Abstract
Background The rapid spread of severe acute respiratory syndrome coronavirus-2 or SARS-CoV-2 necessitated a scaled treatment response to the novel coronavirus disease 2019 (COVID-19).
Objective This study aimed to characterize the design and rapid implementation of a complex, multimodal, technology response to COVID-19 led by the Intermountain Healthcare's (Intermountain's) Care Transformation Information Systems (CTIS) organization to build pandemic surge capacity.
Methods Intermountain has active community-spread cases of COVID-19 that are increasing. We used the Centers for Disease Control and Prevention Pandemic Intervals Framework (the Framework) to characterize CTIS leadership's multimodal technology response to COVID-19 at Intermountain. We provide results on implementation feasibility and sustainability of health information technology (HIT) interventions as of June 30, 2020, characterize lessons learned and identify persistent barriers to sustained deployment.
Results We characterize the CTIS organization's multimodal technology response to COVID-19 in five relevant areas of the Framework enabling (1) incident management, (2) surveillance, (3) laboratory testing, (4) community mitigation, and (5) medical care and countermeasures. We are seeing increased use of traditionally slow-to-adopt technologies that create additional surge capacity while sustaining patient safety and care quality. CTIS leadership recognized early that a multimodal technology intervention could enable additional surge capacity for health care delivery systems with a broad geographic and service scope. A statewide central tracking system to coordinate capacity planning and management response is needed. Order interoperability between health care systems remains a barrier to an integrated response.
Conclusion The rate of future pandemics is estimated to increase. The pandemic response of health care systems, like Intermountain, offers a blueprint for the leadership role that HIT organizations can play in mainstream care delivery, enabling a nimbler, virtual health care delivery system that is more responsive to current and future needs.
Keywords: COVID-19, surge capacity, telemedicine, electronic medical record, health information interoperability
Background and Significance
The rapid spread of severe acute respiratory syndrome coronavirus 2 or SARS-CoV-2 1 necessitates a scaled treatment response for the novel coronavirus disease 2019 (COVID-19) that exceeds a health care system's existing capacity to deliver care. 2 Community mitigation measures, including social distancing, have been implemented to slow the spread of the virus, allowing health care systems time to address system capacity constraints. 2 3 Expanding surge capacity to accommodate rapid increases in suspected COVID-19 cases requires system resilience. 4 5 Given the complexity of health care and the risk of unintended consequences, rapid implementation of interventions during pandemics poses additional safety risks to clinicians and patients if poorly executed. 6 7
The first human cases of COVID-19 were reported in China in December 2019. On March 11, 2020, the World Health Organization (WHO) declared the outbreak of a pandemic. 8 On March 13, the Trump Administration declared a national emergency. 9 The first case of COVID-19 in Utah was diagnosed on March 6, 2020, with the first patient case of community-spread COVID-19 diagnosed on March 14, 2020 with the first confirmed death on March 22, 2020. On March 15, the Governor of Utah introduced community mitigation plans to slow the spread of the virus. 10 Intermountain Healthcare's (Intermountain) health information technology (HIT) organization known as Care Transformation Information Systems (CTIS) moved quickly to support Intermountain's response to a surge in clinical COVID-19 cases, drawing lessons from high-performing health systems outside the United States. 11 A system-wide hospital incident command center (HICS) was operationalized on March 16, 2020 to manage Intermountain's COVID-19 response, working in close coordination with state and local government and other healthcare systems in Utah. This includes virtual incident management, surveillance, and epidemiology to track transmission, laboratory testing, community mitigation, and medical care. 12 As of June 30, 2020, Utah is seeing a significant increase in positive cases as the society reopens.
Objective
We characterize the rapid design and execution of a complex, multimodal, technology response to COVID-19 led by Intermountain's CTIS organization to build surge capacity at Intermountain. We provide initial data on results through June 30, 2020, as infection rates increase in Utah as the society reopens. Further, we characterize lessons learned and identify barriers to implementation of key health technologies.
Methods
The CTIS organization at Intermountain is responsible for assuring that highly reliable, well performing, and secure information systems, including electronic health record (EHR) technologies and related processes, exist that meet the needs of stakeholders systemwide at Intermountain. Intermountain is an integrated, community-based health system that provides services to about half the residents of Utah. Intermountain has 24 hospitals with 2,900 licensed beds and 215 owned or supported clinics. The system has 38,000 employees including 2,400 physicians and advanced practice providers and 3,800 affiliated physicians. The organization services 495,000 emergency department (ED) visits, 136,000 inpatient admissions, and 160,000 inpatient and ambulatory surgeries annually. Intermountain's hospitals house 17 intensive care units (ICUs) and mechanically ventilate approximately 4,300 patients annually. The health system's health plan, SelectHealth, provides insurance products to more than 870,000 members. Intermountain currently uses the Cerner Corporation (North Kansas City, Missouri, United States) EHR.
The Centers for Disease Control and Prevention (CDC) Pandemic Intervals Framework (the Framework) describes the progression of an influenza pandemic using six intervals and is used to guide community pandemic planning and response. 12 The Framework includes eight domains or functional work requirements. Using the Framework, we characterize the CTIS organization's technology response to COVID-19 through the pandemic acceleration interval. We did not specifically analyze the vaccine, risk communication, and state/local coordination domains in the results, as these are primarily a state and local government responsibility or were not underway as of study completion. To assess initial solution feasibility and sustainability, we measured intervention usage volumes during the study period March 1, 2020 to June 30, 2020, unless noted.
Results
The CTIS organization's technology response is outlined below by select Framework domains, with most new capabilities deployed within a 6-week period ending March 31. Framework domains, related clinical/business needs, and EHR integration points are summarized in Table 1 . As of June 30, all functionality continues to support the expanded COVID-19 caseload associated with reopening the state. For each CTIS intervention, we identify the underlying needs and primary stakeholders, the enabling technologies deployed, timing, results, and, as applicable, lessons learned.
Table 1. Select Pandemic Interval Framework (PIF) domain activities, related business/clinical needs and electronic health record integration points.
| PIF domain (paper section) | Primary domain activities through pandemic acceleration | Business or clinical needs | EHR integration points |
|---|---|---|---|
| Incident management | • Activate emergency operations center • Coordinate with state-wide response |
Track enterprise performance and shift resources to address capacity needs | Majority of data drawn from patient and encounter data |
| Community mitigation | • Activate community-level personal protective measures | Disseminate reliable information and assessment tools (e.g., ChatBot) | Not applicable |
| Surveillance and laboratory testing | • Optimize laboratory capacity • Conduct enhanced surveillance • Limit testing using surveillance criteria • Confirm suspected cases • Move to severe disease and syndromic surveillance |
Conduct on-demand COVID-19 testing | Integrated test eligibility algorithm and standard COVID-19 EHR order form; order sets |
| Prioritize COVID-19 laboratory samples for processing | Dynamic screening prioritization algorithm given daily laboratory volumes | ||
| Follow-up on laboratory results using a closed-loop process | Results call back interface | ||
| Auto-generation of results in the physician problem list, including open chart alert | |||
| Patient self-service access to results | |||
| Medical care and countermeasures | • Promptly diagnose and treat ill persons • Implement infection control practices • Provide chemoprophylaxis to exposed persons • Educate clinicians on prompt treatment of ill patients • Assess impact on care facilities • Assess medical resources are sufficient to manage ill person and conduct case-based control efforts • Set up alternate care sites • Monitor surge and assess needs • Monitor shortages • Initiate therapeutic clinical trials |
Track bed capacity, usage and billing | Bed characterization |
| Track physician/nurse availability | Redeploy staff using “switch-roles” function | ||
| Limit personal protective equipment use | Usage data | ||
| Manage ventilator capacity | Usage data | ||
| Manage medication supplies | Restricted medication physician alert | ||
| Provide ongoing clinical visits | Integrate ordering, scheduling and billing | ||
| Rapidly assess and document patients presenting to emergency department | Quick Visit order sets | ||
| Provide ventilation management | Integrated clinical decision support tool | ||
| Other medical care essentials | Simplification of nursing documentation | ||
| Clinical trials | Initiate zero touch for patients using electronic consent |
Abbreviations: COVID-19, novel coronavirus disease 2019; EHR, electronic health record.
Incident Management
CTIS enabled HICS members by providing a single virtual collaboration platform to communicate and collaborate that is more effective than disparate communication modes. The Intermountain systemwide HICS management structure was led by three incident commanders (Chief Operating, Medical and Nursing Officers) and organized into section-level functions including Operations, Planning, Logistics, Information Technology/Services and Finance. Each section-level function was responsible for supporting broad program initiatives categorized by Framework domain in Table 1 . Every HICS section has their own channel and membership in these teams has been relatively open. The education strategy for users involved orientation and education to get incident commanders, executive assistants, and section-level project managers using the tools. The CTIS event management team provided program office support. Incident commanders set system-level priorities responsive to external factors, and escalations from frontline caregivers. The priorities were reviewed and updated daily. The round trip for new issues from escalation to prioritization to execution and was often completed as rapidly as 2 days. The section chiefs for CTIS executed the priorities in a center-led fashion using high reliability management principles. Multidisciplinary teams with decision-making authority were identified to address prioritized projects with clear role assignment for each team member.
Effective surge planning and capacity management requires real time information to key operational and clinical decision makers within the health care system and with community stakeholders. Beginning in early March, CTIS enabled the development of a COVID-19 dashboard using a data visualization tool that contains a variety of COVID-19-related information needed for pandemic response. Data elements in the dashboard, as of June 30, are included in Table 2 . New measures continue to be added as new requirements are identified, and data are made available from the EHR and other data sources. Most dashboard elements are updated on at least a daily basis. As of June 30, the dashboard is the primary “source of truth” for managing the organization's pandemic response.
Table 2. Key Information elements for COVID-19 enterprise operations dashboard at Intermountain.
| Information elements |
|---|
| Test volumes and results |
| Hospitalization rates |
| Inpatient capacity |
| ICU bed capacity |
| Ventilator use and available capacity |
| Telehealth usage |
| Medication and supplies available capacity |
| Projected infection rates |
| Projected hospitalization and death rates |
| Antibody testing |
| Screening symptoms |
| Contact tracing |
| Workforce redeployment |
| Intermountain employees testing positive |
| Clinical appointments—cancelled, rescheduled |
| Ambulatory care volumes (not limited to COVID-19) |
| Daily encounters by type (not limited to COVID-19) |
Abbreviation: ICU, intensive care unit.
Community Mitigation
Given resource constraints, CTIS implemented a COVID-19 ChatBot on Intermountain's public web site in 3 weeks to support initial patient triage. The ChatBot lets individuals self-assess whether they should pursue additional testing or medical care related to COVID-19 given self-reported information on risk factors. A sample ChatBot interaction is noted in Table 3 . As of June 30, more than 200,000 self-assessments were completed by community members. The ChatBot function provides current information on COVID-19 treatment while hopefully decreasing phone calls to providers or the system
Table 3. Sample abbreviated ChatBot interaction.
| ChatBot dialogue box | User selection |
|---|---|
| Hello, my name is Scout. I am your digital helper. I was designed to provide info about COVID-19, also known as the novel coronavirus. | Let's start |
| Here is the latest information as of July 10, 2020 (test updates). How can I help you? | Clicked check me for it |
| The only way to confirm if you have COVID-19 is to get tested with a kit. However, I can still help you figure out if you should seek medical care. I will ask you a few questions and give you some advice. Ready to begin? | Yes/no button Clicked yes |
| Have you been at risk of exposure to COVID-19 by experiencing any of the following? (1) Traveled to an area of high-risk for COVID-19 (2) Been around someone who recently traveled to an area of high-risk and is also sick (3) Been around someone who is known to have COVID-19 (4) Been told by a health official that you may have been exposed to the virus | Yes/no button Clicked yes |
| Have you had a fever recently or think you have a fever? Cough? Fatigue? Body aches? Sore throat? Reduced sense of smell or taste? Diarrhea? Mild to moderate shortness of breath or mild to moderate difficulty breathing? | Yes/no button by question Clicked yes to some |
| Are you experiencing symptoms that feel like a life-threatening medical emergency? Describes possible symptoms? | Yes/no button Clicked no |
| How old are you? | Recorded age |
| Do you have any of the following? high blood pressure, diabetes, heart disease, lung disease, kidney disease, liver disease, history of asthma, history of smoking, obesity | Yes/no button Clicked no |
| COVID-19 can affect people who have weaker immune systems from things like chemotherapy, HIV/AIDS, organ transplant, being pregnant, or prolonged steroid use. Do you have a weakened immune system from a known cause? | Yes/no button Clicked no |
| Result Please call the COVID-19 call center or your doctor. They can help in creating an order for testing and directing you to the nearest testing facility if appropriate. Further instructions on limiting transmission were also provided. | |
Surveillance and Laboratory Testing
Key internal and external stakeholders needed testing scaled across multiple geographies, locations, and facility types, including new community curbside testing facilities. The test process also needed to address test order requests from affiliated and nonaffiliated physicians without access to Intermountain's EHR and ensure that all community members that were tested received results and follow-up actions could be taken. Given early limitations on test availability, testing also had to be prioritized to those most likely to benefit.
As COVID-19 cases emerged, the Intermountain clinicians found the internal manual process for assessing patient COVID-19 test eligibility and the electronic process for ordering onerous. To increase order efficiency, CTIS developed an integrated clinical decision support system (CDSS) and order form in the EHR to limit test ordering to eligible patients. The CDSS includes a novel algorithm that allows the intake clinician to determine patient eligibility for COVID-19 testing in the EHR using patient-reported criteria including symptoms, exposure risks, patient disposition, and other test indications. For patients meeting test eligibility criteria, a unique patient identifier is autogenerated in the EHR to facilitate test taking and processing. Dynamic updates to this algorithm are continually generated from rapid cycle analysis of emerging correlations in symptoms, exposures, and positive results. This revised approach led to meaningful reductions in the time required to assess patient eligibility and place a test order from 10 minutes per patient to less than 1 minute. For affiliated physicians without access to Intermountain's EHR, unaffiliated physicians and the patient community, a community hotline was established to facilitate community questions and to provide an intake point for faxed manual test orders. As of June 30, the hotline takes approximately 2,000 calls a day. Daily COVID-19 test volumes are included in Fig. 1 .
Fig. 1.
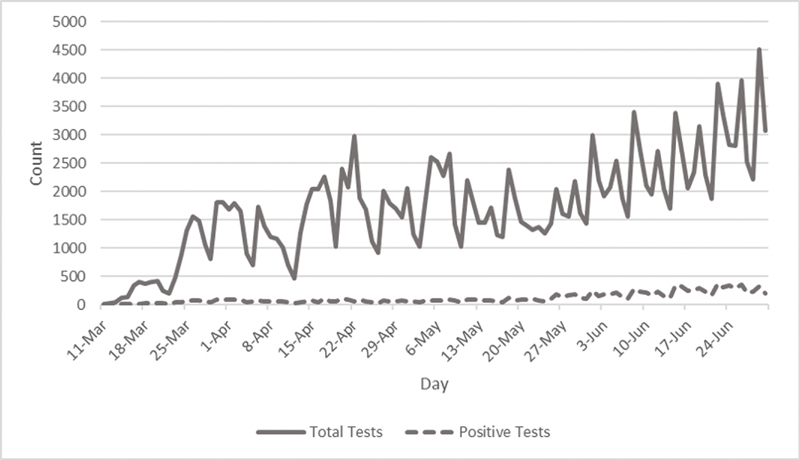
Daily COVID-19 test volumes coordinated by Intermountain healthcare COVID-19 laboratory testing volumes, overall and positive tests. Daily change in COVID-19 testing at Intermountain Healthcare during acceleration of the COVID-19 pandemic in Utah from March 8 to June 30, 2020.
One Central Processing Laboratory (CPL) processes inpatient and outpatient testing across all Intermountain facilities in Utah. Given high-test volumes, CTIS developed a dynamic prioritization algorithm ranking each COVID-19 laboratory test for processing based on location, exposure, and symptoms as noted in Table 4 . The dynamic nature of the algorithm lets the health care system respond to change in testing demand. The prioritization algorithm results are automatically surfaced on all samples enabling each facility to triage shipment of test samples for processing.
Table 4. Test processing prioritization grid as of June 30, 2020.
| Priority 1 = highest | Patient location category | |||
|---|---|---|---|---|
| High risk: ICU, inpatient, skilled nursing facility, nursing home | Medium risk: emergency department, urgent care | Low risk: connect care, drive through collection sites | ||
| Exposure Category | High risk: • Close contact with confirmed case of COVID-19 • Travel to high-risk geographic area within 14 days of symptom onset • Symptomatic healthcare worker with high-risk exposure • Special populations |
Priority 2 | Priority 4 | Priority 5 |
| Medium risk: • Close contact with person under investigation for COVID-19 |
Priority 3 | Priority 6 | Priority 8 | |
| Low risk: • No known exposure or epidemiologic risk |
Priority 9 | |||
| Priority 1 (highest) patients (rapid test with 2 to 4 hour turnaround time) includes symptomatic hospitalized patients, symptomatic labor and delivery patients, all pre-term labor (<37 weeks) labor and delivery patients, all pre-transplant patients and any asymptomatic emergency department patients from high-density residences. Priority 2 patients also includes symptomatic health care workers. Preprocedural screenings and repeat tests given the lowest priority. | ||||
CTIS enabled the tracking and addressing of individual test results through a unified telehealth strategy. For patients admitted to the hospital, the CPL calls the inpatient team directly to relay positive test results. For ambulatory or community testing, a COVID-19 remote patient monitoring (cRPM) team places direct calls to patients for all positive test results. The cRPM team works with COVID-19-positive patients to develop a follow-up care plan. The cRPM team uses an EHR results callback interface for tracking patient callbacks to ensure COVID-19-positive patients seek care through virtual urgent care (UC) visits if symptoms or clinical decompensation occurs. For Intermountain patients, COVID-19 test results are routed via the EHR to the ordering provider and primary care physician of record where a problem is autogenerated in the problem list. Test results are available in near real time via Intermountain's patient portal. This closed-loop COVID-19 testing approach was implemented within 3 weeks.
Medical Care and Countermeasures
For utilization management, Intermountain's HICS team is using a three-tiered surge management framework. 13 Under this model, the organization strives to practice tier 1 (conventional care) and tier 2 (contingency care) for as long as possible. Criteria for moving to tier 2 and tier 3 (crisis care), is driven by evaluating existing bed status, critical resource levels (beds, ventilators, and medications), and critical staffing shortages. As each hospital approaches 85% of ICU occupancy, HICS considers moving to tier 3. Weekly hospitalization rates for COVID-19 through the week of June 21 are included in Fig. 2 .
Fig. 2.
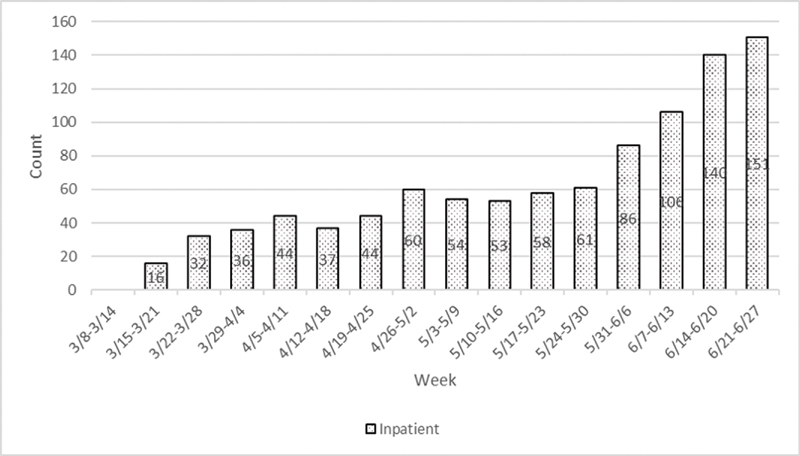
Weekly Nonnegative COVID-19 Encounter Volumes at Intermountain Healthcare by Service Type. Weekly increase in COVID-19 encounters at Intermountain Healthcare inpatient admissions during acceleration of the COVID-19 pandemic in Utah from March 8 to June 27, 2020.
Coordinating Bed and Space Capacity
To meet inpatient bed demand, clinical operations were converting beds from their primary use or relocating them. CTIS worked within the EHR to create a new bed and a new unit in which to place each bed as applicable. Detailed inventory was taken to ensure that each surge space was equipped with all the necessary equipment and hardware. CTIS then worked to ensure that the bed and new location are appropriately interfacing with the needs of the clinical staff based on the type of care being delivered in the new unit. The CTIS interface team ensures that pharmacy deliveries, laboratory and imaging results, vital sign equipment, and billing are all connected to the new bed space and meet the patient's needs. New units are connected with printers, so that blood specimens, patient labels, and prescriptions can be easily printed. This functionality was designed and deployed within 2 weeks. Approximately 20 mobile workstations were purchased to augment existing equipment. Through June 30, the team set up the ability to ramp up to 600 new beds.
Redeploying Physician and Nurse Capacity
Key internal operations and clinical leaders needed a system-wide approach to redeploy staff to support treatment of COVID-19 patients. Information on certifications and unique skills were typically maintained at the local level, making efforts to match trained clinicians with excess capacity, and COVID-19 staffing needs difficult. To address this, CTIS worked with the Intermountain nursing leadership and research to deploy an online survey ( Appendix A ) to 10,000 nurses to gain an immediate inventory of skills and experience (85% response rate). Survey results were integrated with other demographic and certification information to identify gaps in key skills and certification information. The data captured were sufficient to initiate tier-2 assignments with some modifications. CTIS developed a distinct role for redeployed employees in the EHR that allows a single individual to access multiple different workflows using “switch roles” functionality. As of June 30, CTIS had generated more than 1,500 new roles while retaining the employees existing primary role assignment.
Managing Equipment and Supply Capacity Constraints
Shortages of personal protective equipment (PPE) are well documented during the COVID-19 pandemic. 14 Key stakeholders, in coordination with state and local governments, required a robust inventory tracking system that would provide leaders with the visibility to available PPE supply levels. Stakeholders need to be able to adjust company PPE and supplies policies almost daily. Inventories of PPE overall and by site are being maintained using a CTIS-deployed operational dashboard. In-hospital use of a telehealth platform for treatment and management of COVID-19 positive and person-under-investigation pending patients has also been utilized to reduce PPE demand. CTIS is providing tablets to patients for use in telehealth management on a tiered basis, starting with COVID-19-positive patients. Clinical teams connect with patients via the in-room tablet using the standard telehealth platform using from outside the room. CTIS develop and deployed PPE inventory tracking in 3 weeks.
To manage potentially scarce medication supplies, CTIS developed a medication alert in the EHR that alerts a provider who tries to order medications that were restricted or in short supply due to COVID-19, including quinolones, inhalers (e.g., albuterol) and azithromycin. Use of quinolones requires a second approval from the Intermountain Infectious Disease team. For use of inhalers and azithromycin, the alert notes that the medication is restricted and provides additional instructions regarding proper use for confirmed/suspected COVID-19 patients. From March to June, the Intermountain saw a 67% reduction in the use of inhalers and azithromycin over the prior quarter due to seasonal factors, education efforts, and implementation of the alert. Hydroxychloroquine consumption fell by over 50% by June following a brief increase in March 2020 when the drug was initially presented as a potential treatment for COVID-19. Inclusion and removal of alerts by CTIS are ongoing as medication supplies change.
To address ventilator inventory management, an HIT development team deployed a real-time dashboard that reports the total number of ventilators by facility and the number in use. 15 This dashboard was developed and deployed within 3 weeks. As of June 30, 22% of conventional adult ventilators were in use (63 of 287) with 27 COVID-19 patients on ventilator.
During times of high-visitor restrictions, both clinicians and patient stakeholders needed mechanisms to communicate with family members. CTIS implemented a solution to support family interaction using telehealth-enabled tablets for interpreter services during real-time care, for educating patients and families on admit/discharge instructions, and to connect patients with those unable to visit during vulnerable times. CTIS developed and deployed the tablet solution in 3 weeks. As of June 30, CTIS has deployed 1,400 tablets systemwide to support these key patient and community facing activities.
Increasing Safe Primary and Specialty Care Treatment Capacity
Internal stakeholders needed safe alternatives for delivering patient care. Working with CTIS, Intermountain had begun to carefully design and deploy an EHR-integrated solution that allowed scheduled video visits as early as January 2019. The visits using the integrated platform were known as scheduled video visits (SVV) and met all existing privacy and security requirements. SVV penetration in January 2020 was less than 1% of all eligible encounters. On March 17, Federal regulatory and reimbursement policy changes allowed health care systems to deploy alternative video visits (AVV) using more common technologies. 16 17 Successful AVVs required aligning appointment types with the correct billing codes/modifiers, interface integration, and downstream prompts to use the correct change orders. To address these requirements, CTIS repurposed existing SVV visit types for scheduling, coding, and orders configuration needed to rapidly deploy. This allowed CTIS to retain all standard SVV EHR-enabled functionality including automated attestation language to clinical notes, automatic application of correct point of service codes, and modifiers to the clinical encounter use of correct video visit charge orders and dashboard tracking of activity. CTIS enabled full use of AVV within 2 weeks. More than 3,000 caregivers were trained on AVV technologies during the first week of deployment. Weekly video visit data through the week of June 28 are noted in Fig. 3 .
Fig. 3.
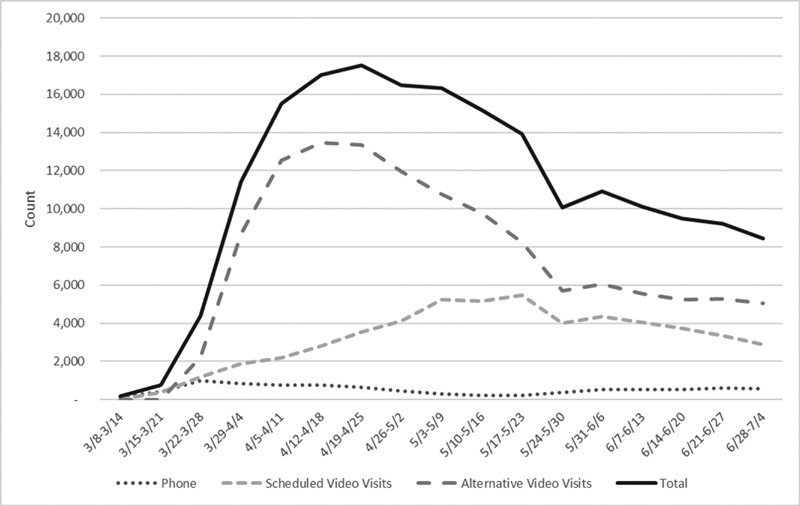
Weekly telehealth encounters at Intermountain healthcare overall and by telehealth services type. Weekly increase in telehealth encounters at Intermountain Healthcare during acceleration of the COVID-19 pandemic in Utah from March 8 to June 28, 2020. Alternative video visits represent visits using technologies made available following the March 17, 2020 “Notification of Enforcement Discretion for Telehealth Remote Communications During the COVID-19 National Public Health Emergency” issued by the Office of Civil Rights at the Department of Health and Human Services.
Increasing Emergency Department and Urgent Care Treatment Capacity
Many of our EDs and UCs responded to pandemic acceleration by segregating patients with respiratory complaints and fever into dedicated clinical unit areas. Most of these patients had similar problem, documentation (history and physical), diagnostic, therapeutic, and follow-up needs. However, each need required a separate workflow with multiple “clicks.” Clinical care teams needed a standardized template to batch these disparate activities into a simple completion checklist to facilitate safe and efficient documentation of presenting patients. CTIS deployed a rapid local design, configuration, and deployment using Quick Visit, a Cerner template that facilitates bundling of a set of activities into a simple, and coordinated workflow (i.e., ED adult COVID-19 visit). Care pathway content for Quick Visit is developed by the Intermountain stakeholder team. When a patient presents, using the Quick Visit, the clinician can bundle care pathway tasks together in parallel and sign them simultaneously versus typical serial processes and workflows. Quick Visits are readily adaptable to keep up with necessary clinical changes. Through June 30, over 400 COVID-19 Quick Visits were completed in EDs, UCs, and ambulatory settings.
Evidence-Based Care for Ventilated Patients with COVID-19 and Acute Respiratory Distress
Acute respiratory distress syndrome (ARDS) is an acute lung injury that can be caused by many factors, including COVID-19, and results in hypoxemic respiratory failure requiring mechanical ventilation for support until lung injury subsides. 18 19 Lung-protective ventilation (LPV) strategies, including low tidal volume ventilation (LTVV), improve outcomes for patients with ARDS in clinical studies. 20 21 22 23 24 25 The ICU Medical Director system needed a mechanism to scale ventilation management and improve the use of LPV in mechanically ventilated patients. CTIS worked with researchers at Intermountain to develop a comprehensive clinical decision support tool that includes four different open-loop computerized protocols integrated with the EHR: an LTVV protocol, an oxygenation protocol with either standard or high-positive expiratory end pressure (PEEP), a weaning assessment, and a spontaneous breathing protocol. Physicians place an electronic order in the EHR for each protocol component. Once ordered, the respiratory therapist (RT) measures patient height and sets parameters for tidal volume, respiratory rate, fraction of inspired oxygen (FiO 2 ) and PEEP. Given the results of an arterial blood gas, oxygenation, and ventilation protocols are then run, generating instructions for the RT to adjust or maintain FiO 2 , PEEP, tidal volume, and respiratory rate. As long as the RT maintains adherence to the protocol instructions, the RT can continue to adjust ventilator settings without a physician order. Intermountain is sustaining high adherence (>80%) to lung-protective ventilation strategies at all hospitals.
Launching Therapeutic Clinical Trials
Intermountain researchers quickly launched several clinical trials including investigator initiative studies (ClinicalTrials.gov: NCT04334382 and NCT04329832). These were designed to discover which therapies may be beneficial to patients early in the pandemic. 26 CTIS developed an electronic consent form to have no-touch procedures in place to obtain informed consent, ensure safety of the staff, and reduce PPE needed for clinical care. As of June 30, 2020, over 1,000 COVID-19 inpatients have been treated in a clinical trial, expanded access use, or clinical off-label use.
Discussion
The rate of future pandemics is estimated to increase given high global travel and integration, urbanization, changes in land use, and environmental impacts. 27 28 Building preparedness and health capacity are critical priorities to stem the cost of current and future pandemics. 29 The CTIS organization's use of HIT to create surge capacity during COVID-19 shares similarities with other health systems of varying profiles, including the ubiquitous use of telehealth in mainstream care. 30 31 32 Important differences in CTIS organization's technology response include use of novel algorithms and EHR-integrated computerized clinical decision support.
CTIS enabled critical integration between telehealth technologies and clinical functions. Historically, patients and providers have been slow to use telehealth capabilities, even in rural locations. 33 For provider–provider interaction, the use of telehealth is associated with increased access to specialty care, reduced wait times, shorter waiting lists, and unnecessary appointments for patients seeking specialist services. 34 Barriers to widespread use include the impact of service change, staff–patient interaction, credibility, and autonomy and technical issues. 35 Despite benefits, patients also remain resistant to telehealth use. 36 Payer changes in reimbursement policies 16 for telehealth and relaxing of communication requirements, 17 remove important barriers to technology use. 36 37 At Intermountain, patients struggled to use the SVV telehealth technology but were given several familiar AVV options that they were more willing to use. This did create additional operational complexity ensuring that support and training was available for multiple AVV platforms. Expanded use of telemedicine in mainstream care during the lengthening pandemic may prove a tipping point to higher use. 38
CTIS rapidly designed and developed EHR-embedded algorithms for prioritizing screening and test processing resources to limit waste by identifying those most likely to benefit from screening and/or from more rapid turnaround of test results. CTIS also found the algorithms responsive to rapidly changing testing criteria present during pandemic acceleration. These algorithms continue to be applied to all individuals presenting for screening at Intermountain-supported community testing locations. Such algorithms can be extended to evaluate value-based resource decisions in mainstream health care activities.
Other important lessons were learned during solution design and deployment. First, use of the single virtual collaboration platform for CTIS coordination was critical to success. Clinical operations teams, such as the COVID-19 cRPM team, responsible for patient follow-up on test results and struggled to coordinate their work. CTIS assisted the team in transitioning to a virtual collaboration platform that noticeably improved their coordination, efficiency, and the patient experience. Second, given the rapidly changing understanding of the disease course and treatment, a centrally maintained knowledge management system with regularly updated and accurate clinical content would have improved efficiency of ongoing tool maintenance. CTIS employees had to manually coordinate and regularly update clinical content and rules in the ChatBot functionality, prioritization algorithms, order sets, and Quick Visits to ensure information remained current. Third, an affiliate clinician order entry system would have improved operational workflow. Affiliated clinicians without access to Intermountain's EHR were required to fax test orders that were then manually entered by the COVID-19 hotline team. Orders were often received that did not meet testing criteria. Staff would then have to go back to affiliated clinicians to resolve the matter, slowing the test process. Finally, clinical data availability and quality problems affected response efforts. For example, an early review of EHR data revealed that some beds being reported no longer existed while other beds were being reported twice. A custom software solution was ultimately required to track these issues on an ongoing basis to ensure bed counts remained accurate for surge planning.
Barriers to interoperability are well documented and persist in the context of our pandemic response. 37 38 39 Order interoperability, or the ability for a medical order created in one EHR to be transmitted to and acted on in another health system's EHR, remains a significant barrier to delivering essential care. Capacity management may need to move from a system level to a community-level response. Multisite health care organizations, including Intermountain, need a central tracking system to coordinate capacity planning of beds, clinician time, and supplies. Such a system should also enable community and state-wide response capacity planning to support management by ensuring full utilizing of existing resources before investing in new capabilities or modifying existing service lines.
Conclusion
The rate of future pandemics is estimated to increase. The pandemic response of health care systems like Intermountain offers a blueprint for the role that HIT organizations can play in mainstream care delivery, enabling a nimbler, and virtual health care delivery system that is more responsive to current and future needs.
Clinical Relevance Statement
Building preparedness and health capacity are critical priorities to stem the cost of current and future pandemics. Scalable, virtual technologies, such as telemedicine, resource use algorithms and clinical decision support tools are key facilitators to COVID-19 surge capacity building and pandemic response with rapid increases in mainstream use to deliver care. The pandemic response of Intermountain's health information technology organization offers a blueprint for the role that technology can play in mainstream care delivery, enabling a nimbler, virtual health care delivery system that is more responsive to current and future needs.
Multiple Choice Questions
-
When responding to COVID-19 as a health care system, which ubiquitous technology is rapidly increasing in use to drive increased system capacity and to support mainstream care?
Tablets
Database management systems
Telehealth
Electronic health records
Correct Answer: The correct answer is option c. While all technologies enable a coordinated system response, telehealth technology is rapidly growing from a niche to a mainstream capability enabling provider–patient interaction in primary, specialty, and inpatient care during the COVID-19 pandemic. Telehealth technology is also enabling patients to interact with family members.
-
In developing a multimodal technology response to the COVID-19 pandemic, what persistent barrier to electronic health record use is limiting an integrated, community response?
Ease of use
Order interoperability
Provider resistance
Patient resistance
Correct Answer: The correct answer is option b. Electronic health record order interoperability is an essential component of an integrated, community response to COVID-19. Sociotechnical barriers to order interoperability persist and limit the level of integration required for a community response to the pandemic.
Acknowledgments
We would like to acknowledge our frontline clinical teams that work tirelessly to treat our patients under such challenging circumstances. We would also like to acknowledge the support and participation of key technology, research, and operations leaders who participated in field interviews to collect information for this manuscript.
Chris Fillmore
Anika Gardenhire
Bert Lopansri
Doug Nelson
Brian Roundy
McKay Perry
Jason McClellan
Perry Gee
Christopher Gourley
Trent Tuckett
Kathryn Kuttler
Michelle Edwards
We also want to acknowledge the contribution of Intermountain Healthcare Delivery Institute interns, Nick Baker and Taylor Throne who assisted in collecting and collating information from rapid field interviews.
Funding Statement
Funding The analysis in this publication was supported (in part or in full) by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR002539. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Research related to the section “Evidence-Based Care for Ventilated Patients with COVID-19 and Acute Respiratory Distress” was supported in part by the National Heart, Lung and Blood Institute (NHLBI) of the National Institutes of Health under award number U-01HL143505 (ClinicalTrials.gov Identifiers NCT:03225807 and NCT:03984175). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest A.J.K. reports grants from National Center for Advancing Translational Sciences, NIH, during the conduct of the study; other from publicly-traded stock, personal fees from St. Catherine's University, St. Paul, Minnesota, United States, outside the submitted work.
Protection of Human and Animal Subjects
We reviewed this work with our Institutional Review Board and concluded it was not human subjects research.
Appendix A: Nurse Survey.
Please Complete** (2 minutes)
Due to the quickly changing nature of our work situations, we are collecting nursing work experience data from all clinical areas. This will help facilitate the sharing of nursing staff between areas in event of COVID-19 urgent needs. You may receive this survey from multiple sources, but you only need to complete it once.
Name:
__________________________________
Employee ID number:
__________________________________
Job title:
__________________________________
-
Which certifications do you currently have?
○ BLS
○ ACLS
○ PALS
○ NRP
○ TNCC
○ Other
○ None of the above
What other current certifications do you have?
__________________________________
-
Indicate which of the following areas you have prior nursing work experience
○ Home health
○ Critical care
○ ED
○ Mom/baby
○ Postpartum
○ Special care nursery
○ Labor and delivery
○ NICU
○ Med/surg
○ Endoscopy
○ PACU
○ Cath laboratory
○ Peri-op
○ Peds
○ None of the above
For each area selected, using branching logic in the survey, the nurse was then asked:
How many years of (selected area) nursing experience do you have?
○< 1 year
○ 1–3 years
○ 4–5 years
○ 6–10 years
○ > 10 years
How many years ago was your (selected area) nursing experience?
○< 1 year
○ 1–5 years
○ > 5 years
Other supplemental questions that were asked based upon the topic area are noted below:
Which of the following postpartum topics do you have nursing work experience with?
○Maternal care
○ Well newborn care
Which of the following special care nursery topics do you have nursing work experience with?
○Severe preterm (ETT, TPN, and pressors)
○ Feeder grower
Which of the following labor and delivery topics do you have nursing experience with?
○Vaginal delivery
○ C/S delivery
○ Antepartum care
○ Fetal monitoring
Which of the following NICU topics do you have nursing work experience?
○Severe preterm (ETT, TPN, and pressors)
○ Feeder grower
With your peds nursing work experience, do you have experience working with <28 day old admits? Yes/No
References
- 1.World Health Organization Naming the coronavirus disease (COVID-19) and the virus that causes itAvailable at:https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it. Accessed October 12, 2020
- 2.Ruoran L, Rivers C, Tan Q, Murray M B, Toner E, Lipsitch M. The demand for inpatient and ICU beds for COVID-19 in the US: lessons from Chinese cities. Version 2. medRxiv. doi: 10.1101/2020.03.09.20033241. [DOI] [Google Scholar]
- 3.Glass R J, Glass L M, Beyeler W E, Min H J. Targeted social distancing design for pandemic influenza. Emerg Infect Dis. 2006;12(11):1671–1681. doi: 10.3201/eid1211.060255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanchet K, Nam S L, Ramalingam B, Pozo-Martin F. Governance and capacity to manage resilience of health systems: towards a new conceptual framework. Int J Health Policy Manag. 2017;6(08):431–435. doi: 10.15171/ijhpm.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanefeld J, Mayhew S, Legido-Quigley H. Towards an understanding of resilience: responding to health systems shocks. Health Policy Plan. 2018;33(10):1144. doi: 10.1093/heapol/czy087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipsitz L A. Understanding health care as a complex system: the foundation for unintended consequences. JAMA. 2012;308(03):243–244. doi: 10.1001/jama.2012.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton C. Heavy tailed distributions of effect sizes in systematic reviews of complex interventions. PLoS One. 2012;7(03):e34222–e22. doi: 10.1371/journal.pone.0034222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization Responding to community spread of COVID-19: interim guidance, March 7, 2020Available at:https://apps.who.int/iris/handle/10665/331421. Accessed October 12, 2020
- 9.Office of the President of the United States Proclamation on Declaring a National Emergency Concerning the Novel Coronavirus Disease (COVID-19) OutbreakAvailable at:https://www.whitehouse.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/. Accessed October 12, 2020
- 10.Governor Gary R.Governor Herbert announces two-week dismissal of Utah's Public SchoolsAvailable at:https://schools.utah.gov/file/d50bddb8-a7bb-417a-9016-20b317a8b32b. Accessed October 12, 2020
- 11.Legido-Quigley H, Asgari N, Teo Y Y.Are high-performing health systems resilient against the COVID-19 epidemic? Lancet 2020395(10227):848–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Center for Immunization and Respiratory Diseases (NCIRD) DoVD Pandemic Intervals FrameworkAvailable at:https://www.cdc.gov/flu/pandemic-resources/national-strategy/intervals-framework.html. Accessed October 12, 2020
- 13.Utah Hospital Association Crisis Standards of Care Workgroup Utah Crisis Standards of Care GuidelinesAvailable at:https://www.utahhospitals.org/images/Final_Utah_Crisis_Standards_of_Care_011719.pdf. Accessed October 12, 2020
- 14.World Health Organization Shortage of personal protective equipment endangering healthcare workers worldwideAvailable at:https://www.who.int/news-room/detail/03-03-2020-shortage-of-personal-protective-equipment-endangering-health-workers-worldwide. Accessed October 12, 2020
- 15.James B C, Edwards D P, James A F. An efficient, clinically-natural electronic medical record system that produces computable data. EGEMS (Wash DC) 2017;5(03):8. doi: 10.5334/egems.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.President Trump Expands Telehealth Benefits for Medicare Beneficiaries During COVID-19 OutbreakAvailable at:https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak#:∼:text=%E2%80%9CThe%20Trump%20Administration%20is%20taking,%2C%E2%80%9D%20said%20Administrator%20Seema%20Verma.&text=On%20March%2013%2C%202020%2C%20President,and%20the%20National%20Emergencies%20Act. Accessed October 12, 2020
- 17.Health Information Privacy. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergencyAvailable at:https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Accessed October 12, 2020
- 18.ARDS Definition Task Force . Ranieri V M, Rubenfeld G D, Thompson B T. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 19.National Center for Immunizationand Respiratory Diseases Interim Clinical Guidelines for Management of Patients with Confirmed Coronavirus Disease (COVID-19)Available at:https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Accessed October 12, 2020
- 20.Acute Respiratory Distress Syndrome Network . Brower R G, Matthay M A, Morris A, Schoenfeld D, Thompson B T, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 21.Needham D M, Yang T, Dinglas V D. Timing of low tidal volume ventilation and intensive care unit mortality in acute respiratory distress syndrome. A prospective cohort study. Am J Respir Crit Care Med. 2015;191(02):177–185. doi: 10.1164/rccm.201409-1598OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Thoracic Society, European Society of Intensive Care Medicine, and Society of Critical Care Medicine . Fan E, Del Sorbo L, Goligher E C. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(09):1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 23.Weiss C H, McSparron J I, Chatterjee R S. Summary for clinicians: mechanical ventilation in adult patients with acute respiratory distress syndrome clinical practice guideline. Ann Am Thorac Soc. 2017;14(08):1235–1238. doi: 10.1513/AnnalsATS.201704-332CME. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson B T, Chambers R C, Liu K D. Acute Respiratory Distress Syndrome. N Engl J Med. 2017;377(06):562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 25.Slutsky A S, Ranieri V M. Ventilator-induced lung injury. N Engl J Med. 2013;369(22):2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 26.Brown S M, Peltan I D, Webb B. Hydroxychloroquine versus azithromycin for hospitalized patients with suspected or confirmed COVID-19 (HAHPS). Protocol for a pragmatic, open-label, active comparator trial. Ann Am Thorac Soc. 2020;17(08):1008–1015. doi: 10.1513/AnnalsATS.202004-309SD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morse S S. Factors in the emergence of infectious diseases. Emerg Infect Dis. 1995;1(01):7–15. doi: 10.3201/eid0101.950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones K E, Patel N G, Levy M A.Global trends in emerging infectious diseases Nature 2008451(7181):990–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madhav N, Oppenheim B, Gallivan M. 3rd Edition ed. Washington, DC: The International Bank for Reconstruction and Development; 2017. Pandemics: risks, impacts and mitigation. [PubMed] [Google Scholar]
- 30.Reeves J J, Hollandsworth H M, Torriani F J. Rapid response to COVID-19: health informatics support for outbreak management in an academic health system. J Am Med Inform Assoc. 2020;27(06):853–859. doi: 10.1093/jamia/ocaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hick J L, Hanfling D, Wynia M K, Pavia A.Duty to plan: health care, crisis standards of care and novel coronavirus SARS-CoV-2. NAM perspectivesAvailable at:https://nam.edu/duty-to-plan-health-care-crisis-standards-of-care-and-novel-coronavirus-sars-cov-2/. Accessed October 12, 2020
- 32.Ravaud P, le Ouay F, Depaulis E, Huckert A, Vegreville B, Tran V.Reconfiguring health services to reduce the workload of caregivers during the COVID-19 outbreak using an open-source scalable platform for remote digital monitoring and coordination of care in hospital Command CentresAvailable at:https://arxiv.org/ftp/arxiv/papers/2003/2003.05873.pdf. Accessed October 12, 2020
- 33.Mehrotra A, Jena A B, Busch A B, Souza J, Uscher-Pines L, Landon B E. Utilization of telemedicine among rural medicare beneficiaries. JAMA. 2016;315(18):2015–2016. doi: 10.1001/jama.2016.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caffery L J, Farjian M, Smith A C. Telehealth interventions for reducing waiting lists and waiting times for specialist outpatient services: a scoping review. J Telemed Telecare. 2016;22(08):504–512. doi: 10.1177/1357633X16670495. [DOI] [PubMed] [Google Scholar]
- 35.Brewster L, Mountain G, Wessels B, Kelly C, Hawley M. Factors affecting front line staff acceptance of telehealth technologies: a mixed-method systematic review. J Adv Nurs. 2014;70(01):21–33. doi: 10.1111/jan.12196. [DOI] [PubMed] [Google Scholar]
- 36.Almathami H KY, Win K T, Vlahu-Gjorgievska E. Barriers and facilitators that influence telemedicine-based, real-time, online consultation at patients' homes: systematic literature review. J Med Internet Res. 2020;22(02):e16407–e07. doi: 10.2196/16407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edwards A, Hollin I, Barry J, Kachnowski S. Barriers to cross–institutional health information exchange: a literature review. J Healthc Inf Manag. 2010;24(03):22–34. [PubMed] [Google Scholar]
- 38.Bashshur R L, Shannon G, Krupinski E A, Grigsby J. Sustaining and realizing the promise of telemedicine. Telemed J E Health. 2013;19(05):339–345. doi: 10.1089/tmj.2012.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross J, Stevenson F, Lau R, Murray E. Factors that influence the implementation of e-health: a systematic review of systematic reviews (an update) Implement Sci. 2016;11(01):146. doi: 10.1186/s13012-016-0510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


