Abstract
STUDY QUESTION
How has the performance of the European regional register of the European IVF-monitoring Consortium (EIM)/European Society of Human Reproduction and Embryology (ESHRE) evolved from 1997 to 2016, as compared to the register of the Centres for Disease Control and Prevention (CDC) of the USA and the Australia and New Zealand Assisted Reproduction Database (ANZARD)?
SUMMARY ANSWER
It was found that coherent and analogous changes are recorded in the three regional registers over time, with a different intensity and pace, that new technologies are taken up with considerable delay and that incidental complications and adverse events are only recorded sporadically.
WHAT IS KNOWN ALREADY
European data on ART have been collected since 1997 by EIM. Data collection on ART in Europe is particularly difficult due to its fragmented political and legal landscape. In 1997, approximately 78.1% of all known institutions offering ART services in 23 European countries submitted data and in 2016 this number rose to 91.8% in 40 countries.
STUDY DESIGN, SIZE, DURATION
We compared the changes in European ART data as published in the EIM reports (2001–2020) with those of the USA, as published by CDC, and with those of Australia and New Zealand, as published by ANZARD.
PARTICIPANTS/MATERIALS, SETTING, METHODS
We performed a retrospective analysis of the published EIM data sets spanning the 20 years observance period from 1997 to 2016, together with the published data sets of the USA as well as of Australia and New Zealand. By comparing the data sets in these three large registers, we analysed differences in the completeness of the recordings together with differences in the time intervals on the occurrence of important trends in each of them. Effects of suspected over- and under-reporting were also compared between the three registers. X2 log-rank analysis was used to assess differences in the data sets.
MAIN RESULTS AND THE ROLE OF CHANCE
During the period 1997–2016, the numbers of recorded ART treatments increased considerably (5.3-fold in Europe, 4.6-fold in the USA, 3.0-fold in Australia and New Zealand), while the number of registered treatment modalities rose from 3 to 7 in Europe, from 4 to 10 in the USA and from 5 to 8 in Australia and New Zealand, as published by EIM, CDC and ANZARD, respectively. The uptake of new treatment modalities over time has been very different in the three registers. There is a considerable degree of underreporting of the number of initiated treatment cycles in Europe. The relationship between IVF and ICSI and between fresh and thawing cycles evolved similarly in the three geographical areas. The freeze-all strategy is increasingly being adopted by all areas, but in Europe with much delay. Fewer cycles with the transfer of two or more embryos were reported in all three geographical areas. The delivery rate per embryo transfer in thawing cycles bypassed that in fresh cycles in the USA in 2012, in Australia and New Zealand in 2013, but not yet in Europe. As a result of these changing approaches, fewer multiple deliveries have been reported. Since 2012, the most documented adverse event of ART in all three registers has been premature birth (<37 weeks). Some adverse events, such as maternal death, ovarian hyperstimulation syndrome, haemorrhage and infections, were only recorded by EIM and ANZARD.
LIMITATIONS, REASONS FOR CAUTION
The methods of data collection and reporting were very different among European countries, but also among the three registers. The better the legal background on ART surveillance, the more complete are the data sets. Until the legal obligation to report is installed in all European countries together with an appropriate quality control of the submitted data the reported numbers and incidences should be interpreted with caution.
WIDER IMPLICATIONS OF THE FINDINGS
The growing number of reported treatments in ART, the higher variability in treatment modalities and the rising contribution to the birth rates over the last 20 years point towards the increasing impact of ART. High levels of completeness in data reporting have been reached, but inconsistencies and inaccuracies still remain and need to be identified and quantified. The current trend towards a higher diversity in treatment modalities and the rising impact of cryostorage, resulting in improved safety during and after ART treatment, require changes in the organization of surveillance in ART. The present comparison must stimulate all stakeholders in ART to optimize surveillance and data quality assurance in ART.
STUDY FUNDING/COMPETING INTEREST(S)
This study has no external funding and all costs are covered by ESHRE. There are no competing interests.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: ART, surveillance, vigilance, registry, data collection, pregnancy, freeze-all, maternal death, oocyte donation, prematurity
Introduction
On behalf of the European IVF-monitoring Consortium (EIM), it was decided to compare recorded data dealing with ART in Europe with those of two other large registers in order not only to assess achievements but also to study potential deficiencies in the registration process. EIM was created 20 years ago by European Society of Human Reproduction and Embryology (ESHRE) and the first set of data for 1997 was published in 2001 (Nygren and Andersen, 2001). Since then, annual reports have appeared covering the European activities in ART from 1997 to 2016, including a survey describing the trends over 15 years of ART in Europe (Ferraretti et al., 2017). Although the first successful treatment with ART was carried out in Europe (Steptoe and Edwards, 1978), systematic data collection only started in Europe in 1997. Europe currently consists of 51 independent countries with very diverse cultural, political, economic and legal systems, often lacking national data registers dealing with reproduction, rendering the Europe-wide registration of activities in ART difficult. Whereas the first report contained data from 18 countries (Nygren and Andersen, 2001), EIM currently manages the largest global register, dealing with ART reported by 40 European countries (Wyns et al., 2020).
One way to analyse the quality of the European data register as managed by EIM is to compare it with large data sets provided by other sizeable registries that have been active during the same time period. Similarities and discrepancies between such registries are likely to be useful to further improve data recording and interpretation. For that reason, the trends observed in Europe during the time interval from 1997 to 2016 were compared with those of other large registers, such as that of the USA (Centres for Disease Control and Prevention (CDC), with the first data set collected in 1996) and of Australia and New Zealand (National Perinatal Epidemiology and Statistics Unit, first data set available online 1996, since 2004 known as the Australia and New Zealand Assisted Reproduction Database (ANZARD)). The retrospective analysis of the data sets spanning the 20-year observance period from 1997 to 2016 could help to identify timely trends in the various techniques of ART.
Materials and methods
Objective and rationale
We performed a retrospective analysis of the published EIM data sets spanning the 20 years observance period from 1997 to 2016 together with the published data sets of the USA and those of Australia and New Zealand, both during the same time interval. The analysis was carried out to assess newly occurring trends in ART in the three registers. By comparing the data sets in the three large registers, we analysed differences in the completeness of the recordings together with differences in the time intervals between the occurrence of important trends in each of the three large registers.
Search methods
Since 2001, a total of 18 EIM reports covering the activities in ART from 1997 to 2014 in all participating European countries have been published in Human Reproduction. The latest reports dealing with ART in Europe in 2015 and 2016 were published in Human Reproduction Open. All data included in the present report have been extracted from the published data sets, all available online. For comparison purposes we chose the annual data sets with similar degrees of completeness and with a similar multinational organization, i.e. ART activity register of the USA, published in English by CDC and available online, and those of Australia and New Zealand, also published in English by ANZARD and available online. The two other large registers in ART are the Latin American registry of Assisted Reproduction (RLA) (Zegers-Hochschild et al., 2020) and the global overview of activities in ART, published by the International Committee for Monitoring Assisted Reproductive Technologies (ICMART, Adamson et al., 2018; De Mouzon et al., 2020).
Organization and validation of data collections
The EIM data register collects data on IVF, ICSI, frozen embryo transfer (FET), egg donation (ED, since 1998), IVM (since 2002), pooled data on preimplantation genetic testing (PGT, since 2002) and frozen oocyte replacement (since 2006). In principle, the national registries of all European countries are welcome to participate. The legal obligation of single institutions offering ART services to report data to the authorities is very different among different European countries and, as a consequence, the methods of reporting vary widely. The degree of participation of the national registries in the EIM-data collection changes every year, as does the participation of institutions towards their respective national registries. The collected data governed by the central office of ESHRE in Grimbergen, Belgium, consists of aggregated data sets delivered by the participating national registries. The cross-sectional data were originally collected through a questionnaire, and later through online software. While in some countries data are reported in a prospective fashion, cycle by cycle, in other countries aggregated data are reported by each institution every year to their national register, which then sends the national data sets to the ESHRE-EIM office. Most countries use their own software systems, if available. Data quality assurance through external auditing was documented in some countries, such as Switzerland, where every institution is visited every second year (Van den Bergh et al., 2005). However, a uniform quality assurance protocol is not available in Europe. The submitted data sets have been consistently validated by Veerle Goossens.
In the USA outcome data in ART must be reported annually to the CDC, which in agreement with the Fertility Clinic Success Rate and Certification Act (FCSRCA, instituted 24 October 1992) since 1995 collect, analyse and publish data online (www.cdc.gov). Starting in 1997, CDC collaborated with the Society for Assisted Reproductive Technology (SART) to publish the incoming data. For the purpose of the current survey, only the data spanning the observation period from 1997 to 2016 were selected. Details of the history and current organization of surveillance in ART in the USA as carried out by CDC have been described recently (Toner et al., 2019). CDC collects data on ART cycles carried out in virtually all institutions offering ART services across the USA. The data are submitted annually by each institution to the National ART Surveillance System (NASS) using an online reporting system. Institutions that are members of SART report their data to NASS via SART, while others report directly to NASS. Validation of data accuracy needs to be performed by the collecting institutions. Periodic unannounced inspections of all institutions, including the records, as part of the accreditation system are imposed by public law.
The Australian and New Zealand database on ART (ANZARD) was initiated in 2004 by the national perinatal epidemiology and statistics unit (NPSU), now located at the University of New South Wales, Australia, in collaboration with the Fertility Society of Australia. Australia was the first country worldwide to establish a data registry in 1992 for the purpose of surveillance in ART. The data on ART starting in 1992 and going up to 2016 are all available online (www.npesu.unsw.edu.au). Details of the history and current settings of surveillance in ART in Australia and New Zealand, as carried out by ANZARD, have been published recently (Chambers et al., 2019). In Australia and New Zealand, all data on ART and on donor insemination cycles were reported by each institution offering IVF services as early as 1983 to the supranational data collecting service ACDC (Assisted Conception Data Collection) and since 2002 to ANZARD (Chambers et al., 2019). Compulsory data collection and reporting have been carried out as part of accreditation cycle by cycle but since 2009 has developed towards a woman-based data collection. Data quality assurance is carried out through annual external auditing of each institution offering ART services. For the purpose of the current survey, only the data spanning the observation period from 1997 to 2016 were selected.
Treatment modalities and reported items
The recorded treatments in the three registers are listed in Table I. Variations in the reporting of treatment outlined together with outcome data and incident complications in the three registers are presented in Table II.
Table I.
Recorded ART treatment modalities between 1997 and 2016 in the three registers.
| Treatment modalities | EIM | CDC | ANZARD |
|---|---|---|---|
| Autologous | |||
| IVF | 1997 | 1995 | 1996 |
| ICSI | 1997 | 1995 | 1996 |
| FET (IVF + ICSI) | 1997 | 1995 | 1996 |
| Freeze-all | 2017 | 2008 | 2010 |
| eSET | not rec.a | 2008 | 1997b |
| PGT | 2002 | 2006 | 2004 |
| IVM | 2002 | not rec. | not rec. |
| FOR | 2006 | not rec. | not rec. |
| GIFT | not rec. | 1995 | 1996 |
| ZIFT | not rec. | 1995 | not rec. |
| Fertility protection | since 2016 | not rec. | not rec. |
| Heterologous | |||
| ED | 1998 | 1995 | 1996 |
| Embryo donation | 2009 | 2015c | 2004 |
| IVF | not rec. | not rec. | 1996 |
| ICSI | not rec. | not rec. | 1996 |
| Surrogacyd | not rec. | 2014 | 2005 |
‘not rec.’ means ‘not recorded’.
Before 2007, the transfer of a single embryo was recorded only in fresh cycles. It was not differentiated between elective and non-elective single embryo transfer.
In the Centres for Disease Control and Prevention (CDC) data sets, the distinction is made between frozen and fresh embryo donation.
The Australia and New Zealand Assisted Reproduction Database (ANZARD) data sets denominate surrogacy by the term ‘surrogacy arrangement cycles’, whereas the CDC denominates surrogacy by the term ‘gestational carrier’.
ED, egg donation; EIM, European IVF-monitoring Consortium; eSET, elective single embryo transfer; FET, frozen embryo transfer; FOR, frozen oocyte replacement; GIFT, gamete intra-Fallopian transfer; PGT, preimplantation genetic testing; ZIFT, zygote intra-Fallopian transfer.
Table II.
Recorded patient characteristics, treatment outline, outcome data and incident complications.
| EIM | CDC | ANZARDa | |
|---|---|---|---|
| Recorded patient characteristics | |||
| Age of the female patient | Yes | Yes | Yes |
| Age of the partner or husband | No | No | Yes |
| Medical indication for treatment | No | Yes | Yes |
| Treatment outline | |||
| Initiated cycles | Yes | Yes | Yes |
| Aspiration done or not | Yes | Yes | Yes |
| Elective single embryo transfer | No | Since 2008 | No |
| Embryo stage at transfer | Yes | No | Yes |
| Freeze-all | Since 2017 | Yes | Yes |
| Cryopreservation technology | No | No | Yes |
| Number of transferred embryos | Yes | Yes | Yes |
| Outcome data | |||
| Pregnancy | Yes | Yes | Yes |
| Miscarriage | No | No | Yes |
| Other abnormal pregnancy outcomes | No | No | Yes |
| Delivery | Yes | Yes | Yes |
| Mode of delivery | No | No | Yes |
| Cumulative outcome data | Yes, 1 year | No | Yes |
| Multiple delivery | Yes | Yes | Yes |
| Preterm delivery <37 weeks | Yes | Since 2011 | Yes |
| Neonatal birthweight | No | Yes | Yes |
| Neonatal malformation | No | No | Yes |
| Perinatal mortality | No | No | Yes |
| Complications | |||
| OHSS | Yes | No | Yes |
| Maternal deaths | Yes | No | 1999b |
| Haemorrhage | Yes | No | No |
| Infections | Yes | No | No |
| Foetal reduction | Yes | No | Yesc |
See also Table 15.1 in Chambers et al. (2019).
Six cases of maternal death were mentioned 1999.
The number of foetal reductions is combined with terminations of pregnancy.
OHSS, ovarian hyperstimulation syndrome.
Data extraction
European data were extracted manually from the published EIM reports, which contain cross-sectional data sets published annually in Human Reproduction and in Human Reproduction Open (De Geyter et al., 2020; Wyns et al., 2020). Only the data sets needed to calculate the proportion of children born after ART and the entire population of neonates in each European country reporting to EIM were calculated from the raw data sets, made available by the ESHRE office. The total numbers of newborn children and of inhabitants of each participating European country each year were taken from Wikipedia.
The data from the USA were taken from the national summary reports made available by the CDC on the internet (www.cdc.gov), where an extended list of reports on annual data (since 2005) are archived. Between 1997 and 2004, data were taken from the Morbidity and Mortality Weekly Report (surveillance summaries), published by CDC. The number of PGT cycles was calculated from percentage values given in the annual reports.
Annual reports with cross-sectional data on ART in Australia and New Zealand were obtained from the ANZARD website (www.npesu.unsw.edu.au) under the title ‘surveillance reports’. Between 1998 and 2001, reports were published bi-annually and the distinction between events occurring in 1 year and those in the next year was not always clear, most particularly with respect to pregnancies and deliveries after ART.
Statistical analysis
Differences in occurrences in the three geographical areas were analysed with non-parametric X2 analysis using contingency tables. In most instances the differences between populations in the three registries were considerable. In order to better appreciate the degree of difference, X2 values were given in addition to the P-values. Higher X2 values correspond to higher levels of difference between populations. Changes in practices over time were analysed with log-rank statistics, using the following formula and using absolute numbers.
The estimated numbers were calculated with the following formula:
The term ‘events’ refers to a particular treatment or adverse event, whereas the term ‘cases’ refers to the overall population, in which the event may have occurred.
In all comparisons the degree of freedom = 1.
Results
Contributions of individual ART institutions and national registries over time
During the observation period (1997–2016), the number of institutions offering ART services has increased in all three geographical areas. During this time period, 100% coverage of all treatments was reported to be maintained continuously only in Australia and New Zealand (Table III). Between 1997 and 2016, the number of institutions has increased 2.8-fold in Europe (as published by EIM), 2.7-fold in Australia and New Zealand (as published by ANZARD) and only 1.4-fold in the USA (as published by CDC). Only EIM provides explicit information concerning the distribution of the size of institutions offering ART in participating countries. Over time the number of these institutions with more than 1000 treatment cycles per year has nearly doubled (from 11.2% in 2000 to 19.4% in 2016). The number of smaller institutions with fewer than 200 treatment cycles per year decreased from 33.7% in 2000 to 25.2% in 2016 (Supplementary Fig. S1).
Table III.
Degree of completeness of data sets.
| EIM |
CDC |
ANZARD |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Countries with ART | Reporting countries | % | Registered institutions | Participating institutions | % | Registered institutions | Participating institutions | % | Registered institutions | Participating institutions | % |
| 1997 | 40 | 18 | 45.0 | 482 | 335 | 335 | 100 | 35 | 35 | 100 | ||
| 1998 | 41 | 18 | 43.9 | 521 | 360 | 360 | 100 | 38 | 38 | 100 | ||
| 1999 | 41 | 22 | 53.7 | 537 | 370 | 370 | 100 | 38 | 38 | 100 | ||
| 2000 | 41 | 22 | 53.7 | 729 | 569 | 78.1 | 383 | 383 | 100 | 41 | 41 | 100 |
| 2001 | 41 | 23 | 56.1 | 740 | 579 | 78.2 | 385 | 385 | 100 | 41 | 41 | 100 |
| 2002 | 41 | 22 | 53.7 | 770 | 631 | 81.9 | 428 | 391 | 91.4 | 29 | 29 | 100 |
| 2003 | 41 | 28 | 68.3 | 1008 | 725 | 71.9 | 437 | 399 | 91.3 | 29 | 29 | 100 |
| 2004 | 41 | 29 | 70.7 | 1121 | 785 | 70.0 | 461 | 411 | 89.2 | 34 | 34 | 100 |
| 2005 | 41 | 30 | 73.2 | 1134 | 923 | 81.4 | 475 | 422 | 88.8 | 36 | 36 | 100 |
| 2006 | 41 | 32 | 78.0 | 1160 | 998 | 86.0 | 483 | 426 | 88.2 | 39 | 39 | 100 |
| 2007 | 41 | 33 | 80.5 | 1204 | 1029 | 88.7 | 485 | 430 | 88.7 | 43 | 43 | 100 |
| 2008 | 41 | 36 | 87.8 | 1245 | 1051 | 84.4 | 475 | 436 | 91.8 | 43 | 43 | 100 |
| 2009 | 41 | 34 | 82.9 | 1179 | 1005 | 85.2 | 481 | 451 | 93.8 | 37 | 37 | 100 |
| 2010 | 39 | 31 | 79.5 | 1202 | 991 | 82.4 | 474 | 443 | 93.5 | 37 | 37 | 100 |
| 2011 | 41 | 33 | 80.5 | 1314 | 1064 | 81.0 | 481 | 451 | 93.8 | 37 | 37 | 100 |
| 2012 | 41 | 36 | 87.8 | 1354 | 1111 | 82.1 | 486 | 456 | 93.8 | 43 | 43 | 100 |
| 2013 | 41 | 38 | 92.7 | 1369 | 1169 | 85.4 | 497 | 467 | 94.0 | 43 | 43 | 100 |
| 2014 | 42 | 38 | 90.5 | 1419 | 1280 | 90.2 | 498 | 458 | 92.0 | 48 | 48 | 100 |
| 2015 | 43 | 38 | 88.4 | 1483 | 1343 | 90.6 | 499 | 464 | 93.0 | 93 | 93 | 100 |
| 2016 | 44 | 40 | 90.9 | 1467 | 1347 | 91.8 | 501 | 463 | 92.2 | 94 | 94 | 100 |
More European countries have progressively become members of EIM: whereas in 1997 only 18 of all 51 European countries (35.3%) provided data to EIM, the number of participating countries rose to 40 in 2016 (78.4%). As, however, seven (small) European countries do not harbour any known ART institutions, the degree of completeness reached 93.0% in 2016 (Wyns et al., 2020). The number of European countries with complete coverage rose from 10 countries in 1997 to 20 in 2016. Among the 20 European countries with complete coverage in 2016, 17 reported to have a compulsory registration system (85%), whereas three had a voluntary registration system (15%). In contrast, among the 20 European countries with incomplete reporting only four had a compulsory registration (20%) and 16 a voluntary registration (80%) (P = 0.00039, X2 statistic: 16.942).
Comparison of the recorded ART treatment modalities 1997–2016
Over the observation period, the array of registered treatment modalities has significantly increased over time in all three registries (Table I). Whereas some treatment modalities have virtually disappeared, such as gamete intra-Fallopian transfer and zygote intra-Fallopian transfer, many more treatment modalities have been added over time, such as IVM, PGT, ED, surrogacy, and more recently, elective single embryo transfer (eSET), freeze-all strategy and cell and gonadal tissue freezing for fertility preservation. ICSI has been recorded by CDC since 1996, by ANZARD since 1996 and by EIM since 1997. The use of PGT was first recorded by EIM in 2002, by ANZARD in 2004 and by CDC in 2006. CDC initiated registration of eSET in 2008 and ANZARD in 2010. The freeze-all strategy was first registered by CDC in 2008, by ANZARD in 2011 and by EIM in 2017.
Numbers of ART treatments 1997–2016
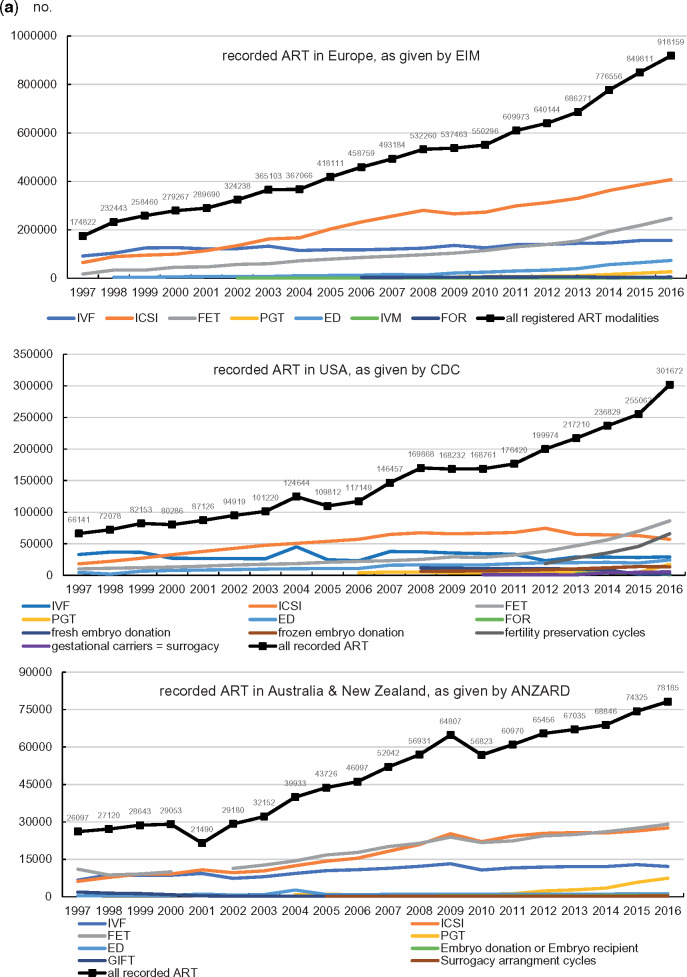
For each therapeutic modality, the treatment numbers recorded by EIM in Europe, by CDC in the USA and by ANZARD in Australia and New Zealand are presented in Fig. 1a and in Supplementary Table SI. In all geographical areas not only the number of treatment modalities has increased over time, but also the total number of therapeutic interventions. Whereas IVF, ICSI and FET have remained the dominant treatment modalities in all three registries, ED became the fourth most used treatment modality in Europe and in the USA (in 2016: 8.1% in Europe, 11.0% in the USA), whereas in 2016, PGT became the fourth most used technology in Australia and New Zealand (9.5%).
Figure 1.
The number of treatments with various forms of ART from 1997 to 2016. ANZARD, Australia and New Zealand Assisted Reproduction Database; CDC, the Centres for Disease Control and Prevention; ED, egg donation; EIM, the European IVF-monitoring Consortium; FET, frozen embryo transfer; FOR, frozen oocyte replacement; PGT, preimplantation genetic testing.
Figure 1.
Continued.
The availability of ART in society has previously been defined by the number of treated couples per million inhabitants and an appropriate number was set at 1500 treatments per million inhabitants (ESHRE Capri Workshop Group, 2001; Collins 2002). In 2016, the utilization of ART in Australia and New Zealand reached 2688 ART treatments per million inhabitants (78 185 reported treatments per 29 082 100 inhabitants), in the USA 934 (301 672 reported treatments per 323 100 000 inhabitants), in Europe including all countries participating in the EIM data collection 1220 (918 159 reported treatments per 752 265 824 inhabitants). In 2016, the European countries with complete coverage of all ART treatments reached a utilization level of 1410 (458 404 reported treatments per 325 078 700 inhabitants); in the European countries with incomplete coverage 1076 (459 755 reported treatments per 323 100 000 inhabitants) (Wyns et al., 2020).
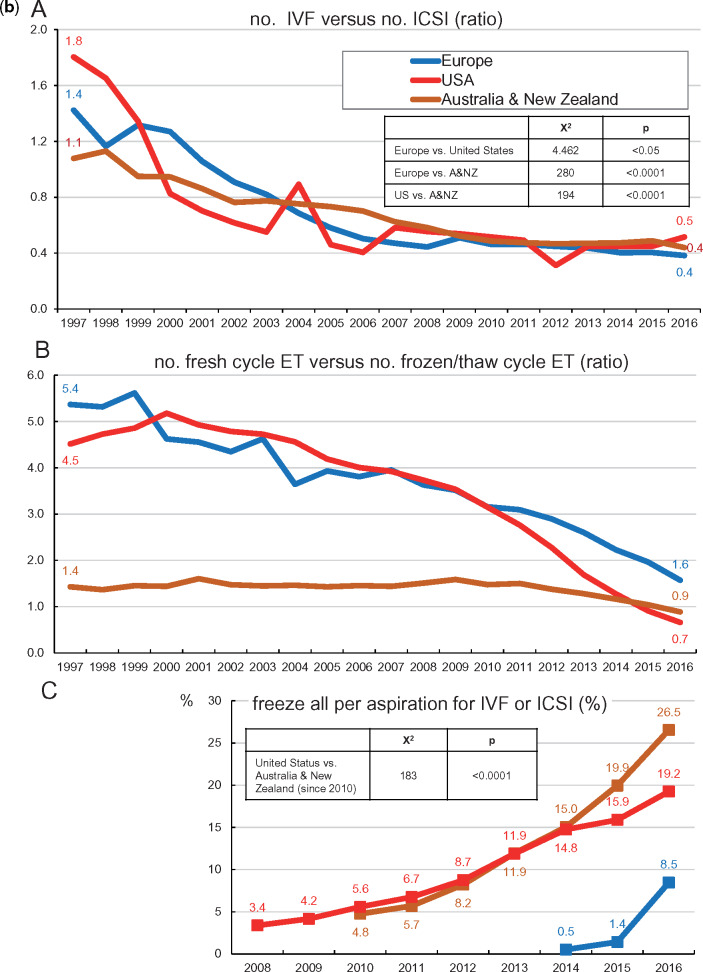
A shift towards more ICSI instead of conventional IVF gradually took place in all three geographical areas (Fig. 1b; A): in Australia and New Zealand, ICSI became more frequently used than IVF in 1999, in the USA in 2000 and in Europe in 2002. Using log-rank statistical analysis the differences in the evolution of the use of IVF with respect to ICSI in the three geographical areas are significant: between Europe and USA, the X2 test statistic was 4.462 (P < 0.05), between Europe and Australia and New Zealand 280 (P < 0.0001) and between the USA and Australia and New Zealand 194 (P < 0.0001).
In addition, the ratio of fresh over thawing cycles is evolving similarly in the three geographical areas. The number of FET cycles started to prevail over fresh treatments in 2016 in Australia and New Zealand and in 2015 in the USA (Fig. 1b; B). In Europe, the number of FET cycles was still less prevalent than fresh transfers in 2016.
Finally, a sharp rise in the number of freeze-all cycles is observed both in Australia and New Zealand (reaching 26.5% of all oocyte retrieval cycles for IVF and ICSI in 2016) and in the USA (19.2% in 2016). Although the evolution of the numbers of freeze-all cycles in the USA and in Australia and New Zealand seems similar, log-rank statistics revealed a statistically significant difference (X2 analysis 183.5, P < 0.0001). The pickup of freeze-all is taking place in Europe as well, although with a clear delay (8.5% in 2016) (Fig. 1b; C).
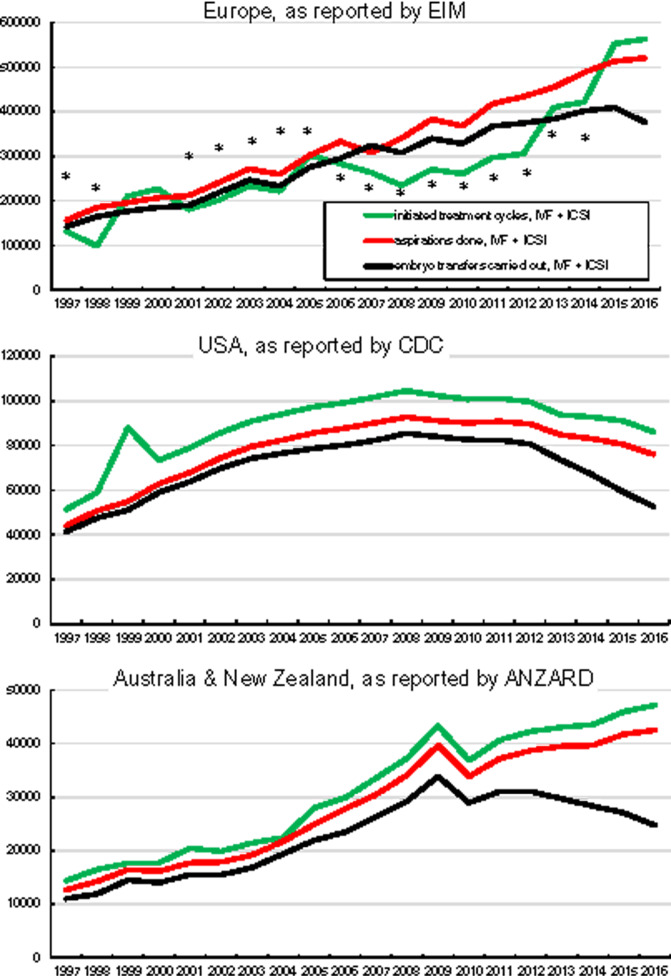
Relationship between the reported numbers of initiated treatments, aspirations for oocyte collection and embryo transfers
During the observation period, the recorded numbers of initiated treatments, oocyte retrievals (aspirations), and embryo transfers in each of the three geographical regions were compared in order to assess the validity of submitted data on treatment numbers (Supplementary Table SII). In the European data sets, the logic in the sequence of events (higher numbers of initiated cycles compared to the numbers of aspirations and higher numbers of aspirations compared to numbers of embryo transfers) was true in only 4 of 20 years. In 16 reported years, the number of initiated cycles did not fit to the number of reported aspirations or to the number of embryo transfers (as marked by asterisks in Fig. 2 and Table IV). In 2007, the reported number of embryo transfers even exceeded the number of initiated cycles and the number of aspirations (Fig. 2). This is caused by a number of large European countries in which the number of initiated cycles has consistently not been reported. Both in the USA and in Australia and New Zealand this requirement was fulfilled during all 20 years of reporting.
Figure 2.
The number of initiated fresh cycles with IVF + ICSI, the number of aspirations of oocytes and the number of cycles with embryo transfers.
Table IV.
European countries reporting IVF + ICSI initiated fresh cycles.
| Year | Reporting countries (no) | Countries, reporting initiated fresh cycles (no) | % |
|---|---|---|---|
| 1997* | 18 | 16 | 88.9 |
| 1998* | 18 | 13 | 72.2 |
| 1999 | 22 | 19 | 86.4 |
| 2000 | 22 | 22 | 100 |
| 2001* | 23 | 21 | 91.3 |
| 2002* | 25 | 22 | 88.0 |
| 2003* | 28 | 25 | 89.3 |
| 2004* | 29 | 23 | 79.3 |
| 2005* | 30 | 26 | 86.4 |
| 2006* | 32 | 29 | 90.6 |
| 2007* | 33 | 27 | 81.8 |
| 2008* | 36 | 31 | 86.1 |
| 2009* | 34 | 30 | 88.2 |
| 2010* | 31 | 29 | 93.5 |
| 2011* | 33 | 33 | 100 |
| 2012* | 34 | 26 | 76.5 |
| 2013* | 38 | 34 | 89.5 |
| 2014* | 39 | 32 | 82.1 |
| 2015 | 38 | 36 | 94.7 |
| 2016 | 40 | 38 | 95.0 |
The asterisks (*) mark the years in which the number of initiated cycles is underreported (Fig. 2).
The reported numbers of initiated treatment cycles varied among European countries (Table IV). In the 4 years with higher reported numbers of initiated treatment cycles, the proportion of reporting European countries varied between 86.4% and 100%.
In the USA fewer embryo transfers during fresh cycles were reported since 2010, in Australia and New Zealand since 2012 and in Europe since 2016 (Fig. 2).
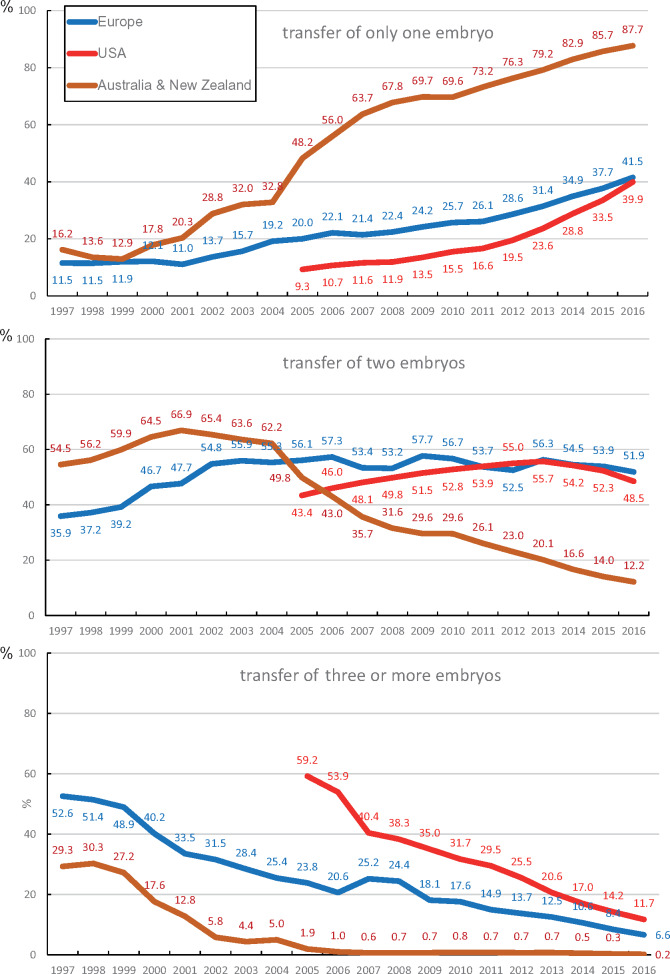
Embryo transfer policy 1997–2016
Changes over time in the number of embryos transferred per treatment cycle are depicted in Fig. 3 and full data are provided in Supplementary Table SIII. Whereas in the European data sets the number of embryos transferred is given both in fresh and in FET cycles, the Australian and New Zealand data sets provide those data from 2007 onwards. Before 2007, the number of embryos per transfer was available from fresh cycles only. The US data sets provide information about the number of embryos transferred in fresh cycles from 2005 onwards (not FET cycles).
Figure 3.
Proportion of single embryo transfers, transfers with two embryos and transfers with three or more embryos.
In all three geographical areas, there is a clear trend towards the transfer of fewer embryos (Fig. 3). In Australia and New Zealand, the trend towards the transfer of a single embryo started as early as in 2000, whereas this shift was taking place in Europe and in the USA more gradually. The transfer of three or more embryos per treatment was virtually abandoned in Australia and New Zealand in 2006. The practice of transferring three or more embryos per treatment has prevailed only in the USA.
Pregnancy outcomes 1997–2016
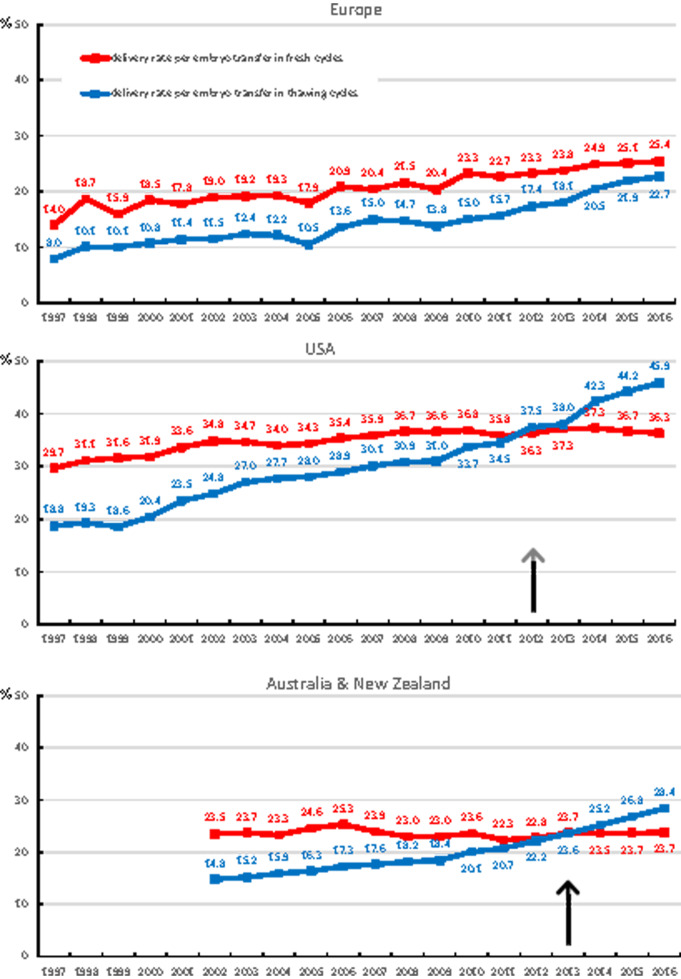
Changes over time in the delivery rates both in fresh and in FET cycles are given in Fig. 4 and Supplementary Table SIV. The Australian and New Zealand registry provided data on the number of deliveries after FET cycles systematically from 2002 onward. The outcome results were similar in Europe and in Australia and New Zealand. The delivery rates in fresh and FET cycles, achieved in the USA were consistently higher than in the two other geographical areas (Fig. 4). The delivery rate per number of embryos transferred was not obtained in this survey.
Figure 4.
Deliveries per embryo transfer in fresh cycles and in thawing cycles. The arrows point towards the year in which the number of deliveries after thawing cycles exceed the number of deliveries after fresh cycles.
In all three geographical areas, the numbers of deliveries achieved after FET cycles have been on the rise. The number of deliveries after FET cycles started to exceed those after fresh cycles in 2012 in the USA, and in 2013 also in Australia and New Zealand. In Europe, this marking point has not yet been reached.
In parallel, the number of multiple deliveries, both of twins and triplets, has decreased in all three geographical areas during 1997–2016 (Supplementary Table SV and Supplementary Fig. S2). Whereas triplet deliveries were the first to virtually disappear from the recorded data sets, the delivery rates of twins started to decrease much later.
Neonatal and infant outcomes 1997–2016
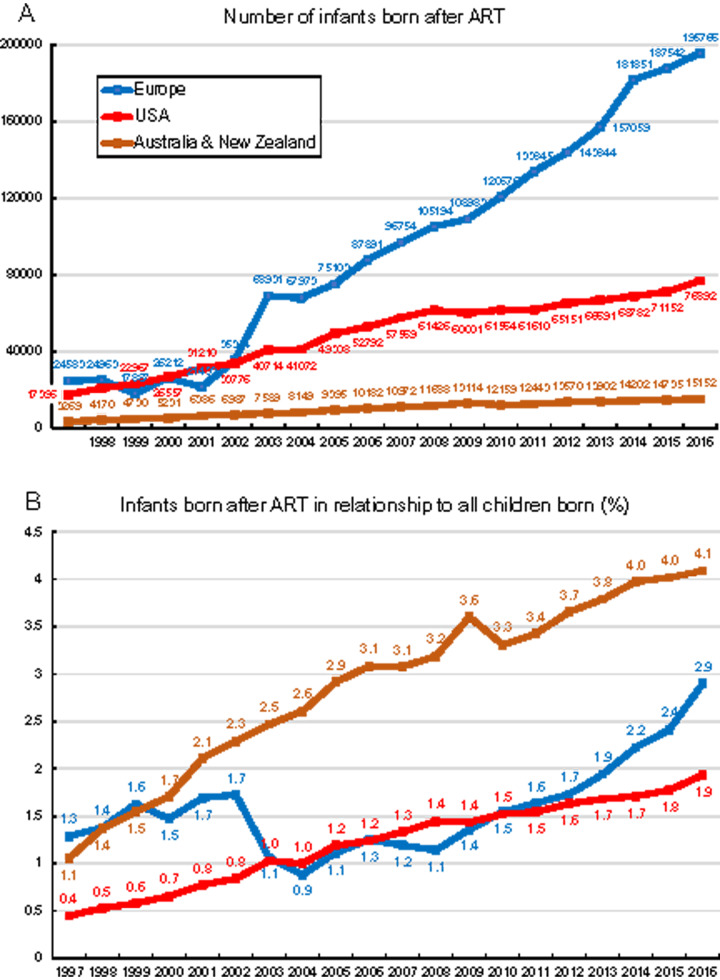
In all three geographical areas, the number of children born after ART was registered (in numbers) along with the ratio between children born after ART and all children born in the respective geographical areas (in %). As depicted in Fig. 5, the absolute number of children born (Fig. 5A) and the ratio between the number of neonates from ART over all neonates (Fig. 5B) tended to rise constantly during the 20-year observation period. The steepest increase in the number of newborn children after ART is observed in Europe, with the lowest increase in Australia and New Zealand. The highest proportion of newborn children after ART is being observed in Australia and New Zealand, reaching 4.1%. The European countries with the highest proportion of newborn children after ART in 2016 were Spain (7.7%) and Austria (6.2%, Wyns et al., 2020). In the same year, the European countries with the lowest proportion of children after ART were Lithuania (0.1%) and Serbia (0.2%, Wyns et al., 2020).
Figure 5.
Changes in the numbers of infants born after IVF, ICSI and FET.
Except for the gender of the children, which is continuously being recorded in the Australia and New Zealand register, none of the three registers record any information about the long-term health status of the children born after ART beyond the moment of birth.
Adverse events 1997–2016
Adverse events are recorded as complications observed during or after ART, such as ovarian hyperstimulation syndrome (OHSS), infections and haemorrhage after oocyte collection, foetal reduction and maternal death (Table V). CDC does not deliver any information concerning these complications but mentions the rare occurrence of maternal deaths. In the Austrialia and New Zealand data sets both the incidence of OHSS (only those cases that had to be admitted to a hospital) and of foetal reduction (combined with termination of pregnancy) are provided, albeit intermittently. Over the entire observation period the mean incidence of OHSS in Europe was reported to be 0.61% and in Australia and New Zealand 0.71% (X2 statistic is 171, P < 0.00001). In recent years the incidence of OHSS has decreased both in Europe and in Australia and New Zealand (Table V). The incidence of haemorrhage after oocyte collection has been fairly constant both in Europe and in Australia and New Zealand. The reported numbers of infections and of foetal reductions have been fluctuating throughout the observation period. Maternal deaths were reported systematically only in Europe and these were rare events.
Table V.
Recorded complications after ART.
| EIM |
ANZARD |
||||||
|---|---|---|---|---|---|---|---|
| Year | OHSS | Haemorrhage | Infection | Foetal reduction | Maternal death | OHSS | Foetal reduction |
| 1997 | 18 | ||||||
| 1998 | 153 (1.1) | 6 | |||||
| 1999 | 1083 (0.6) | 84 (0.04) | 30 (0.02) | 121 (0.7) | |||
| 2000 | 1586 (0.8) | 388 (0.19) | 36 (0.02) | 256 | 0 | 113 (0.7) | 14 |
| 2001 | 1851 (0.9) | 394 (0.19) | 24 (0.01) | 391 | 1 | ||
| 2002 | 2148 (0.9) | 622 (0.26) | 227 (0.09) | 461 | 2 | 192 (1.1) | 58 |
| 2003 | 2646 (1.1) | 799 (0.29) | 135 (0.05) | 480 | 2 | 218 (1.1) | 50 |
| 2004 | 2858 (1.1) | 520 (0.20) | 362 (0.14) | 526 | 4 | 300 (1.4) | 49 |
| 2005 | 3347 (1.1) | 523 (0.17) | 207 (0.07) | 436 | 0 | 306 (1.2) | 60 |
| 2006 | 2753 (0.8) | 544 (0.16) | 42 (0.01) | 466 | 2 | 240 (0.9) | 76 |
| 2007 | 2470 (0.7) | 574 (0.17) | 64 (0.02) | 364 | 3 | 244 (0.8) | |
| 2008 | 2947 (0.6) | 652 (0.13) | 49 (0.01) | 394 | 1 | 198 (0.6) | |
| 2009 | 2137 (0.6) | 415 (0.11) | 61 (0.02) | 484 | 1 | 259 (0.7) | |
| 2010 | 1500 (0.4) | 641 (0.17) | 53 (0.01) | 441 | 2 | 206 (0.6) | |
| 2011 | 1705 (0.5) | 711 (0.17) | 59 (0.01) | 343 | 1 | 229 (0.7) | 99 |
| 2012 | 1953 (0.5) | 848 (0.20) | 101 (0.02) | 485 | 3 | 266 (0.7) | 105 |
| 2013 | 1845 (0.4) | 793 (0.18) | 78 (0.02) | 416 | 2 | 294 (0.7) | 106 |
| 2014 | 2039 (0.4) | 919 (0.19) | 108 (0.02) | 526 | 3 | 274 (0.7) | 94 |
| 2015 | 2167 (0.4) | 946 (0.19) | 114 (0.02) | 501 | 2 | 211 (0.5) | 115 |
| 2016 | 1928 (0.4) | 983 (0.19) | 117 (0.02) | 553 | 0 | 215 (0.5) | 177 |
CDC did not deliver any numbers on adverse events occurring in the USA.
Premature deliveries 1998–2016
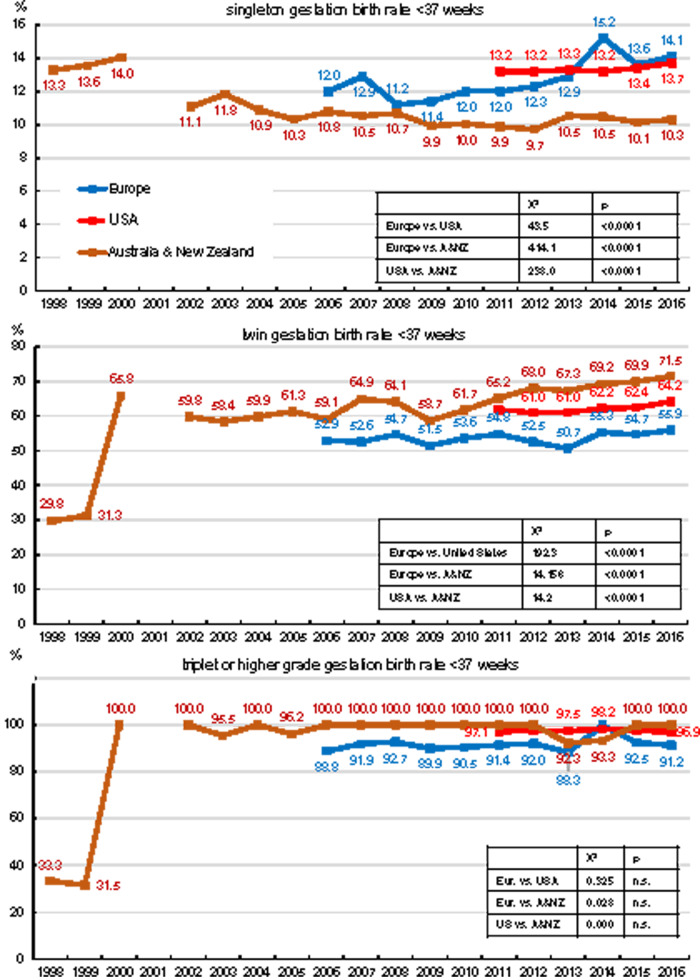
Data on the incidence of premature delivery (before 37th week of gestation) have been recorded for variable time periods in the three geographical areas (Fig. 6). The percentages of prematurity were taken from the data in Supplementary Table SV of each published EIM report (which also includes premature deliveries after ED) and recalculated from the delivery numbers after IVF, ICSI and FET, thereby assuming that premature delivery rates were similar in all four treatment modalities. The incidence of prematurity evidently depends on the degree of multigestation, but differences in the reported incidences of prematurity are observed in the three geographical areas. Because the premature delivery rates in the three areas were becoming more similar from 2014 to 2016, we analysed the quantitative differences during that time interval using log-rank analysis (insets in Fig. 6). Whereas the prematurity rates in triplet deliveries were similar in the three geographical areas, reported singleton and twin prematurity rates were highly distinct in the three geographical areas. Australia and New Zealand had the lowest rate of premature births for singleton gestations, but the highest rate for twins and triplets. Since 2006, the reported prematurity rates of singletons in the European data sets rose to incidence levels similar to those in the USA, whereas the reported prematurity rates in twin and triplet pregnancies remained the lowest of the three regions.
Figure 6.
Changes in the proportion of premature deliveries (in singleton, twin and triplet (or higher grade)) pregnancies after ART.
Discussion
In 1999, ESHRE created the EIM and published in 2001 the first data set of the European survey on ART, performed in 1997 (Nygren and Andersen, 2001). Since then, annual reports have appeared with data sets that have become increasingly more complete over time (Table III). Although these surveys remain largely descriptive, the ultimate aim of data recording in medicine in general, and in ART in particular, is to establish both surveillance and vigilance (De Geyter and Adashi, 2019; De Geyter, 2019). ART is still a young technology and prone to many changes that are in many instances swiftly and widely adopted by the subspecialist community, but often without adequate proof of safety. Although prospective randomized trials are the best instrument to demonstrate improvements in treatment strategies, it takes much time to plan and carry them out and they are often underpowered for small effects or subpopulations. Registry data are a valuable adjunct to randomized studies and to meta-analyses, as they have the potential to demonstrate ongoing changes under real-life conditions based on data sets arising from large cohorts. However, the quality of the recorded data must be optimized, particularly in Europe with its very fragmented political and legal landscape (Calhaz-Jorge et al., 2020).
After 20 years of data collection the steering committee of EIM decided, on behalf of ESHRE, to compare the existing and published EIM data sets with those of two other comparable regional registries, namely CDC and ANZARD, using the annual cross-sectional data sets, all published and freely available online. Whereas in the USA (FCSRCA) and in Australia and New Zealand (Reproductive Technology Accreditation Committee) data registration is compulsory, the regulations in the various European countries continue to be highly diverse (Calhaz-Jorge et al., 2020).
The comparison of the three registries revealed a number of deficiencies that are closely linked to the methods used in national and supranational data registries. Although an increasing array of novel technology has been introduced and adopted by the three registries (Tables I and II), large national or supranational registries are slow to initiate recording them. Most if not all emerging novel treatment modalities were initiated after key publications, such as the first birth after ICSI (Palermo et al., 1992). ICSI was recorded by all registries from their early beginnings, for example by CDC since 1996, by ANZARD since 1996 and by EIM since 1997. PGT was first introduced for genetic analysis of the X-chromosome in a human embryo (Handyside et al., 1990) and its use was first recorded by EIM in 2002, by ANZARD in 2004 and by CDC in 2006. The feasibility of eSET was first suggested as an appropriate treatment in 2001 (Tiitinen et al., 2001) and its use was registered by CDC in 2008, and by ANZARD in 2010. In the European data sets, the transfer of one embryo is being recorded, but not whether this embryo stems from eSET in particular. Freeze-all was first described 1999 (Ferraretti et al., 1999) and was first registered by CDC in 2008, by ANZARD in 2011 and by EIM in 2017. In general, new developments in ART have been picked up by EIM later than CDC and ANZARD. There is no indication that the adoption of novel technology into the recording of data is orchestrated among the three registers.
In times in which many adjuncts, with limited or no evidence of success, are constantly being introduced into ART (Harper et al., 2017), the complexity of present-day ART is barely reflected by current simplified data recordings. It is crucial that the structure of the published cross-sectional data sets remains comparable from one year to another. Furthermore, the ever more complex mixture of new evolving techniques and treatment options does pose a challenge to any data registry. Gamete and embryo donation, fresh or FET, transfer at different stages of embryonic development, genetic testing options at different levels of gamete and embryonic development, and the extraction of gametes at different stages of development out of the ovaries or from testicular tissue all contribute to the current variability of treatment options. In addition to a constantly updated and unified glossary (Zegers-Hochschild et al., 2017), we need to set up a well-structured and systematic catalogue of present-day (and possibly foreseeable) treatment modalities that can be adapted easily with time while maintaining the overall structure of the ongoing data collections and annual reports.
The first aim of all national and supranational registers has traditionally been to record completely and accurately all possible treatment numbers and outcomes at different stages of the process and to come up with a coherent descriptive analysis (Adamson et al., 2018). Intention-to-treat is an important variable that allows us to understand and evaluate the effects of dropouts or cycle cancellations and of conversions to other treatment modalities: both impact on the final outcome results. In contrast to the Australian and New Zealand and the US data sets, the number of reported treatments, most particularly initiated treatments, in the European data sets was incomplete during 16 of the 20 years of this survey (Fig. 2), resulting in an underrepresentation of the number of initiated cycles in comparison to the number of oocyte retrievals. As a result of incomplete data reporting, the outcome of ART for these years can only be related to the number of retrievals, not to the number of initiated cycles. The EIM registry consists of the national representatives of all participating European countries. Europe is made up of a high diversity of countries with very different cultural, political and legal systems. The lack of unifying structures and, often, the absence of any requirement for national data registration, renders the undertaking of a systematic collection of data on ART particularly difficult. In a number of European countries, the number of initiated cycles remained unreported for many years. Owing to better awareness and improved data collection, more countries are now delivering more complete and validated data sets to EIM (Table IV).
In the USA, the highest number of initiated cycles per year was reported in 2008 and these numbers have declined since then (Fig. 2). These changes are paralleled by rising numbers of oocyte, and fresh and frozen embryo donations (Fig. 1a), suggesting a shift of conventional IVF and ICSI to donation cycles in a significant proportion of patients since 2008. Although the selection towards good prognosis cases may be linked to the higher delivery rates seen in the USA in conventional fresh IVF and ICSI, as discussed elsewhere (Kushnir et al., 2016, 2017), other factors, such as the number of embryos transferred per cycle, may be involved in contributing to the observed higher delivery rates in the USA. The delivery rate per number of transferred embryos was not analysed in this survey.
Since 2010–2012, in all three regions the number of initiated cycles (as far as available in the EIM data set), the number of oocyte retrievals and the number of embryo transfers in fresh IVF and ICSI cycles has declined (Fig. 2). In agreement, the proportion of FET cycles started to increase around the same time period (Fig. 1b; B), Concomitantly, the practice of freeze-all emerged in the USA and in Australia and New Zealand (Fig. 1b; C). This shift towards more freezing and thawing cycles resulted in more deliveries after FET cycles, which first exceeded the number of deliveries after fresh cycles in the USA, then in Australia and New Zealand, and probably soon in Europe as well (Fig. 4). Despite all of the problems with data registration and recording, these trends can clearly be visualized in all three registries and establishes their high value in demonstrating ongoing trends in ART.
Under- and over-reporting of adverse events lead to either over- or under-estimation, respectively, of the safety of interventions. The comparison of the recorded data on complications and the distinct incidence of various adverse events in the three registries during the 20-year observation period are likely related to over- and under-reporting. The incidence of OHSS has been determined prospectively and the degree of severity of the syndrome may vary, but approximately 2.5–2.9% of all women undergoing treatment with conventional ART suffer of at least one episode of OHSS (Malchau et al., 2019). Both EIM and ANZARD reported a mean incidence of OHSS between 0.61% and 0.71% per treatment cycle (Table V), which suggests under-reporting. In addition to over- and under-reporting of adverse events, the lack of standardization in defining complications in ART is a major obstacle. The international glossary on definitions may help to standardize the reporting of adverse events (Zegers-Hochschild et al., 2017).
Another example of potential under-reporting is given by the significantly lower reported incidence of premature deliveries in twin and triplet gestations in the European register. In recent years, however, the number of recorded premature twin and triplet deliveries in Europe is now approaching the long-standing incidences reported by CDC and ANZARD (Fig. 6). In the beginning of ART data recordings in Australia and New Zealand, some degree of under-reporting of premature twin and triplet pregnancies may have prevailed as well.
Over-reporting may also occur. A comparison of recording ART in the case of normal pregnancies in birth certificates with national ART surveillance data in FL and MA, USA, has demonstrated a clear discordance between both data registries: ART was more often reported for women at risk of adverse pregnancy outcome, but much less so in normal pregnancies (Cohen et al., 2014). The higher incidence of premature deliveries in singleton pregnancies in the USA than in Australia and New Zealand may perhaps be explained by some under-reporting of normal delivery outcomes in the US data sets.
Even worse than over- or under-reporting, a number of known complications of ART are, even today, still not being registered systematically, such as accidental loss of gametes and embryos (not registered at all), infections and bleedings (recorded only by EIM) and maternal death (recorded annually only by EIM). Complications during ongoing pregnancies achieved with ART, such as hypertensive disorders after infertility treatment (Monseur et al., 2019), are not being recorded systematically by any register. The health status of the infants is recorded only at the moment of birth, although minor and major malformations or health impairments are more often determined during further infant development. Long-term outcomes of children conceived with ART might be better evaluated through linking of ART data sets with the data sets of other health services, as done in the USA and in the European Nordic countries (Luke et al., 2019).
Despite these data recording deficiencies, which become apparent when comparing the results of the three registries over a prolonged time interval, much has been achieved in the last 20 years. Between 1997 and 2016, the number of recorded treatments with ART by EIM in Europe has multiplied by a factor of 5.3 (in the USA by CDC: 4.6, in Australia and New Zealand by ANZARD: 3.0) (Fig. 1a). During the same time period, the number of recorded treatment modalities has increased in Europe from three to seven. The high impact of ART in Europe together with a level of utilization reaching 1410 treatments per 1 million inhabitants in countries with complete coverage (from a medical point of view the optimal goal being at least 1500 treatments per 1 million inhabitants, ESHRE Capri Workshop Group, 2001; Collins 2002) resulted in 2.9% of infants born after ART in Europe in 2016 (in the USA: 1.9%, in Australia and New Zealand: 4.1%) (Fig. 5). Many ongoing trends can be observed, both in time and in quantity, such as the shift from conventional IVF to ICSI (Fig. 1b; A), the increasing preponderance of FET or cryo-cycles versus fresh cycles (Fig. 1b; B) and the progressive adoption of ‘freeze-all’ (Fig. 1b; C). Those changes in attitudes in the three geographical areas impact on treatment outcomes, such as more deliveries now taking place after thawing cycles than after fresh cycles in the USA and in Australia and New Zealand, but not yet in Europe (Fig. 4). These results demonstrate a rising reliance on freezing and thawing of embryos as a technique, which has been recorded from the early beginnings of the three registers. In consequence, fewer embryos are now being replaced per treatment trial (Fig. 3) resulting in fewer twin and triplet deliveries (Supplementary Table SV and Supplementary Fig. S2). Although these developments can be observed as they take place in the three geographical areas, the pace is set by Australia and New Zealand, which was the first to predominantly adopt a SET policy and which was the first to push the twin delivery rates below 5% in 2013. In all three regions, triplet deliveries have practically vanished since 2012. Unfortunately, none of the registers allow us to check this change in practice on the health status of the infants. For that we have to rely on studies linking ART surveillance data sets with other health services data sets (Luke et al., 2019).
To summarize, and to provide suggestions for improvements, the comparison of three large registers in ART clearly demonstrates that surveillance in ART is working. In all three registers, the number of main developments in ART are both identified and quantified, and some of these developments result in lowering the incidence of adverse events or in improving the outcome of pregnancies. However, the current concept of surveillance in ART through national or supranational registries is not without deficiencies. Instead of the current cross-sectional approach, the cumulative approach of modern ART requires more continuous recording systems in which the various therapeutic and outcome steps of each couple or individual can be linked together over prolonged time periods, in different institutions and across borders. It will be crucial to install a central European data collection system based on individual patients and/or couples to monitor their path through different treatments and institutions prospectively (De Geyter et al., 2016). This may be achieved by installing electronic healthcare records of individual couples or persons, which store all the complex data arising from ART and that ultimately can be used to add pregnancy-related and obstetric outcome data (Pandey et al., 2012). Incorporating electronic health records has been demonstrated to be beneficial in surveillance and vigilance (Kruse et al., 2018). Such an approach may be time-saving, by avoiding having to write extensive documentation and by improving the workflow, thereby resulting in better productivity (Kruse et al., 2018). Whereas the health authorities and/or professional societies of some European countries have already developed adapted software systems to collect data from their respective institutions offering ART (including plausibility testing of the incoming data), others have not. Therefore, the centralized European data collecting office must use a software system that can be linked to those of the national authorities and/or professional societies. Comparisons of outcome data on an individual patient level highlight the limitations of registries (De Neubourg et al., 2016), necessitating appropriate validation standards of submitted data sets (Wilkinson et al., 2017; Bacal et al., 2019). Of course, confidentiality and protection of privacy must be safeguarded, and traceability of submitted data should be organized in full compliance with legal regulations. Electronic health data resulting from extensive diagnostics and treatments in medically assisted reproduction must be made the property of the patients (and their offspring) for documentation purposes and for long-term outcome monitoring of both the patients and their offspring. Not only professional societies should be interested in such an accurate, far reaching and transgenerational healthcare monitoring system in ART, but also governmental organizations and the lay public.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Authors’ roles
Ch.D.G.: conception of the study, data analysis, preparation of the manuscript. C.W.: conception of the study, data analysis, preparation of the manuscript. C.C.-J.: conception of the study, preparation of the manuscript. J.d.M.: conception of the study, preparation of the manuscript. A.P.F.: conception of the study, preparation of the manuscript. M.K.: conception of the study, preparation of the manuscript. A.N.A.: conception of the study, preparation of the manuscript. K.G.N.: conception of the study, preparation of the manuscript. V.G.: data analysis, preparation of the manuscript.
Funding
This study did not receive any particular funding.
Conflict of interest
All contributing authors declare no conflict of interest.
Supplementary Material
Contributor Information
Ch De Geyter, Reproductive Medicine and Gynecological Endocrinology (RME), University Hospital, University of Basel, Basel, Switzerland.
C Wyns, Cliniques universitaires Saint-Luc, Université Catholique de Louvain, Brussels, Belgium.
C Calhaz-Jorge, Faculdade de Medicina da Universidade de Lisboa, Lisbon, Portugal.
J de Mouzon, Institut National de Santé et de la Recherche Médicale, Service de Gynécologie Obstétrique II et de Médecine de la Procréation, Groupe Hospitalier Cochin-Saint Vincent de Paul, Paris, France.
A P Ferraretti, S.I.S.Me.R. Reproductive Medicine Unit, Bologna, Italy.
M Kupka, Fertility Center—Gynaekologicum, Hamburg, Germany.
A Nyboe Andersen, The Fertility Clinic, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark.
V Goossens, ESHRE Central Office, Meerstraat 60, Grimbergen, Belgium.
References
- Adamson GD, de Mouzon J, Chambers GM, Zegers-Hochschild F, Mansour R, Ishihara O, Banker M, Dyer S. International Committee for Monitoring Assisted Reproductive Technology: world report on assisted reproductive technology, 2011. Fertil Steril 2018;110:1067–1080. [DOI] [PubMed] [Google Scholar]
- Bacal V, Russo M, Fell DB, Shapiro H, Walker M, Gaudet LM. A systematic review of database validation studies among fertility populations. Hum Reprod Open 2019;2019:doi:10.1093/humrep/hoz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhaz-Jorge C, De Geyter CH, Kupka MS, Wyns C, Mocanu E, Motrenko T, Scaravelli G, Smeenk J, Vidakovic S, Goossens V. Survey on ART and IUI: legislation, regulation, funding and registries in European countries: The European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum Reprod Open 2020;2020:doi:10.1093/humrep/hoz044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers GM, Lancaster P, Ilingworth P. ART surveillance in Australia and New Zealand In: Kissin DM, Adamson GD, Chambers GM, De Geyter C. (eds). Assisted Reproductive Technology Surveillance, Chapter 15. Cambridge, UK: Cambridge University Press, 2019. [Google Scholar]
- Cohen B, Bernson D, Sappenfield W, Kirby RS, Kissin D, Zhang Y, Copeland G, Zhang Z, Macaluso M; States Monitoring Assisted Reproductive Technology (SMART) Collaborative. Accuracy of assisted reproductive technology information on birth certificates: Florida and Massachusetts, 2004-06. Paediatr Perinat Epidemiol 2014;28:181–190. [DOI] [PubMed] [Google Scholar]
- Collins J. An international survey of the health economics of IVF and ICSI. Hum Reprod Update 2002;8:265–277. [DOI] [PubMed] [Google Scholar]
- De Geyter C. Assisted reproductive technology: impact on society and need for surveillance. Best Pract Res Clin Endocrinol Metab 2019;33:3–8. [DOI] [PubMed] [Google Scholar]
- De Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, Scaravelli G, Smeenk J, Vidakovic S, Goossens V; European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). ART in Europe, 2015: results generated from European registries by ESHRE. Hum Reprod Open 2020;2020:doi:10.1093/humrep/hoz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Geyter C, Wyns C, Mocanu E, de Mouzon J, Calhaz-Jorge C. Data collection systems in ART must follow the pace of change in clinical practice. Hum Reprod 2016;31:2160–2163. [DOI] [PubMed] [Google Scholar]
- De Geyter C, Adashi EY. Future directions ART surveillance and monitoring novel technology In: Kissin DM, Adamson GD, Chambers GM, De Geyter C. (eds). Assisted Reproductive Technology Surveillance, Section 2. Cambridge, UK: Cambridge University Press, 2019. [Google Scholar]
- De Mouzon J, Chambers GM, Zegers-Hochschild F, Mansour R, Ishihara O, Banker M, Dyer S, Kupka M, Adamson GD. International Committee for Monitoring Assisted Reproductive Technologies world report: assisted reproductive technology 2012. Hum Reprod 2020;35:1900–1913. [DOI] [PubMed] [Google Scholar]
- De Neubourg D, Bogaerts K, Blockeel C, Coetsier T, Delvigne A, Devreker F, Dubois M, Gillain N, Gordts S, Wyns C. How do cumulative live birth rates and cumulative multiple live birth rates over complete courses of assisted reproductive technology treatment per woman compare among registries? Hum Reprod 2016;31:93–99. [DOI] [PubMed] [Google Scholar]
- ESHRE Capri Workshop Group. Social determinants of human reproduction. Hum Reprod 2001;16:1518–1526. [DOI] [PubMed] [Google Scholar]
- Ferraretti AP, Gianaroli L, Magli C, Fortini D, Selman HA, Feliciani E. Elective cryopreservation of all pronucleate embryos in women at risk of ovarian hyperstimulation syndrome: efficiency and safety. Hum Reprod 1999;14:1457–1460. [DOI] [PubMed] [Google Scholar]
- Ferraretti AP, Nygren K, Andersen AN, de Mouzon J, Kupka M, Calhaz-Jorge C, Wyns C, Gianaroli L, Goossens V; European IVF-Monitoring Consortium (EIM), for the European Society of Human Reproduction and Embryology (ESHRE). Trends over 15 years in ART in Europe: an analysis of 6 million cycles. Hum Reprod Open 2017;2017:doi:10.1093/humrep/hox012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handyside AH, Kontogianni EH, Hardy K, Winston RM. Pregnancies from biopsied human preimplantation embryos sexed by Y-specific DNA amplification. Nature 1990;344:768–770. [DOI] [PubMed] [Google Scholar]
- Harper J, Jackson E, Sermon K, Aitken RJ, Harbottle S, Mocanu E, Hardarson T, Mathur R, Viville S, Vail A. et al. Adjuncts in the IVF laboratory: where is the evidence for ‘add-on’ interventions? Hum Reprod 2017;32:485–491. [DOI] [PubMed] [Google Scholar]
- Kruse CS, Stein A, Thomas H, Kaur H. The use of electronic health records to support population health: a systematic review of the literature. J Med Syst 2018;42:214–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnir VA, Barad DH, Albertini DF, Darmon SK, Gleicher N. Effect of Embryo Banking on U.S. National Assisted Reproductive Technology live birth rates. PLoS One 2016;11:e0154620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnir VA, Barad DH, Albertini DF, Darmon SK, Gleicher N. Systematic review of worldwide trends in assisted reproductive technology 2004-2013. Reprod Biol Endocrinol 2017;15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke B, Boulet SL, Henningsen A-K. Monitoring long-term outcomes of ART: linking ART surveillance data with other datasets In: Kissin DM, Adamson GD, Chambers GM, De Geyter C. (eds). Assisted Reproductive Technology Surveillance, Section 9. Cambridge, UK: Cambridge University Press, 2019. [Google Scholar]
- Malchau SS, Henningsen AA, Forman J, Loft A, Nyboe Andersen A, Pinborg A. Cumulative live birth rate prognosis based on the number of aspirated oocytes in previous ART cycles. Hum Reprod 2019;34:171–180. [DOI] [PubMed] [Google Scholar]
- Monseur BC, Morris JR, Hipp HS, Berghella V. Hypertensive disorders of pregnancy and infertility treatment: a population-based survey among United States women. J Assist Reprod Genet 2019;36:1449–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygren KG, Andersen AN. Assisted reproductive technology in Europe, 1997. Results generated from European registers by ESHRE. European IVF-Monitoring Programme (EIM), for the European Society of Human Reproduction and Embryology (ESHRE). Hum Reprod 2001;16:384–391. [DOI] [PubMed] [Google Scholar]
- Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992;340:17–18. [DOI] [PubMed] [Google Scholar]
- Pandey S, Shetty A, Hamilton M, Bhattacharya S, Maheshwari A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis. Hum Reprod Update 2012;18:485–503. [DOI] [PubMed] [Google Scholar]
- Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet 1978;312:366. [DOI] [PubMed] [Google Scholar]
- Tiitinen A, Halttunen M, Härkki P, Vuoristo P, Hyden-Granskog C. Elective single embryo transfer: the value of cryopreservation. Hum Reprod 2001;16:1140–1144. [DOI] [PubMed] [Google Scholar]
- Toner JP, Lanes A, Kissin D. ART surveillance in North America In: Kissin DM, Adamson GD, Chambers G, De Geyter C. (eds). Assisted Reproductive Technology Surveillance. Cambridge, UK: Cambridge University Press, 2019:172–181. [Google Scholar]
- Van den Bergh M, Hohl MK, De Geyter C, Stalberg AM, Limoni C. Ten years of Swiss National IVF Register FIVNAT-CH. Are we making progress? Reprod Biomed Online 2005;11:632–640. [DOI] [PubMed] [Google Scholar]
- Wilkinson J, Vail A, Roberts SA. Direct-to-consumer advertising of success rates for medically assisted reproduction: a review of national clinic websites. BMJ Open 2017;7:e012218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyns C, De Geyter Ch C-JC, Kupka MS, Motrenko T, Smeenk J, Bergh C, Tandler-Schneider A, Rugescu IA, Vidakovic S, Goossens V; for the European IVF Monitoring consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). ART in Europe, 2016: results generated from European registries by ESHRE. Hum Reprod Open 2020;2020:doi:10.1093/humrep/hoaa32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID. et al. The international glossary on infertility and fertility care, 2017. Hum Reprod 2017;32:1786–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Crosby JA, Musri C, de Souza MDCB, Martinez AG, Silva AA, Mojarra JM, Masoli D, Posada N; Latin American Network of Assisted Reproduction. Assisted reproductive technology in Latin America: the Latin American Registry, 2017. Reprod Biomed Online 2020;41:44–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.