Abstract
This study used Electron Cryo-tomography (ECT) and fluorescent images to evaluate antimicrobial photodynamic therapy (aPDT) on the envelope architecture of a Gram-negative bacteria and the effects of combined therapy of aPDT and antibiotics. Standard and clinical suspension of Escherichia coli were submitted to photodynamic treatment with methylene blue solution (100μM) and a 100mW LED emitting at 660nm with 3 and 18J of energy. As a control group, a suspension of E. coli was submitted to penicillin V for 60 min at 30 °C, to compare the damage in cell wall structure. After treatment, ECT images were collected and E. coli biofilms were grown in glass-cover slides and stained with live/dead staining for fluorescence analysis before and after treatments. Bacteria were also submitted to disc diffusion and MIC50 tests with Ampicillin, Amoxicillin + Clavulanic acid, Clindamycin and Erythromycin. For in vivo experiment Galleria mellonella larvae were infected with E. coli and treated with antibiotics, aPDT or combined therapy. ECT images presented damage to cell walls and vesicles structures inside and outside the bacteria and fluorescent images showed dose dependent effect of aPDT. Antibiotic or aPDT alone did not improve the survival of caterpillars, but the combined therapy significantly increased survival curve. ECT and fluorescent images shows that aPDT seems to promote micro-damages to cell envelope and causes the production of membrane vesicles permeabilizing cell membranes. The results showed that pre-treating bacterial cells with a photosensitizer and light make them more susceptible to antibiotics and could be an alternative to local infection treatment by resistant bacteria.
Keywords: Electron Cryo-tomography, methylene blue, PDT, antibiotics
Introduction:
The discovery of antibiotics in the 19th century led to a medical revolution in preventing and controlling infectious diseases [1], leading many to believe that the infectious diseases chapter was closed. However, it is now estimated that 700,000 people die annually due to drug-resistant bacteria mainly the group known as ESKAPE pathogens which includes: Enterococcus spp., Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp., such as Escherichia coli. [2]. To mitigate this problem, alternative strategies either with or without antibiotics have been considered: the use of new antibiotics [3], or antibiotics in combination with adjuvant therapies such as bacteriophage therapy [4], antimicrobial peptides [5], photodynamic therapy [6], phytochemicals and nanoparticles as antibacterial agents [7].
Antimicrobial Photodynamic therapy (aPDT) is a potential alternative method to fight resistant microorganisms, including bacteria, fungi, parasites and viruses [8]. It combines a non-toxic dye (called a photosensitizer - PS) with low-power-visible light to kill biological entities by the production of reactive oxygen species (ROS) [9]. Generally speaking, a low-power light source excites the PS which reacts with molecular oxygen to produce ROS, initiating the injury and death of targeted cells such as microorganisms and cancer cells [10] or age-related macular degeneration [11]. More recently, aPDT has also been used as an alternative approach to antibiotics [12] against resistant microorganisms, especially in dental [13] and dermatological infections [14]. Importantly, cationic PS can rapidly bind or infiltrate bacterial membranes thus exhibiting a high degree of selectivity and little toxicity toward host cells [15]. Previous studies showed that aPDT is equally effective against antibiotics-resistant or sensitive bacterial strains such as methicillin-resistant Staphylococcus aureus (MRSA) and native ones [16] or resistant E. coli and sensitive strains [17].
Compared to conventional antibiotic therapy, aPDT works in a short time against localized infections, killing the microorganisms that are present in the infection site without influencing the normal microbiota [18]. Also, there is currently no indication that aPDT leads to the development of resistant bacteria [19]. Recent studies have shown that sub-lethal aPDT doses do not result in the development of resistance, since ROS oxidation occurs due to a number of targets presented on bacterial cell structure and components and not only in one major target as in the case of antibiotics [20,21]. Nevertheless, the precise effect of aPDT on bacterial cells structure remains elusive and it is still unknown the change in the morphology of aPDT-treated cells compared to antibiotics-treated bacterial cells.
In the present study, we examined the effects of aPDT using methylene blue (MB) as a PS with low-power light emitting diode (LED) on E. coli cells and to compare aPDT to conventional antibiotics (penicillin) using electron cryotomography (cryo-ET), fluorescent microscopy and by an in vivo animal experiment, in order to understand the mechanism of bacterial death by aPDT. Our results suggest that, at the cryo-ET resolution, cell morphology is preserved after aPDT treatment, in contrast to antibiotics which resulted in cell damage and lysis. Thus, aPDT therapy induces membrane damage in the bacterial cells which renders them more susceptible to antibiotics treatment.
Materials and Methods:
Cell strains and growth conditions
E. coli (standard and clinical) strains were grown at 30°C in LB broth for 24hs to reach exponential phase. Suspensions of each E. coli were diluted in 3ml of PBS solution to a cell density of 107 per mL, in a glass test tube. E. coli standard strain used was an ATCC 25922 and clinical strain was isolated from oral samples of patients treated at São Leopoldo Mandic Dental School and identified using chromogenic agar broth (CPS, BioMérieux) and VITEK2 system (BioMérieux).
Antimicrobial PhotodynamicTherapy
A LED (LumiLEDs, Phillips - Netherlands), with a peak wavelength at 660nm, was used as the light source (power output of 100 mW, spot size of 1cm2 and power density of 0.1W/cm2).
Two different energies consisted on 3J and 18J, resulted from 30s and 180s of LED irradiation, were used as sub-lethal and lethal dose of aPDT, respectively [15,22].
For the in vitro experiments, irradiations were performed from the bottom to the top of the glass test tube and 96 wells plate and for in vivo analysis, larvae were irradiated with a LED (LumiLEDs, Phillips - Netherlands) in a Petri dish plate in a dorsal position.
As a photosensitizer (PS) an aqueous solution of Methylene blue (Sigma Aldrich, Milwaukee, USA) at 100μM were prepared and kept at dark conditions before use.
Electron cryotomography sample preparation and image analysis
E. coli cells in suspension were incubated in a glass test tube (16×100mm) with 100 mM of PS for 5 min in the absence of light and irradiated with sub-lethal and lethal dose of light.
Another sample of E. coli was resuspended in PBS solution as reported previously and incubated with 0.3μg/ml penicillin V (Phenoxymethylpenicillin) for 1 hour at 30°C with shaking.
An untreated control group (no aPDT and antibiotics) was imaged using electron cryotomography (cryo-ET) to evaluate in a macromolecular level the morphological changes in E. coli envelope induced by each treatment.
Samples from control, antibiotic, aPDT sub-lethal and lethal dose groups were mixed with colloidal gold particles, plunge freezing and analyzed using cryo-ET images.
Data collection and image analysis was done as described previously in the literature [25–27].
Microbiological analysis
Experiments were performed as described in Ref. [28]. Briefly, suspensions of E. coli were diluted in PBS, and 200μl aliquots were added to a 96-well plate followed by removal of 10ml aliquots for serial dilution and streaking on BHI agar plates for colony forming units (CFUs) enumeration. Bacteria were challenged by aPDT (MB+LED with 3J and 18J) or penicillin V (0.3μg/ml) for 1 hour as described previously. Survival fractions were determined from the CFUs in the initial innoculum and compared with the remaining after aPDT or antibiotic treatment.
Fluorescent analysis
E. coli biofilms were grown in glass-cover slides submerged into a BHI broth contaminated with 100ml of bacterial suspension (aprox.1 × 108 cfu/mL) for 72 h at 37 °C. After 72 h biofilm was washed with 1ml of PBS solution to detach planktonic cells and visualized using the LIVE/DEAD fluorescent microscopy showing a single layer of cells with the clusters covering approximately 70% of the glass surface.
The cover slides were incubated with PS for 5 minutes and irradiated with lethal (18J) and sub-lethal dose (3J) of aPDT.
Live/Dead BacLight (Bacterial Viability Kit, Invitrogen, USA) was then added according to the manufacturer’s instructions. The Syto9 stain was used with 480-nm excitation and 500-nm emission, to stain living bacterial cells in green. The propidium iodide stain (490-nm excitation and 635-nm emission) was used to stain dead bacterial cells in red [29].
Antibiotics resistance
To test if a dose of aPDT could promote damage to bacteria envelope and facilitate antibiotic penetration into the cell, an antibiotic resistance/susceptibility test was performed using antibiotic discs of Ampicillin – 10μg, Amoxicillin + Clavulanic acid – 20μg, Clindamycin – 2μg and Ertromycin 15μg (Laborclin, Pinhais Brazil) and MIC50 test, based on CSLI [30] and EUCAST reference values [31]. For combined therapy, aPDT was applied with parameters of lethal and sublethal dose, before antibiotic challenge. For this experiment ATCC strain was used as recommended by CSLI and EUCAST tests.
In vivo Antimicrobial experiments
To analyze E. coli virulence in G. mellonella, bacterial suspensions of E. coli clinical strain was adjusted in PBS to 10−6 cfu/mL by spectrophotometer at 660nm according to the methodology proposed by Chibebe Jr et al[32].
Twenty randomly chosen G. mellonella larvae with weight around 300mg were used per group. Larvae were obtained from the Microbiology and Immunology invertebrate laboratory, UNESP Dental School (São José dos Campos, Brazil).
Two control groups were included in the study: one inoculated with sterile PBS to evaluate the results of physical trauma, and the other group received no injection to access general viability. A 10μl Hamilton syringe (Hamilton Inc, EUA) was used to inject 10μl of E. coli inoculum aliquots into the hemocoel of each larvae.
For a survival curve analysis, larvae were incubated at 37°C in plastic petri dishes for 7 days or until death. They were considered dead when presented no movement in response to light touch.
Antibiotic treatment
For antimicrobial treatment, Amoxicillin+Clavulanic acid (20μg) and Clindamycin (2μg) were selected since E. coli clinical strain showed resistance during disc diffusion test and some degree of improvement after aPDT in in vitro test. Antibiotic solutions were prepared by diluting the antibiotic in sterile PBS solution to reach the recommended concentration of each compound. Ten microliters of the solution were injected 60min after infection of larvae with a lethal dose of E. coli [32]. The antibiotics was injected at the opposite proleg of each infected larvae. As control group, the caterpillars received PBS injections. Posteriorly, survival curves were calculated during the 7 days of the experiment.
aPDT
The experiments were performed as follows: G. mellonella received the PS injection (10μL) 30min after bacterial infection. The caterpillars were kept in the dark for 10 minutes to allow PS dispersion into the body of the caterpillars and then irradiated with lethal dose. After irradiation, survival curves were calculated.
Photodynamic Therapy + Antibiotic treatment
Same methodologies were applied as described above. After irradiation, each animal received the antibiotic dose as previously detailed and a control group received PBS injection. Survival curves were then calculated.
Statistical analysis
Survival curve was determined using the Kaplan-Meier method, and the level of significance between the survival curves was calculated using the log-rank test (Mantel-Cox). Microbiological analysis was submitted to ANOVA followed by the Tukey’s test. All tests were carried out using GraphPad Prism 5.0, where 5% was considered a significant difference.
Results:
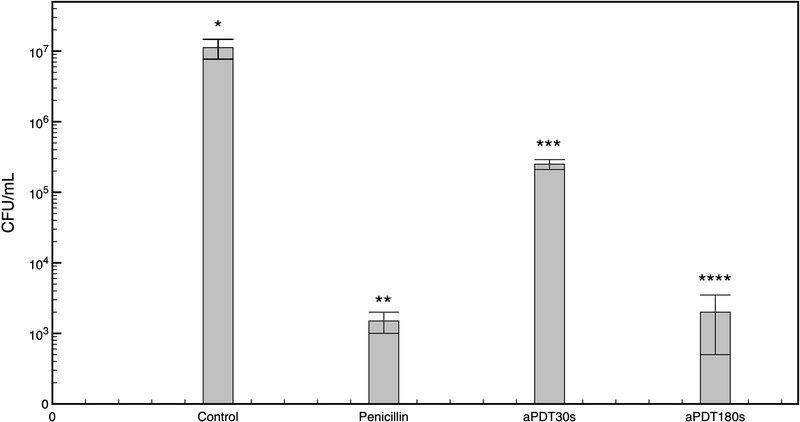
The results of the effect of aPDT on the viability of E. coli cells (ATCC 25922, see materials and methods) performed by calculating the colony-forming unit (CFU)/ml of E. coli control cells, LED-treated (without MB), or with 100 μM MB (incubated for 5 minutes in the dark) showed that in all these cases, no significant reduction (p>0.05) in cell viability was observed (Figure 1). On the other hand, applying a sub-lethal (30s, corresponding to 3J) or lethal (180s, corresponding to 18J) dose of LED on E. coli (with MB), or treating the cells with penicillin for 1 hour resulted in a substantial reduction in cell viability, with the lethal dose having a comparable level of reduction to that of penicillin-treated cells (Figure 1).
Figure 1:
Mean and standard deviation of viable bacteria (CFU) after each treatment. Asterisks indicate differences between groups (p < 0.05). Note that light alone or MB alone did not show significative bacterial reduction. Also, lethal dose of aPDT showed similar results than antibiotic treatment.
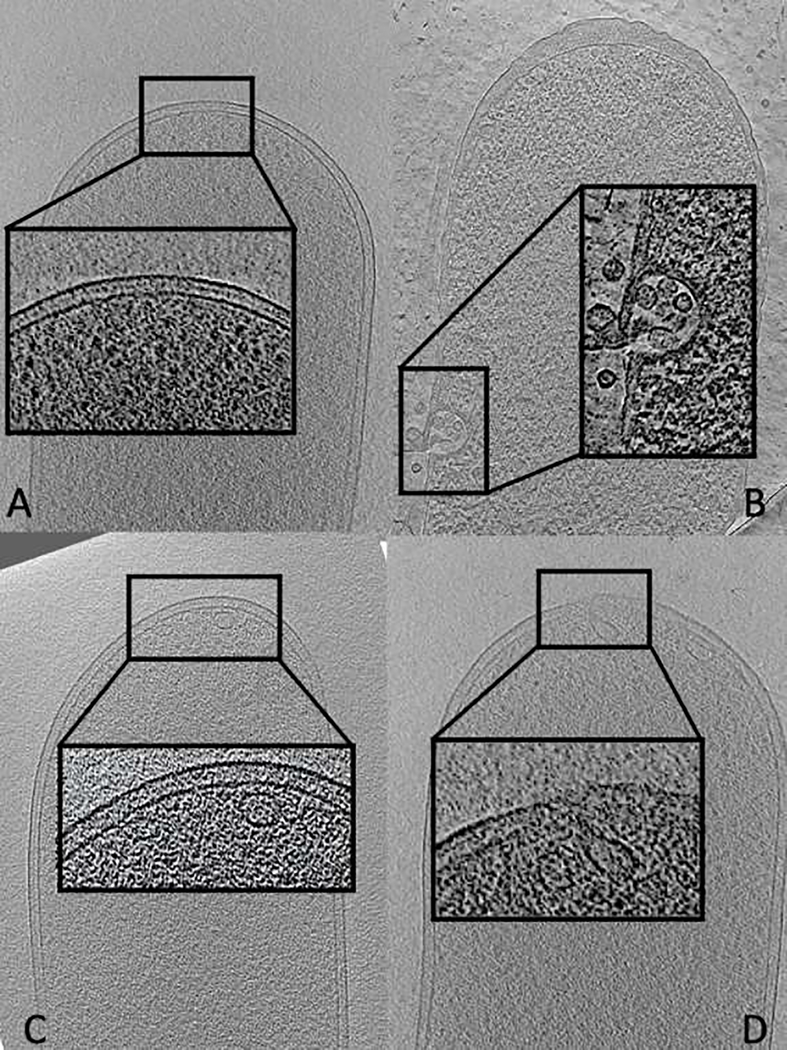
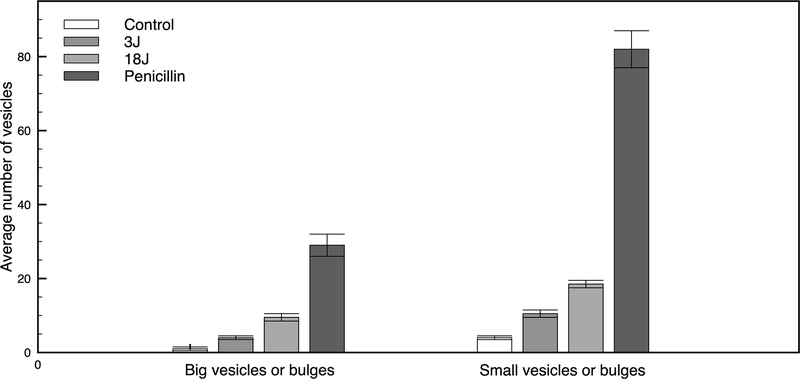
Later, it was investigated the morphological changes at the macromolecular level in E. coli cells as induced by penicillin, lethal- and sub-lethal-aPDT treatments using cryo-ET which allows the visualization of macromolecular complexes in native cellular contexts at the nanometer resolution [21]. The cell envelope of Gram-negative bacteria consists of an outer membrane, an inner (cytoplasmic) membrane and a peptidoglycan layer in between (Figure 2). Penicillin, a beta-lactam antibiotics, acts by inhibiting the synthesis of the peptidoglycan layer resulting in a weakened cell wall. Cryo-ET of E. coli cells treated with penicillin revealed an altered cell morphology due to cell rupture and lysis and the presence of membrane vesicles as a sign. On the contrary, cells treated with sub-lethal and lethal doses of aPDT stayed intact with no visible significant changes to the cell morphology (at the cryo-ET resolution). However, there was a tendency of the numbers of membrane vesicles, bulges and invaginations in the images of each sample to increase in these structures proportionally to the applied aPDT dose, but at a notably lower rate than that observed in penicillin-treated samples (Figure 3).
Figure 2:
Representative tomographic slices of cryo-section through E. coli cell treated with methylene blue and irradiated with 3J or 18J and treated with penicillin. A) Control group – no treatments. Note the presence of intact inner and outer membrane, such as cell wall B) Penicillin treated. Note the rupture of cell envelope with the presence of multiples vesicles C) aPDT 3J and D) aPDT 18J, presence of vesicles and buds. Zoom-in of cell envelope of each group.
Figure 3:
Average number of vesicles present in tomograms of each group. Asterisks mean significant difference (p> 0.05). Note the dose dependence of vesicles production, both for small and big vesicles.
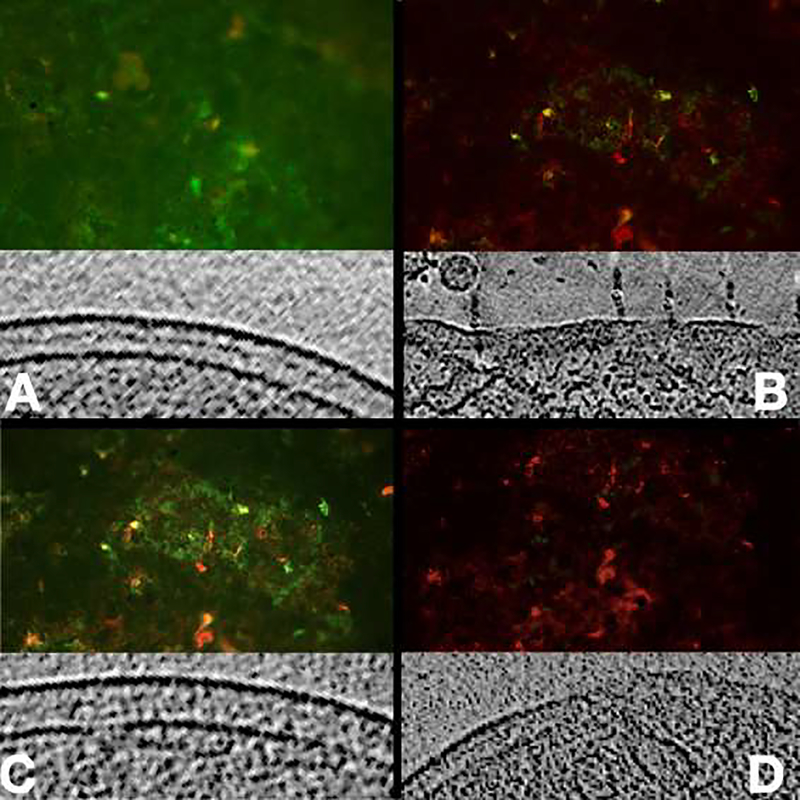
In order to test whether applying aPDT has an effect on bacterial cellular membranes, we performed a fluorescent analysis using live/dead staining with propidium iodide (PI) before and after exposing bacterial cells to sub-lethal and lethal doses of aPDT. Permeabilized bacterial membranes would facilitate the penetration of the PI dye into the cell for DNA staining [33,34]. In both cases, (lethal or sub-lethal doses of aPDT), an increased number of PI stained cells was observed proportionally to the applied dose (Figure 4), suggesting again that aPDT affects the cellular membranes and rendering them more permeable.
Figure 4:
Fluorescence images of biofilm on glass cover slides using live/dead stain and a representative tomographic slice of cryosection. A) Most of the bacteria are viable (green), note the intact envelope; B) Treatment with penicillin, significative damage to cell envelope; C) Sub-lethal dose of aPDT, some of the bacteria are dead, note the vesicles formation; D) Lethal aPDT dose, most of the bacteria are dead (red), vesicles and damage to cell envelope.
Membrane vesicles are usually formed when the outer membrane expands faster than the underlying peptidoglycan layer, resulting in a localized detachment of the peptidoglycan from the outer membrane. Subsequently, if a difference in local pressure persists, areas of detachment will ‘bulge’ and be released from the outer membrane, forming vesicles and damaging the cell envelope [32,34]. Hence, we hypothesized based on the above-mentioned observation that aPDT induce a damage to the cell envelope which would promote an antibiotics-resistant bacteria to be more sensitive to antibiotics treatment. This hypothesis was scrutinized using a clinical E. coli strain resistant to antibiotics (see Materials and Methods) by in vitro disc diffusion test and minimum inhibitory concentration (MIC50) test, and in vivo experiments on the animal model of Galleria mellonella.
To test the degree of E. coli strain resistance, different antibiotics were investigated in the disc diffusion tests: Amoxicillin + Clavulanic acid, Ampicillin, Erythromycin, and Clindamycin. For this experiment, it was assumed that the inhibition of bacterial growth on an agar plate is proportional to how sensitive the bacterial strain is to the present antibiotic. After aPDT treatment (180s), there was an increase in the sensitivity of E. coli cells for both Amoxicillin + Clavulanic acid and Ampicillin. The size of the inhibition zones for Amoxicillin + Clavulanic acid changed from 16.4 mm (intermediate resistance) to 18.1 mm (susceptible), while for Ampicillin, the inhibition zone size changed from 15.3 mm to 17.2 mm. On the contrary, no significant change in the size of the inhibition zone was observed for the remaining antibiotics (Table 1). Furthermore, an improvement was observed in MIC50 only for Clindamycin but not for the other examined antibiotics.
Table 1:
Susceptibility to antibiotic for disc diffusion test with and without the previous application of aPDT (in mm)
| No aPDT (mm) | aPDT (mm) | |
|---|---|---|
| Ampicilin | 15.3 (±0,1) | 17.2 (±0,05) |
| Amoxilin+Clavulinic Acid | 16.4 (±0,06) | 18.1 (±0,1) |
| Clindamicin | 13.1 (±0,12) | 15.56 (±0,16) |
| Eritromycin | 0 | 0 |
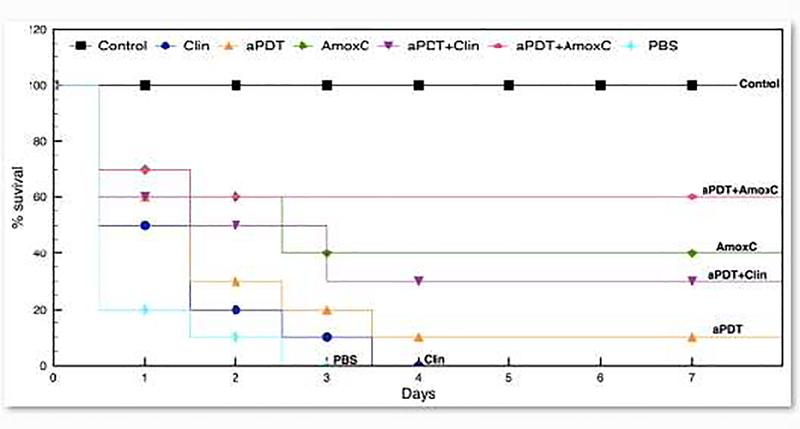
The result of the synergistic effect of aPDT with antibiotics on infections caused by the clinical E. coli strain was subsequently investigated in vivo on the caterpillar Galleria mellonella. The infected G. mellonella were treated with either individual aPDT (180s) and antibiotics, or aPDT (180s) combined with antibiotics. None of the therapies (aPDT or antibiotics) significantly prolonged larvae survival compared to the control group (infected animals treated with PBS). Survival rate showed that after three days, all samples from the control group died (n=20) and animals treated with Clindamycin died after four days. Two animals survived after aPDT treatment and 8 animals survived after Amoxicillin + Clavulanic acid injection. On the other hand, applying aPDT followed by antibiotics treatment decreased significantly the mortality rate of the larvae where 6 animals survived when using Clindamycin after aPDT and 12 animals remained alive when Amoxicillin + Clavulanic acid was used (Figure 5).
Figure 5:
Survival curve of G. mellonella after inoculation of E. coli clinical strain and different therapies. Groups: Control - no infection and no treatment; PBS – infection and administration of saline solution buffer; Clin and AmoxC - infection and administration of 2μg/ml of clindamycin or 20μg/ml of Amoxicilin+Clavulinic acid; aPDT - infection and treatment with MB + 18J light; aPDT+Clin and aPDT+AmoxC - combined therapy, i.e, aPDT followed by antibiotic therapy.
Discussion:
The emergence of antibiotic-resistant bacteria represents one of the major challenges for modern medicine, hence, different alternative therapies, either alone or in combination with antibiotics, have been proposed. One alternative method that has been proposed is aPDT which is useful against local infections and can be used efficiently against resistant and non-resistant strains [19,20]. Previous studies have shown that combining aPDT with antibiotics treatment can render resistant bacteria susceptible to antibiotics [32,35,36]. While some studies have suggested that this effect of aPDT is due to an induced DNA damage in the cell, others have presumed that aPDT induces a damage to the bacterial cell wall resulting in the leakage of cellular contents [37].
Although many studies have concluded that DNA damage occurs, it may not be the primary target of aPDT. For instance, Deinococcus radiodurans which is a bacteria that have a very efficient DNA repair mechanism and one of the most radiation-resistant organisms can be easily killed by aPDT[38]. For this reason, other studies suggested damage at membrane level, especially using short pre-irradiation time and lower energy [28,39].
In the present study, we used different imaging techniques (cryo-ET and fluorescent microscopy) together with other biochemical and in vivo animal experiments to explore the effects of aPDT on antibiotic-resistant Gram-negative bacteria (E. coli). Cryo-ET results showed a tendency of increasing the number of membrane vesicles, bulges and invaginations after aPDT treatment, arguing that an increased in membrane permeability might help killing the cells and/or inducing resistant bacteria to be more susceptible to antibiotics. Interestingly, the cells remained generally intact (at the cryo-ET resolution) after aPDT, unlike the evidently cell lysis that is shown after penicillin treatment. The resolution of our data does not allow us to attest whether there is any aPDT-induced DNA-damage in the cells or not.
The observation of aPDT-induced membrane damage was further supported by fluorescent microscopy and staining with PI. Numerous reports used PI to differentiate the dead or nonviable prokaryotic cells from living ones, since PI is used to stain only cells with irreparable damaged membranes that can be described as nonviable or dead [33,40,41]. Our results showed an increased number of bacteria stained with PI after 1 hour of beta-lactam antibiotic treatment, as well as, after aPDT. The red fluorescence signal was significantly higher when a 18J total energy was applied compared to 3J of total energy (Figure 4). These results are in agreement with the hypothesis of damage on the membrane may occur after aPDT.
One explanation of the membrane damage is the peroxidation of the membrane lipids upon the binding of the cationic MB with the negatively-charged lipopolysaccharide [42]. In addition, our disc diffusion experiments showed that aPDT pre-treatment of resistant bacteria increased their susceptibility to different antibiotics. This last observation was corroborated by in vivo animal experiments which indicated that aPDT followed by antibiotics prolonged larval survival after infection compared to animals treated with antibiotics or aPDT solely. Previous studies of the combined effects of aPDT and antibiotics on Gram positive bacteria also have hypothesized that aPDT could permeabilize the bacterial cell envelope or inactivate cell enzymes, facilitating the penetration and action of antibiotics, leading to the enhancement of effectiveness for both therapies[32,35,36]. However, for the first time, cryo-ET images could show the effects on membrane cell damage, helping to explain the mechanism of envelope damage after aPDT.
The development of new strategies to combat antibiotic-resistant bacteria, especially ESKAPE, has become a worldwide challenge and aPDT is one of the promising methods to alleviate this problem. Although aPDT can be applied only locally, recent evidence has been suggested in combination with other methods, such as conventional antibiotics treatment, it might be an efficient alternative therapy [32,35]. The combined therapy might be helpful during root canal treatment, periimplantitis or even burned wounds repair.
More studies are required to investigate the structural effect of aPDT on the thick cell wall of Gram-positive bacteria and compare it to Gram-negative bacteria investigated in this study. In addition, novel treatment alternatives in the field of dentistry and dermatology should be proposed since it is frequent to encounter localized, superficial or shallow infections that can be affected by resistant bacteria and are potential targets for photodynamic therapy and associated therapies.
Highlights.
aPDT with sub-lethal and lethal doses promote envelope damage in bacteria
aPDT renders the membranes more permeable and thus more sensitive to antibiotics
Electron Cryo-tomography revealed that the effects of photodynamic therapy on Gram negative bacteria promote formation of vesicles and bulges on outer and inner membrane.
Acknowledgements:
This work was supported in part by NIH grant R35 GM122588 (to G.J.J.). M.K. acknowledges a Rubicon postdoctoral fellowship from the Nederlandse Organisatie voor Wetenschappelijk Onderzoek. Cryo-ET work was performed in the Beckman Institute Resource Center for Transmission Electron Microscopy at the California Institute of Technology.
Footnotes
Conflicts of interest/Competing interests: The authors inform no conflit of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].St. Denis TG, Dai T, Izikson L, Astrakas C, Anderson RR, Hamblin MR, Tegos GP, All you need is light, Virulence. 2 (2011) 509–520. doi: 10.4161/viru.2.6.17889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhen X, Lundborg CS, Sun X, Hu X, Dong H, Economic burden of antibiotic resistance in ESKAPE organisms: a systematic review, Antimicrob. Resist. Infect. Control. 8 (2019) 137. doi: 10.1186/s13756-019-0590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Skinner K, Sandoe JAT, Rajendran R, Ramage G, Lang S, Efficacy of rifampicin combination therapy for the treatment of enterococcal infections assessed in vivo using a Galleria mellonella infection model, Int. J. Antimicrob. Agents. 49 (2017) 507–511. doi: 10.1016/j.ijantimicag.2016.12.006. [DOI] [PubMed] [Google Scholar]
- [4].Duplessis C, Biswas B, Hanisch B, Perkins M, Henry M, Quinones J, Wolfe D, Estrella L, Hamilton T, Refractory Pseudomonas Bacteremia in a 2-Year-Old Sterilized by Bacteriophage Therapy, J. Pediatric Infect. Dis. Soc 7 (2018) 253–256. doi: 10.1093/jpids/pix056. [DOI] [PubMed] [Google Scholar]
- [5].Rishi P, Vashist T, Sharma A, Kaur A, Kaur A, Kaur N, Kaur IP, Tewari R, Efficacy of designer K11 antimicrobial peptide (a hybrid of melittin, cecropin A1 and magainin 2) against Acinetobacter baumannii-infected wounds, Pathog. Dis 76 (2018) fty072. doi: 10.1093/femspd/fty072. [DOI] [PubMed] [Google Scholar]
- [6].Garcez AS, Nuñez SC, Hamblin MR, Ribeiro MS, Antimicrobial Effects of Photodynamic Therapy on Patients with Necrotic Pulps and Periapical Lesion, J. Endod 34 (2008) 138–142. doi: 10.1016/j.joen.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mekkawy A, El-Mokhtar M, Nafady N, Yousef N, Hamad M, El-Shanawany S, Ibrahim E, Elsabahy M, In vitro and in vivo evaluation of biologically synthesized silver nanoparticles for topical applications: effect of surface coating and loading into hydrogels, Int. J. Nanomedicine. Volume 12 (2017) 759–777. doi: 10.2147/IJN.S124294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hamblin MR, Antimicrobial photodynamic inactivation: a bright new technique to kill resistant microbes, Curr. Opin. Microbiol. 33 (2016) 67–73. doi: 10.1016/j.mib.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dai T, Huang Y-Y, Hamblin MR, Photodynamic therapy for localized infections—State of the art, Photodiagnosis Photodyn. Ther. 6 (2009) 170–188. doi: 10.1016/j.pdpdt.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wainwright M, Maisch T, Nonell S, Plaetzer K, Almeida A, Tegos GP, Hamblin MR, Photoantimicrobials—are we afraid of the light?, Lancet Infect. Dis. 17 (2017) e49–e55. doi: 10.1016/S1473-3099(16)30268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Allison RR, Photodynamic therapy: oncologic horizons, Futur. Oncol. 10 (2014) 123–124. doi: 10.2217/fon.13.176. [DOI] [PubMed] [Google Scholar]
- [12].Garcez AS, Nuñez SC, Hamblim MR, Suzuki H, Ribeiro MS, Photodynamic Therapy Associated with Conventional Endodontic Treatment in Patients with Antibiotic-resistant Microflora: A Preliminary Report, J. Endod 36 (2010) 1463–1466. doi: 10.1016/j.joen.2010.06.001. [DOI] [PubMed] [Google Scholar]
- [13].Azaripour A, Dittrich S, Van Noorden CJF, Willershausen B, Efficacy of photodynamic therapy as adjunct treatment of chronic periodontitis: a systematic review and meta-analysis, Lasers Med. Sci. 33 (2018) 407–423. doi: 10.1007/s10103-017-2383-7. [DOI] [PubMed] [Google Scholar]
- [14].Ozog DM, Rkein AM, Fabi SG, Gold MH, Goldman MP, Lowe NJ, Martin GM, Munavalli GS, Photodynamic Therapy, Dermatologic Surg 42 (2016) 804–827. doi: 10.1097/DSS.0000000000000800. [DOI] [PubMed] [Google Scholar]
- [15].Garcez AS, Hamblin MR, Methylene Blue and Hydrogen Peroxide for Photodynamic Inactivation in Root Canal - A New Protocol for Use in Endodontics, Eur. Endod. J 2 (2017) 29–29. doi: 10.5152/eej.2017.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].García I, Ballesta S, Gilaberte Y, Rezusta A, Pascual Á, Antimicrobial photodynamic activity of hypericin against methicillin-susceptible and resistant Staphylococcus aureus biofilms, Future Microbiol. 10 (2015) 347–356. doi: 10.2217/fmb.14.114. [DOI] [PubMed] [Google Scholar]
- [17].Hanakova A, Bogdanova K, Tomankova K, Pizova K, Malohlava J, Binder S, Bajgar R, Langova K, Kolar M, Mosinger J, Kolarova H, The application of antimicrobial photodynamic therapy on S. aureus and E. coli using porphyrin photosensitizers bound to cyclodextrin, Microbiol. Res. 169 (2014) 163–170. doi: 10.1016/j.micres.2013.07.005. [DOI] [PubMed] [Google Scholar]
- [18].Garcez AS, Hamblin MR, A New Light on Antibiotic Resistance, EC Microbiol 5 (2017) 201–202. [Google Scholar]
- [19].Cassidy CM, Donnelly RF, Tunney MM, Effect of sub-lethal challenge with Photodynamic Antimicrobial Chemotherapy (PACT) on the antibiotic susceptibility of clinical bacterial isolates, J. Photochem. Photobiol. B Biol. 99 (2010) 62–66. doi: 10.1016/j.jphotobiol.2010.02.004. [DOI] [PubMed] [Google Scholar]
- [20].Maisch T, Resistance in antimicrobial photodynamic inactivation of bacteria, Photochem. Photobiol. Sci. 14 (2015) 1518–1526. doi: 10.1039/C5PP00037H. [DOI] [PubMed] [Google Scholar]
- [21].Oikonomou CM, Chang Y-W, Jensen GJ, A new view into prokaryotic cell biology from electron cryotomography, Nat. Rev. Microbiol. 14 (2016) 205–220. doi: 10.1038/nrmicro.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Garcez AS, Núñez SC, Baptista MS, Daghastanli NA, Itri R, Hamblin MR, Ribeiro MS, Antimicrobial mechanisms behind photodynamic effect in the presence of hydrogen peroxide, Photochem. Photobiol. Sci. 10 (2011) 483–490. doi: 10.1039/c0pp00082e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Iancu CV, Tivol WF, Schooler JB, Dias DP, Henderson GP, Murphy GE, Wright ER, Li Z, Yu Z, Briegel A, Gan L, He Y, Jensen GJ, Electron cryotomography sample preparation using the Vitrobot, Nat. Protoc. 1 (2006) 2813–2819. doi: 10.1038/nprot.2006.432. [DOI] [PubMed] [Google Scholar]
- [24].Tivol WF, Briegel A, Jensen GJ, An Improved Cryogen for Plunge Freezing, Microsc. Microanal. 14 (2008) 375–379. doi: 10.1017/S1431927608080781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kaplan M, Subramanian P, Ghosal D, Oikonomou CM, Pirbadian S, Starwalt-Lee R, Mageswaran SK, Ortega DR, Gralnick JA, El-Naggar MY, Jensen GJ, In situ imaging of the bacterial flagellar motor disassembly and assembly processes, EMBO J. 38 (2019). doi: 10.15252/embj.2018100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kaplan M, Ghosal D, Subramanian P, Oikonomou CM, Kjaer A, Pirbadian S, Ortega DR, Briegel A, El-Naggar MY, Jensen GJ, The presence and absence of periplasmic rings in bacterial flagellar motors correlates with stator type, Elife. 8 (2019). doi: 10.7554/eLife.43487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kaplan M, Sweredoski MJ, Rodrigues JPGLM, Tocheva EI, Chang Y-W, Ortega DR, Beeby M, Jensen GJ, Bacterial flagellar motor PL-ring disassembly subcomplexes are widespread and ancient, Proc. Natl. Acad. Sci. (2020) 201916935. doi: 10.1073/pnas.1916935117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Garcez AS, Núñez SC, Azambuja N, Fregnani ER, Rodriguez HMH, Hamblin MR, Suzuki H, Ribeiro MS, Effects of Photodynamic Therapy on Gram-Positive and Gram-Negative Bacterial Biofilms by Bioluminescence Imaging and Scanning Electron Microscopic Analysis, Photomed. Laser Surg 31 (2013) 519–525. doi: 10.1089/pho.2012.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Soares RB, Costa DH, Miyakawa W, Delgado MGT, Garcez AS, Yoshimura TM, Ribeiro MS, Nunez SC, Photodynamic Activity on Biofilm in Endotracheal Tubes of Patients Admitted to an Intensive Care Unit, Photochem. Photobiol. (2020) php.13239. doi: 10.1111/php.13239. [DOI] [PubMed] [Google Scholar]
- [30].CLSI, Performance Standards for Antimicrobial Susceptibility Testing;, 2013. [Google Scholar]
- [31].Matuschek E, Brown DFJ, Kahlmeter G, Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories, Clin. Microbiol. Infect. 20 (2014) O255–O266. doi: 10.1111/1469-0691.12373. [DOI] [PubMed] [Google Scholar]
- [32].Chibebe Junior J, Fuchs BB, Sabino CP, Junqueira JC, Jorge AOC, Ribeiro MS, Gilmore MS, Rice LB, Tegos GP, Hamblin MR, Mylonakis E, Photodynamic and Antibiotic Therapy Impair the Pathogenesis of Enterococcus faecium in a Whole Animal Insect Model, PLoS One. 8 (2013) e55926. doi: 10.1371/journal.pone.0055926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yang Y, Xiang Y, Xu M, From red to green: the propidium iodide-permeable membrane of Shewanella decolorationis S12 is repairable, Sci. Rep 5 (2016) 18583. doi: 10.1038/srep18583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wensink J, Witholt B, Outer-Membrane Vesicles Released by Normally Growing Escherichia coli Contain Very Little Lipoprotein, Eur. J. Biochem. 116 (1981) 331–335. doi: 10.1111/j.1432-1033.1981.tb05338.x. [DOI] [PubMed] [Google Scholar]
- [35].Di Poto A, Sbarra MS, Provenza G, Visai L, Speziale P, The effect of photodynamic treatment combined with antibiotic action or host defence mechanisms on Staphylococcus aureus biofilms, Biomaterials. 30 (2009) 3158–3166. doi: 10.1016/j.biomaterials.2009.02.038. [DOI] [PubMed] [Google Scholar]
- [36].Barra F, Roscetto E, Soriano A, Vollaro A, Postiglione I, Pierantoni G, Palumbo G, Catania M, Photodynamic and Antibiotic Therapy in Combination to Fight Biofilms and Resistant Surface Bacterial Infections, Int. J. Mol. Sci 16 (2015) 20417–20430. doi: 10.3390/ijms160920417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hamblin MR, Hasan T, Photodynamic therapy: a new antimicrobial approach to infectious disease?, Photochem. Photobiol. Sci. 3 (2004) 436. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].SCHAFER M, High sensitivity of Deinococcus radiodurans to photodynamically-produced singlet oxygen, Int. J. Radiat. Biol. 74 (1998) 249–253. doi: 10.1080/095530098141636. [DOI] [PubMed] [Google Scholar]
- [39].Bertoloni G, Rossi F, Valduga G, Jori G, Ali H, van Lier JE, Photosensitizing activity of water- and lipid-soluble phthalocyanines on prokaryotic and eukaryotic microbial cells., Microbios. 71 (1992) 33–46. http://www.ncbi.nlm.nih.gov/pubmed/1406343. [PubMed] [Google Scholar]
- [40].Sträuber H, Müller S, Viability states of bacteria-Specific mechanisms of selected probes, Cytom. Part A. 77A (2010) 623–634. doi: 10.1002/cyto.a.20920. [DOI] [PubMed] [Google Scholar]
- [41].Davey HM, Life, Death, and In-Between: Meanings and Methods in Microbiology, Appl. Environ. Microbiol. 77 (2011) 5571–5576. doi: 10.1128/AEM.00744-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mertins O, Bacellar IOL, Thalmann F, Marques CM, Baptista MS, Itri R, Physical Damage on Giant Vesicles Membrane as a Result of Methylene Blue Photoirradiation, Biophys. J. 106 (2014) 162–171. doi: 10.1016/j.bpj.2013.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]