open and editor for the Cochrane Gynaecology and Fertility Group. J.L.H.E. reports being the Editor Emeritus of Human Reproduction. A.W.H. reports research sponsorship from the Chief Scientist’s Office, Ferring, Medical Research Council, National Institute for Health Research and Wellbeing of Women and consultancy fees from AbbVie, Ferring, Nordic Pharma and Roche Diagnostics. M.L.H. reports grants from Merck, grants from Myovant, grants from Bayer, outside the submitted work and ownership in Embrace Fertility, a private fertility company. N.P.J. reports research sponsorship from AbbVie and Myovant Sciences and consultancy fees from Guerbet, Myovant Sciences, Roche Diagnostics and Vifor Pharma. J.M.L.K. reports research sponsorship from Ferring and Theramex. R.S.L. reports consultancy fees from AbbVie, Bayer, Ferring, Fractyl, Insud Pharma and Kindex and research sponsorship from Guerbet and Hass Avocado Board. B.W.M. reports consultancy fees from Guerbet, iGenomix, Merck, Merck KGaA and ObsEva. E.H.Y.N. reports research sponsorship from Merck. C.N. reports being the Co Editor-in-Chief of Fertility and Sterility and Section Editor of the Journal of Urology, research sponsorship from Ferring and retains a financial interest in NexHand. J.S. reports being employed by a National Health Service fertility clinic, consultancy fees from Merck for educational events, sponsorship to attend a fertility conference from Ferring and being a clinical subeditor of Human Fertility. A.S. reports consultancy fees from Guerbet. J.W. reports being a statistical editor for the Cochrane Gynaecology and Fertility Group. A.V. reports that he is a Statistical Editor of the Cochrane Gynaecology & Fertility Review Group and the journal Reproduction. His employing institution has received payment from Human Fertilisation and Embryology Authority for his advice on review of research evidence to inform their ‘traffic light’ system for infertility treatment ‘add-ons’. N.L.V. reports consultancy and conference fees from Ferring, Merck and Merck Sharp and Dohme. The remaining authors declare no competing interests in relation to the present work. All authors have completed the disclosure form.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: Consensus science methods, infertility, modified Delphi method, modified Nominal Group Technique, reproductive medicine, research priorities
Introduction
The ultimate aim of infertility research is to improve clinical practice and optimize the chances of people with fertility problems achieving parenthood. For this to be possible, research needs to address questions that are pertinent to people with infertility, be conducted using appropriate methods, and be reported in a comprehensive, transparent and accessible manner (Duffy et al., 2017). The first step in research production is to identify appropriate questions. Traditionally, research funding organizations and researchers have identified, refined and prioritized their own research agenda. It is unlikely that such prioritization has used formal consensus methods, engaged wider stakeholders, including people with fertility problems, and was independent of commercial interests. There has been modest improvement in some countries, including the Netherlands, the UK and the USA, which has emphasized the importance of including patients and the public in developing research priorities (Graham et al., 2020).
Sir Iain Chalmers, founder of the Cochrane Collaboration, has advocated for research priorities to be jointly identified by healthcare professionals, patients and communities (Chalmers and Glasziou, 2009). He established the James Lind Alliance, which brings together healthcare professionals, patients and others, in priority setting partnerships. Using formal consensus methods, each priority setting partnership engages in an open and transparent process to identify and prioritize unanswered research questions, known as research uncertainties, in a particular area of health care (James Lind Alliance, 2018). The expectation is that prioritized research uncertainties will establish the future research agenda of funding organizations and researchers. As a result, it is hoped that the gap will close between what research is needed and what research is pursued (Wilkinson et al., 2019a).
An international collaboration has brought healthcare professionals, people with fertility problems and others together within a Priority Setting Partnership for Infertility to develop future research priorities for male infertility, female and unexplained infertility, medically assisted reproduction and ethics, access and organization of care.
Materials and methods
An international multidisciplinary steering group, including healthcare professionals, people with fertility problems and researchers, was established to provide a diverse range of perspectives to inform key methodological decisions. The steering group was convened during the development of the study protocol, before the launch of the initial survey and interim prioritization survey, and before the consensus development meeting. A systematic review of registered, progressing and completed priority setting research settings was completed to assist with the planning and delivery of the study (Graham et al., 2020).
Research uncertainties related to infertility associated with endometriosis, miscarriage and polycystic ovary syndrome were not considered because of other current or completed research prioritization initiatives (Horne et al., 2017; Prior et al., 2017).
Research priorities were developed in a three-stage process using consensus methods advocated by the James Lind Alliance (2018). Potential research uncertainties were gathered through an online survey of healthcare professionals, people with fertility problems and others. Healthcare professionals, including embryologists, fertility specialists and gynecologists, were recruited through the British Fertility Society, Core Outcomes in Women’s Health (CROWN) initiative, Cochrane Gynaecology and Fertility Group, Fertility and Sterility Forum, Reproductive Medicine Clinical Study Group and Royal College of Obstetricians and Gynecologists. People with fertility problems were recruited through Fertility Europe, an umbrella organization of more than 20 European patient organizations, including Fertility Network UK and Freya, Fertility New Zealand, RESOLVE: The National Infertility Association, and the Women’s Voices Involvement Panel hosted by the Royal College of Obstetricians and Gynecologists. Other people could register to participate, including healthcare funders, healthcare regulators and researchers. Recruitment was supported by an active social media campaign. Potential participants received an explanatory video abstract, a plain-language summary and survey instructions. Before completing the survey, participants provided demographic details, including age, gender and geographical location, and information pertaining to their professional or personal experience of infertility. Participants were invited to suggest up to five research questions related to infertility that they considered unanswered.
After the survey had closed, the survey responses were examined in detail within an iterative process. Individual responses were reviewed by at least two members of the steering group. Responses were excluded if they included questions that did not fit the scope of the study, were not answerable by research, related to a specific person or situation or were ambiguous. Incomplete responses were also excluded. The remaining responses were formatted into appropriate research questions.
In addition, research recommendations were identified from a systematic review of clinical practice guidelines and Cochrane systematic reviews. Clinical practice guidelines relevant to infertility were identified by searching bibliographical databases, including Embase, International Guideline Library and MEDLINE, from 2007 to July 2017. Research recommendations were extracted verbatim from clinical practice guidelines. Using a data extraction tool available to the Cochrane Gynaecology and Fertility Group, research recommendations were extracted from individual Cochrane reviews evaluating potential fertility treatments. Research recommendations from clinical practice guidelines and Cochrane systematic reviews were reviewed by two members of the steering group and formatted into appropriate research questions. Differences in opinion were resolved by discussion with the steering group.
The long list of potential research questions was organized by allocating individual research questions in four categories: male infertility; female and unexplained infertility, including age-related infertility, ovarian cysts, uterine cavity abnormalities and tubal factor infertility; medically assisted reproduction including ovarian stimulation, IUI and IVF; and ethics, access and organization of care. These categories were identified in consultation with the steering group. Duplicate research questions were removed. Research questions were checked against the published research evidence, including clinical practice guidelines, Cochrane systematic reviews and randomized trials, and those questions considered to be already answered were removed.
The long list of confirmed research uncertainties was entered into an interim prioritization survey. Initial survey participants were invited to participate in the survey. In addition, healthcare professionals, people with fertility problems and others were recruited using the same methods as the initial survey. Before completing the survey, participants provided demographic details, including age, gender and geographical location, and information pertaining to their professional or personal experience of infertility. Participants were invited to select the research uncertainties they considered most important. After the survey had closed, questions were ranked based on the frequency they had been chosen by participants.
The top 15 research uncertainties in each category were discussed during a consensus development meeting (data are presented in the Supplementary Table S1). A formal consensus development method, the modified nominal group technique, was used to identify the top 10 research uncertainties for each category (James Lind Alliance, 2018). Healthcare professionals, people with fertility problems and others who had completed the initial or interim prioritization survey were invited to participate. The modified nominal group technique does not depend on statistical power. In consultation with the steering group, the aim was to recruit between 15 and 30 participants, as this number has yielded sufficient results and assured validity in other settings (Murphy et al., 1998).
Before the consensus development meeting, participants provided demographic details, including age, gender and geographical location, and information pertaining to their professional or personal experience of infertility. Following an introductory session, participants were assigned to one of two groups, each with a facilitator, to discuss the ranking of prioritized research uncertainties. The assignments were pre-specified to ensure a mixture of healthcare professionals, people with fertility problems and others. The groups were provided with a set of cards with an individual research uncertainty printed on each. Each participant was asked to contribute their opinions on the research uncertainties they felt most and least strongly about. Following this initial discussion, participants were invited to discuss the ordering of the research uncertainties. By the end of the session, the research uncertainties were placed in ranked order. The rankings from the two groups were aggregated into a single ranking order and presented to the entire group. Participants were invited to discuss the ordering of the research uncertainties. By the end of the discussion, the research uncertainties were placed in a final ranked order.
The National Research Ethics Service, UK, advised the study did not require formal review.
Results
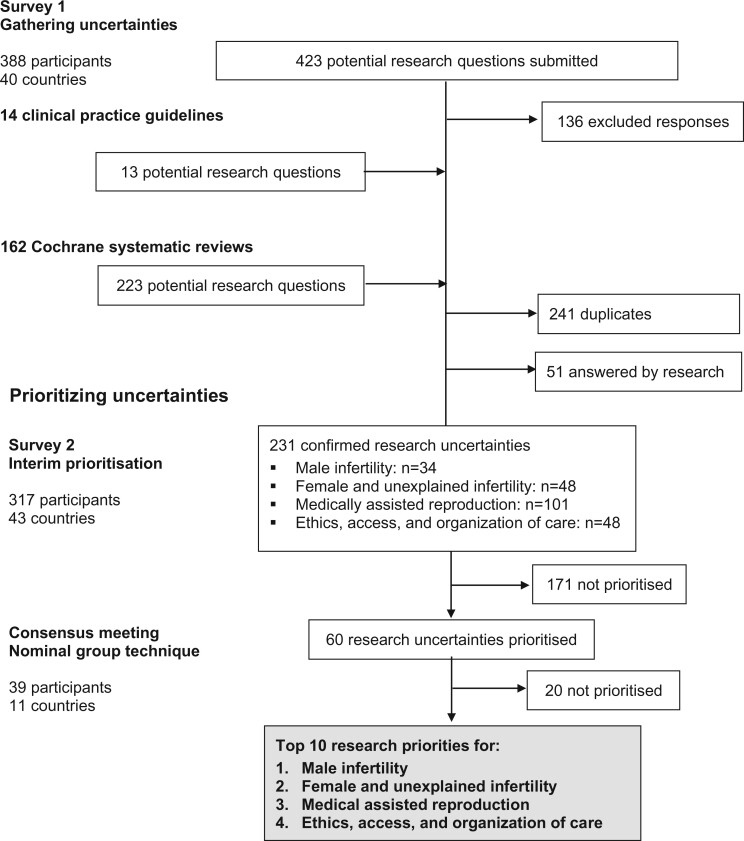
The initial survey was completed by 179 healthcare professionals (46%), 153 people with fertility problems (39%) and 56 others (14%), from 40 countries (Table I). Four hundred and twenty-three responses were submitted (Fig. 1). Following review, 136 responses (32%) were excluded. Clinical practice guidelines relevant to infertility were identified by searching bibliographical databases; the search strategy identified 3680 records. After excluding 731 duplicate records, 2949 titles and abstracts were screened. Thirty-two potentially relevant clinical practice guidelines were evaluated. Fourteen clinical practice guidelines met the inclusion criteria, including two guidelines related to infertility in general (Loh et al., 2014; National Institute for Health and Care Excellence, 2017), five guidelines related to male infertility (American Urological Association, 2010; Jarvi et al., 2010; Jungwirth et al., 2018), five guidelines related to uterine anomalies (Kroon et al., 2011; American Association of Gynecologic Laparoscopists, 2012; Carranza-Mamane et al., 2015; Practice Committee of the American Society for Reproductive Medicine, 2016a, 2017) and two guidelines related to medically assisted reproduction (Practice Committee of the American Society for Reproductive Medicine, 2016b; Penzias et al., 2017). Thirteen research recommendations were extracted from the clinical practice guidelines. The Cochrane Gynaecology and Fertility Group provided research recommendations from 162 Cochrane systematic reviews. Two hundred and twenty-three potential research questions were extracted from these research recommendations. A long list of 533 potential research uncertainties was reviewed, 241 duplicate research uncertainties were removed and 51 research uncertainties which had been answered by research were also removed.
Table I.
Characteristics of the participants in a survey to identify the priorities for future infertility research.
| Survey 1 | Survey 2 | Consensus meeting | |
|---|---|---|---|
| Initial survey | Interim prioritization | Final prioritization | |
| n = 388 | n = 317 | n = 41 | |
| Stakeholder group, n | |||
| People with fertility problems | 153 | 119 | 14 |
| Healthcare professionals | 179 | 143 | 19 |
| Embryologists | 39 | 26 | 4 |
| Fertility specialists | 71 | 64 | 6 |
| Gynecologists | 44 | 28 | 6 |
| Others | 25 | 25 | 3 |
| Researchers | 28 | 28 | 7 |
| Others | 15 | 10 | 1 |
| Prefer not to say | 13 | 17 | 0 |
| Gender, n | |||
| Female | 223 | 176 | 25 |
| Male | 129 | 119 | 16 |
| Prefer not to say | 36 | 22 | 0 |
| Age (years), n | |||
| Below 30 | 47 | 26 | 2 |
| 30–39 | 118 | 85 | 12 |
| 40–49 | 61 | 60 | 5 |
| 50–59 | 73 | 61 | 13 |
| Over 60 | 42 | 29 | 5 |
| Prefer not to say | 47 | 56 | 4 |
| Geographical location, n | |||
| Africa | 15 | 14 | 0 |
| Asia | 57 | 34 | 3 |
| Australia and New Zealand | 61 | 51 | 22 |
| Europe | 115 | 117 | 13 |
| North America | 82 | 54 | 3 |
| South America | 27 | 19 | 0 |
| Prefer not to say | 31 | 28 | 0 |
Figure 1.
Overview of the process of identifying research uncertainties.
A rationalized list of 231 confirmed research uncertainties was developed, which included 34 research uncertainties related to male infertility, 48 research uncertainties related to female and unexplained infertility, 101 research uncertainties related to medically assisted reproduction and 48 research uncertainties related to ethics, access and organization of care. These confirmed research uncertainties were entered into an interim prioritization survey, which was completed by 143 healthcare professionals, 119 people with fertility problems and 55 others, from 43 countries.
Nineteen healthcare professionals, 14 people with personal experience of infertility and 8 others, from 11 countries, participated in the consensus development meeting. The modified nominal group technique was used to prioritize the top 10 research uncertainties for male infertility, female and unexplained infertility, medically assisted reproduction and ethics, access and organization of care. Fifteen highly prioritized research uncertainties for each category were discussed during the consensus development meeting (Supplementary Table SI). The 15 highly prioritized research uncertainties were initially discussed by two separate groups and at the end of the discussion, they ranked the research uncertainties. The first-round ranking is presented in Supplementary Table SI. The rankings from the two groups were aggregated into a single ranking order and discussed by the entire group (Supplementary Table SI). Participants were encouraged to discuss and finalize the rank order of the research priorities. The top 10 research priorities are presented in Fig. 2.
Figure 2.
The top 10 priorities for future infertility research in each of the four categories.
Discussion
The Priority Setting Partnership for infertility has brought together healthcare professionals, people with fertility problems and others to identify the top 10 research priorities for future infertility research. These research priorities are diverse and seek answers to questions regarding prevention, treatment and the longer-term impact, as well as wider contextual issues related to access and public health policy. They highlight the importance of pursuing research which has often been overlooked, including addressing the emotional and psychological impact of infertility, improving access to fertility treatment, particularly in lower resource settings, and securing appropriate regulation. Addressing these priorities will require diverse research methodologies, including laboratory-based science, qualitative and quantitative research and population science.
Strengths and limitations
The James Lind Alliance (2018) has published guidance to inform the design of research priority setting studies. This study has followed this guidance to ensure the research priorities were developed using a clear and transparent process using formal consensus development methods. The study design, development and delivery were also informed by a systematic review of research priority setting studies relevant to women’s health (Graham et al., 2020). With 388 respondents from 40 countries participating in the initial survey, 317 respondents from 43 countries participating in the interim prioritization survey, and 41 participants from 11 countries included in the consensus development meeting, the global participation achieved in this study should secure the generalizability of the results within an international context. The study included people with fertility problems and they were able to suggest potential research uncertainties during the initial survey, share their views regarding the importance of research uncertainties during the interim prioritization survey and participate fully in the consensus development meeting which prioritized the final research priorities.
This consensus study is not without limitations. Consideration should be given to the representativeness of the study’s participants. For example, when considering the initial survey, there was a higher response from participants who identified as living in Europe (115 participants; 30%). To participate in the initial survey and interim prioritization survey, English proficiency and literacy, a computer and internet access were required. We appreciate that limitations in the representativeness of the sample could impact upon the research uncertainties suggested and prioritized. There is uncertainty regarding the optimal consensus development method to prioritize research uncertainties, and methodological research is required to evaluate different approaches to priority setting and the use of different consensus methods. Further contextual information, including the number of people the research priority impacts upon, the feasibility of answering the research priority, and the resources required to address the research uncertainty could have assisted participants to prioritize research uncertainties. Future methodological research should evaluate the use of contextual information in research priority studies.
Reflections on the research priorities
Reproductive medical care for men has lagged behind that for women. Setting impactful and tractable priorities for male reproduction is consequently a critically important task. For diagnosis, the variation in morphology is extraordinary and counting sperm is challenging, severely limiting our ability to make predictions of male reproductive potential from the standard semen analysis, and begging the question: are there other, better tests of sperm? We need to explore how overall health affects male fertility and whether treating other diseases improves it. Because a man does not live in a vacuum, we need to understand how the environment affects male reproduction. When considering the treatment of male infertility, men often ask what they can do to improve their fertility, and well-conducted studies into diet and nutraceuticals are essential. The endocrine system drives the making of sperm and further evidence is required to understand if hormonal therapy could improve the production of sperm and improve live birth rates.
The priorities for unexplained infertility seek answers to several challenging and long-standing questions, including the prevention of age-related infertility and exploring the role of fibroids, polyps, intrauterine adhesions and uterine septa in unexplained infertility. It is also surprising that it remains unclear what the first-line treatment is for couples with unexplained infertility, IVF or IUI, and the timing of the superior treatment for that couple.
When considering medically assisted reproduction, new large prospective cohorts that consider all variables and use advance methodology will be required to address casual relationships related to implantation failure. Similar complexity will exist when studying oocyte yield and quality over subsequent IVF cycles, even though similar stimulation protocols have been used. The three research priorities concerning the effectiveness of IVF are seeking to identify optimal ovarian stimulation protocols in poor responders, sperm selection techniques and embryo selection. These contrast with the research priorities which explore if, when, and how IUI should be used. To answer these effectiveness questions, well-designed randomized controlled trials will be required (Wilkinson et al., 2019b). The psychological impact of fertility treatment is brought into sharper focus with research priorities related to the emotional and psychological impact of repeated fertility treatment failure and in children following gamete donation. Strong involvement of patient representatives, psychologists and behavioral scientists will be required to establish the appropriate qualitative and quantitative studies to address these important priorities.
The research priorities for ethics, access and organization of care broadly fall into two overarching themes: access and infertility as a public health issue. When considering access, cost is a major barrier to appropriate care, which is reflected in the research priorities aiming to explore interventions to reduce the cost of fertility treatment and increase the availability of fertility treatment in lower-resources settings. Turning to infertility as a public health issue, prevention of infertility should be a key priority for public health initiatives. We need to determine the minimum standard of care that people with fertility problems should expect, especially if we are seeking reimbursements for this care.
Wider context
A prioritized list of research uncertainties, developed to specifically highlight the most pressing clinical needs as perceived by healthcare professionals, people with fertility problems and others, should help funding organizations and researchers to set their future research agenda. The selected list of research uncertainties should serve to focus a discussion regarding the allocation of limited resources.
Many of the research priorities will require national and international collaboration. Several countries, including China, the Netherlands, the UK and the USA, have developed national networks to undertake infertility research (Devall et al., 2020). Further development of national infrastructure is required. Collaboration should spread beyond national boundaries and develop within an international context. It is hoped the development of a prioritized research agenda could be an important enabler to deepen international collaboration. Development of generic infrastructure could help foster collaboration, including the use of minimum data sets, known as core outcome sets, low-cost data repositories and standardized approaches to the reporting of research. A core outcome set has recently been developed for future infertility trials (Duffy et al., 2018). Over 400 healthcare professionals, researchers and patients, from 40 countries, have used formal consensus development methods to identify a core outcome set for infertility (Duffy et al., 2020a). Consensus definitions have also been agreed for individual core outcomes (Duffy et al., 2020b). It is hoped the core outcome set will provide generic tools to collect outcomes during research, provide concise guidance regarding statistical analysis and standardize the approach to research reporting (Duffy et al., 2019).
Research priorities identified in this study correspond with research priorities identified by the Priority Setting Partnership for Miscarriage, including determining the emotional and psychological impact of miscarriage, investigating the modifiable risk factors which cause miscarriage and identifying specific comorbidities which cause miscarriage (Prior et al., 2017). Other similarities exist when considering the research uncertainties prioritized by the Priority Setting Partnership for Endometriosis and International Polycystic Ovary Syndrome Network (Horne et al., 2017).
Answering the prioritized research questions would represent a significant step forward for our specialty. The steering group recognizes the important role of research which stems from the intellectual curiosity of individuals, fundamental research which does not have an immediate clinical application and research which is funded by special interest groups raising funding for the topic of their particular interest. A blended research strategy should offer the optimal pathway to improving clinical care and patient outcomes.
Perhaps the most important part of this process has been the strengthening of relationships between partner organizations, healthcare professionals and people with lived experience of infertility. The prioritized list of uncertainties that require research should help funding organizations and researchers to set their future research agenda. Our approach should ensure that future research has the necessary reach and relevance to inform clinical practice and to improve patient outcomes.
Supplementary data
Supplementary data are available at Human Reproduction online.
Supplementary Material
Acknowledgments
We would like to thank the initial survey, interim prioritization survey and consensus development meeting participants, and colleagues at the Cochrane Gynaecology and Fertility Group, University of Auckland, New Zealand.
Authors’ roles
Study concept and design: J.M.N.D., S.B., B.C., C.C., J.L.H.E., R.G.F., S.F., L.C.G., A.W.H., N.P.J., Y.K., J.M.L.K., R.S.L., S.L., B.W.M., H.N., E.H.Y.N., C.N., A.S.O., L.P., S.R.H., M.S., J.S., A.S., C.S., A.V., M.v.W., M.A.V., N.L.V., A.Y.W., R.W., J.W. and C.M.F. Acquisition of data: J.M.N.D., S.B., K.B., C.B., C.C., J.L.H.E., R.G.F., A.F., S.F., L.C.G., A.W.H., N.P.J., Y.K., J.M.L.K., R.S.L., S.L., B.W.M., E.H.Y.N., C.N., A.S.O., L.P., S.R.H., M.S., J.S., A.S., C.S., A.V., M.v.W., M.A.V., N.L.V., A.Y.W., R.W., J.W. and C.M.F. Analysis and interpretation of data: J.M.D., G.D.A., E.B., S.B., S.B., M.B., K.B., B.C., C.C., J.L.H.E., R.G.F., A.F., S.F., L.C.G., E.G., M.H., A.W.H., M.L.H., N.P.J., V.J., Y.K., J.M.L.K., R.S.L., S.L., J.M., D.M., B.W.M., D.E.M., H.N., E.H.Y.N., C.N., A.S.O., L.P., S.R.H., L.S., I.S., M.S., J.S., A.S., C.S., A.V., M.v.W., M.V., N.L.V., A.Y.W., R.W., J.W., K.W., T.W. and C.M.F. Drafting of the manuscript: J.M.D., B.C., S.L., H.N., C.N., M.S., M.v.W., M.V., R.W., J.W. and C.M.F. Critical revision of the manuscript for important intellectual content: G.D.A., E.B., S.B., S.B., M.B., B.K., C.C., J.L.H.E., R.G.F., A.F., S.F., L.C.G., E.G., M.H., A.W.H., M.L.H., N.P.J., V.J., Y.K., J.M.L.K., R.S.L., J.M., M.M., D.M., B.W.M., D.M., E.H.Y.N., A.S.O., L.P., S.R.H., L.S., I.S., J.S., A.S., C.S., A.V., N.L.V., A.Y.W., K.W. and T.W. Statistical analysis: J.M.D., J.W. and A.V. Study supervision: C.M.F.
Funding
This research was funded by the Catalyst Fund, Royal Society of New Zealand, Auckland Medical Research Foundation and Maurice and Phyllis Paykel Trust. The funders had no role in the design and conduct of the study, the collection, management, analysis or interpretation of data or manuscript preparation. B.W.M. is supported by a National Health and Medical Research Council Practitioner Fellowship (GNT1082548). Siladitya Bhattacharya was supported by the Auckland Foundation Seelye Travelling Fellowship.
Conflict of interest
G.D.A. reports research sponsorship from Abbott, personal fees from Abbott and LabCorp, a financial interest in Advanced Reproductive Care, committee membership of the FIGO Committee on Reproductive Medicine, International Committee for Monitoring Assisted Reproductive Technologies, International Federation of Fertility Societies and World Endometriosis Research Foundation, and research sponsorship of the International Committee for Monitoring Assisted Reproductive Technologies from Abbott and Ferring. Siladitya Bhattacharya reports being the Editor-in-Chief of Human Reproduction Open and editor for the Cochrane Gynaecology and Fertility Group. J.H.L.E. reports being the Editor Emeritus of Human Reproduction. A.W.H. reports research sponsorship from the Chief Scientist’s Office, Ferring, Medical Research Council, National Institute for Health Research and Wellbeing of Women and consultancy fees from AbbVie, Ferring, Nordic Pharma and Roche Diagnostics. M.L.H. reports grants from Merck, grants from Myovant, grants from Bayer, outside the submitted work and ownership in Embrace Fertility, a private fertility company. N.P.J. reports research sponsorship from AbbVie and Myovant Sciences and consultancy fees from Guerbet, Myovant Sciences, Roche Diagnostics and Vifor Pharma. J.M.L.K. reports research sponsorship from Ferring and Theramex. R.S.L. reports consultancy fees from AbbVie, Bayer, Ferring, Fractyl, Insud Pharma and Kindex and research sponsorship from Guerbet and Hass Avocado Board. B.W.M. reports consultancy fees from Guerbet, iGenomix, Merck, Merck KGaA and ObsEva. E.H.Y.N. reports research sponsorship from Merck. C.N. reports being the Co Editor-in-Chief of Fertility and Sterility and Section Editor of the Journal of Urology, research sponsorship from Ferring and retains a financial interest in NexHand. J.S. reports being employed by a National Health Service fertility clinic, consultancy fees from Merck for educational events, sponsorship to attend a fertility conference from Ferring and being a clinical subeditor of Human Fertility. A.S. reports consultancy fees from Guerbet. J.W. reports being a statistical editor for the Cochrane Gynaecology and Fertility Group. A.V. reports that he is a Statistical Editor of the Cochrane Gynaecology & Fertility Review Group and the journal Reproduction. His employing institution has received payment from Human Fertilisation and Embryology Authority for his advice on review of research evidence to inform their ‘traffic light’ system for infertility treatment ‘add-ons’. N.L.V. reports consultancy and conference fees from Ferring, Merck and Merck Sharp and Dohme. The remaining authors declare no competing interests in relation to the present work. All authors have completed the disclosure form.
Appendix
Priority Setting Partnership for Infertility
Dr Hisham AlAhwany, University of Nottingham, UK; Ofra Balaban, CHEN: Patient Fertility Association, Israel; Faith Barton, UK; Dr Yusuf Beebeejaun, King’s Fertility, Fetal Medicine Research Institute, UK; Professor Jacky Boivin, Cardiff University, UK; Professor Jan J. A. Bosteels, Imelda Hospital, Belgium; Professor Carlos Calhaz-Jorge, Faculdade de Medicina da Universidade de Lisboa, Portugal; Dr Arianna D’Angelo, Wales Fertility Institute, UK; Dr Leona F. Dann, Health Quality and Safety Commission, New Zealand; Professor Christopher J. De Jonge, University of Minnesota Medical Center, United States; Elyce du Mez, University of Auckland, New Zealand; Professor Rui A. Ferriani, University of Sao Paulo, Brazil; Dr Marie-Odile Gerval, Chelsea and Westminster Hospital NHS Foundation Trust, UK; Lynda J. Gingel, UK; Dr Ellen M. Greenblatt, Mount Sinai Fertility, University of Toronto, Toronto; Professor Geraldine Hartshorne, University of Warwick, UK; Charlie Helliwell, New Zealand; Charlotte Helliwell, New Zealand; Lynda J. Hughes, The Fertility Clinic, London Health Sciences Centre, Canada; Dr Junyoung Jo, Conmaul Hospital of Korean Medicine, Republic of Korea; Jelena Jovanović, Serbia; Professor Ludwig Kiesel, University of Münster, Germany; Dr Chumnan Kietpeerakool, Khon Kaen University, Thailand; Dr Elena Kostova, Cochrane Gynaecology and Fertility, New Zealand; Professor Tansu Kucuk, Acibadem Maslak Hospital, Turkey; Rajesh Kumar, National Foundation for the Deaf, New Zealand; Robyn L. Lawrence, The Liggins Institute, The University of Auckland, New Zealand; Nicole Lee, Canada; Katy E. Lindemann, UK; Professor Olabisi M. Loto, Obafemi Awolowo University, Nigeria; Associate Professor Peter J. Lutjen, Monash University, Australia; Michelle MacKinven, Fertility New Zealand; New Zealand; Dr Mariano Mascarenhas, Leeds Teaching Hospital NHS Trust, UK; Helen McLaughlin, Endometriosis UK, UK; David J. Mills, UK; Dr Selma M. Mourad, Isala Hospital Zwolle, The Netherlands; Linh K. Nguyen, Vietnam; Professor Robert J. Norman, Robinson Research Institute, University of Adelaide, Adelaide; Maja Olic, NGO Counselling Center for In Vitro Fertilisation, Serbia; Kristine L. Overfield, NISIG: National Infertility Support and Information Group, Ireland; Maria Parker-Harris, UK; David G. Ramos, Spain; Aleksandra Rendulic, Serbia; Sjoerd Repping, Amsterdam University Medical Centres, The Netherlands; Professor Roberta Rizzo, University of Ferrara, Italy; Professor Pietro Salacone, Italy; Catherine H. Saunders, The Dartmouth Institute for Health Policy and Clinical Practice, USA; Dr Rinku Sengupta, UK; Dr Ioannis A. Sfontouris, Eugonia: Assisted Reproduction Unit, Greece; Natalie R. Silverman, The Fertility Podcast, UK; Dr Helen L. Torrance, University Medical Center Utrecht, The Netherlands; Dr Eleonora P. Uphoff, UK; Dr Sarah A. Wakeman, Fertility Associates, New Zealand; Professor Tewes Wischmann, Heidelberg University, Germany; Dr Bryan J. Woodward, UK; and Mohamed A. Youssef, Cairo University, Egypt.
Footnotes
This article has not been externally peer reviewed.
This article has been published simultaneously in Fertility and Sterility.
Contributor Information
J M N Duffy, King’s Fertility, Fetal Medicine Research Institute, London, UK; Institute for Women’s Health, University College London, London, UK.
G D Adamson, ARC Fertility, Cupertino, CA, USA.
E Benson, Patient and Public Participation Group, Priority Setting Partnership for Infertility, University of Auckland, Auckland, New Zealand.
S Bhattacharya, Institute of Applied Health Sciences, University of Aberdeen, Aberdeen, UK.
S Bhattacharya, Institute of Applied Health Sciences, University of Aberdeen, Aberdeen, UK.
M Bofill, Department of Obstetrics and Gynaecology, University of Auckland, Auckland, New Zealand.
K Brian, Women’s Network, Royal College of Obstetricians and Gynecologists, London, UK.
B Collura, Resolve: The National Infertility Association, VA, USA.
C Curtis, School of Psychology, University of Waikato, Hamilton, New Zealand.
J L H Evers, Centre for Reproductive Medicine and Biology, University Medical Centre Maastricht, Maastricht, The Netherlands.
R G Farquharson, Department of Obstetrics and Gynaecology, Liverpool Women's NHS Foundation Trust, Liverpool, UK.
A Fincham, Fertility Europe, Belgium.
S Franik, Department of Obstetrics and Gynaecology, Münster University Hospital, Münster, Germany.
L C Giudice, Center for Research, Innovation and Training in Reproduction and Infertility, Center for Reproductive Sciences, University of California, San Francisco, CA, USA; International Federation of Fertility Societies, Mount Royal, NJ, USA.
E Glanville, Auckland District Health Board, Auckland, New Zealand.
M Hickey, Department of Obstetrics and Gynaecology, University of Melbourne, Victoria, Australia.
A W Horne, MRC Centre for Reproductive Health, University of Edinburgh, Edinburgh, UK.
M L Hull, Robinson Research Institute and Adelaide Medical School, University of Adelaide, Adelaide, Australia.
N P Johnson, Robinson Research Institute and Adelaide Medical School, University of Adelaide, Adelaide, Australia.
V Jordan, Department of Obstetrics and Gynaecology, University of Auckland, Auckland, New Zealand.
Y Khalaf, Department of Women and Children’s Health, Kings College London, London, UK.
J M L Knijnenburg, Freya, Gorinchem, The Netherlands.
R S Legro, Department of Obstetrics and Gynaecology, Penn State College of Medicine, PA, USA.
S Lensen, Department of Obstetrics and Gynaecology, University of Melbourne, Victoria, Australia.
J MacKenzie, Fertility Plus, Auckland, New Zealand.
D Mavrelos, Reproductive Medicine Unit, University College Hospital, London, UK.
B W Mol, Department of Obstetrics and Gynaecology, Monash University, Melbourne, Australia.
D E Morbeck, Department of Obstetrics and Gynaecology, University of Auckland, Auckland, New Zealand; Fertility Associates, Auckland, New Zealand.
H Nagels, Cochrane Gynaecology and Fertility, University of Auckland, Auckland, New Zealand.
E H Y Ng, Department of Obstetrics and Gynaecology, The University of Hong Kong, Hong Kong; Shenzhen Key Laboratory of Fertility Regulation, The University of Hong Kong-Shenzhen Hospital, China.
C Niederberger, Department of Urology, University of Illinois at Chicago College of Medicine, Chicago, IL, USA.
A S Otter, Osakidetza OSI, Bilbao, Spain.
L Puscasiu, ARC Fertility, Cupertino, CA, USA; Institute for Women’s Health, University College London, London, UK; Center for Reproductive Medicine, Amsterdam Reproduction and Development Institute, Amsterdam University Medical Centres, Amsterdam, The Netherlands.
S Rautakallio-Hokkanen, Fertility Europe, Belgium.
L Sadler, Department of Obstetrics and Gynaecology, University of Auckland, Auckland, New Zealand; Auckland District Health Board, Auckland, New Zealand.
I Sarris, King’s Fertility, Fetal Medicine Research Institute, London, UK.
M Showell, Cochrane Gynaecology and Fertility, University of Auckland, Auckland, New Zealand.
J Stewart, British Fertility Society, Middlesex, UK.
A Strandell, Sahlgrenska Academy, Department of Obstetrics and Gynecology, University of Gothenburg, Sahlgrenska University Hospital, Göteborg, Sweden.
C Strawbridge, Fertility Network UK, London, UK.
A Vail, Centre for Biostatistics, University of Manchester, Manchester Academic Health Science Centre, Manchester, UK.
M van Wely, Center for Reproductive Medicine, Amsterdam Reproduction and Development Institute, Amsterdam University Medical Centres, Amsterdam, The Netherlands.
M Vercoe, Cochrane Gynaecology and Fertility, University of Auckland, Auckland, New Zealand.
N L Vuong, Department of Obstetrics and Gynaecology, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam.
A Y Wang, Australian Centre for Public and Population Health Research, Faculty of Health, University of Technology, Sydney, Australia.
R Wang, Department of Obstetrics and Gynaecology, Monash University, Melbourne, Australia.
J Wilkinson, Centre for Biostatistics, University of Manchester, Manchester Academic Health Science Centre, Manchester, UK.
K Wong, School of Psychology, University of Waikato, Hamilton, New Zealand.
T Y Wong, Auckland District Health Board, Auckland, New Zealand.
C M Farquhar, Department of Obstetrics and Gynaecology, University of Auckland, Auckland, New Zealand; Cochrane Gynaecology and Fertility, University of Auckland, Auckland, New Zealand.
Priority Setting Partnership for Infertility:
Hisham AlAhwany, Ofra Balaban, Faith Barton, Yusuf Beebeejaun, Jacky Boivin, Jan J A Bosteels, Carlos Calhaz-Jorge, Arianna D’Angelo, Leona F Dann, Christopher J De Jonge, Elyce du Mez, Rui A Ferriani, Marie-Odile Gerval, Lynda J Gingel, Ellen M Greenblatt, Geraldine Hartshorne, Charlie Helliwell, Charlotte Helliwell, Lynda J Hughes, Junyoung Jo, Jelena Jovanović, Ludwig Kiesel, Chumnan Kietpeerakool, Elena Kostova, Tansu Kucuk, Rajesh Kumar, Robyn L Lawrence, Nicole Lee, Katy E Lindemann, Olabisi M Loto, Peter J Lutjen, Michelle MacKinven, Mariano Mascarenhas, Helen McLaughlin, David J Mills, Selma M Mourad, Linh K Nguyen, Robert J Norman, Maja Olic, Kristine L Overfield, Maria Parker-Harris, David G Ramos, Aleksandra Rendulic, Sjoerd Repping, Roberta Rizzo, Pietro Salacone, Catherine H Saunders, Rinku Sengupta, Ioannis A Sfontouris, Natalie R Silverman, Helen L Torrance, Eleonora P Uphoff, Sarah A Wakeman, Tewes Wischmann, Bryan J Woodward, and Mohamed A Youssef
References
- American Association of Gynecologic Laparoscopists. Practice guidelines for the diagnosis and management of submucous leiomyomas. J Minim Invasive Gynecol 2012;19:152–171. [DOI] [PubMed] [Google Scholar]
- American Urological Association. The Management of Obstructive Azoospermia. Linthicum, MD: American Urological Association, 2010.
- Carranza-Mamane B, Havelock J, Hemmings R, Cheung A, Sierra S, Carranza-Mamane B, Case A, Cathie D, Graham J, Havelock J et al. The management of uterine fibroids in women with otherwise unexplained infertility. J Obstet Gynaecol Can 2015;37:277–285. [DOI] [PubMed] [Google Scholar]
- Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet 2009;374:86–89. [DOI] [PubMed] [Google Scholar]
- Devall AJ, Out JH, Mol BWJ, Duffy JMN, Collura B, Dyer S. Coordination and planning of clinical research on a national and global level. Fertil Steril 2020;113:1100–1106. [DOI] [PubMed] [Google Scholar]
- Duffy JMN, AlAhwany H, Bhattacharya S, Collura B, Curtis C, Evers JLH, Farquharson RG, Franik S, Giudice LC, Khalaf Y et al. Developing a core outcome set for future infertility research: an international consensus development study. Hum Reprod 2020. a;35: 2725–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JMN, Bhattacharya S, Bhattacharya S, Bofill M, Collura B, Curtis C, Evers JLH, Giudice LC, Farquharson RG, Franik S et al. Standardizing definitions for the infertility core outcome set: an international consensus development study. Hum Reprod 2020. b;35:2735–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JMN, Bhattacharya S, Curtis C, Evers JLH, Farquharson RG, Franik S, Khalaf Y, Legro RS, Lensen S, Mol BW, COMMIT: Core Outcomes Measures for Infertility Trials et al. A protocol developing, disseminating and implementing a core outcome set for infertility. Hum Reprod Open 2018;2018:hoy007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JMN, Bhattacharya S, Herman M, Mol B, Vail A, Wilkinson J, Farquhar C, the Cochrane Gynaecology and Fertility Group. Reducing research waste in benign gynaecology and fertility research. BJOG: Int J Obstet Gy 2017;124:366–369. [DOI] [PubMed] [Google Scholar]
- Duffy JMN, Ziebland S, von Dadelszen P, McManus RJ. Tackling poorly selected, collected, and reported outcomes in obstetrics and gynecology research. Am J Obstet Gynecol 2019;220:71.e71–71.e74. [DOI] [PubMed] [Google Scholar]
- Graham L, Illingworth B, Showell M, Vercoe M, Crosbie E, Gingel L, Farquhar C, Horne A, Prior M, Stephenson J et al. Research priority setting in women’s health: a systematic review. BJOG: Int J Obstet Gy 2020;127:694–700. [DOI] [PubMed] [Google Scholar]
- Horne AW, Saunders PTK, Abokhrais IM, Hogg L. Top ten endometriosis research priorities in the UK and Ireland. Lancet 2017;389:2191–2192. [DOI] [PubMed] [Google Scholar]
- James Lind Alliance. The James Lind Alliance Guidebook. Southampton, UK: National Institute for Health Research Evaluation, Trials and Studies Coordinating Centre, 2018. [Google Scholar]
- Jarvi K, Lo K, Fischer A, Grantmyre J, Zini A, Chow V, Mak VC. Guideline the workup of azoospermic males. Can Urol Assoc J 2010;4:163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungwirth A, Diemer T, Kopa Z, Krausz C, Minhas S, Tournaye H. EAU Guidelines on Male Infertility. Arnhem, The Netherlands: European Association of Urology, 2018. [Google Scholar]
- Kroon B, Johnson N, Chapman M, Yazdani A, Hart R, on behalf of the Australasian CREI Consensus Expert Panel on Trial evidence (ACCEPT) group. Fibroids in infertility–consensus statement from ACCEPT (Australasian CREI Consensus Expert Panel on Trial evidence). Aust N Z J Obstet Gynaecol 2011;51:289–295. [DOI] [PubMed] [Google Scholar]
- Loh SF, Agarwal R, Chan JK, Chia SJ, Cho LW, Lim LH, Lau MSK, Loh SKE, Hendricks MS, Nair S et al. Academy of Medicine-Ministry of Health Clinical Practice Guidelines: assessment and management of infertility at primary healthcare level. Singapore Med J 2014;55–58; quiz 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CF, Askham J, Marteau T. Consensus development methods, and their use in clinical guideline development. Health Technol Assess 1998;2:1–88, i-iv, [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. Fertility Problems: Assessment and Treatment. London, UK: National Institute for Health and Care Excellence, 2017 [PubMed] [Google Scholar]
- Penzias A, Bendikson K, Butts S, Coutifaris C, Falcone T, Fossum G, Gitlin S, Gracia C, Hansen K, La Barbera A et al. Performing the embryo transfer: a guideline. Fertil Steril 2017;107:882–896. [DOI] [PubMed] [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. Uterine septum: a guideline. Fertil Steril 2016. a;106:530–540. [DOI] [PubMed] [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril 2016. b;106:1634–1647. [DOI] [PubMed] [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. Removal of myomas in asymptomatic patients to improve fertility and/or reduce miscarriage rate: a guideline. Fertil Steril 2017;108:416–425. [DOI] [PubMed] [Google Scholar]
- Prior M, Bagness C, Brewin J, Coomarasamy A, Easthope L, Hepworth-Jones B, Hinshaw K, O'Toole E, Orford J, Regan L et al. Priorities for research in miscarriage: a priority setting partnership between people affected by miscarriage and professionals following the James Lind Alliance methodology. BMJ Open 2017;7:e016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson J, Bhattacharya S, Duffy JMN, Kamath MS, Marjoribanks J, Repping S, Vail A, Wely M, Farquhar CM. Reproductive medicine: still more ART than science? BJOG: Int J Obstet Gy 2019. a;126:138–141. [DOI] [PubMed] [Google Scholar]
- Wilkinson J, Brison DR, Duffy JMN, Farquhar CM, Lensen S, Mastenbroek S, van Wely M, Vail A. Don’t abandon RCTs in IVF. We don’t even understand them. Hum Reprod 2019. b;34:2093–2098. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.