Abstract
Background and Purpose:
Clot perviousness in large vessel occlusion (LVO) has been shown to be associated with improved recanalization outcomes with mechanical thrombectomy and intravenous thrombolysis. We sought to evaluate the association between clot perviousness based on thrombus attenuation increase (TAI) on CT, and histologic composition of clots in acute ischemic stroke (AIS).
Methods:
A retrospective review was completed of patients with AIS secondary to large-vessel occlusion, NCCT and CTA images, and histologic analysis of the retrieved clot. TAI was measured by subtracting clot attenuation on NCCT from the attenuation on CTA. Up to 3 regions of interest (ROIs) were evaluated on each clot; the average attenuation was used for analysis if multiple ROIs were assessed. Pervious clots were defined as TAI ≥10 HU; impervious clots had TAI <10 HU. Histopathologic analyses of clots were assessed for relative compositions of RBCs, WBCs, fibrin, and platelets/other.
Results:
57 patients were included. Pervious clots were more likely to be RBC rich (p=0.04); impervious clots were more likely to be fibrin and WBC rich (p=0.01 for both). Pervious clots also had greater RBC composition than impervious clots (49.8% and 33.0%, respectively; p=0.006); fibrin composition of pervious clots was lower than impervious clots (17.8% and 23.2%, respectively; p=0.02).
Conclusion:
Clot perviousness assessed on NCCT and CTA imaging is associated with higher RBC density and lower fibrin density, offering a possible explanation for the higher rates of successful thrombectomy and favorable clinical outcome seen in such patients.
Introduction:
The use of endovascular thrombectomy in patients with acute ischemic stroke (AIS) has allowed for histopathological analysis of the inciting clots.[1] The bulk of these retrieved clots are made up of four major components: RBCs, WBCs, fibrin and platelets conglomerations.[2] Histologically, however, significant diversity is seen amongst these clots; relative densities of the major components, type of fibrin network, and presence or absence of atheromatous gruel can vary widely.[1][3] This cellular variability has implications for treatment: RBC-rich clots are thought to be more amenable to thrombolysis and thrombectomy, whereas fibrin-rich clots are less responsive to revascularization attempts.[4]
Clot permeability (also known as perviousness), the degree to which blood is able to flow through a clot’s structure, is increasingly being recognized as an important predictor of responsiveness to therapy.[5][6] CT imaging can be used to estimate clot perviousness by comparing clot attenuation on NCCT to that on CTA – known as “thrombus attenuation increase” (TAI).[6] High TAI implies increased perviousness, and is associated with better functional outcome and response to recanalization in acute ischemic stroke (AIS).[6][7][8] To date, however, scant research has directly compared the degree of TAI with the histologic composition of a clot retrieved during mechanical thrombectomy.
The purpose of this study was to assess if TAI is associated with the histologic characteristics of retrieved clots. Because both increased perviousness on CT imaging and high levels of RBCs within clots predict better responses to therapy and favorable patient outcomes, we hypothesize that clots with increased TAIs will have RBC-rich compositions.
Materials and Methods:
Patient Selection:
Institutional review board approval was obtained for the purposes of this study. A retrospective review was completed of consecutive patients who presented to our institution with stroke-like symptoms between 1/1/2016 and 10/1/2018. Included patients: 1) had AIS caused by an occlusive intracranial clot, 2) had pre-thrombectomy NCCT and CTA imaging, and 3) underwent successful mechanical thrombectomy with histopathological analysis of the retrieved clot available. Patients were excluded if the inciting clot was too small to be visualized on NCCT or if diagnostic quality was poor (e.g. degraded by motion artifact).
CT Imaging
The vast majority of NCCT and CTA imaging was completed on a 128-slice multi- detector scanner (SOMATOM Definition Flash; Siemens Healthcare, Erlangen, Germany). Slice thickness for both NCCT and CTA was 0.75 mm. Tube voltage and tube current were set to 120 kV and 350 mAs for NCCT, respectively, and set to 120 kV and 415 mAs for CTA. CTAs were performed nearly immediately after acquisition of NCCT images, per our institution’s stroke code protocol. In cases where IV thrombolysis (IVT) was administered, all patients were treated with IVT after acquisition of CTA images.
Imaging Review and Interpretation:
A neuroradiology fellow, who had been blinded to clot histology, reviewed the images. TAI was used to assess the degree of clot permeability based on NCCT and CTA imaging, as this technique has been utilized in multiple prior studies.[6][8] To measure the TAI, the Hounsfield units (HUs) of multiple regions of interest (ROIs) within each clot were measured on both CTA and corresponding NCCT images. NCCT and CTA images were not co-registered, but instead read side-by-side within the PACS system to ensure accurate placement of ROIs within the thrombus location on the NCCT images. The placement, and number, of ROIs was based on a subjective analysis of how well the artery and/or clot could be visualized on both CTA and NCCT images. Up to 3 ROIs were placed over each clot; fewer (1 or 2) ROIs were used for smaller clots. The diameters of ROIs measured approximately 2–3 mm. If multiple ROIs were obtained, the average value of the measured HUs was used. Intra-clot attenuation on NCCT was subtracted from that measured on CTA to yield the TAI (Figs. 1–3). Clot perviousness on NCCT/CTA was based on absolute TAI: pervious clots were defined as having TAI ≥10 HU; impervious clots had TAI <10 HU. 10 HU was chosen as the cutoff for clot perviousness using Youden’s index in conjunction with a receiver operating characteristic analysis.
Fig. 1:
Elderly patient who presented with right hemiplegia, gaze preference, homonymous hemianopsia, found to have an occlusion of the left MCA. Acquired (A) and digitally zoomed (B) NCCT and CTA (C and D – also acquired and digitally zoomed, respectively) images show an occlusive clot in the M1 segment of the left MCA.
Fig. 3:
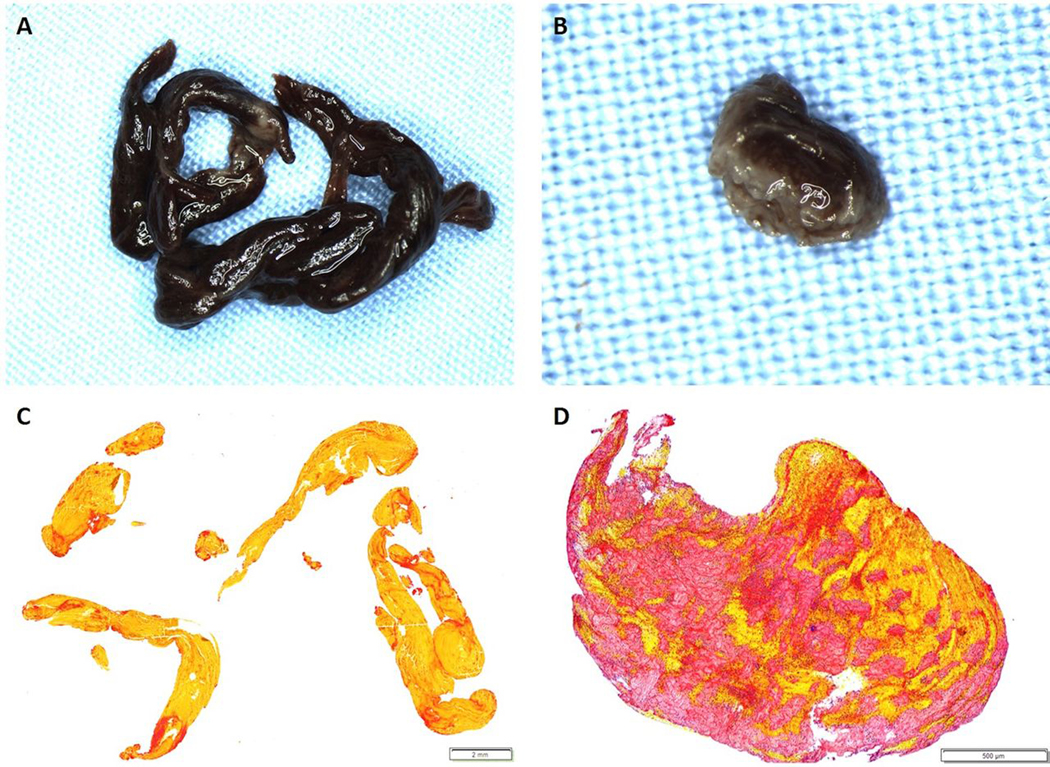
Gross photographs and histological staining of removed clots. Gross photographs of a RBC-rich and Fibrin-rich clot (A (1.25x) and B (3.2x), respectively) were taken after formalin-fixation, prior to embedding. C and D are the corresponding MSB-stained slides demonstrating the presence of Red Blood Cells (Yellow), White Blood Cells (Blue), fibrin strands (Red) and platelets/other (Grey). C is an example of an RBC-rich clot (83.6% RBC, 0.6x) and D is an example of a fibrin-rich clot (62.9% fibrin, 4x).
Thrombectomy and Histologic Analysis:
TICI scores were obtained by local operators within our institution. All histopathological specimens were immediately fixed in 10% phosphate-buffered formalin, processed using a standard tissue processing protocol and embedded in paraffin. The formalin-fixed paraffin-embedded clot material was cut into 3–5μm sections and representative slides from each clot were stained with Hematoxylin and Eosin (H&E) and Martius Scarlett Blue (MSB). Representative MSB stained slides from each case were sent for whole slide scanning (Aperio ScansScope AT Turbo, Leica Biosystems). Histologic quantification was performed using Orbit Image Analysis Software (Orbit Image Analysis, Idorsia Ltd.) and clots were assessed for relative densities of RBCs, WBCs, fibrin, and platelets. The median of each clot component was calculated and if the proportion of a given component in a clot was higher than the overall median value the clot was considered rich in that component. Thus, RBC-rich clots were defined as having greater than >49% RBC composition; WBC-rich clots were composed of >3.9% of WBC, fibrin-rich clots were composed of >28.7% of fibrin, and platelet-rich clots were composed of >16.3% of platelets.
Statistical Analysis:
Statistical analyses were performed with the SAS-based statistical software package JMP 13.0 (http://www.jmp.com, Cary, NC). Clot histologic compositions and perviousness based on TAI were statistically analyzed using students t-test for continuous variables and chi-squared tests for categorical variables. R-squared values were calculated examining the association between TAI and clot composition. The threshold for significance was set to p<0.05. The data were normally distributed for the student’s t-test. No expected counts of 0 were noted in the chi-squared test.
Results:
Baseline Characteristics
57 patients were included, all of whom underwent both pre-thrombectomy NCCT and CTA imaging prior to mechanical thrombectomy; 27/57 (47.4%) were female (Fig. 4). Average patient age at time of presentation was 68.3±12.8. All patients were treated with IV tPA prior to endovascular thrombectomy. By location, most clots were found in the MCA (n=38; 66.7%), followed by the ICA (n=6; 10.5%), both the ICA and MCA (n=5; 8.8%), basilar artery (n=3; 5.3%), PCA (n=2; 3.5%), the ICA, MCA, and ACA (n=2; 3.5%), and extending from the CCA to the MCA (n=1; 1.8%). Of clots within part of the MCA, the most common arterial segment involved was the M1 (35/46; 76.1%). The most common etiology of observed clots was cardiac origin (n=34; 59.7%), followed by large artery atherosclerosis (n=12; 21.1%), other (n=7; 12.3%), and unknown (n=4; 7.0%).
Fig. 4:
Flowchart of patient cohort selection, before and after exclusions.
Five patients (8.8%) were treated with stent-retriever alone, 39 patients (68.4%) were treated with aspiration alone and 13 patients (22.8%) were treated with a combination of aspiration and stent retrievers. Mean number of passes made during thrombectomy was 2.2±1.6. Following thrombectomy, the most frequently observed thrombolysis in cerebral infarction (TICI) grade was 2b (n=30; 53.6%), followed by 3 (n=20; 35.1%), 2a (n=3; 5.3%), 0 (n=2; 3.5%), and 2c (n=1; 1.8%). Both patients with TICI 0 had some clot aspirated and available for histologic analysis, but had residual clot without revascularization of the affected territory.
Clot Perviousness and Composition
Of all clots, the average attenuation (HU) was 46.4±15.6 HU on NCCT and 65.6±21.3 HU on CTA. Multiple ROIs were used in 33 clots and a single TOI was used in 24 clots. When multiple ROIs were used on the NCCT, the median difference in attenuation between various clot segments was 9 (IQR=4.5–14). For the CTA, the median difference was 10 (IQR=7–21). Measured TAIs range from 0–81 HU.
38/57 clots (66.7%) were pervious based on TAI ≥10. Clots that appeared pervious on imaging had higher RBC density than impervious clots (49.8% and 33.0%, respectively; p=0.006); fibrin density within pervious clots was lower than impervious clots (17.8% and 23.2%, respectively; p=0.02) (Table 1). Pervious clots were more likely to be RBC-rich (23/38; 60.5%) compared to impervious clots (6/19; 31.6%) (p=0.04). Conversely, impervious clots were more likely to be WBC-rich (14/19; 73.7%) and fibrin-rich (14/19; 73.7%) than pervious clots (15/38 (39.5%) and 15/38 (39.5%), respectively) (p=0.01 for both). No significant difference was found between the frequency of pervious and impervious platelet-rich clots although there was a trend towards increased platelet content in impervious clots (p=0.06). When examining the linear association between clot perviousness and composition, we found a positive association between TAI and RBC composition (P=0.04), a negative association between TAI and fibrin composition (P=0.01) and no association between TAI and platelet or WBC composition.
Table 1:
Histologic makeup of pervious and impervious clots.
| Pervious clots (n, %) | Impervious clots (n, %) | p value | |
|---|---|---|---|
| RBC-rich | 23 (60.5%) | 6 (31.6%) | 0.04 |
| WBC-rich | 15 (39.5%) | 14 (73.7%) | 0.01 |
| Fibrin-rich | 15 (39.5%) | 14 (73.7%) | 0.01 |
| Platelet rich | 16 (42.1%) | 13 (68.4%) | 006 |
No association was noted between clot density on NCCT and density on CTA (p=0.22). In addition, no association was found between the clot perviousness and the clot age (p=0.36). No association was found between the first-pass effect and clot perviousness (p=0.99) or between the first-pass effect and TAI (p=0.65).
The majority of thrombi were treated with aspiration thrombectomy; there was insufficient statistical power to detect a difference in the composition of thrombi retrieved with and without a stent-retriever.
Discussion:
This study sought to find an association between clot perviousness based on TAI and clot histologic composition. The results indicate that there is an association between clot permeability and relative density of RBCs and fibrin within a clot. An absolute arterial attenuation increase of ≥10 HU on CTA compared to NCCT was associated with relatively higher RBC density and lower fibrin density within the retrieved clots.
The relative densities of these RBCs, WBCs, and fibrin/platelet conglomerations within a clot are predictive of its responsiveness to treatment: clots with denser fibrin have impaired lysability during attempted revascularization with tPA, while RBC-rich clots have better responsiveness to intravenous revascularization therapy.[4][9][10] There also seems to be an emerging consensus in the neurointerventional literature that fibrin rich clots are more difficult to remove than RBC rich clots due to factors such as a higher static coefficient of friction resulting in increased adherence to the vessel wall as well as decreased compressibility of the fibrin rich clots resulting in poorer device integration with stent retrievers.
Multiple prior studies have established a correlation between clot histology and clot appearance on NCCT.[11][12] RBC-rich clots tend to have higher attenuation than those that are platelet or fibrin-rich, likely related to the hemoglobin concentration within the clot.[11] In addition, the presence of a hyperdense middle cerebral artery sign (HMCAS) on NCCT is predictive of a RBC-rich clot and early-phase clot composition; per Liebeskind et al., absence of a HMCAS is an indicator that the clot may be fibrin-predominant.[13][14][15] These findings have implications for clot retrieval: increased clot density on NCCT and high RBC density on histologic analysis have been shown to be associated with successful recanalization after thrombectomy and reduced thrombectomy procedure time.[16][17][18] Conversely, Sporns et al. found that fibrin-rich clots are associated with increased intervention time, and are more susceptible to embolism during thrombectomy, possibly reflecting fragility of clots with high fibrin levels.[19]
Most previous investigations of clot permeability based on CT imaging have focused on the association between clot imaging characteristics and responsiveness to treatment or clinical outcome. Correlations found between such variables have been attributed to multiple factors: clots that appear pervious on CT may be relatively porous, allowing the passage of residual arterial flow and preserving at least some oxygenation to downstream tissues.[7] Additionally, pervious clots may be more amenable to intravenous thrombolysis as the perviousness of a clot to contrast may be a reasonable biomarker to tPA drug delivery to the clot itself. Regarding endovascular recanalization therapy, most studies examining the association between perviousness and thrombectomy outcomes have concluded that patients with pervious clots are more likely to be successfully recanalized and will have better outcomes, however this notion has been refuted in at least one subsequent study[6][20][21]. By correlating the TAI with clot composition, the current study offers a histologic explanation for the association between clot perviousness on imaging and favorable outcomes.
Only one prior study, by Berndt et al, has directly investigated the association between clot perviousness based on TAI and the histologic composition of clots.[22] The results of that study, which performed histological analysis on 32 clots, were opposite to those reported here; the authors found an inverse correlation between TAI and RBC density, and a positive correlation between clot perviousness and fibrin/platelets. Such contrasting conclusions may draw to question the reproducibility of results, and further research into this topic may be required before reaching a finalized conclusion. Still, the results of the current study offer a reasonable association between two variables previously shown to be associated with positive response to recanalization: RBC-rich histology and high perviousness based on CTA.
This study has limitations. First, clinical outcome was not assessed as part of this study as our main interest was in finding an association between perviousness with histopathology. Hence, it is uncertain if the observed relationship between TAI and clot histopathology also correlates with clinical outcome, as has been reported in prior studies. Also, the majority of cases were treated with aspiration thrombectomy. It is possible that the relatively high number of thrombi treated with aspiration could affect the composition of the retrieved clots. Similarly, only clots that were successfully retrieved could be histologically analyzed, which is a potential source of bias in any such analysis. Next, because patients were excluded whose clot was below the level of NCCT imaging, the results from this study are confined to patients with intracranial arterial occlusion. Finally, as stated above, the conflicting results obtained between Berndt et al.’s recent study and the current study suggests that further research will be required to continue to investigate this topic in the future.
Summary
Increased clot perviousness based on NCCT and CTA imaging is associated with higher RBC density and lower fibrin density of mechanically retrieved clots. This histologic association offers a possible explanation for the superior recanalization rates with IV-thrombolysis and mechanical thrombectomy and favorable clinical outcomes of patients with permeable clots seen on CT imaging.
Supplementary Material
Fig. 2:
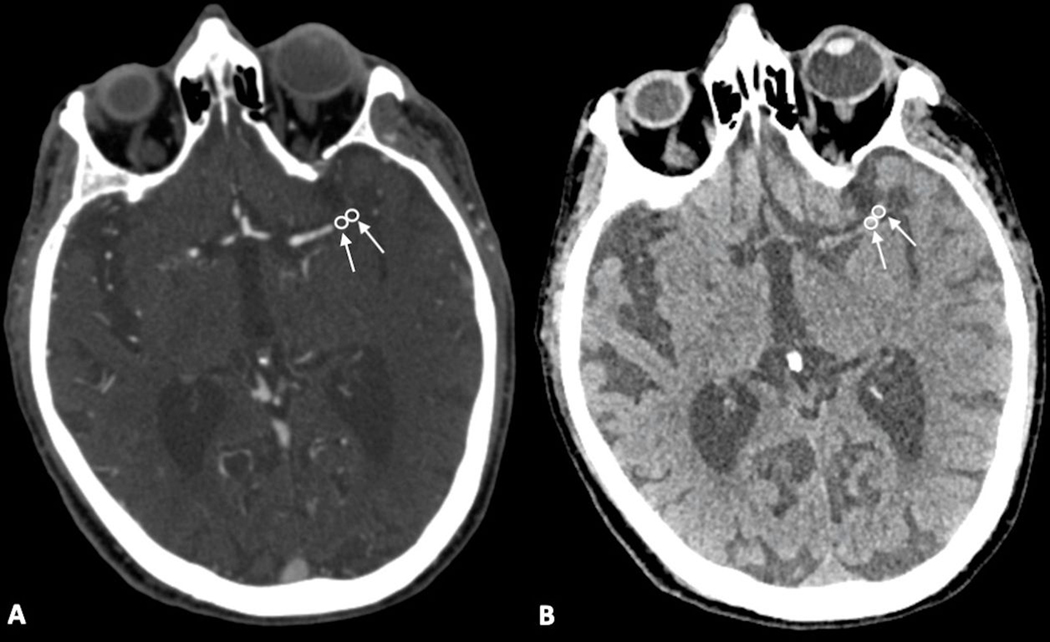
Elderly patient with a history of hyperlipidemia and atrial fibrillation who presented with right-sided weakness. CTA images demonstrated an occlusive clot in the distal M1 segment of the left MCA. ROIs of the clot were measured on CTA and NCCT (arrows and circles in A and B, respectively).
Abbreviations:
- AIS
Acute ischemic stroke
- HMCAS
Hyperdense middle cerebral artery sign
- ROI
Region of interest
- TAI
Thrombus attenuation increase
Footnotes
Disclosures: None
References:
- [1].Hashimoto T, Hayakawa M, Funatsu N, Yamagami H, Satow T, Takahashi JC, et al. Histopathologic Analysis of Retrieved Thrombi Associated With Successful Reperfusion After Acute Stroke Thrombectomy. Stroke 2016;47:3035–7. [DOI] [PubMed] [Google Scholar]
- [2].Berndt M, Prothmann S, Maegerlein C, Oberdieck P, Zimmer C, Hegge B, et al. Artificial Stroke Clots: How Wide is the Gap to the Real World? World Neurosurg 2018;110:e90–9. [DOI] [PubMed] [Google Scholar]
- [3].Undas A, Slowik A, Wolkow P, Szczudlik A, Tracz W. Fibrin clot properties in acute ischemic stroke: relation to neurological deficit. Thromb Res 2010;125:357–61. [DOI] [PubMed] [Google Scholar]
- [4].Choi MH, Park GH, Lee JS, Lee SE, Lee S-J, Kim J-H, et al. Erythrocyte Fraction Within Retrieved Thrombi Contributes to Thrombolytic Response in Acute Ischemic Stroke. Stroke 2018;49:652–9. [DOI] [PubMed] [Google Scholar]
- [5].Voronov RS, Stalker TJ, Brass LF, Diamond SL. Simulation of intrathrombus fluid and solute transport using in vivo clot structures with single platelet resolution. Ann Biomed Eng 2013;41:1297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Santos EMM, Dankbaar JW, Treurniet KM, Horsch AD, Roos YB, Kappelle LJ, et al. Permeable Thrombi Are Associated With Higher Intravenous Recombinant Tissue-Type Plasminogen Activator Treatment Success in Patients With Acute Ischemic Stroke. Stroke 2016;47:2058–65. [DOI] [PubMed] [Google Scholar]
- [7].Santos EMM, Marquering HA, den Blanken MD, Berkhemer OA, Boers AMM, Yoo AJ, et al. Thrombus Permeability Is Associated With Improved Functional Outcome and Recanalization in Patients With Ischemic Stroke. Stroke 2016;47:732–41. [DOI] [PubMed] [Google Scholar]
- [8].Chen Z, Shi F, Gong X, Zhang R, Zhong W, Zhang R, et al. Thrombus Permeability on Dynamic CTA Predicts Good Outcome after Reperfusion Therapy. Am J Neuroradiol 2018;39:1854–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bembenek JP, Niewada M, Siudut J, Plens K, Członkowska A, Undas A. Fibrin clot characteristics in acute ischaemic stroke patients treated with thrombolysis: the impact on clinical outcome. Thromb Haemost 2017;117:1440–7. [DOI] [PubMed] [Google Scholar]
- [10].Pera J, Undas A, Topor-Madry R, Jagiella J, Klimkowicz-Mrowiec A, Slowik A. Fibrin Clot Properties in Acute Stroke. Stroke 2012;43:1412–4. [DOI] [PubMed] [Google Scholar]
- [11].Niesten JM, van der Schaaf IC, van Dam L, Vink A, Vos JA, Schonewille WJ, et al. Histopathologic Composition of Cerebral Thrombi of Acute Stroke Patients Is Correlated with Stroke Subtype and Thrombus Attenuation. PLoS One 2014;9:e88882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kirchhof K, Welzel T, Mecke C, Zoubaa S, Sartor K. Differentiation of white, mixed, and red thrombi: value of CT in estimation of the prognosis of thrombolysis phantom study. Radiology 2003;228:126–30. [DOI] [PubMed] [Google Scholar]
- [13].Brinjikji W, Duffy S, Burrows A, Hacke W, Liebeskind D, Majoie CBLM, et al. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: a systematic review. J Neurointerv Surg 2017;9:529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Simons N, Mitchell P, Dowling R, Gonzales M, Yan B. Thrombus composition in acute ischemic stroke: A histopathological study of thrombus extracted by endovascular retrieval. J Neuroradiol 2015;42:86–92. [DOI] [PubMed] [Google Scholar]
- [15].Liebeskind DS, Sanossian N, Yong WH, Starkman S, Tsang MP, Moya AL, et al. CT and MRI Early Vessel Signs Reflect Clot Composition in Acute Stroke. Stroke 2011;42:1237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shin JW, Jeong HS, Kwon H-J, Song KS, Kim J. High red blood cell composition in clots is associated with successful recanalization during intra-arterial thrombectomy. PLoS One 2018;13:e0197492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mokin M, Morr S, Natarajan SK, Lin N, Snyder KV, Hopkins LN, et al. Thrombus density predicts successful recanalization with Solitaire stent retriever thrombectomy in acute ischemic stroke: Table 1. J Neurointerv Surg 2015;7:104–7. [DOI] [PubMed] [Google Scholar]
- [18].Maekawa K, Shibata M, Nakajima H, Mizutani A, Kitano Y, Seguchi M, et al. Erythrocyte-Rich Thrombus Is Associated with Reduced Number of Maneuvers and Procedure Time in Patients with Acute Ischemic Stroke Undergoing Mechanical Thrombectomy. Cerebrovasc Dis Extra 2018;8:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sporns PB, Hanning U, Schwindt W, Velasco A, Buerke B, Cnyrim C, et al. Ischemic Stroke: Histological Thrombus Composition and Pre-Interventional CT Attenuation Are Associated with Intervention Time and Rate of Secondary Embolism. Cerebrovasc Dis 2017;44:344–50. [DOI] [PubMed] [Google Scholar]
- [20].Menon BK, Al-Ajlan FS, Najm M, Puig J, Castellanos M, Dowlatshahi D, et al. Association of Clinical, Imaging, and Thrombus Characteristics With Recanalization of Visible Intracranial Occlusion in Patients With Acute Ischemic Stroke. JAMA 2018;320:1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Borst J, Berkhemer OA, Santos EMM, Yoo AJ, den Blanken M, Roos YBWEM, et al. Value of Thrombus CT Characteristics in Patients with Acute Ischemic Stroke. Am J Neuroradiol 2017;38:1758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Berndt M, Friedrich B, Maegerlein C, Moench S, Hedderich D, Lehm M, et al. Thrombus Permeability in Admission Computed Tomographic Imaging Indicates Stroke Pathogenesis Based on Thrombus Histology. Stroke 2018;49:2674–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.