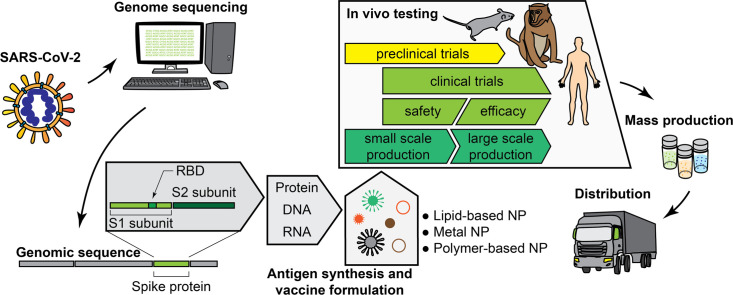
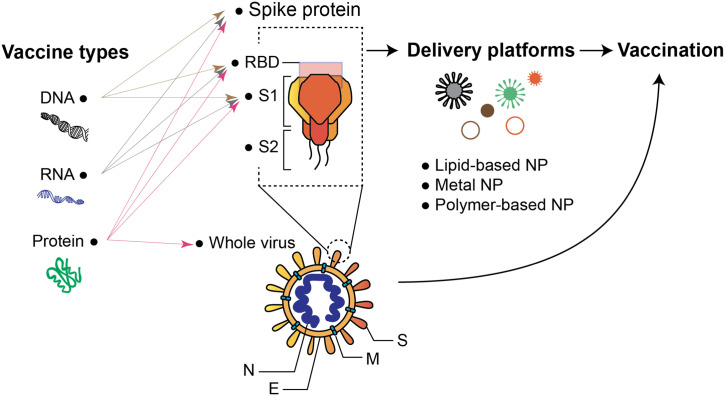
Abstract
The novel corona virus termed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread throughout the globe at a formidable speed, causing tens of millions of cases and more than one million deaths in less than a year of its report in December 2019. Since then, companies and research institutions have raced to develop SARS-CoV-2 vaccines, ranging from conventional viral and protein-based vaccines to those that are more cutting edge, including DNA- and mRNA-based vaccines. Each vaccine exhibits a different potency and duration of efficacy, as determined by the antigen design, adjuvant molecules, vaccine delivery platforms, and immunization method. In this review, we will introduce a few of the leading non-viral vaccines that are under clinical stage development and discuss delivery strategies to improve vaccine efficacy, duration of protection, safety, and mass vaccination.
Graphical abstract
1. Introduction
A novel coronavirus, termed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in Wuhan, China as first reported in December 2019. SARS-CoV-2 resembles SARS-CoV, which was responsible for the 2002 epidemic, and MERS-CoV, which attracted global concern due to its high mortality rate in 2012. Whereas both SARS-CoV and MERS-CoV were managed to be contained, SARS-CoV-2 has spread globally, leading the World Health Organization to declare coronavirus disease 2019 (COVID-19) as a pandemic in March 2020 [1]. As of November 2020, SARS-CoV-2 has caused more than 54 million cases and 1.3 million deaths around the globe. The United States alone has reported more than 11 million cases and suffered >250,000 lives lost since the first reported case in March 2020.
There are worldwide efforts to develop therapies and prophylactic vaccines against SARS-CoV-2. Therapeutics aim to shorten the hospitalization period and increase survival of infected patients, while prophylactic vaccines aim to generate protective immunity against SARS-CoV-2. Given the urgent pandemic setting with its associated consequences, such as limited ventilators and hospital capacity, it is critical to develop successful prophylactic vaccines against SARS-CoV-2. SARS-CoV-2 is a single stranded RNA virus that has a structure of betacorona viruses composed of spike (S) protein, envelope protein, membrane protein, nucleocapsid protein, and accessory proteins. SARS-CoV-2 shares high sequence similarity with SARS-CoV and takes a similar entry route to infect human cells [2]. That is, the receptor binding domain (RBD) of S1 subunit of S protein interacts with human angiotensin-converting enzyme 2 (ACE2), followed by membrane fusion mediated by S2 subunit [3]. Thus, S protein is a critical component of SARS-CoV-2 for cellular infection. Also, functional neutralizing antibodies (NAbs) generated in COVID-19 patients were found to mostly target epitopes within S protein, suggesting S protein as a promising target for vaccination against SARS-CoV-2.
The process of developing a vaccine consists of two key steps: 1) identifying an antigen and 2) developing a delivery approach for said antigen to achieve robust cellular and humoral immunity. In the case of SARS-CoV-2, previous experiences with SARS-CoV and MERS-CoV have enabled rapid development of vaccine candidates. As of November 2020, more than 170 vaccines against SARS-CoV-2 are under development as tracked by the World Health Organization, and of those, 13 are undergoing Phase III human clinical trials. These vaccine candidates can be categorized into four vaccine platforms: viral vector-, RNA-, DNA-, and protein-based vaccines [4] (Fig. 1 ). Notably, as of this writing, BioNTech/Pfizer and Moderna have recently reported that their mRNA vaccines exhibited > 90% protection efficacy based on their first interim analyses of the ongoing Phase III trials [5,6]. BioNTech/Pfizer and Moderna have been granted emergency authorization for their COVID-19 vaccines by he Food and Drug Administration (FDA).
Fig. 1.
Various vaccine platforms for vaccination against SARS-CoV-2.
Whole virus is a conventional vaccine type historically used for major diseases such as smallpox, tuberculosis, and yellow fever. This vaccine type can be split into two major types: live attenuated and live inactivated whole virus. Inactivated viruses are more commonly used due to their inability to induce viral reversion. Spearheaded by researchers in China, Wuhan Institute of Biological Products/Sinopharm and Sinovac Biotech have successfully proceeded to Phase III clinical trials using inactivated whole SARS-CoV-2 [7]. In addition, there is a vaccine based on chimpanzee adenovirus called ChAdOx1 developed by the University of Oxford and AstraZeneca as well as Adenovirus 26 vector-based vaccines of Johnson & Johnson that elicit potent immune responses [8].
In addition to these viral vector-based vaccines, non-viral vaccine platforms, enabled by advances in nanomedicine and vaccine delivery technologies, are in the late stages of clinical trials (Table 1 ). They include mRNA and DNA encoding protein antigens of SARS-CoV-2 as well as protein antigen-based vaccines. The membrane-bound glycoproteins on corona viruses, called spike proteins, are responsible for viral entry into host cells, hence presenting an ideal target for vaccines against SARS-CoV-2 [9,10]. Vaccines based on mRNA and DNA focus on the ways to deliver genetic materials encoding antigen candidates into the host cells, but due to limited cellular uptake and instability of naked mRNA and DNA, they require the use of delivery vehicles or electroporation devices. On the other hand, protein-based vaccines require the synthesis and characterization of protein antigens as the part of vaccine production. Since protein antigens are synthesized by cells and secreted into culture medium in soluble forms, it is often challenging to ensure proper protein folding and maintain their antigenicity. Therefore, recombination techniques are often involved when designing genetic vectors to endow stability to antigen proteins before the vectors are transfected to the target cells. Compared with whole virus-based or viral vector-based vaccines, subunit antigens used in mRNA-, DNA- and protein-based vaccines elicit weaker immune responses, thus requiring co-administration of adjuvants. Adjuvants are immunostimulatory agents added to vaccines to enhance the immune response, and they are usually in the forms of ligands that interact with pattern recognition receptors (PRRs) on antigen-presenting cells (APCs). Once engaged, downstream signaling within APCs triggers various immune pathways that lead to stronger immune activation.
Table 1.
Non-viral COVID19 vaccines currently under development.
| Vaccine type | Developer | Vaccine | Antigen | Formulation/Delivery system | Delivery route | adjuvant | Status | Clinical trial |
|---|---|---|---|---|---|---|---|---|
| Virus-like particles (VLP) | Medicago | CoVLP | Self-assembled VLP from S protein | Recombinant Coronavirus Virus-Like Particle (CoVLP) | IM | AS03 or CpG 1018 | Phase 1 | NCT04450004 |
| Recombinant protein | Novavax | NVX-CoV2373 | Prefusion state full-length S protein | Spontaneous nanoparticle formation | IM | Matrix-M (saponin-based) | Phase 2b | NCT04368988 |
| Clover Biopharmaceuticals | SCB-2019 | S protein trimer | Patented Trimer-Tag technology used for trimer formation | IM | AS03 or CpG 1018 | Phase 1 | NCT04405908 | |
| University of Queensland | UQ-CSL V451 | Prefusion state S protein trimer | "Molecular clamp" used for trimer formation | IM | MF59 (Squalene-based) | Phase 1 | ISRCTN51232965 | |
| University of Pittsburgh | PittCoVacc | S1 subunit protein | Microneedle patch | cutaneous | none | Preclinical | NA | |
| DNA | Inovio Pharmaceuticals/International Vaccine Institute | INO-4800 | S protein | Eectroporation | ID | None | Phase 1/2 | NCT04336410 |
| AnGes, Inc. | AG0301-COVID19 | S protein | Plasmid | IM | None | Phase 1/2 | NCT04447781 | |
| Symvivo | bacTRL-Spike | S protein | Bifidobacterium longum | Oral | None | Phase 1 | NCT04334980 | |
| Genexine Consortium | GX-19 | S protein | Electroporation | IM | None | Phase 1/2 | NCT04445389 | |
| Osaka University/AnGes/ Takara Bio | AG0301-COVID19 | S protein | Plasmid | IM | unknown | Phase 1/2 |
NCT04463472 NCT04527081 |
|
| mRNA | Moderna/NIAID | mRNA-1273 | A full-length, prefusion stabilized spike (S) protein of COVID-19 | Lipid nanoparticle | IM | None | Phase 3 | NCT04470427 |
| BioNTech/Fosun Pharma/Pfizer | BNT-162b2 | Optimized SARS-CoV-2 full-length spike protein or optimized SARS-CoV-2 receptor-binding domain (RBD) | Lipid nanoparticle | IM | None | Phase 3 | NCT04368728 | |
| BNT-162a1 | Phase 1/2 | |||||||
| BNT-162b1 | ||||||||
| BNT-162c2 | ||||||||
| Arcturus/Duke-NUS | ARCT-021 | Self-replicating mRNA encoding the prefusion spike protein of 2019-nCoV | LUNAR® lipid nanoparticle | IM | None | Phase 1/2 | NCT04480957 | |
| People's Liberation Army (PLA) Academy of Military Sciences/Walvax Biotech. | ARCoV | SARS-CoV-2 receptor-binding domain (RBD) | Lipid nanoparticle | IM | None | Phase 1 | ChiCTR2000034112 | |
| Imperial College London | SARS-CoV-2 saRNA LNP | SARS-CoV-2 spike protein | Lipid nanoparticle | IM | None | Phase 1 | ISRCTN17072692 | |
| Curevac AG | CVnCoV | full-length spike protein of SARS-CoV-2 | Lipid nanoparticle | IM | None | Phase 1 | NCT04449276 |
Source: World Health Organization (WHO)
In this review, we focus on the leading non-viral vaccine candidates currently under development against SARS-CoV-2. We discuss the role of their delivery platforms and present factors crucial for the development of successful non-viral vaccines against SARS-CoV-2, including immunogenicity, adjuvants, in vivo delivery, safety, as well as hurdles to overcome for rapid deployment and mass vaccination.
2. mRNA vaccines
2.1. Clinical stage mRNA vaccines
mRNA vaccines are the most advanced COVID-19 vaccines in Phase III clinical trials [[11], [12], [13], [14], [15]]. Although mRNA vaccines are a newly emerging technology without an approved product on the market, mRNA vaccines has a number of advantages over other platforms [16,17]: 1) mRNA vaccines are safe [17]. Compared with DNA-based vaccines, mRNA does not integrate into the host’s genome, thus minimizing the genotoxicity issue. Compared with inactivated virus or live vector-based vaccines, production of synthetic mRNA vaccine is amenable for quality control with reduced chance of biological contamination during production [18]. Moreover, mRNA itself as well as the lipid-based mRNA carriers are biodegradable. 2) mRNA vaccines have demonstrated its efficacy to induce strong humoral and cellular immune response in preclinical [11,19,20] and clinical studies [12,17,21]; 3) mRNA vaccine can be designed and manufactured within a short time scale to meet the need of pandemic outbreak. The well-defined chemistry makes mRNA vaccine suitable for the state of art manufacturing process employed in the pharmaceutical industry. The ability for rapid scale-up production up to billion doses make mRNA vaccines the front-runners in the COVID-19 vaccine development [16,17,22]. However, there are still hurdles for mRNA vaccines to overcome. First, mRNA without a proper formulation is unstable and can be quickly degraded by ubiquitous RNases in vitro and in vivo. Second, mRNA is impermeable to cell membranes and cannot be efficiently internalized into cytosol for translation. Third, mRNA could activate the innate immune system and potentially induce inflammation and toxicity. Fourth, the current mRNA vaccines require a strict cold-chain condition for storage and distribution, thus posing a logistics challenge for deployment in developing countries. To address these problems, it is crucial to design appropriate mRNA delivery technologies that can protect mRNA from degradation, shuttle mRNA to target cell cytosol, and increase mRNA vaccine thermal stability, safety profiles, and efficacy.
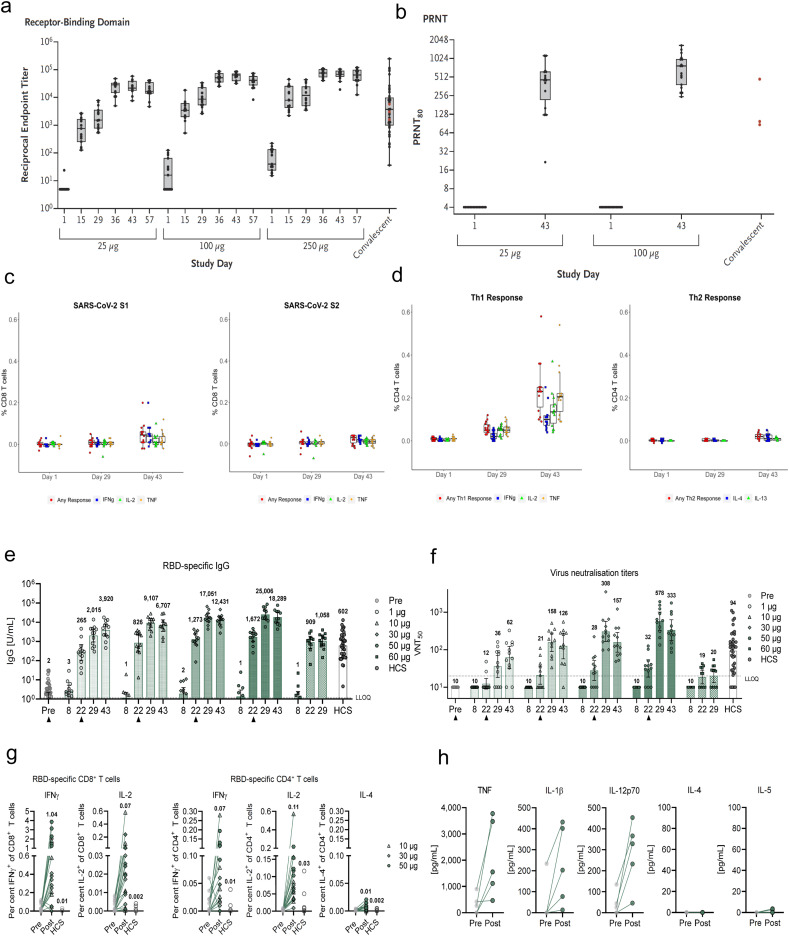
The speed of vaccine development against SARS-CoV-2 has been unprecedented [12,13], and this is well reflected in the progress of mRNA vaccine development against SARS-CoV-2. As early as Jan 13, Moderna and NIH finalized the mRNA-1273 sequence encoding prefusion-stabilized spike protein of SARS-CoV-2 and moved toward clinical manufacturing, only 4 days after WHO announced an unknown pneumonia case found in Wuhan and 2 days after a Chinese team reported the genetic sequence of COVID-19. A short 88 days later, mRNA-1273 doses were administered to the first human clinical trials participants. This rapid pace is in stark contrast to the normal vaccine development process that takes years to reach clinical trials. Due to the speed at which mRNA vaccines progressed, there are now two ongoing Phase III clinical trials on mRNA vaccines from BioNTech and Moderna, and they have recently announced in November 2020 that their vaccines showed > 90% protective efficacy against SARS-CoV-2 infection from their ongoing Phase III trials. In addition, there are many more mRNA vaccines currently in phase I/II clinical trials (Table 1). Based on preclinical and clinical and studies reported so far, mRNA vaccines are well-tolerated and induce efficient immune responses [12,13]. For mRNA-1273 [12], the Phase I clinical trial of 47 participants revealed robust NAb responses after two vaccinations, achieving similar levels of neutralizing activity as observed in convalescent sera of COVID-19 patients. Additionally, Th1-skewed CD4 T cell responses and low level CD8 T cell response to S-2P were observed (Fig. 2a-d). No severe adverse effects were observed, but mild systemic and local adverse effect were noted. Consistent with the human results, mRNA-1273 vaccination of non-human primates induced robust NAb and Th1-skewed CD4 T cell responses, protecting animals from SARS-CoV-2 challenge. For BioNTech’s BTN-162b1 [13], prime-boost vaccinations in Phase I/II clinical trial induced strong NAb responses, which were higher than the levels observed in COVID-19 convalescent human sera. In particular, BTN-162b1 vaccination generated strong RBD-specific CD4+ and CD8+ T cell responses, which may target and lyse infected cells with long term memory (Fig. 2e-h). Similar to Moderna’s mRNA-1273, there were no severe adverse effects but mild side effects were noted for BioNTech’s BTN-162b1. Overall, the early-stage clinical results indicate that mRNA vaccines are well tolerated and induce strong humoral and cellular immune responses against COVID19.
Fig. 2.
Humoral and cellular immune response of mRNA-1273 and BTN-162b1. a-d, humoral immune response (a-b) and cellular immune response (c-d) of mRNA-1273 against SARS-CoV-2. e-h, humoral immune response (e-f) and cellular immune response (g-h) of mRNA-1273 against SARS-CoV-2. Reproduced with permission from [12] (a-d) and [13] (e-h).
2.2. Design considerations for mRNA vaccines
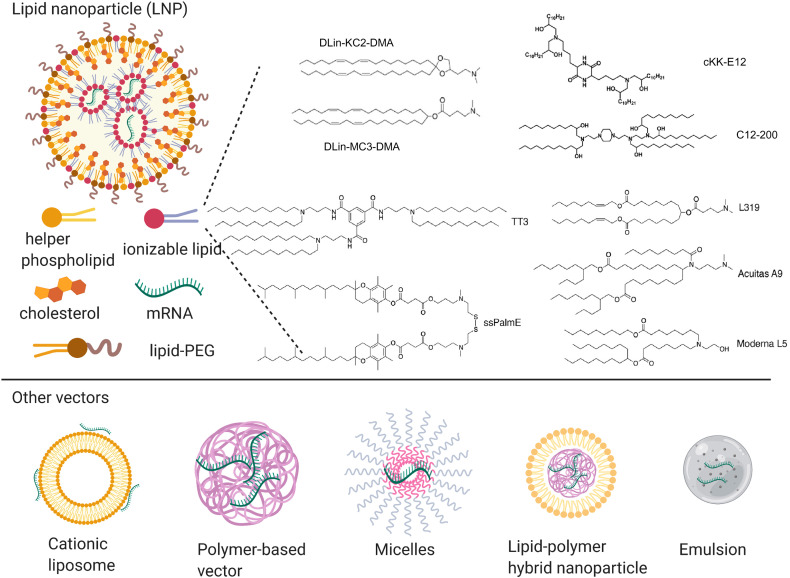
As shown in Table 1, interestingly, all COVID-19 mRNA vaccines under clinical development are delivered by lipid nanoparticles (LNP). LNPs, encapsulating mRNA within a solid lipid structure, are composed of four components [17,23] (Fig. 3 ): cationic or ionizable lipids for mRNA complexation, cholesterol to stabilize the nanoparticle, helper phospholipids to aid the formation and intracellular release, and PEGylated lipids to reduce non-specific interactions. LNPs have the following advantages as non-viral mRNA vectors: 1) LNP efficiently encapsulates and condenses mRNA; 2) LNP promotes intracellular delivery of mRNA to cytosol by increasing cellular uptake and triggering endosomal escape; 3) LNP increases mRNA stability by protecting them from degradation in extracellular spaces; 4) LNP are composed of biocompatible materials suitable for human use; 5) GMP-grade LNP are synthesized in a large scale.
Fig. 3.
mRNA vaccine delivery systems applicable for COVID-19 vaccine development.
To develop an effective LNP vector for delivery of COVID-19 mRNA vaccine, two critical factors should be taken into consideration. The first factor is choosing an appropriate cationic or ionizable lipid [16]. Even though both cationic and ionizable lipids could effectively complex RNA, ionizable lipids may be preferred given their favorable safety profiles. Ionizable lipids are a class of lipid bearing neutral or mild positive charge at physiological pH and expose high cationic groups at acidic conditions. In this setting, the mRNA encoding SARS-CoV-2 antigens could be encapsulated into LNPs at low pH, and LNPs could maintain a neutral surface charge in the extracellular space to reduce non-specific interactions. Once LNPs are internalized into cells, the acidic environment within endosomes could turn the surface charge of LNPs to positive, facilitating endosomal escape and mRNA release in the cytosol. As the critical component of LNPs, great efforts have been devoted to identify optimal ionizable lipids [24,25]. Representative ionizable lipids include: DLin-KC2-DMA [26] and DLin-MC3-DMA [27], which were based on rational design; C12-200 [28] and cKK-E12 [29], which were identified by high throughout screenings of large combinatorial libraries; L319 [30], TT3 [31], ssPalmE [32], Acuitas (A9) [33] and Moderna (L5) [34], which are biodegradable. Given the state of pandemic emergency, repurposing these high-performer ionizable lipids for a COVID-19 mRNA vaccine formulation would be a good option. The second factor is optimizing cholesterol, helper phospholipids, and the lipid-PEG composition along with their relative ratio in LNPs. These factors could greatly affect the efficacy and performance of mRNA vaccines. However, the optimization process for formulations usually involves many more variables and requires extensive resources. To make the optimization process more efficient, Design of Experiment (DOE) methodologies have been applied, including both Fractional Factorial Designs and Definitive Screening [25]. Through these methods, multiple parameters could be tuned simultaneously, such as the lipid ratio and lipid structure, leading them to find that incorporating DOPE and increasing ionizable lipid:mRNA ratio could increase the efficacy of mRNA delivery. Based on this, C12-200-containing LNP was optimized for erythropoietin mRNA delivery and a 7-fold potency improvement was observed. Similar approaches could also be applied for optimizing LNP for COVID-19 vaccine delivery.
Apart from LNPs, cationic liposomes could also be used for mRNA-based vaccine applications, such as widely employed transfection agent, lipofectamine composed of (N-(1-(2,3-dioleyloxy)propyl)-N-(2-(sperminecarboxamido)ethyl)-N,N-dimethylammonium trifluoroacetate (DOSPA) and (DOPE) [35,36] as well as liposomes containing 1,2-di-O-octadecenyl-3-trimethylammonium-propane (DOTMA) and 1,2-dioleoyl-3-trimethylammoniumpropane (DOTAP) [37,38]. In particular, a recent study reported a ready-to-use melanoma mRNA vaccine that requires simple mixing of the RNA component with a cationic liposome suspension prior to injection [39]. The optimized mRNA delivery system activated APCs and encoded various cancer antigens to induce strong antigen-specific T-cell responses, leading to tumor regression. These advances would allow for rapid design and development of ready-to-use mRNA vaccines targeting new emerging pathogens.
Another important category of mRNA delivery system is based on polymer and polymer/lipid hybrid particles. Even though these delivery systems are not as clinically advanced as the lipid-based systems, their functional diversity and flexibility of polymers make them a very attractive candidate for mRNA delivery. For example, a PEI-based polyplex system has been used to deliver self-amplifying replicon RNA (RepRNA) vaccines encoding influenza virus hemagglutinin and nucleocapsid [40]. A dendrimer-mRNA nanoparticle with lipid-PEG coating was used as single dose vaccine against multiple lethal infections, including Ebola virus, H1N1 influenza, and Toxoplasma gondii. Poly(lactic-co-glycolic acid) (PLGA) polymer has also been incorporated into a lipid mRNA delivery system, called TT3-LLNs, for mRNA delivery in human cell lines [41]. In addition to these classic polymers, charge-altering releasable transporters (CARTs) have been designed for mRNA delivery [42]. CARTs is a class of cationic oligo(carbonate-β-α-amino ester) polymers, which complex with mRNA and facilitate intracellular delivery. After mRNA-CARTs complex is internalized, CARTs undergo a self-immolate degradation to release mRNA for protein translation. This design promoted endosomal escape and protein translation both in vitro and in vivo. Similarly, poly(b-amino esters) (PBAEs) have also been extensively used for mRNA delivery. For example, a polymer brush nanoparticle formed by mixing poly(β-amino esters) (PBAEs) with cholesterol, DSPC (1,2-distearoyl-sn-glycero-3-phosphocholine), mPEG2000-DMG (1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000]), and mRNA has been reported for mRNA delivery applications [43]. Co-formulation of PBAEs and lipid-PEG effectively delivered mRNA to the lungs [44], and a core-shell structure formed by encapsulation of PBAE/mRNA complex in a double-layered liposome has been reported for mRNA-based vaccine applications [45].
Other delivery systems, such as micelles and emulsions, have been used for mRNA delivery applications. For example, a nano micelle PEG-PAsp(TEP)-Chol has been used for systemic delivery of mRNA vaccine as a pancreatic cancer treatment [46], and a micelle system based on branched PEI-stearic acid conjugates (PSA) [47] has been used for HIV mRNA vaccine delivery. Emulsions are well-established as potent vaccine adjuvants. Based on MF59 emulsion adjuvant from Novartis, a cationic emulsion (CNE) has been designed by adding DOTAP into the water phase [48]. The positive charge allows electrostatic adsorption of a 9 kb self-amplifying mRNA encoding protein antigens from respiratory syncytial virus (RSV), human cytomegalovirus (hCMV), and human immunodeficiency virus (HIV). In mice, rats, rabbits, and non-human primates, CNE formulated with self-amplifying mRNA vaccine induced effective humoral and T cell responses.
Overall, mRNA vaccine is a powerful technology to fight against a pandemic outbreak, such as COVID-19. Despite the overall optimism on mRNA vaccines against COVID-19, more studies are needed for effective countermeasures against other emerging pathogens and for potential cases of mutations or seasonal reoccurrences of SARS-CoV-2. Ready-to-use standardized mRNA vaccine delivery systems should be optimized to 1) effectively package and protect antigen-encoding mRNA; 2) enhance the vaccine thermal stability, enabling room temperature storage and shipping; 3) have appropriate adjuvant properties, effectively inducing both cellular and humoral immune response against wide range of pathogens; and 4) be able to achieve quality-controlled mass production for global needs with affordable cost.
3. DNA vaccines
3.1. Clinical stage DNA vaccines
DNA vaccines are based on bacterial plasmids that encode vaccine antigens driven by eukaryotic promoters [49]. Unlike protein antigens, the plasmid (DNA vaccine) must enter the nucleus of locally transfected cells, including APCs. Once inside the nucleus, expression of plasmid-encoded genes allows the synthesis of foreign antigens, which are subject to immune surveillance and subsequently initiate humoral and cellular immune responses [50]. The advantages of DNA vaccines are their ease of manufacturing, storage, and safety. Manufacturing of plasmid DNA is rapid, with one batch completed in 2-4 weeks [51]. In terms of storage, plasmid DNA, unlike chromosomal DNA, readily and quickly renatures under many conditions with no loss of biological activity, making it more similar to a small molecule in terms of storage and analysis than a biologic [52]. DNA vaccines have shown no significant adverse effects in clinical trials [50]. Each of these advantages lend to the fact that DNA vaccines can be developed and deployed rapidly. However, DNA vaccines also have potential disadvantages as well. DNA vaccines could activate oncogenes due to incorporation of plasmids into the host genome and also elicit anti-DNA antibodies, thus potentially causing autoimmune responses. Additionally, chronic inflammation may occur due to the vaccine continuously stimulating a humoral immune response or induction of immunologic tolerance to the protein antigen produced. Much research must still be done to further explore the safety concerns, which leads to the last issue – DNA vaccines are still new and there are not any FDA-approved DNA vaccines for human use. Human clinical trials will likely be the best way to assess and understand DNA vaccine safety, making the current COVID vaccine trials a major step forward. Moreover, another major challenge for DNA vaccines is that DNA vaccines in general have induced limited immunogenicity in non-human primates and humans, compared with results observed in mice [50]. An avenue of research focused on solutions to this challenge includes addition of immune modulatory adjuvants and delivery strategies. Co-delivery of DNA plasmids and plasmids encoding cytokines, chemokines, or co-stimulatory molecules has had a positive effect on immune response [53,54]. Nanocarriers are able to efficiently package the DNA plasmids, shield it from serum protein aggregation, and prevent endo/lysosomal degradation [55]. Applications of jet injectors and bacterial delivery are generally safe and easy to administer. Following, we will look at COVID-19 vaccine candidates that address these concerns.
Some of the earliest examples of DNA vaccines were those against influenza [56,57], and the first Phase I clinical trial with a DNA vaccine was for a HIV-1 vaccine [58]. Since then, there have been numerous clinical trials utilizing DNA vaccines against cancer, cardiovascular, infectious, neurological, and ocular diseases and conditions [50], highlighting their versatility. In response to the COVID-19 pandemic, DNA vaccines are explored as one of the primary vaccine technologies. A series of DNA vaccine candidates expressing six different forms of the SARS-CoV-2 Spike (S) protein has been evaluated in 35 rhesus macaques [59]. Animals received 5 mg DNA vaccines by intramuscular route at week 0, 3, and 5. Despite variations among the different candidates, vaccinated macaques induced NAb titers comparable in magnitude to cohorts of SARS-CoV-2 convalescent macaques and humans, and vaccinated animals also exhibited S-specific and RBD-specific antibodies of diverse subclasses and effector functions and Th1-biased cellular immune responses [59]. After the macaques were challenged with SARS-CoV-2 intranasally and intratracheally, viral load was reduced by >3.1 and >3.7 log10 in the bronchoalveolar lavage and nasal mucosa, respectively. Not only does this work demonstrate DNA vaccine protection in non-human primates, but it defines NAb titers as an immune correlate of protection. Additionally, Phase I clinical studies were conducted using plasmids encoding the S proteins from SARS-CoV-1, which were well-tolerated and produced cellular and NAb responses in healthy adults [60], providing more evidence of potential efficacy in humans.
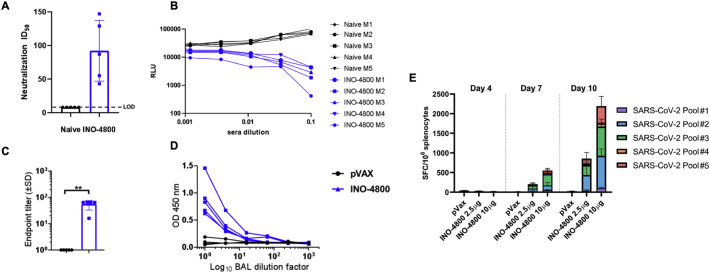
Multiple companies and research development groups, including Inovio Pharmaceuticals, Symvivo, AnGes, Genexine, and Zydus Cadila, have taken this next step by developing and testing DNA-based vaccine candidates in clinical trials. Inovio Pharmaceuticals developed a vaccine and delivery device targeting the SARS-CoV-2 S protein (INO-4800) (Fig. 4 ) [61]. Inovio leveraged prior experiences in developing a MERS-CoV vaccine (INO-4700), which shares a global protein fold structure with SARS-CoV-2 [61]. Subjects treated with INO-4700 in Phase I/IIa clinical trials exhibited durable NAbs, T cell immune responses, and a seroconversion rate of 96% [62]. Both vaccine candidates use the company’s proprietary CELLECTRA devices for delivery of DNA directly into the skin. Unlike other DNA and nucleic acid approaches, CELLECTRA overcomes a key limitation by using a brief electrical pulse to reversibly open small pores in cells that allow plasmids to enter and produce the targeted antigen. Additionally, the CELLECTRA 3PSP is a small, portable, and hand-held device that can manufactured and stockpiled in large quantities with minimal maintenance, characteristics that are desirable in a pandemic setting [63]. Pre-clinical studies in INO-4800-immunized mice and guinea pigs showed that anti-SARS-CoV-2 binding Abs blocked ACE2 binding, the primary receptor for SARS-CoV-2 cellular entry. Animals elicited Abs in the lungs after a single intradermal immunization, preventing viral replication in the lungs when challenged with SARS-CoV-2 [64]. In the Phase I clinical trial with INO-4800, 40 healthy adults aged 18 – 50 received two 1 mg or 2 mg doses four weeks apart. INO-4800 vaccine induced balanced humoral and cellular responses, with 94% of participants exhibiting immunological responses based on humoral (binding and neutralizing) and T cell immune responses [65]. Based on these positive data and no seriously adverse events, Phase I trials have expanded to include older participants, and a Phase II/III efficacy trial is planned to initiate upon FDA regulatory clearance.
Fig. 4.
Inovio Pharmaceutical’s INO-4800 DNA vaccine elicits immune response in BALB/c mice. (A) Neutralization ID50 in naïve and INO-4800 immunized mice and (B) relative luminescence units (RLU) for sera from naïve and vaccinated mice. (C) Bronchoalveolar lavage fluid assayed for SARS-CoV-2 Spike protein-specific IgG antibodies by ELISA. (D) BAL dilution curves with raw OD 450 values. (E) T cell responses measured by IFN-γ ELISpot in splenocytes with overlapping peptide pools spanning the SARS-CoV-2 Spike protein. Reproduced with permission from [61].
Other DNA-based vaccine candidates are being explored, some of which have interesting delivery strategies. Oral vaccine delivery is attractive because it results in improved safety, patient compliance, and reduced costs [66,67], and the gastrointestinal tract with over 300 m2 of mucosal surface could serve as an immune inductive organ [66,68]. For example, Symvivo has developed a bacTRL-Spike vaccine candidate, which is a lyophilized gel capsule of genetically modified probiotic bacteria (Bifidobacteria) that can colonize the gut, bind directly to intestinal epithelial cells, and constitutively replicate, secrete, and deliver plasmid DNA molecules encoding the SARS-CoV-2 S protein [69]. Because the vaccine is a living medicine, the gene delivery rates are sustained throughout the life of the bacterial colony, resulting in extensive gene expression throughout the epithelial lining [69,70]. Oral vaccine is highly advantages for mass vaccination, and this platform is one of two orally administered COVID-19 vaccine candidates currently in human clinical trials. Another DNA vaccine candidate with a delivery strategy without needle is Genexine’s GX-19, expected to start a Phase IIa clinical trial soon [71]. GX-19 is delivered by a PharmaJet’s jet injector, which uses a high-pressure, narrow stream of fluid to penetrate the skin instead of a needle [72]. Pharmjet technology has been shown to be safe and effective with commercial vaccines, including influenza, MMR, HPV, and polio vaccines [73]. Furthermore, delivery of DNA-based swine influenza vaccine using PharmaJet Tropis Needle-free Jet Injector resulted in comparable immune responses as vaccine administered via needle and syringe, followed by electroporation [74]. More classic examples of DNA vaccine candidates include those from AnGes and Zydus that use DNA plasmids encoding the SARS-CoV-2 S and membrane proteins, respectively [[75], [76], [77]]. AnGes vaccine is given intramuscularly and currently in Phase I/II clinical trials. Zydus’ vaccine is given intradermally and currently in a Phase II clinical trial and likely entering Phase III clinical trials by late 2020 or early 2021.
3.2. Alternative strategies for DNA vaccine delivery
There are additional DNA delivery strategies in pre-clinical stages that are applicable to COVID-19. One method to enhance efficacy in vivo is through the addition of adjuvants or adjuvant-encoded plasmids. When co-delivered with antigen-encoded plasmids, adjuvants are secreted into the surrounding region, where they are able to stimulate both local APCs and cells in draining lymph nodes (LNs), resulting in stronger and more durable immune responses [53,[78], [79], [80], [81]]. Another method is to apply microneedles (MN) transcutaneously, which allows the DNA vaccine to be deposited at the immune-cell-rich epidermis and dermis [66,82,83]. Compared with the soluble DNA vaccine, MN vaccine elicited 3-fold greater frequencies of antigen-specific IgG1 serum antibodies, 3-fold excess cytotoxic CD8 T cells, and inhibited melanoma lung metastasis [83]. Alternatively, oral delivery of DNA vaccine has been examined in preclinical studies. Unlike Symvivo’s bacTRL-Spike vaccine, M1 DNA plasmids encoding the matrix protein in H1N1 virus were encapsulated in cationic liposomes and orally administered, leading to significantly increased IgG titers, T cell activity, and immunological memory [66]. When challenged intranasally with homologous influenza A virus, viral levels were below the detectable level in the lungs of mice immunized with the oral M1 pDNA liposomes, indicating protection against respiratory challenge [66]. Given respiratory SARS-CoV-2 infection, vaccine candidates that offer enhanced respiratory protection are of particular interest.
Taken together, DNA vaccines are a promising vaccine strategy against SARS-CoV-2. They have a simple design, facile and rapid manufacturing process, and they are cost-efficient and stable with a long shelf life. The primary disadvantage with DNA vaccines is their relatively weaker immunogenicity, compared with other vaccine platforms, but more studies are being conducted to improve this aspect through various delivery strategies. While there is no approved DNA-based vaccine on the market, DNA vaccines may prove to be an effective platform for vaccination against SARS-CoV-2.
4. Other vaccine platforms
4.1. Protein vaccines
Compared with DNA and RNA-based vaccines, which only require the genetic sequence of the virus, protein-based vaccines require other technical and purification steps. In addition, protein vaccines require multiple dosing regimens with adjuvants to achieve strong immune responses. Despite these hurdles, there are many protein-based vaccines processes before the antigen is prepared, such as in vitro cell-based protein expression system against SARS-CoV-2 currently under Phase I, II, and III clinical trials [84,85].
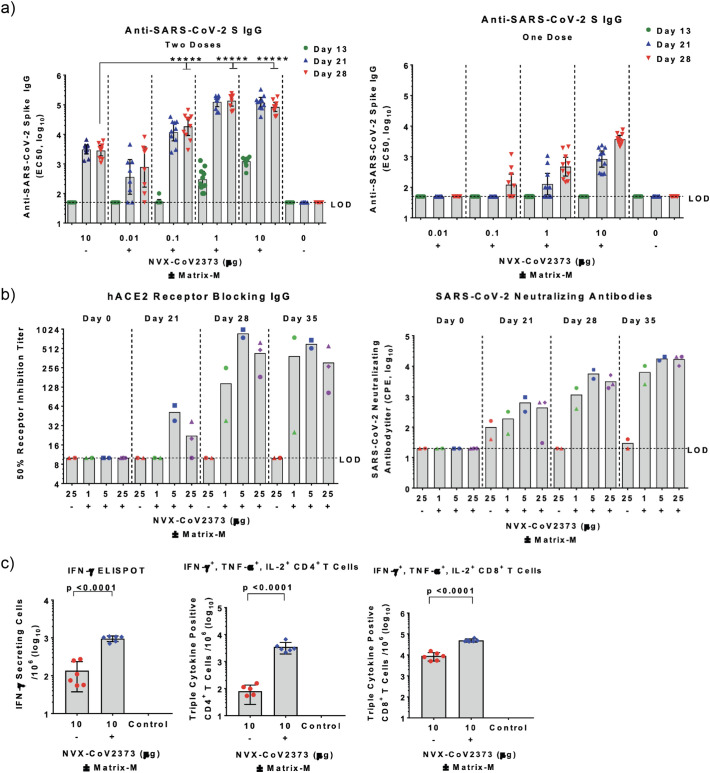
Novavax is one of the leading vaccine companies in a race against SARS-CoV-2. Novavax’s vaccine employs a full-length S protein (NVX-CoV2373) genetically modified for greater structural stability compared with the wild-type (WT) S protein. The S protein variant used in the study had a smaller hydrodynamic size of 27.2 nm as well as a more uniform size, mass, and shape distribution (polydispersity index of 0.25-0.29) compared to the WT size of 69.53 nm and PDI of 0.46, despite the two having the same molecular weight [86,87]. Additionally, mutations generated in the genetic sequence are thought to be crucial for reliable production of a stable pre-fusion S protein structure and ability to withstand stress from pH and temperature post in vivo administration, all contributing to a more effective immune response. NVX- CoV2373 is formulated with Novavax’s proprietary Matrix M, an adjuvant composed of lipid molecules and saponin [88]. Preclinical studies performed with NVX- CoV2373 in mice and baboons as well as a Phase I/II human clinical trial showed elevated anti-S protein IgG (anti-S IgG) titers with potent neutralizing activity (Fig. 5a) [86,89]. The Phase I/II clinical trial showed the vaccine was well tolerated in healthy adults between the ages of 18 and 59 when used in a two-dose regimen of 5 μg and 25 μg of S protein and the Matrix M adjuvant, respectively. Of note, co-injection with the adjuvant significantly improved vaccine efficacy, allowing for potential dose-sparing, which is a crucial advantage for mass vaccination in the future. Serum antibodies blocked the binding between hACE2 and S protein and were also able to neutralize the virus (Fig. 5b). In addition to the humoral response, strong cellular responses were observed, as shown by ELISPOT and intracellular staining assays performed in mice (Fig. 5c).
Fig. 5.
Humoral and cellular immune responses observed in mice and baboons after vaccination with NVX-CoV2373 plus Matrix-M. (a) Two-dose regimen of NVX-CoV2373 (left) elicited stronger serum IgG response in mice, compared with single-dose regimen (right). Addition of Matrix-M further amplified the responses. (b) Vaccination study in baboons indicated generation of neutralizing serum IgG that blocked interaction between ACE2 and S protein (left) and viral infection to Vero E6 cells in vitro (right). (c) Cellular immune responses examined by ELISPOT assay (left) and intracellular staining assay (right). Reproduced with permission from [86].
In addition to Novavax, Clover Biopharmaceuticals, based in China, focuses on the trimeric structure of native S proteins on SARS-CoV-2. Clover Biopharmaceuticals is using their proprietary Trimer-Tag technology to link three S proteins into a conformation that mimics the native S protein trimer. Using this trimeric S protein in combination with either AS03 or CpG 1018 plus Alum as adjuvants, the company recently started a Phase I clinical trial (NCT04405908).
In addition to the aforementioned vaccines in clinical trials, there are other studies currently in the pre-clinical trial phase. A study that used microneedle array (MNA) for delivery of MERS-CoV and SARS-CoV-2 subunit proteins is one of them [90]. The MNA was synthesized with carboxymethyl cellulose (CMC), which is designed to dissolve intracutaneously after applying to the skin, thus releasing the protein content. When tested in mice, both MERS-CoV and SARS-CoV-2 MNA vaccines were able to induce significant levels of serum IgG. Antibodies induced by the MERS-CoV vaccine were able to neutralize the virus in vitro, indicating the effectiveness of the MNA for S protein-based vaccine delivery. Following these promising preclinical studies, the University of Pittsburgh is developing a SARS-CoV-2 vaccine (PittCoVacc) to be tested for clinical trials.
While administering soluble proteins in combination with adjuvant(s) has shown efficacy in vivo, further improvements can be made through the use of nanotechnology. Nanotechnology can increase stability of soluble proteins, while achieving targeted delivery to LNs for improved immunogenicity. In a pandemic setting, improving targeted delivery can translate to reducing the amount of antigen in a dose, which is advantageous when trying to maximize vaccine distribution. Similar to soluble proteins, co-delivery with adjuvants further improves nanoparticle-based vaccine efficacy. For example, a multi-layered lipid-based nanoparticle system was used to co-deliver protein antigen and an adjuvant monophosphoryl lipid A (MPLA), a Toll-like receptor-4 agonist used in other FDA-approved vaccines [91]. Compared with the soluble formulation, the nanoparticle vaccine elicited significantly more potent humoral and cellular immune responses upon subcutaneous injection in preclinical studies. Along the same vein, calcium phosphate (CaP)-based nanoparticles were used to deliver CpG and a viral antigen derived from the influenza A virus hemagglutinin, which prevented viral infection in mice [92]. When the vaccine was administered via intraperitoneal or intranasal routes, the nanoparticles were efficiently taken up by DCs, subsequently leading to T cell-mediated immune responses. Another study used the CaP nanoparticles as a tumor antigen/CpG carrier to treat colorectal cancer in a murine model [93]. The vaccine induced a type-I interferon mediated immune response that increased the frequency of model tumor antigen-specific CD8+ T cells and exerted greater tumor control, compared with the soluble formulation. These examples indicate the versatility of nanoparticle systems as vaccine delivery platforms and show their potential for vaccination against other emerging pathogens.
4.2. Virus-like particle (VLP) vaccines
As discussed, live-attenuated or inactivated viruses elicit effective immune activation, but due to potential viral reversion and batch-to-batch variation, an alternative technology is being pursued to mimic viral structures. Virus-like particles (VLPs) can be designed to express the surface proteins or nucleic acid sequences of the native virus without the risk of replication or infection. Although VLPs can be categorized as a recombinant protein vaccine, they generally maintain the native conformation of viral proteins, which is advantageous over other subunit proteins vaccines in terms of antigenicity and immunogenicity [94]. Additionally, depending on how the particle is designed, it can potentially carry other types of immunopotentiators to enhance the immune response.
Medicago, a Canadian pharmaceutical company, generated VLP using a plant-based method where a synthetic gene containing a part of SARS-CoV-2 genes was transfected to a species of tobacco using a bacterial vector. These plants then express VLPs that can be purified through multiple processing steps. The company has started a Phase I clinical trial (NCT04450004), testing intramuscular injection of Corona virus-like particles (CoVLP) in combination with either CpG 1018 or AS03 adjuvants. The company previously used a similar technology to synthesize VLPs for influenza vaccines. The VLPs had oblate spheroidal structure with sizes of ~75 nm, which showed strong humoral and cellular immune responses in murine models as well as in Phase II and III clinical trials [95]. One of the Phase III clinical trials tested efficacy of their influenza vaccine on participants above the age of 65 (NCT03739112) and demonstrated the effectiveness of the VLP-based vaccine system in an age group that is more vulnerable to COVID-19 [96,97].
4.3. Peptide-based vaccines
Another strategy is to use peptides as the immunogen, which have a relatively simple and stable structure compared to proteins. However, peptides often suffer from suboptimal immune activation due to the short length of amino acid sequences providing epitopes to the immune cells. This limitation can be improved by identifying the immunodominant region of an antigen, e.g. the subunit or receptor binding domain of S protein, followed by generation of peptides from those regions. Generally, the antigen amino acid sequence is segmented into multiple short sequences, and each segment is investigated for its immunogenicity. This technique has been applied to investigate the reactivity of antibodies generated against segments of SARS-CoV S and nucleocapsid proteins, providing significant information about the regional immunodominance of each protein [98]. A similar study was undergone recently to examine the regional immunogenicity of SARS-CoV-2 S protein [99]. Also, there have been attempts to design peptide-based multi-epitope vaccines through in silico approaches. Candidate epitopes of the SARS-CoV-2 subunit proteins are screened by computation which allows prediction of their stability, interactions with major histocompatibility complex (MHC) molecules, and immunogenicity [[100], [101], [102]]. Although these studies require the actual synthesis and testing of the vaccines to validate their efficacies, they suggest a possible alternative to larger-sized protein vaccines, which may provide better safety and specificity.
5. Future outlook on vaccine delivery systems
5.1. Adjuvant delivery systems
Adjuvants trigger PRRs on adaptive immune cells, and depending on which type of PRR an adjuvant aims to activate, different routes of immune responses are elicited. Therefore, the use of a potent and well-matched adjuvant can greatly enhance the vaccine efficacy. Currently, many COVID-19 vaccine developers employ adjuvants in their vaccines, including AS03 (GSK’s α-tocopherol and squalene in an oil-in-water emulsion), CpG 1018 (Dynavax’s DNA-based TLR-9 agonist), and MF59 (Novartis’s squalene in an oil-in-water emulsion), which have shown to greatly improve the vaccine efficacy in preclinical and clinical studies [[103], [104], [105]].
For mRNA vaccines, mRNA itself could bind to some pattern recognition receptors (PRRs), such as TLR 3, 7, 8, RIG-I, PKR, OAS, and MDA5, which in turn induce innate immune activation, type-I interferon, and proinflammatory cytokine production, providing adjuvant effects. However, activation of these receptors could trigger natural antiviral mechanism to inhibit exogenous mRNA translation via phosphorylation of eiF2α and overexpression of RNase L. Thus, there is a balance between for mRNA-induced innate immune activation and mRNA translation. Alternatively, the delivery system could be tuned as adjuvant or additional adjuvant could be co-delivered. For example, a combinatorial library of lipidoids has been screened for the lipid component of LNP that could induce both effective antigen expression and appropriate innate stimulation for mRNA vaccine. After screening of over 1000 lipid formulations, the authors identified a class of top-performing lipids with an unsaturated lipid tail, a dihydroimidazole linker, and cyclic amine head groups. These lipids induced DC activation via the stimulator of interferon genes (STING) pathway, independent of TLR or RIG pathway, and elicited robust antigen-specific T cell responses with a therapeutic effect in murine tumor models.
DNA vaccines also have a potential to induce innate immune activation since many DNA plasmids produced in bacteria may contain unmethylated CpG motifs [106]. In fact, CpG motifs had been intentionally added to the DNA plasmid backbones as adjuvants to enhance the vaccine efficacy. It has been shown that inclusion of CpG greatly improves the antigen-specific T cell-mediated immune response, which protected mice from challenge with mouse melanoma cells [107]. On the other hand, similar to mRNA, there are DNA vaccine delivery systems that have immunostimulatory properties. Vaxfectin is one of these, which is a cationic lipid-based delivery platform. It has been reported that intramuscular delivery of DNA vaccine via Vaxfectin enhanced antigen-specific IgG1 and IgG2a responses in mice [108]. A mechanistic study performed in another study revealed modulation of genes in mice after intramuscular injection of DNA vaccine with Vaxfectin, resulting in significant enrichment of transcripts related to antigen processing, presentation, and the TLR-pathway within the muscle cells [109], thus explaining the adjuvanticity of Vaxfectin.
In addition, there are many adjuvants under development with a great potential to improve protein-based vaccines. A synthetic TLR-7/8 agonist, 3M-052, has been used as an adjuvant together with HIV-1 clade C 1086.C–derived gp140 envelope protein (Env) for vaccination against HIV-1 [110]. Once vaccinated to rhesus macaques, Env plus 3M-052 induced higher levels of antibody response and long-lived plasma cells in bone marrow, compared with vaccination with conventional adjuvants, such as alum, R848 (a TLR-7/8 agonist), MPL, and GLA (a TLR-4 agonists). In fact, many studies are currently examining ways to deliver TLR7/8 agonists, based on their potential to boost immune responses from immunologically vulnerable populations, including children and elderly [111].
5.2. Routes of vaccination
Although COVID-19 vaccines under development are mainly using intramuscular (IM) injection (Table 1), other vaccination routes should be considered, including intradermal (ID), subcutaneous (SC), intranasal (IN), and intravenous (IV) injection.
There are only a few reports that have compared different routes for mRNA vaccination. In terms of protein expression, LNP-mediated delivery of firefly luciferase mRNA was examined in mice after injection via 6 different routes [112]. Interestingly, at a dose of 5 μg mRNA/injection, whereas IV injection produced highest total amount of protein, ID and IM injection prolonged the duration of protein expression for up to 10 days, and ID injection worked better at a low dose of mRNA (0.1 μg/dose). This study also reported that intratracheal (IT) administration induced a high level of protein expression in the lungs, which are the target organ of SARS CoV-2 infection. An IT vaccine and similarly an intranasal vaccine would likely provide better immune protection in the lungs. Recently, Ugar Sahin, et al. reported the first-in-human data of an intravenously administered liposomal mRNA (RNA-LPX) vaccine (BNT111) for melanoma treatment [113]. A clinical response with strong and durable antigen-specific CD8+ T cell and CD4+ T cell responses were observed. These findings could shed new insights into mRNA vaccine delivery via various routes.
There have been many studies that investigated the effects of delivery routes on the efficacy of DNA vaccines. When several invasive or non-invasive administration routes were tested by delivering a hepatitis B surface antigen-encoding DNA plasmid to mice and non-human primates, it was found that the administration routes had a great influence on the potency and nature of the immune responses [114]. For DNA vaccines that are under clinical trials, IM or ID injections, followed by electroporation are the most commonly used delivery methods [106]. However, there are reports indicating different magnitude and duration of immune responses between the two administration routes. In a murine study, ID injection of EGFP-encoding DNA elicited higher but only transient antibody and cytotoxic CD8 T cell responses to EGFP, whereas IM injection induced more weaker yet more durable immune responses up to 5 weeks after the last vaccination [115]. Also, when nanoparticle delivery technologies are applied to DNA vaccine, various vaccination routes can be employed, including IM, ID, IV, and SC [116,117].
One of the reasons mRNA and DNA vaccines are often delivered via IM route is due to prolonged protein expression by muscle cells, compared with transient expression in other cell types targeted after injection via different routes [118,119]. However, proteins bypass the need for such consideration since they are readily processed by APCs without the need to be transcribed and translated. Therefore, for protein-based vaccines, various administration routes other than the traditional SC and IM routes could offer additional advantages. For example, when an influenza vaccine was administered via IM or IN route, both achieved increases in antibody secreting cells in secondary lymphoid organs, whereas IgA production in the mucosal tissues and localization in the lungs were only prominent for IN vaccination [120]. Also, when non-ionic surfactant vesicles incorporating bile salts (bilosome) and influenza A antigen were delivered through oral administration, Th1 or Th2 immune responses were induced depending on the size of the vesicles [121,122]. Since the mucosal tissues are where most infections and transmissions occur, these studies highlight the advantage of protein-based vaccines for mucosal route of vaccination and induction of mucosal immunity.
5.3. Thermal stability
Another active area of mRNA formulation is to increase the thermal stability of mRNA vaccines, so as to allow for storage and shipping in less restricted conditions. For long-term storage and transportation, the current mRNA vaccines from BioTech/Pfizer and Moderna in Phase III trials require -80°C and -20°C, respectively, and this cold-chain requirement greatly limits their distribution and increases the cost. However, prior research has shown that mRNA vaccines can be stored in an unfrozen form. After freeze-drying, a naked mRNA vaccine was reported to be stable for as long as 10 months in 4°C storage [123]. Using an appropriate delivery system, this can be further improved. The lyophilized RNA platform from CureVac can be stored for 3 years at 5-25°C and 6 months at 40°C [124]. Recently, a thermally stable COVID-19 mRNA vaccine, ARCoV, has been reported to induce promising humoral and cellular immune responses against SARS-CoV-2 in mice and non-human primates [14]. This LNP mRNA vaccine formulation could be stored for one week at room temperature. Even though unfrozen mRNA vaccines are not ready for the clinical use at this time for COVID-19, future studies on mRNA protection by the use of delivery systems or co-delivery with RNase inhibitors may make this possible.
DNA vaccines are considered to be relatively more thermally stable than mRNA. Lyophilized DNA are mostly stable at room temperature, and as mentioned above, they maintain their original structure after renaturation before use [52]. A DNA vaccine encoding Ebola glycoprotein tested in a clinical trial was shown to be temperature stable, demonstrating the relative ease of distributing DNA-based vaccines to the parts of the world that do not have the equipment to maintain the low temperatures required [125].
In contrast, protein-based vaccines are vulnerable to structural deformation or degradation by both temperature fluctuations and storage in ambient temperatures. Most FDA-approved protein-based vaccines, in solution or lyophilized form, are recommended for storage at 2-8°C or at sub-zero temperature [126]. In an attempt to prevent thermal denaturation of antigen proteins, a tuberculosis antigen (Ag85b) was encapsulated in a silica cage resulting in significant increases in antigen stability outside the refrigerated or lyophilized conditions [127]. In another study, a thin-film platform was used to preserve live virus, bacteria, and enzymes in room temperature for a long term, ranging from 30 days to 3 years [128].
Since cold-chain shipping of vaccines is going to be a major challenge for world-wide deployment of COVID-19 vaccines, solving the thermal stability issues of vaccines with the goal of room temperature storage is a crucial factor to consider in COVID-19 vaccine development.
5.4. Lymph node targeted delivery of vaccines
Lymph nodes are critical target tissues for vaccine delivery. For cellular immune response, T cells are primed by DCs and activated in LNs. For humoral immune response, germinal centers in the LNs are crucial for antibody affinity maturation and isotype switching. Therefore, targeting LNs may greatly improve immune responses.
To this end, mRNA and DNA vaccines could be directly injected into LNs or delivered by nanoparticles of an appropriate size, surface properties, and charge for effective LN draining. Direct intra LN injection requires an ultrasound imaging guide and has shown promising results in clinical trials [129]. Similarly, when DNA vaccines were directly injected into peripheral LNs, immune responses were significantly greater, offering improved protection of mice against tumor challenge, compared with vaccines delivered via conventional routes such as IM and ID [130]. For LN draining of nanoparticles, when different sizes of PLGA-PEG were examined, 30 nm PLGA-PEG was found to be more efficient for LN draining, retention, and APC uptake [131]. Interestingly, for liposomal vaccines, smaller than 150 nm in diameter could drain to LN and trigger immune responses [132], suggesting that the ideal size for LN targeted delivery depends on the vaccine platforms employed.
There are also studies of LN targeted delivery of peptide or protein vaccines. Use of an amphipathic component to deliver peptides or adjuvants through interaction with albumin post-injection followed by LN draining has proven to significantly enhance vaccine efficacies [133]. Elicio Therapeutics has recently tested amphiphile-bound CpG admixed with SARS-CoV-2 RBD protein to vaccinate mice, which resulted in 25-fold higher antigen-specific T cell responses and Th1 favored antibody responses (IgG2bc and IgG3) [134]. In another study, hydroxy-poly(ethylene glycol)-based cylindrical-shaped nanoparticles were used to deliver a model antigen, ovalbumin, which facilitated antigen draining to LN upon SC injection in mice [135]. These studies demonstrate the benefits of using delivery platforms to improve both LN targeted vaccine delivery and efficacy.
6. Conclusions
Previous experience with similar diseases caused by SARS-CoV and MERS-CoV have laid the groundwork for expedited SARS-CoV-2 vaccine development. Our current global situation requires an urgent need for rapid development of vaccines while adhering to strict guidelines on the safety of SARS-CoV-2 vaccines. While there is strong optimism for late stage vaccine candidates and imminent regulatory approval, the field should be prepared for potential mutation of SARS-CoV-2 as well as its seasonal recurrences. In addition, we should develop effective countermeasures against other emerging pathogens. In particular, the structural fragility and suboptimal immunogenicity of many vaccine candidates should be addressed. In terms of stability, this is especially true when considering the worldwide distribution, cold-chain, and transportation of vaccines, which may increase the cost and limit the distribution. Finally, part of what has made some of these more cutting-edge vaccine types possible are the strides made over the last two decades related to nanoparticle-mediated vaccine delivery. Future studies should be focused on streamlining nanoparticle vaccine delivery systems so that the final vaccine products can be readily produced and formulated. Physicochemical properties of vaccine formulations, especially during long-term storage and transportation should be optimized for rapid deployment and mass vaccination across the globe.
Declaration of Competing Interest
Authors declare no conflict of interest.
Acknowledgments
Acknowledgement
This work was supported in part by NIH (R01AI127070, R01CA210273, R01DK125087, and U01CA210152). J.J.M. is supported by NSF CAREER Award (1553831). K.S.P. acknowledges financial support from the UM TEAM Training Program (DE007057 from NIDCR).
References
- 1.Lau H., Khosrawipour V., Kocbach P., Mikolajczyk A., Ichii H., Zacharski M., Bania J., Khosrawipour T. The association between international and domestic air traffic and the coronavirus (COVID-19) outbreak. J. Microbiol. Immunol. Infect. 2020;53:467–472. doi: 10.1016/j.jmii.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94 doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang T., Bidon M., Jaimes J.A., Whittaker G.R., Daniel S. Coronavirus membrane fusion mechanism offers a potential target for antiviral development. Antivir. Res. 2020;178:104792. doi: 10.1016/j.antiviral.2020.104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Riel D., de Wit E. Next-generation vaccine platforms for COVID-19. Nat. Mater. 2020;19:810–812. doi: 10.1038/s41563-020-0746-0. [DOI] [PubMed] [Google Scholar]
- 5.BioNTech Press release: Pfizer and BioNTech Announce Vaccine Candidate Against COVID-19 Achieved Success in First Interim Analysis from Phase 3 Study. 2020. https://investors.biontech.de/news-releases/news-release-details/pfizer-and-biontech-announce-vaccine-candidate-against-covid-19
- 6.Moderna Press release: Moderna’s COVID-19 Vaccine Candidate Meets its Primary Efficacy Endpoint in the First Interim Analysis of the Phase 3 COVE Study. 2020. https://investors.modernatx.com/news-releases/news-release-details/modernas-covid-19-vaccine-candidate-meets-its-primary-efficacy
- 7.Koirala A., Joo Y.J., Khatami A., Chiu C., Britton P.N. Vaccines for COVID-19: The current state of play. Paediatr. Respir. Rev. 2020;35:43–49. doi: 10.1016/j.prrv.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le T.T., Andreadakis Z., Kumar A., Roman R.G., Tollefsen S., Saville M., Mayhew S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020;19:305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 9.Du L., He Y., Zhou Y., Liu S., Zheng B.J., Jiang S. The spike protein of SARS-CoV--a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009;7:226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292.e286. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbett K.S., Flynn B., Foulds K.E., Francica J.R., Boyoglu-Barnum S., Werner A.P., Flach B., O'Connell S., Bock K.W., Minai M., Nagata B.M., Andersen H., Martinez D.R., Noe A.T., Douek N., Donaldson M.M., Nji N.N., Alvarado G.S., Edwards D.K., Flebbe D.R., Lamb E., Doria-Rose N.A., Lin B.C., Louder M.K., O'Dell S., Schmidt S.D., Phung E., Chang L.A., Yap C., Todd J.M., Pessaint L., Van Ry A., Browne S., Greenhouse J., Putman-Taylor T., Strasbaugh A., Campbell T.A., Cook A., Dodson A., Steingrebe K., Shi W., Zhang Y., Abiona O.M., Wang L., Pegu A., Yang E.S., Leung K., Zhou T., Teng I.T., Widge A., Gordon I., Novik L., Gillespie R.A., Loomis R.J., Moliva J.I., Stewart-Jones G., Himansu S., Kong W.P., Nason M.C., Morabito K.M., Ruckwardt T.J., Ledgerwood J.E., Gaudinski M.R., Kwong P.D., Mascola J.R., Carfi A., Lewis M.G., Baric R.S., McDermott A., Moore I.N., Sullivan N.J., Roederer M., Seder R.A., Graham B.S. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in Nonhuman Primates. N. Engl. J. Med. 2020;383:1544–1555. doi: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson Lisa A., Anderson Evan J., Rouphael Nadine G., Roberts Paul C., Makhene Mamodikoe, Coler Rhea N., McCullough Michele P., Chappell James D., Denison Mark R., Stevens Laura J., Pruijssers Andrea J., McDermott Adrian, Flach Britta, Doria-Rose Nicole A., Corbett Kizzmekia S., Morabito Kaitlyn M., O’Dell Sijy, Schmidt Stephen D., Swanson Phillip A., Padilla Marcelino, Mascola John R., Neuzil Kathleen M., Bennett Hamilton, Sun Wellington, Peters Etza, Makowski Mat, Albert Jim, Cross Kaitlyn, Buchanan Wendy, Pikaart-Tautges Rhonda, Ledgerwood Julie E., Graham Barney S., Beigel John H. An mRNA vaccine against SARS-CoV-2 - preliminary report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S.P., Neuzil K., Raabe V., Bailey R., Swanson K.A.J.M. Phase 1/2 study to describe the safety and immunogenicity of a COVID-19 RNA vaccine candidate (BNT162b1) in adults 18 to 55 years of age: interim report. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 14.Zhang N.-N., Li X.-F., Deng Y.-Q., Zhao H., Huang Y.-J., Yang G., Huang W.-J., Gao P., Zhou C., Zhang R.-R.J.C. A thermostable mRNA vaccine against COVID-19. Cell. 2020;182:1271–1283.e16. doi: 10.1016/j.cell.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M., Baum A., Pascal K., Quandt J., Maurus D.J.M. Concurrent human antibody and TH1 type T-cell responses elicited by a COVID-19 RNA vaccine. MedRxiv. 2020 doi: 10.1101/2020.07.17.20140533. [DOI] [Google Scholar]
- 16.Hajj K.A., Whitehead K.A. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2017;2:1–17. [Google Scholar]
- 17.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines - a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pardi N., Muramatsu H., Weissman D., Kariko K. In vitro transcription of long RNA containing modified nucleosides. Methods Mol. Biol. 2013;969:29–42. doi: 10.1007/978-1-62703-260-5_2. [DOI] [PubMed] [Google Scholar]
- 19.Pardi N., Hogan M.J., Pelc R.S., Muramatsu H., Andersen H., DeMaso C.R., Dowd K.A., Sutherland L.L., Scearce R.M., Parks R., Wagner W., Granados A., Greenhouse J., Walker M., Willis E., Yu J.S., McGee C.E., Sempowski G.D., Mui B.L., Tam Y.K., Huang Y.J., Vanlandingham D., Holmes V.M., Balachandran H., Sahu S., Lifton M., Higgs S., Hensley S.E., Madden T.D., Hope M.J., Kariko K., Santra S., Graham B.S., Lewis M.G., Pierson T.C., Haynes B.F., Weissman D. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017;543:248–251. doi: 10.1038/nature21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petsch B., Schnee M., Vogel A.B., Lange E., Hoffmann B., Voss D., Schlake T., Thess A., Kallen K.J., Stitz L., Kramps T. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat. Biotechnol. 2012;30:1210–1216. doi: 10.1038/nbt.2436. [DOI] [PubMed] [Google Scholar]
- 21.Alberer M., Gnad-Vogt U., Hong H.S., Mehr K.T., Backert L., Finak G., Gottardo R., Bica M.A., Garofano A., Koch Sven Dominik, Fotin-Mleczek Mariola, Hoerr Ingmar, Clemens Ralf, von Sonnenburg Frank. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet. 2017;390:1511–1520. doi: 10.1016/S0140-6736(17)31665-3. [DOI] [PubMed] [Google Scholar]
- 22.Reichmuth A.M., Oberli M.A., Jaklenec A., Langer R., Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016;7:319–334. doi: 10.4155/tde-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan L., Sun X. Recent advances in mRNA vaccine delivery. Nano Res. 2018;11:5338–5354. [Google Scholar]
- 24.Witzigmann D., Kulkarni J.A., Leung J., Chen S., Cullis P.R., van der Meel R. Lipid nanoparticle technology for therapeutic gene regulation in the liver. Adv. Drug Deliv. Rev. 2020;159:344–363. doi: 10.1016/j.addr.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kauffman K.J., Dorkin J.R., Yang J.H., Heartlein M.W., DeRosa F., Mir F.F., Fenton O.S., Anderson D.G. Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Lett. 2015;15:7300–7306. doi: 10.1021/acs.nanolett.5b02497. [DOI] [PubMed] [Google Scholar]
- 26.Semple S.C., Akinc A., Chen J., Sandhu A.P., Mui B.L., Cho C.K., Sah D.W., Stebbing D., Crosley E.J., Yaworski E., Hafez I.M., Dorkin J.R., Qin J., Lam K., Rajeev K.G., Wong K.F., Jeffs L.B., Nechev L., Eisenhardt M.L., Jayaraman M., Kazem M., Maier M.A., Srinivasulu M., Weinstein M.J., Chen Q., Alvarez R., Barros S.A., De S., Klimuk S.K., Borland T., Kosovrasti V., Cantley W.L., Tam Y.K., Manoharan M., Ciufolini M.A., Tracy M.A., de Fougerolles A., MacLachlan I., Cullis P.R., Madden T.D., Hope M.J. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 27.Jayaraman M., Ansell S.M., Mui B.L., Tam Y.K., Chen J., Du X., Butler D., Eltepu L., Matsuda S., Narayanannair J.K., Rajeev K.G., Hafez I.M., Akinc A., Maier M.A., Tracy M.A., Cullis P.R., Madden T.D., Manoharan M., Hope M.J. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew. Chem. Int. Ed. Eng. 2012;51:8529–8533. doi: 10.1002/anie.201203263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akinc A., Zumbuehl A., Goldberg M., Leshchiner E.S., Busini V., Hossain N., Bacallado S.A., Nguyen D.N., Fuller J., Alvarez R., Borodovsky A., Borland T., Constien R., de Fougerolles A., Dorkin J.R., Narayanannair Jayaprakash K., Jayaraman M., John M., Koteliansky V., Manoharan M., Nechev L., Qin J., Racie T., Raitcheva D., Rajeev K.G., Sah D.W., Soutschek J., Toudjarska I., Vornlocher H.P., Zimmermann T.S., Langer R., Anderson D.G. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat. Biotechnol. 2008;26:561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong Y., Love K.T., Dorkin J.R., Sirirungruang S., Zhang Y., Chen D., Bogorad R.L., Yin H., Chen Y., Vegas A.J., Alabi C.A., Sahay G., Olejnik K.T., Wang W., Schroeder A., Lytton-Jean A.K., Siegwart D.J., Akinc A., Barnes C., Barros S.A., Carioto M., Fitzgerald K., Hettinger J., Kumar V., Novobrantseva T.I., Qin J., Querbes W., Koteliansky V., Langer R., Anderson D.G. Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proceed. Nation. Acad. Sci. U.S.A. 2014;111:3955–3960. doi: 10.1073/pnas.1322937111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maier M.A., Jayaraman M., Matsuda S., Liu J., Barros S., Querbes W., Tam Y.K., Ansell S.M., Kumar V., Qin J., Zhang X., Wang Q., Panesar S., Hutabarat R., Carioto M., Hettinger J., Kandasamy P., Butler D., Rajeev K.G., Pang B., Charisse K., Fitzgerald K., Mui B.L., Du X., Cullis P., Madden T.D., Hope M.J., Manoharan M., Akinc A. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. 2013;21:1570–1578. doi: 10.1038/mt.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li B., Luo X., Deng B., Wang J., McComb D.W., Shi Y., Gaensler K.M., Tan X., Dunn A.L., Kerlin B.A., Dong Y. An orthogonal array optimization of lipid-like nanoparticles for mRNA delivery in vivo. Nano Lett. 2015;15:8099–8107. doi: 10.1021/acs.nanolett.5b03528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akita H., Ishiba R., Togashi R., Tange K., Nakai Y., Hatakeyama H., Harashima H. A neutral lipid envelope-type nanoparticle composed of a pH-activated and vitamin E-scaffold lipid-like material as a platform for a gene carrier targeting renal cell carcinoma. J. Control. Release. 2015;200:97–105. doi: 10.1016/j.jconrel.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 33.Conway A., Mendel M., Kim K., McGovern K., Boyko A., Zhang L., Miller J.C., DeKelver R.C., Paschon D.E., Mui B.L., Lin P.J.C., Tam Y.K., Barbosa C., Redelmeier T., Holmes M.C., Lee G. Non-viral delivery of zinc finger nuclease mRNA enables highly efficient in vivo genome editing of multiple therapeutic gene targets. Mol. Ther. 2019;27:866–877. doi: 10.1016/j.ymthe.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabnis S., Kumarasinghe E.S., Salerno T., Mihai C., Ketova T., Senn J.J., Lynn A., Bulychev A., McFadyen I., Chan J., Almarsson O., Stanton M.G., Benenato K.E. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 2018;26:1509–1519. doi: 10.1016/j.ymthe.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kormann M.S., Hasenpusch G., Aneja M.K., Nica G., Flemmer A.W., Herber-Jonat S., Huppmann M., Mays L.E., Illenyi M., Schams A., Griese M., Bittmann I., Handgretinger R., Hartl D., Rosenecker J., Rudolph C. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat. Biotechnol. 2011;29:154–157. doi: 10.1038/nbt.1733. [DOI] [PubMed] [Google Scholar]
- 36.Zangi L., Lui K.O., von Gise A., Ma Q., Ebina W., Ptaszek L.M., Spater D., Xu H., Tabebordbar M., Gorbatov R., Sena B., Nahrendorf M., Briscoe D.M., Li R.A., Wagers A.J., Rossi D.J., Pu W.T., Chien K.R. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat. Biotechnol. 2013;31:898–907. doi: 10.1038/nbt.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hess P.R., Boczkowski D., Nair S.K., Snyder D., Gilboa E. Vaccination with mRNAs encoding tumor-associated antigens and granulocyte-macrophage colony-stimulating factor efficiently primes CTL responses, but is insufficient to overcome tolerance to a model tumor/self antigen. Cancer Immunol. Immunother. 2006;55:672–683. doi: 10.1007/s00262-005-0064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kranz L.M., Diken M., Haas H., Kreiter S., Loquai C., Reuter K.C., Meng M., Fritz D., Vascotto F., Hefesha H., Grunwitz C., Vormehr M., Husemann Y., Selmi A., Kuhn A.N., Buck J., Derhovanessian E., Rae R., Attig S., Diekmann J., Jabulowsky R.A., Heesch S., Hassel J., Langguth P., Grabbe S., Huber C., Tureci O., Sahin U. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 39.Grabbe S., Haas H., Diken M., Kranz L.M., Langguth P., Sahin U. Translating nanoparticulate-personalized cancer vaccines into clinical applications: case study with RNA-lipoplexes for the treatment of melanoma. Nanomedicine (London) 2016;11:2723–2734. doi: 10.2217/nnm-2016-0275. [DOI] [PubMed] [Google Scholar]
- 40.Demoulins T., Milona P., Englezou P.C., Ebensen T., Schulze K., Suter R., Pichon C., Midoux P., Guzman C.A., Ruggli N., McCullough K.C. Polyethylenimine-based polyplex delivery of self-replicating RNA vaccines. Nanomedicine. 2016;12:711–722. doi: 10.1016/j.nano.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Zhao W., Zhang C., Li B., Zhang X., Luo X., Zeng C., Li W., Gao M., Dong Y. Lipid polymer hybrid nanomaterials for mRNA delivery. Cell. Mol. Bioeng. 2018;11:397–406. doi: 10.1007/s12195-018-0536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKinlay C.J., Vargas J.R., Blake T.R., Hardy J.W., Kanada M., Contag C.H., Wender P.A., Waymouth R.M. Charge-altering releasable transporters (CARTs) for the delivery and release of mRNA in living animals. Proceed. Nation. Acad. Sci. U.S.A. 2017;114:E448–E456. doi: 10.1073/pnas.1614193114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong Y., Dorkin J.R., Wang W., Chang P.H., Webber M.J., Tang B.C., Yang J., Abutbul-Ionita I., Danino D., DeRosa F., Heartlein M., Langer R., Anderson D.G. Poly(glycoamidoamine) brushes formulated nanomaterials for systemic siRNA and mRNA delivery in vivo. Nano Lett. 2016;16:842–848. doi: 10.1021/acs.nanolett.5b02428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaczmarek J.C., Patel A.K., Kauffman K.J., Fenton O.S., Webber M.J., Heartlein M.W., DeRosa F., Anderson D.G. Polymer-lipid nanoparticles for systemic delivery of mRNA to the lungs. Angew. Chem. Int. Ed. Eng. 2016;55:13808–13812. doi: 10.1002/anie.201608450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Persano S., Guevara M.L., Li Z., Mai J., Ferrari M., Pompa P.P., Shen H. Lipopolyplex potentiates anti-tumor immunity of mRNA-based vaccination. Biomaterials. 2017;125:81–89. doi: 10.1016/j.biomaterials.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uchida S., Kinoh H., Ishii T., Matsui A., Tockary T.A., Takeda K.M., Uchida H., Osada K., Itaka K., Kataoka K. Systemic delivery of messenger RNA for the treatment of pancreatic cancer using polyplex nanomicelles with a cholesterol moiety. Biomaterials. 2016;82:221–228. doi: 10.1016/j.biomaterials.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 47.Zhao M., Li M., Zhang Z., Gong T., Sun X. Induction of HIV-1 gag specific immune responses by cationic micelles mediated delivery of gag mRNA. Drug Deliv. 2016;23:2596–2607. doi: 10.3109/10717544.2015.1038856. [DOI] [PubMed] [Google Scholar]
- 48.Brito L.A., Chan M., Shaw C.A., Hekele A., Carsillo T., Schaefer M., Archer J., Seubert A., Otten G.R., Beard C.W., Dey A.K., Lilja A., Valiante N.M., Mason P.W., Mandl C.W., Barnett S.W., Dormitzer P.R., Ulmer J.B., Singh M., O'Hagan D.T., Geall A.J. A cationic nanoemulsion for the delivery of next-generation RNA vaccines. Mol. Ther. 2014;22:2118–2129. doi: 10.1038/mt.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L., Petrovsky N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert. Rev. Vaccines. 2016;15:313–329. doi: 10.1586/14760584.2016.1124762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kutzler M.A., Weiner D.B. DNA vaccines: ready for prime time? Nat. Rev. Genet. 2008;9:776–788. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cai Y., Rodriguez S., Hebel H. DNA vaccine manufacture: scale and quality. Expert. Rev. Vaccines. 2009;8:1277–1291. doi: 10.1586/erv.09.84. [DOI] [PubMed] [Google Scholar]
- 52.Middaugh C.R., Evans R.K., Montgomery D.L., Casimiro D.R. Analysis of plasmid DNA from a pharmaceutical perspective. J. Pharm. Sci. 1998;87:130–146. doi: 10.1021/js970367a. [DOI] [PubMed] [Google Scholar]
- 53.Garg R., Kaur M., Saxena A., Prasad R., Bhatnagar R. Alum adjuvanted rabies DNA vaccine confers 80% protection against lethal 50 LD. Mol. Immunol. 2017;85:166–173. doi: 10.1016/j.molimm.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 54.Hemmi H., Takeuchi O., Kawai T., Kaisho T., Sato S., Sanjo H., Matsumoto M., Hoshino K., Wagner H., Takeda K., Akira S. A toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 55.Hobernik D., Bros M. DNA vaccines-how far from clinical use? Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ulmer J.B., Donnelly J.J., Parker S.E., Rhodes G.H., Felgner P.L., Dwarki V.J., Gromkowski S.H., Deck R.R., DeWitt C.M., Friedman A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 57.Fynan E.F., Webster R.G., Fuller D.H., Haynes J.R., Santoro J.C., Robinson H.L. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc. Natl. Acad. Sci. U. S. A. 1993;90:11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.MacGregor R.R., Boyer J.D., Ugen K.E., Lacy K.E., Gluckman S.J., Bagarazzi M.L., Chattergoon M.A., Baine Y., Higgins T.J., Ciccarelli R.B., Coney L.R., Ginsberg R.S., Weiner D.B. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J. Infect. Dis. 1998;178:92–100. doi: 10.1086/515613. [DOI] [PubMed] [Google Scholar]
- 59.Yu J., Tostanoski L.H., Peter L., Mercado N.B., McMahan K., Mahrokhian S.H., Nkolola J.P., Liu J., Li Z., Chandrashekar A., Martinez D.R., Loos C., Atyeo C., Fischinger S., Burke J.S., Slein M.D., Chen Y., Zuiani A., Lelis F.J.N., Travers M., Habibi S., Pessaint L., Van Ry A., Blade K., Brown R., Cook A., Finneyfrock B., Dodson A., Teow E., Velasco J., Zahn R., Wegmann F., Bondzie E.A., Dagotto G., Gebre M.S., He X., Jacob-Dolan C., Kirilova M., Kordana N., Lin Z., Maxfield L.F., Nampanya F., Nityanandam R., Ventura J.D., Wan H., Cai Y., Chen B., Schmidt A.G., Wesemann D.R., Baric R.S., Alter G., Andersen H., Lewis M.G., Barouch D.H. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369:806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin J.E., Louder M.K., Holman L.A., Gordon I.J., Enama M.E., Larkin B.D., Andrews C.A., Vogel L., Koup R.A., Roederer M., Bailer R.T., Gomez P.L., Nason M., Mascola J.R., Nabel G.J., Graham B.S., Team V.S. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a phase I clinical trial. Vaccine. 2008;26:6338–6343. doi: 10.1016/j.vaccine.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith T.R.F., Patel A., Ramos S., Elwood D., Zhu X., Yan J., Gary E.N., Walker S.N., Schultheis K., Purwar M., Xu Z., Walters J., Bhojnagarwala P., Yang M., Chokkalingam N., Pezzoli P., Parzych E., Reuschel E.L., Doan A., Tursi N., Vasquez M., Choi J., Tello-Ruiz E., Maricic I., Bah M.A., Wu Y., Amante D., Park D.H., Dia Y., Ali A.R., Zaidi F.I., Generotti A., Kim K.Y., Herring T.A., Reeder S., Andrade V.M., Buttigieg K., Zhao G., Wu J.M., Li D., Bao L., Liu J., Deng W., Qin C., Brown A.S., Khoshnejad M., Wang N., Chu J., Wrapp D., McLellan J.S., Muthumani K., Wang B., Carroll M.W., Kim J.J., Boyer J., Kulp D.W., Humeau L.M.P.F., Weiner D.B., Broderick K.E. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020;11:2601. doi: 10.1038/s41467-020-16505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Modjarrad K., Roberts C.C., Mills K.T., Castellano A.R., Paolino K., Muthumani K., Reuschel E.L., Robb M.L., Racine T., Oh M.D., Lamarre C., Zaidi F.I., Boyer J., Kudchodkar S.B., Jeong M., Darden J.M., Park Y.K., Scott P.T., Remigio C., Parikh A.P., Wise M.C., Patel A., Duperret E.K., Kim K.Y., Choi H., White S., Bagarazzi M., May J.M., Kane D., Lee H., Kobinger G., Michael N.L., Weiner D.B., Thomas S.J., Maslow J.N. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect. Dis. 2019;19:1013–1022. doi: 10.1016/S1473-3099(19)30266-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Diehl M.C., Lee J.C., Daniels S.E., Tebas P., Khan A.S., Giffear M., Sardesai N.Y., Bagarazzi M.L. Tolerability of intramuscular and intradermal delivery by CELLECTRA((R)) adaptive constant current electroporation device in healthy volunteers. Hum. Vaccin. Immunother. 2013;9:2246–2252. doi: 10.4161/hv.24702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith T.R.F., Patel A., Ramos S., Elwood D., Zhu X., Yan J., Gary E.N., Walker S.N., Schultheis K., Purwar M., Xu Z., Walters J., Bhojnagarwala P., Yang M., Chokkalingam N., Pezzoli P., Parzych E., Reuschel E.L., Doan A., Tursi N., Vasquez M., Choi J., Tello-Ruiz E., Maricic I., Bah M.A., Wu Y., Amante D., Park D.H., Dia Y., Ali A.R., Zaidi F.I., Generotti A., Kim K.Y., Herring T.A., Reeder S., Andrade V.M., Buttigieg K., Zhao G., Wu J.M., Li D., Bao L., Liu J., Deng W., Qin C., Brown A.S., Khoshnejad M., Wang N., Chu J., Wrapp D., McLellan J.S., Muthumani K., Wang B., Carroll M.W., Kim J.J., Boyer J., Kulp D.W., Humeau L., Weiner D.B., Broderick K.E. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020;11:2601. doi: 10.1038/s41467-020-16505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Inovio 2020. http://ir.inovio.com/news-releases/news-releases-details/2020/INOVIO-Announces-Positive-Interim-Phase-1-Data-For-INO-4800-Vaccine-for-COVID-19/default.aspx
- 66.Liu J., Wu J., Wang B., Zeng S., Qi F., Lu C., Kimura Y., Liu B. Oral vaccination with a liposome-encapsulated influenza DNA vaccine protects mice against respiratory challenge infection. J. Med. Virol. 2014;86:886–894. doi: 10.1002/jmv.23768. [DOI] [PubMed] [Google Scholar]
- 67.Yuki Y., Kiyono H. Mucosal vaccines: novel advances in technology and delivery. Expert. Rev. Vaccines. 2009;8:1083–1097. doi: 10.1586/erv.09.61. [DOI] [PubMed] [Google Scholar]
- 68.Lee J.W., Kim H. Fragmentation of dimyristoylphosphatidylcholine vesicles by apomyoglobin. Arch. Biochem. Biophys. 1992;297:354–361. doi: 10.1016/0003-9861(92)90684-o. [DOI] [PubMed] [Google Scholar]
- 69.Alturki S.O., Alturki S.O., Connors J., Cusimano G., Kutzler M.A., Izmirly A.M., Haddad E.K. The 2020 pandemic: current SARS-CoV-2 vaccine development. Front. Immunol. 2020;11:1880. doi: 10.3389/fimmu.2020.01880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Symvivo COVID-10 Program Vision. 2020. https://www.symvivo.com/covid-19
- 71.Ho D., Lee G. South Korea’s Genexine Begins Phase I/IIa Trials for COVID-19 Vaccine. BioWorld. 2020. https://www.bioworld.com/articles/435995-south-koreas-genexine-begins-phase-iiia-trials-for-covid-19-vaccine
- 72.McAllister L., Anderson J., Werth K., Cho I., Copeland K., Le Cam Bouveret N., Plant D., Mendelman P.M., Cobb D.K. Needle-free jet injection for administration of influenza vaccine: a randomised non-inferiority trial. Lancet. 2014;384:674–681. doi: 10.1016/S0140-6736(14)60524-9. [DOI] [PubMed] [Google Scholar]
- 73.Coughlin M.M., Collins M., Saxon G., Jarrahian C., Zehrung D., Cappello C., Dhere R., Royals M., Papania M., Rota P.A. Effect of jet injection on infectivity of measles, mumps, and rubella vaccine in a bench model. Vaccine. 2015;33:4540–4547. doi: 10.1016/j.vaccine.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 74.Bernelin-Cottet C., Urien C., Fretaud M., Langevin C., Trus I., Jouneau L., Blanc F., Leplat J.J., Barc C., Boulesteix O., Riou M., Dysart M., Mahé S., Studsrub E., Nauwynck H., Bertho N., Bourry O., Schwartz-Cornil I. A DNA prime immuno-potentiates a modified live vaccine against the porcine reproductive and respiratory syndrome virus but does not improve heterologous protection. Viruses. 2019;11 doi: 10.3390/v11060576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akst J. COVID-19 Vaccine Prontrunners, The Scientist. 2020. https://www.the-scientist.com/news-opinion/covid-19-vaccine-frontrunners-67382
- 76.News K. Anges Starts Japan’s 1st COVID-19 Vaccine Clinical Trial on Humans. 2020. https://english.kyodonews.net/news/2020/06/0b4d42d1c638-update1-anges-starts-japans-1st-covid-19-vaccine-clinical-test-on-humans.html
- 77.Firstpost Race for COVID-19 Vaccine: Covaxin and ZyCoV-D Begin Human Clinical Trials in India, Moderna Publishes Preliminary Data from Phase 1. 2020. https://www.firstpost.com/health/race-for-covid-19-vaccine-covaxin-and-zycov-d-begin-human-trials-in-india-moderna-publishes-preliminary-data-from-phase-1-8600211.html
- 78.Qiu Z., Xing L., Zhang X., Qiang X., Xu Y., Zhang M., Zhou Z., Zhang J., Zhang F., Wang M. CpG oligodeoxynucleotides augment antitumor efficacy of folate receptor α based DNA vaccine. Oncol. Rep. 2017;37:3441–3448. doi: 10.3892/or.2017.5633. [DOI] [PubMed] [Google Scholar]
- 79.Kim J.J., Yang J.S., Montaner L., Lee D.J., Chalian A.A., Weiner D.B. Coimmunization with IFN-gamma or IL-2, but not IL-13 or IL-4 cDNA can enhance Th1-type DNA vaccine-induced immune responses in vivo. J. Interf. Cytokine Res. 2000;20:311–319. doi: 10.1089/107999000312450. [DOI] [PubMed] [Google Scholar]
- 80.Liu C., Xie Y., Sun B., Geng F., Zhang F., Guo Q., Wu H., Yu B., Wu J., Yu X., Kong W., Zhang H. MUC1- and survivin-based DNA vaccine combining immunoadjuvants CpG and interleukin-2 in a bicistronic expression plasmid generates specific immune responses and antitumour effects in a murine colorectal carcinoma model. Scand. J. Immunol. 2018;87:63–72. doi: 10.1111/sji.12633. [DOI] [PubMed] [Google Scholar]
- 81.Yang Y., Shao Z., Gao J. Antitumor effect of a DNA vaccine harboring prostate cancer-specific antigen with IL-12 as an intramolecular adjuvant. J. Mol. Microbiol. Biotechnol. 2017;27:168–174. doi: 10.1159/000477245. [DOI] [PubMed] [Google Scholar]
- 82.Duong H.T.T., Kim N.W., Thambi T., Giang Phan V.H., Lee M.S., Yin Y., Jeong J.H., Lee D.S. Microneedle arrays coated with charge reversal pH-sensitive copolymers improve antigen presenting cells-homing DNA vaccine delivery and immune responses. J. Control. Release. 2018;269:225–234. doi: 10.1016/j.jconrel.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 83.Duong H.T.T., Yin Y., Thambi T., Nguyen T.L., Giang Phan V.H., Lee M.S., Lee J.E., Kim J., Jeong J.H., Lee D.S. Smart vaccine delivery based on microneedle arrays decorated with ultra-pH-responsive copolymers for cancer immunotherapy. Biomaterials. 2018;185:13–24. doi: 10.1016/j.biomaterials.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 84.Shin M.D., Shukla S., Chung Y.H., Beiss V., Chan S.K., Ortega-Rivera O.A., Wirth D.M., Chen A., Sack M., Pokorski J.K., Steinmetz N.F. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020;15:646–655. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- 85.Thanh Le T., Andreadakis Z., Kumar A., Gomez Roman R., Tollefsen S., Saville M., Mayhew S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020;19:305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 86.Tian J.-H., Patel N., Haupt R., Zhou H., Weston S., Hammond H., Lague J., Portnoff A.D., Norton J., Guebre-Xabier M. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 elicits immunogenicity in baboons and protection in mice. BioRxiv. 2020 doi: 10.1101/2020.06.29.178509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bangaru S., Ozorowski G., Turner H.L., Antanasijevic A., Huang D., Wang X., Torres J.L., Diedrich J.K., Tian J.-H., Portnoff A.D. Structural analysis of full-length SARS-CoV-2 spike protein from an advanced vaccine candidate. BioRxiv. 2020;370:1089–1094. doi: 10.1126/science.abe1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Magnusson S.E., Reimer J.M., Karlsson K.H., Lilja L., Bengtsson K.L., Stertman L. Immune enhancing properties of the novel Matrix-M™ adjuvant leads to potentiated immune responses to an influenza vaccine in mice. Vaccine. 2013;31:1725–1733. doi: 10.1016/j.vaccine.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 89.Keech C., Albert G., Reed P., Neal S., Plested J.S., Zhu M., Cloney-Clark S., Zhou H., Patel N., Frieman M.B. First-in-human trial of a SARS CoV 2 recombinant spike protein nanoparticle vaccine. medRxiv. 2020 doi: 10.1101/2020.08.05.20168435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim E., Erdos G., Huang S., Kenniston T.W., Balmert S.C., Carey C.D., Raj V.S., Epperly M.W., Klimstra W.B., Haagmans B.L., Korkmaz E., Falo L.D., Gambotto A. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine. 2020;55:102743. doi: 10.1016/j.ebiom.2020.102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moon J.J., Suh H., Bershteyn A., Stephan M.T., Liu H., Huang B., Sohail M., Luo S., Um S.H., Khant H., Goodwin J.T., Ramos J., Chiu W., Irvine D.J. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat. Mater. 2011;10:243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Knuschke T., Sokolova V., Rotan O., Wadwa M., Tenbusch M., Hansen W., Staeheli P., Epple M., Buer J., Westendorf A.M. Immunization with biodegradable nanoparticles efficiently induces cellular immunity and protects against influenza virus infection. J. Immunol. 2013;190:6221–6229. doi: 10.4049/jimmunol.1202654. [DOI] [PubMed] [Google Scholar]
- 93.Heße C., Kollenda S., Rotan O., Pastille E., Adamczyk A., Wenzek C., Hansen W., Epple M., Buer J., Westendorf A.M., Knuschke T. A tumor-peptide-based nanoparticle vaccine elicits efficient tumor growth control in antitumor immunotherapy. Mol. Cancer Ther. 2019;18:1069–1080. doi: 10.1158/1535-7163.MCT-18-0764. [DOI] [PubMed] [Google Scholar]
- 94.Noad R., Roy P. Virus-like particles as immunogens. Trends Microbiol. 2003;11:438–444. doi: 10.1016/s0966-842x(03)00208-7. [DOI] [PubMed] [Google Scholar]
- 95.Hodgins B., Pillet S., Landry N., Ward B.J. Prime-pull vaccination with a plant-derived virus-like particle influenza vaccine elicits a broad immune response and protects aged mice from death and frailty after challenge. Immun. Ageing. 2019;16:27. doi: 10.1186/s12979-019-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lindsay B.J., Bonar M.M., Costas-Cancelas I.N., Hunt K., Makarkov A.I., Chierzi S., Krawczyk C.M., Landry N., Ward B.J., Rouiller I. Morphological characterization of a plant-made virus-like particle vaccine bearing influenza virus hemagglutinins by electron microscopy. Vaccine. 2018;36:2147–2154. doi: 10.1016/j.vaccine.2018.02.106. [DOI] [PubMed] [Google Scholar]
- 97.Pillet S., Couillard J., Trépanier S., Poulin J.F., Yassine-Diab B., Guy B., Ward B.J., Landry N. Immunogenicity and safety of a quadrivalent plant-derived virus like particle influenza vaccine candidate-Two randomized Phase II clinical trials in 18 to 49 and ≥50 years old adults. PLoS One. 2019;14 doi: 10.1371/journal.pone.0216533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu M., Stevens V., Berry J.D., Crameri G., McEachern J., Tu C., Shi Z., Liang G., Weingartl H., Cardosa J., Eaton B.T., Wang L.F. Determination and application of immunodominant regions of SARS coronavirus spike and nucleocapsid proteins recognized by sera from different animal species. J. Immunol. Methods. 2008;331:1–12. doi: 10.1016/j.jim.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang J.-D., Zhang B.-z., Hu Y.-f. Mapping the immunodominance landscape of SARS-CoV-2 spike protein for the design of vaccines against COVID-19. bioRxiv. 2020 doi: 10.1101/2020.04.23.056853. [DOI] [Google Scholar]
- 100.Kalita P., Padhi A.K., Zhang K.Y.J., Tripathi T. Design of a peptide-based subunit vaccine against novel coronavirus SARS-CoV-2. Microb. Pathog. 2020;145:104236. doi: 10.1016/j.micpath.2020.104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kar T., Narsaria U., Basak S., Deb D., Castiglione F., Mueller D.M., Srivastava A.P. A candidate multi-epitope vaccine against SARS-CoV-2. Sci. Rep. 2020;10:10895. doi: 10.1038/s41598-020-67749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abdelmageed M.I., Abdelmoneim A.H., Mustafa M.I., Elfadol N.M., Murshed N.S., Shantier S.W., Makhawi A.M. Design of a multiepitope-based peptide vaccine against the E protein of human COVID-19: an immunoinformatics approach. Biomed. Res. Int. 2020;2020 doi: 10.1155/2020/2683286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Garçon N., Vaughn D.W., Didierlaurent A.M. Development and evaluation of AS03, an Adjuvant System containing α-tocopherol and squalene in an oil-in-water emulsion. Expert. Rev. Vaccines. 2012;11:349–366. doi: 10.1586/erv.11.192. [DOI] [PubMed] [Google Scholar]
- 104.O'Hagan D.T., Ott G.S., Nest G.V., Rappuoli R., Giudice G.D. The history of MF59(®) adjuvant: a phoenix that arose from the ashes. Expert. Rev. Vaccines. 2013;12:13–30. doi: 10.1586/erv.12.140. [DOI] [PubMed] [Google Scholar]
- 105.Campbell J.D. Development of the CpG adjuvant 1018: a case study. Methods Mol. Biol. 2017;1494:15–27. doi: 10.1007/978-1-4939-6445-1_2. [DOI] [PubMed] [Google Scholar]
- 106.Grunwald T., Ulbert S. Improvement of DNA vaccination by adjuvants and sophisticated delivery devices: vaccine-platforms for the battle against infectious diseases. Clin. Exp. Vaccine Res. 2015;4:1–10. doi: 10.7774/cevr.2015.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schneeberger A., Wagner C., Zemann A., Lührs P., Kutil R., Goos M., Stingl G., Wagner S.N. CpG motifs are efficient adjuvants for DNA cancer vaccines. J. Invest. Dermatol. 2004;123:371–379. doi: 10.1111/j.0022-202X.2004.23208.x. [DOI] [PubMed] [Google Scholar]
- 108.Reyes L., Hartikka J., Bozoukova V., Sukhu L., Nishioka W., Singh G., Ferrari M., Enas J., Wheeler C.J., Manthorpe M., Wloch M.K. Vaxfectin enhances antigen specific antibody titers and maintains Th1 type immune responses to plasmid DNA immunization. Vaccine. 2001;19:3778–3786. doi: 10.1016/s0264-410x(01)00090-1. [DOI] [PubMed] [Google Scholar]
- 109.Vilalta A., Shlapobersky M., Wei Q., Planchon R., Rolland A., Sullivan S. Analysis of biomarkers after intramuscular injection of Vaxfectin-formulated hCMV gB plasmid DNA. Vaccine. 2009;27:7409–7417. doi: 10.1016/j.vaccine.2009.08.075. [DOI] [PubMed] [Google Scholar]
- 110.Kasturi S.P., Rasheed M.A.U., Havenar-Daughton C., Pham M., Legere T., Sher Z.J., Kovalenkov Y., Gumber S., Huang J.Y., Gottardo R., Fulp W., Sato A., Sawant S., Stanfield-Oakley S., Yates N., LaBranche C., Alam S.M., Tomaras G., Ferrari G., Montefiori D., Wrammert J., Villinger F., Tomai M., Vasilakos J., Fox C.B., Reed S.G., Haynes B.F., Crotty S., Ahmed R., Pulendran B. 3M-052, a synthetic TLR-7/8 agonist, induces durable HIV-1 envelope-specific plasma cells and humoral immunity in nonhuman primates. Sci. Immunol. 2020:5. doi: 10.1126/sciimmunol.abb1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dowling D.J. Recent advances in the discovery and delivery of TLR7/8 agonists as vaccine adjuvants. Immunohorizons. 2018;2:185–197. doi: 10.4049/immunohorizons.1700063. [DOI] [PubMed] [Google Scholar]
- 112.Pardi N., Tuyishime S., Muramatsu H., Kariko K., Mui B.L., Tam Y.K., Madden T.D., Hope M.J., Weissman D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release. 2015;217:345–351. doi: 10.1016/j.jconrel.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sahin U., Oehm P., Derhovanessian E., Jabulowsky R.A., Vormehr M., Gold M., Maurus D., Schwarck-Kokarakis D., Kuhn A.N., Omokoko T. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. 2020;585:107–112. doi: 10.1038/s41586-020-2537-9. [DOI] [PubMed] [Google Scholar]
- 114.McCluskie M.J., Brazolot Millan C.L., Gramzinski R.A., Robinson H.L., Santoro J.C., Fuller J.T., Widera G., Haynes J.R., Purcell R.H., Davis H.L. Route and method of delivery of DNA vaccine influence immune responses in mice and non-human primates. Mol. Med. 1999;5:287–300. [PMC free article] [PubMed] [Google Scholar]
- 115.Ito K., Shinohara N., Kato S. DNA immunization via intramuscular and intradermal routes using a gene gun provides different magnitudes and durations on immune response. Mol. Immunol. 2003;39:847–854. doi: 10.1016/s0161-5890(03)00024-5. [DOI] [PubMed] [Google Scholar]
- 116.Pati R., Shevtsov M., Sonawane A. Nanoparticle vaccines against infectious diseases. Front. Immunol. 2018;9:2224. doi: 10.3389/fimmu.2018.02224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gulla S.K., Rao B.R., Moku G., Jinka S., Nimmu N.V., Khalid S., Patra C.R., Chaudhuri A. In vivo targeting of DNA vaccines to dendritic cells using functionalized gold nanoparticles. Biomater. Sci. 2019;7:773–788. doi: 10.1039/c8bm01272e. [DOI] [PubMed] [Google Scholar]
- 118.Wolff J.A., Malone R.W., Williams P., Chong W., Acsadi G., Jani A., Felgner P.L. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 119.Fazio V.M. "Naked" DNA transfer technology for genetic vaccination against infectious disease. Res. Virol. 1997;148:101–108. doi: 10.1016/s0923-2516(97)89892-5. [DOI] [PubMed] [Google Scholar]
- 120.Joo H.M., He Y., Sundararajan A., Huan L., Sangster M.Y. Quantitative analysis of influenza virus-specific B cell memory generated by different routes of inactivated virus vaccination. Vaccine. 2010;28:2186–2194. doi: 10.1016/j.vaccine.2009.12.058. [DOI] [PubMed] [Google Scholar]
- 121.Mann J.F., Shakir E., Carter K.C., Mullen A.B., Alexander J., Ferro V.A. Lipid vesicle size of an oral influenza vaccine delivery vehicle influences the Th1/Th2 bias in the immune response and protection against infection. Vaccine. 2009;27:3643–3649. doi: 10.1016/j.vaccine.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 122.Conacher M., Alexander J., Brewer J.M. Oral immunisation with peptide and protein antigens by formulation in lipid vesicles incorporating bile salts (bilosomes) Vaccine. 2001;19:2965–2974. doi: 10.1016/s0264-410x(00)00537-5. [DOI] [PubMed] [Google Scholar]
- 123.Jones K.L., Drane D., Gowans E.J. Long-term storage of DNA-free RNA for use in vaccine studies. Biotechniques. 2007;43:675–681. doi: 10.2144/000112593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alberer M., Gnad-Vogt U., Hong H.S., Mehr K.T., Backert L., Finak G., Gottardo R., Bica M.A., Garofano A., Koch S.D., Fotin-Mleczek M., Hoerr I., Clemens R., von Sonnenburg F. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet. 2017;390:1511–1520. doi: 10.1016/S0140-6736(17)31665-3. [DOI] [PubMed] [Google Scholar]
- 125.Tebas P., Kraynyak K.A., Patel A., Maslow J.N., Morrow M.P., Sylvester A.J., Knoblock D., Gillespie E., Amante D., Racine T., McMullan T., Jeong M., Roberts C.C., Park Y.K., Boyer J., Broderick K.E., Kobinger G.P., Bagarazzi M., Weiner D.B., Sardesai N.Y., White S.M. Intradermal SynCon® Ebola GP DNA vaccine is temperature stable and safely demonstrates cellular and humoral immunogenicity advantages in healthy volunteers. J. Infect. Dis. 2019;220:400–410. doi: 10.1093/infdis/jiz132. [DOI] [PubMed] [Google Scholar]
- 126.Kumru O.S., Joshi S.B., Smith D.E., Middaugh C.R., Prusik T., Volkin D.B. Vaccine instability in the cold chain: mechanisms, analysis and formulation strategies. Biologicals. 2014;42:237–259. doi: 10.1016/j.biologicals.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 127.Wahid A.A., Doekhie A., Sartbaeva A., van den Elsen J.M.H. Ensilication improves the thermal stability of the tuberculosis antigen Ag85b and an Sbi-Ag85b vaccine conjugate. Sci. Rep. 2019;9:11409. doi: 10.1038/s41598-019-47657-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bajrovic I., Schafer S.C., Romanovicz D.K., Croyle M.A. Novel technology for storage and distribution of live vaccines and other biological medicines at ambient temperature. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aau4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ribas A., Weber J.S., Chmielowski B., Comin-Anduix B., Lu D., Douek M., Ragavendra N., Raman S., Seja E., Rosario D., Miles S., Diamond D.C., Qiu Z., Obrocea M., Bot A. Intra-lymph node prime-boost vaccination against Melan A and tyrosinase for the treatment of metastatic melanoma: results of a phase 1 clinical trial. Clin. Cancer Res.: Off. J. Am. Assoc. Cancer Res. 2011;17:2987–2996. doi: 10.1158/1078-0432.CCR-10-3272. [DOI] [PubMed] [Google Scholar]
- 130.Maloy K.J., Erdmann I., Basch V., Sierro S., Kramps T.A., Zinkernagel R.M., Oehen S., Kündig T.M. Intralymphatic immunization enhances DNA vaccination. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3299–3303. doi: 10.1073/pnas.051630798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Howard G.P., Verma G., Ke X., Thayer W.M., Hamerly T., Baxter V.K., Lee J.E., Dinglasan R.R., Mao H.-Q. Critical size limit of biodegradable nanoparticles for enhanced lymph node trafficking and paracortex penetration. Res. Article. 2019;12:837–844. doi: 10.1007/s12274-019-2301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Carstens M.G., Camps M.G., Henriksen-Lacey M., Franken K., Ottenhoff T.H., Perrie Y., Bouwstra J.A., Ossendorp F., Jiskoot W. Effect of vesicle size on tissue localization and immunogenicity of liposomal DNA vaccines. Vaccine. 2011;29:4761–4770. doi: 10.1016/j.vaccine.2011.04.081. [DOI] [PubMed] [Google Scholar]
- 133.Liu H., Moynihan K.D., Zheng Y., Szeto G.L., Li A.V., Huang B., Van Egeren D.S., Park C., Irvine D.J. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507:519–522. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Steinbuck M.P., Seenappa L.M., Jakubowski A., McNeil L.K., Haqq C.M., DeMuth P.C. A lymph node targeted amphiphile vaccine induces potent cellular and humoral immunity to SARS-CoV-2. bioRxiv. 2020 doi: 10.1101/2020.08.17.251728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mueller S.N., Tian S., DeSimone J.M. Rapid and persistent delivery of antigen by lymph node targeting PRINT nanoparticle vaccine carrier to promote humoral immunity. Mol. Pharm. 2015;12:1356–1365. doi: 10.1021/mp500589c. [DOI] [PMC free article] [PubMed] [Google Scholar]