Abstract
We present 2 patients with locally advanced conjunctival melanoma for whom definitive surgery would mean an orbital exenteration with its associated inherent total visual loss and major facial disfigurement. Instead both patients were treated with immune checkpoint inhibitor therapy. In one patient neoadjuvant pembrolizumab was used for approximately 12 months and the patient experienced near total clinical resolution of the conjunctival melanoma. Multiple surgical biopsies of very small residual pigmentation showed pigmented macrophages and a complete pathologic response. In the second patient who presented with a locally advanced and metastatic conjunctival melanoma significant shrinkage of conjunctival mass was observed after treatment with a combination of ipilimumab and nivolumab for 5 months and this allowed preservation of the eye and ocular function.
Keywords: conjunctival melanoma, pembrolizumab, nivolumab, ipilimumab, Immunotherapy, PD1 protein
Precis
Immune checkpoint inhibitors such as nivolumab, pembrolizumab, and ipilimumab could be used effectively to decrease the size of locally advanced conjunctival melanoma and avoid orbital exenteration.
Introduction
Conjunctival melanoma is a rare melanoma with an incidence of approximately 2 to 10 cases per 10 million individuals per year, approximately one-tenth the incidence of uveal melanoma.1–3 Initial treatment involves surgery to remove the conjunctival lesion followed by adjuvant treatment, which may include cryotherapy and/or topical chemotherapy. The frequency of local recurrence after initial treatment has been reported to be as high as 30% to 40%, and the risk of nodal metastasis is approximately 15%; the reported disease-specific mortality rate is approximately 20%.4–8 In some patients, eye-preserving surgery is impossible because the conjunctival melanoma involves wide areas of the ocular surface; wide areas of the palpebral conjunctiva; or both eyelids and the caruncle, making eye-preserving surgery impossible. In such patients, an orbital exenteration has historically been the surgical choice, but exenteration leaves the patient with not only visual impairment but also total loss of the eye and surrounding soft tissue and orbitofacial disfigurement.9
Immune checkpoint inhibitors are a relatively new class of drugs that prevent the host’s immune evasion mechanisms by blocking receptors on the surface of activated T-lymphocytes, such as the proteins programmed cell death protein-1 (PD-1) and cytotoxic T-lymphocyte antigen-4 (CTLA-4). Immune checkpoint inhibitors promote an effective immune response to cancer cells. Ipilimumab (Yervoy, Bristol-Myers Squibb, a CTLA-4 inhibitor), pembrolizumab (Keytruda, Merck, a PD-1 inhibitor), and nivolumab (Opdivo, Bristol-Myers Squibb, a PD-1 inhibitor) are FDA approved in the United States for the treatment of metastatic cutaneous melanoma, and Nivolumab and Ipilimumab are also approved for treatment of unresectable cutaneous melanoma.10–12 Pembrolizumab and nivolumab have demonstrated efficacy against locally recurrent and metastatic conjunctival melanoma in a handful of case reports.13–17 Here, we present 2 previously not reported patients with multifocal and locally advanced conjunctival melanoma whose only curative surgical option was orbital exenteration and who were successfully treated with immune checkpoint blockade and were able to avoid an orbital exenteration. This report was prepared according to the guidelines of the Declaration of Helsinki and adhered to the standards set forth by the Health Insurance Portability and Accountability Act.
Case Reports
Patient 1
A 53-year-old otherwise healthy Hispanic woman presented to an outside ophthalmologist with a pigmented lesion in the right lower eyelid palpebral conjunctiva that had grown over the past 8 years. Examination showed additional pigmentation of the right upper and lower bulbar, tarsal, and forniceal conjunctiva as well as the upper punctum, semilunar fold, and caruncle. The AJCC criteria per 8th edition Manual at this presentation was T3bN0M0.
Several conjunctival incisional biopsies were performed and showed primary acquired melanosis with severe atypia / melanoma in situ (Figure 1A–D) with some cells associated with inflammation. Unequivocal invasion was not identified, but subjacent dense lymphohistiocytic inflammatory infiltrate and pigmented macrophages were identified and interpreted as associated regression (Figure 1C, D).
Figure 1.
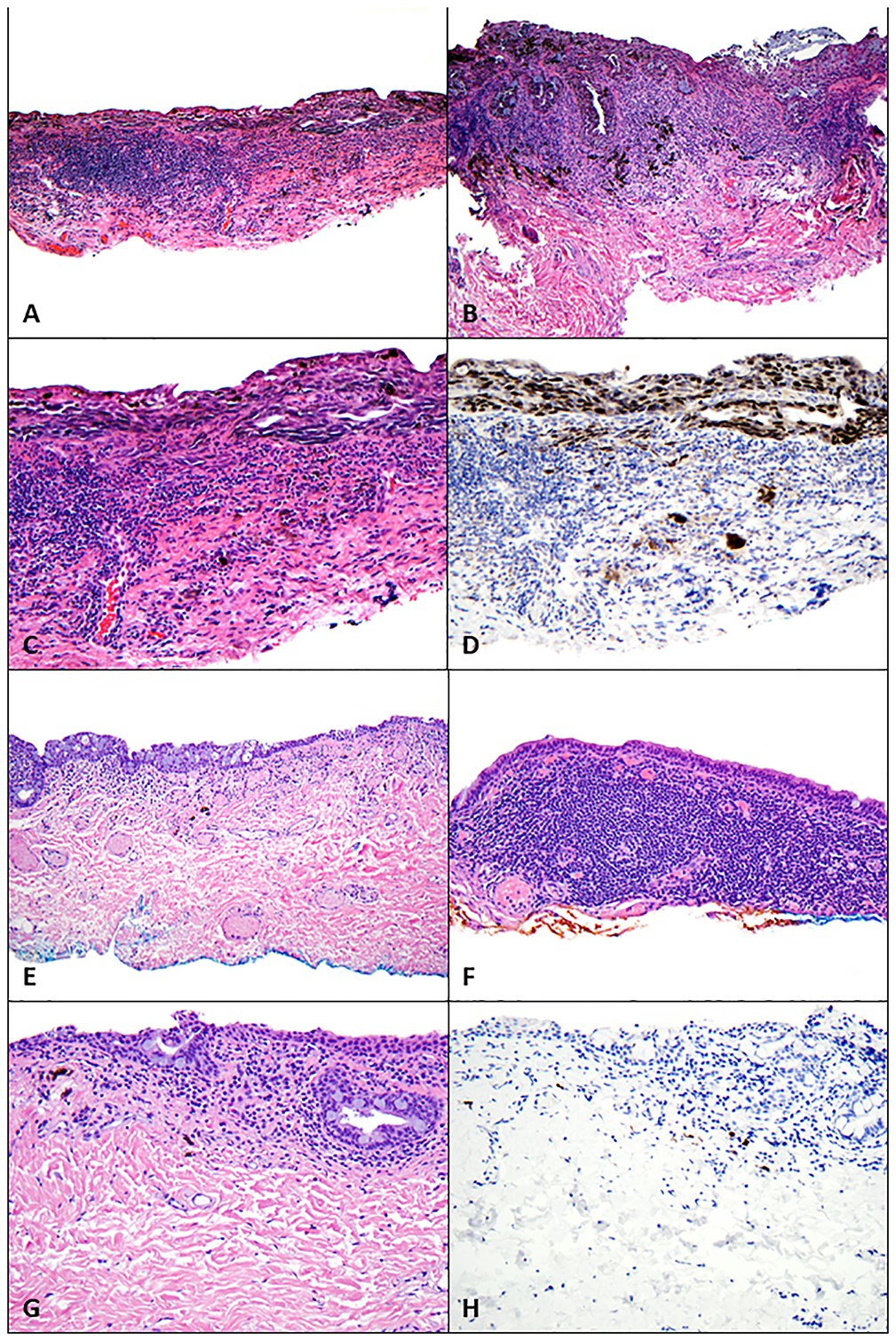
Findings on pathologic examination of conjunctival melanoma at least in situ with underlying regression before and after treatment with immune checkpoint blockade. (A-D) Before treatment with immune checkpoint blockade, biopsies revealed melanoma at least in situ with a dense subjacent lymphohistiocytic inflammatory infiltrate and pigmented macrophages (regression). (A) Scanning magnification shows extensive disease involving the conjunctiva (hematoxylin-eosin [H&E], 40x). (B) A confluent intraepithelial proliferation of atypical melanocytes extends along the glandular epithelium; also present are a dense underlying lymphohistiocytic inflammatory infiltrate and pigmented macrophages (H&E, 100x). (C, D) Atypical melanocytes line the basilar epithelium (C, H&E, 200x) and are highlighted with antibodies for Sox-10 (D, Sox-10, 200x); also present are scattered melanocytes amid the lymphohistiocytic inflammatory infiltrate, which were associated with keratin-positive epithelial cells and thus not interpreted as definitive invasion. (E-H) Following treatment with immune checkpoint blockade, biopsies revealed (E) conjunctiva with a variably dense lymphoplasmacytic inflammatory infiltrate and scattered pigmented macrophages (H&E, 40x). (F) The underlying lymphoplasmacytic inflammatory infiltrate was quite dense in some areas (H&E, 100x). (G) Higher-power magnification did not reveal melanocytes (H&E, 200x), and (I) absence of melanocytes was confirmed with immunohistochemical studies against Sox-10 (Sox-10, 200x).
The patient was subsequently referred to our center for further evaluation and disease management (Figure 2 A,B). Baseline imaging studies were negative for regional or distant metastasis. Given the extent of local disease, the definitive surgery favored to achieve best local control of this lesion was an orbital exenteration. However, after consultation with colleagues in the Department of Melanoma Medical Oncology at our center and discussion of therapy choices with the patient, we proceeded with neoadjuvant systemic pembrolizumab (200 mg intravenously every 3 weeks) prior to any surgical intervention in an attempt to preserve the eye and avoid an orbital exenteration.
Figure 2.
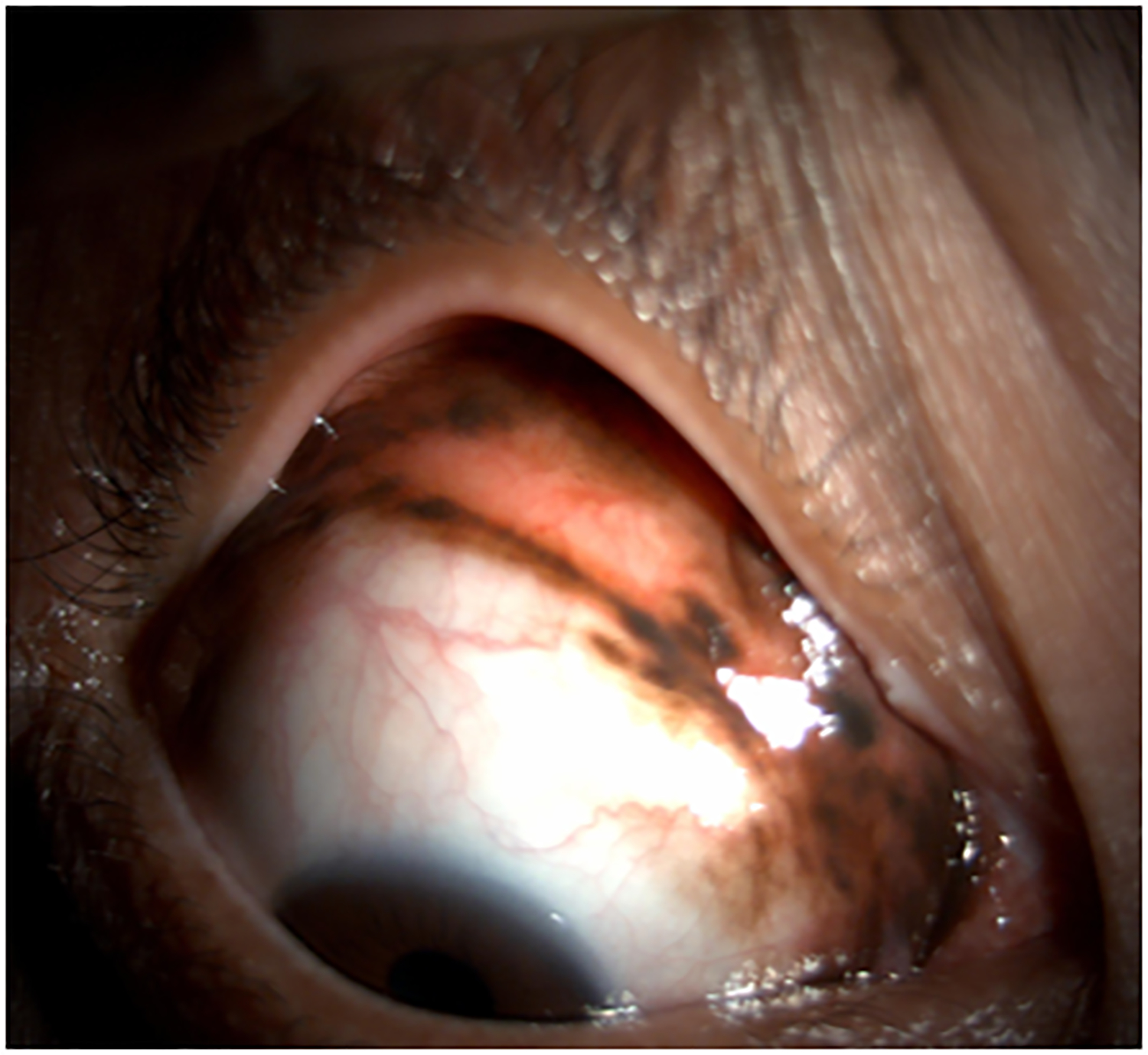
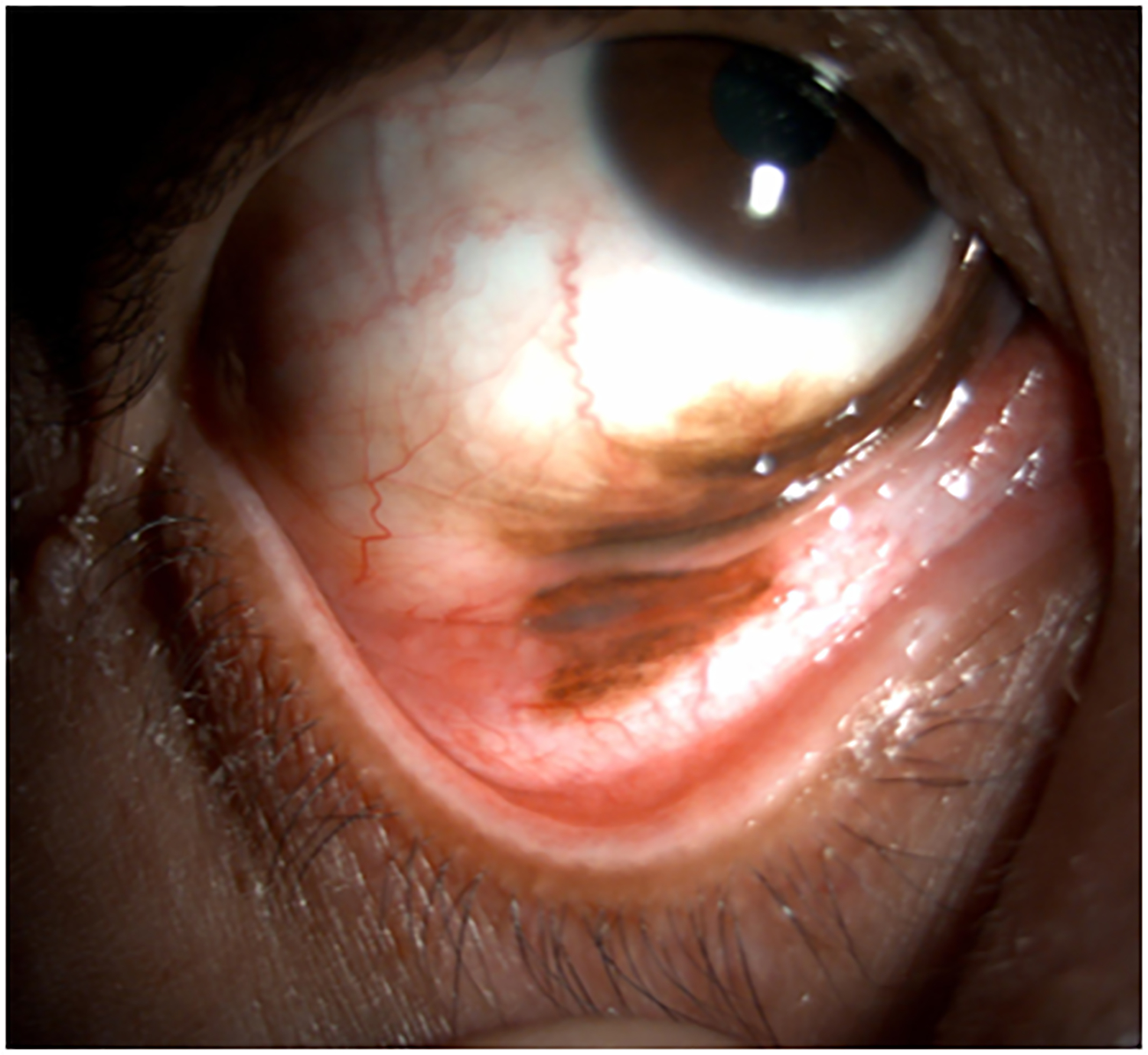
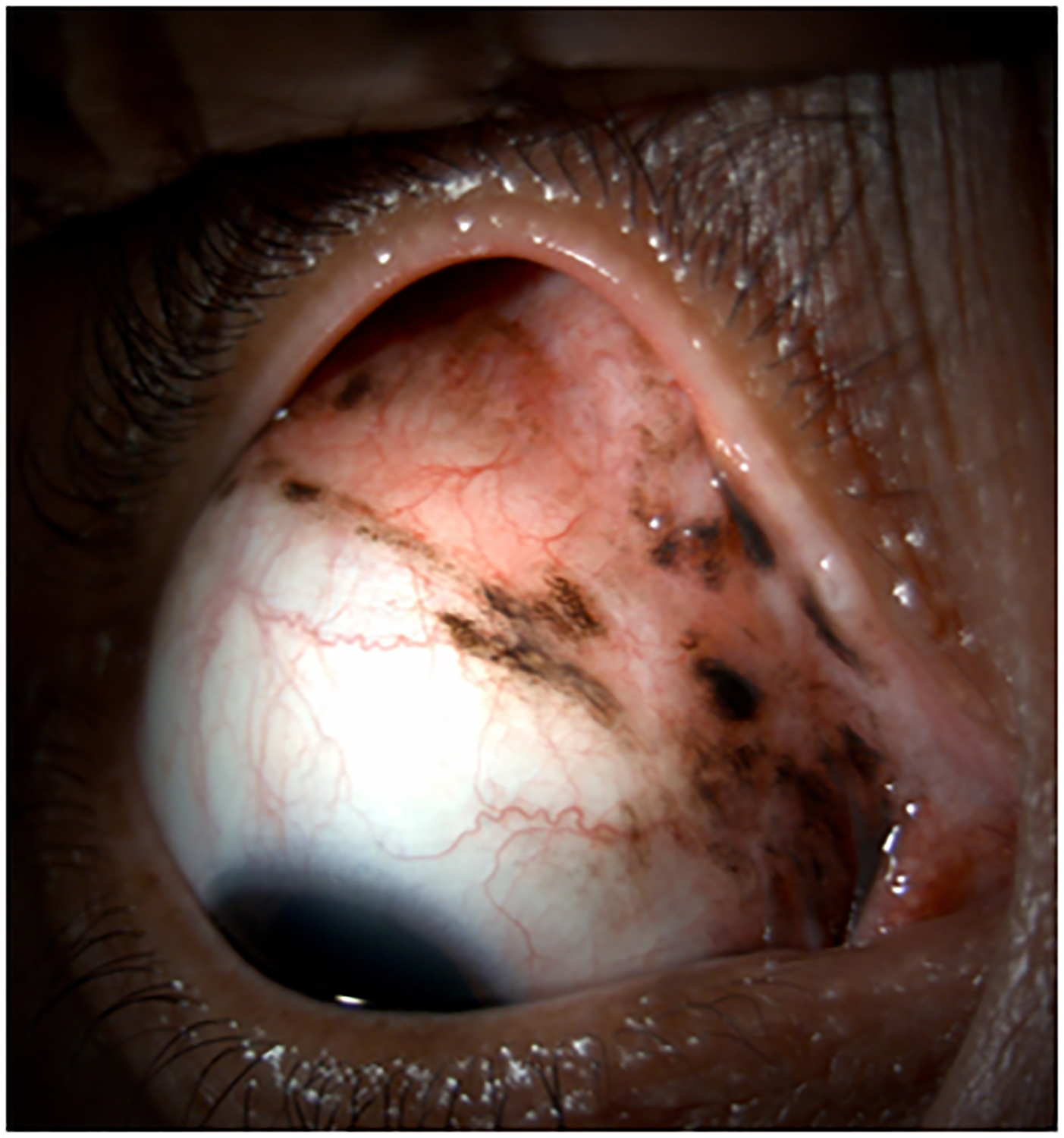
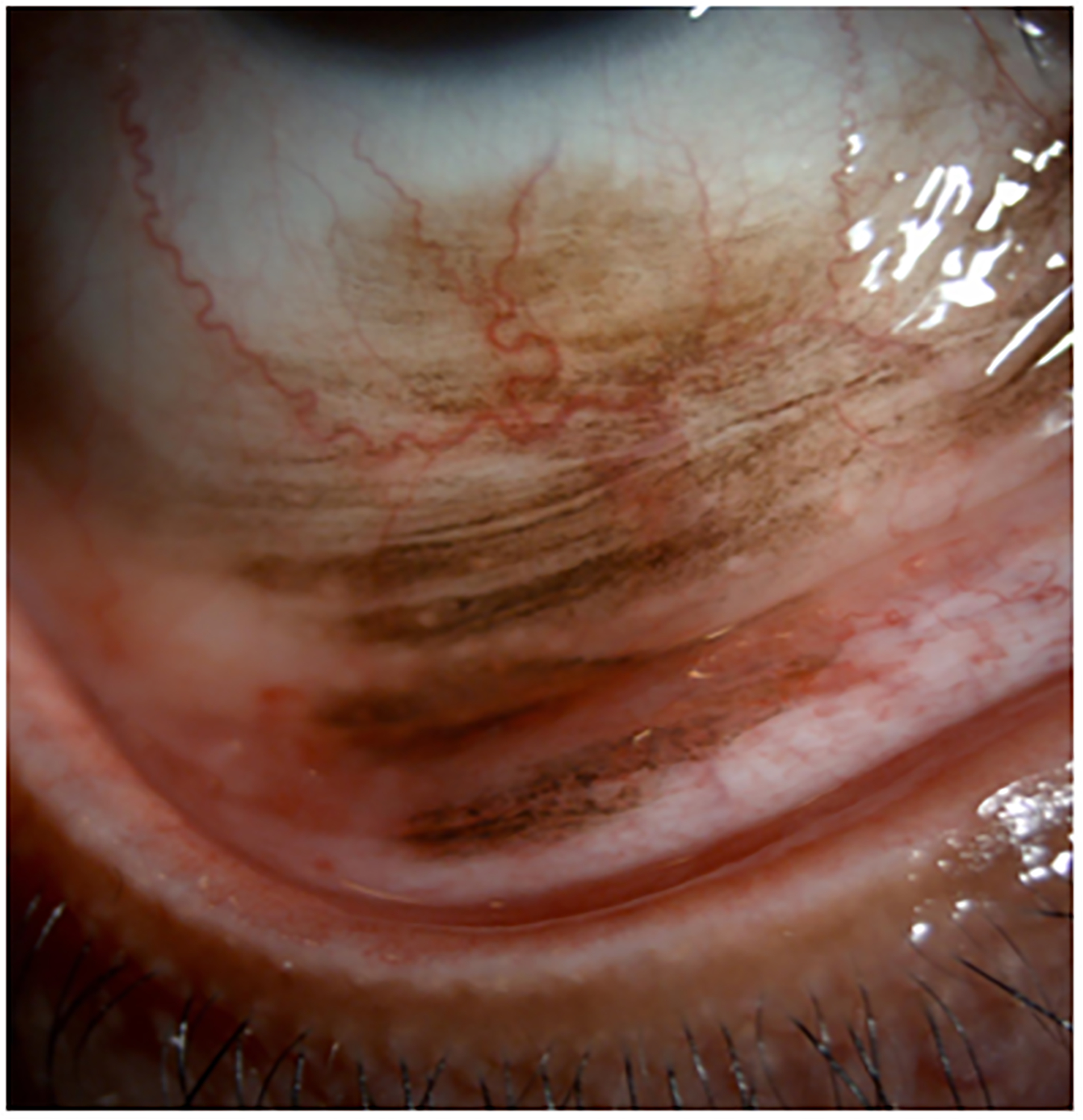
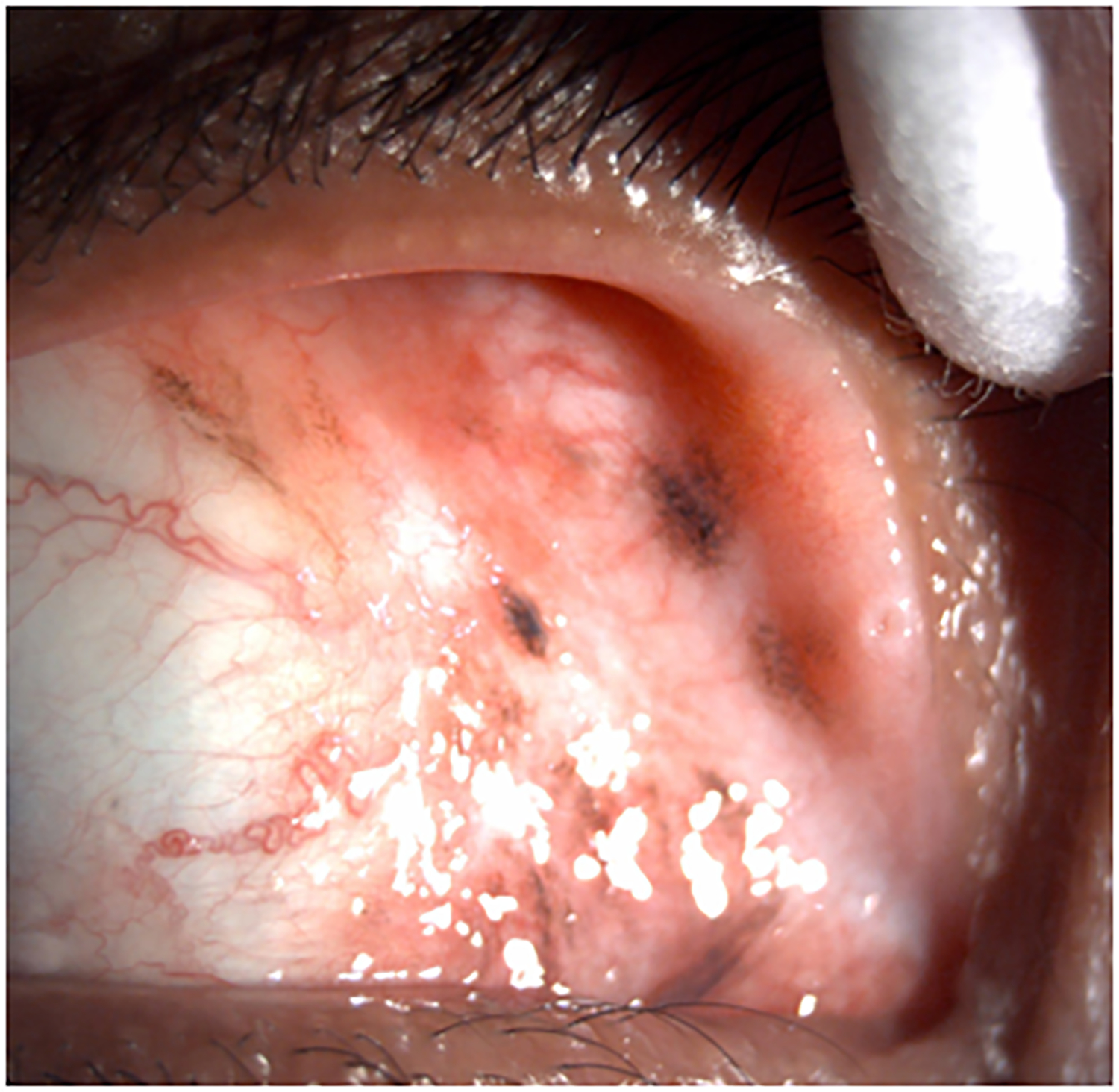
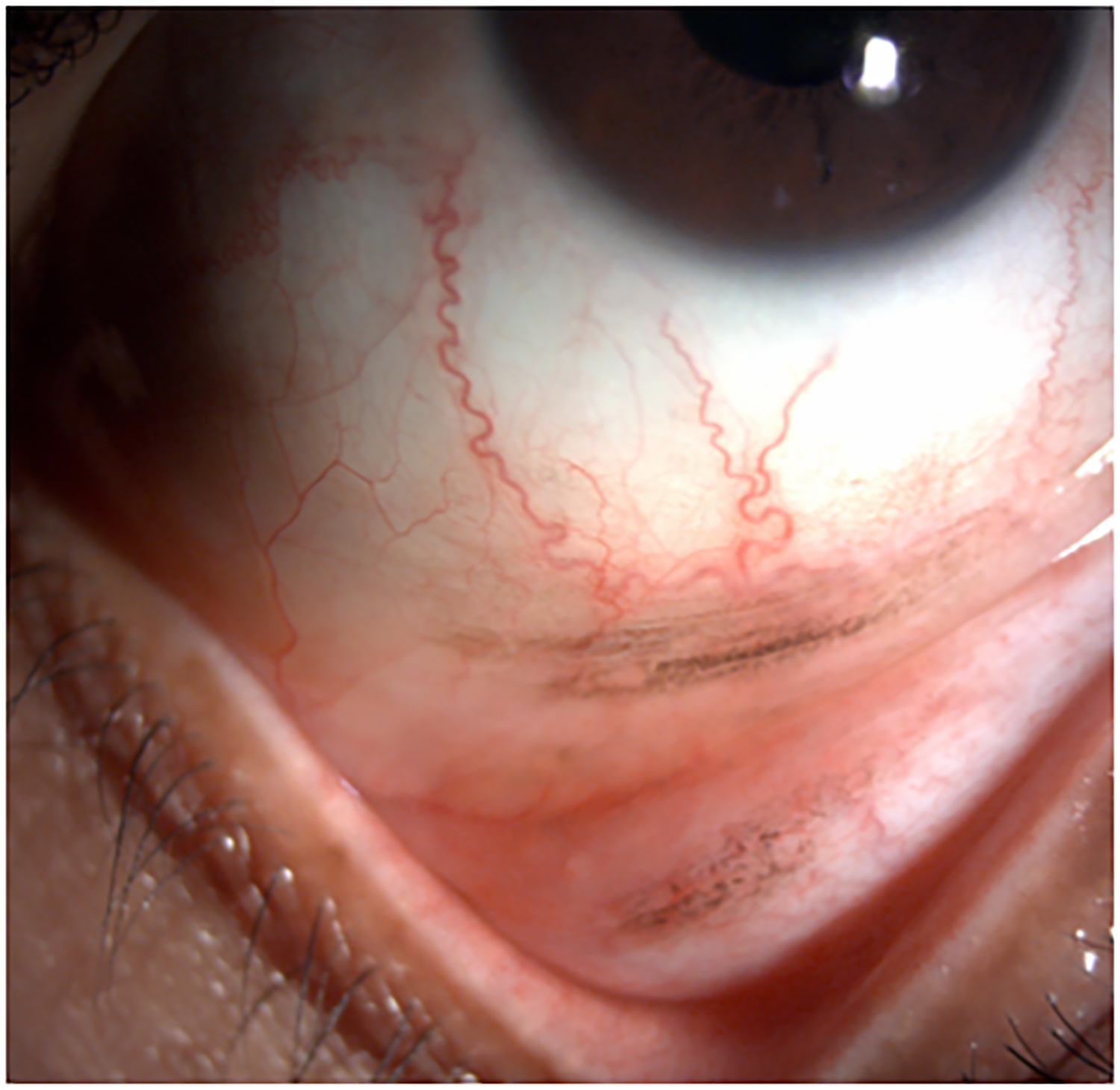
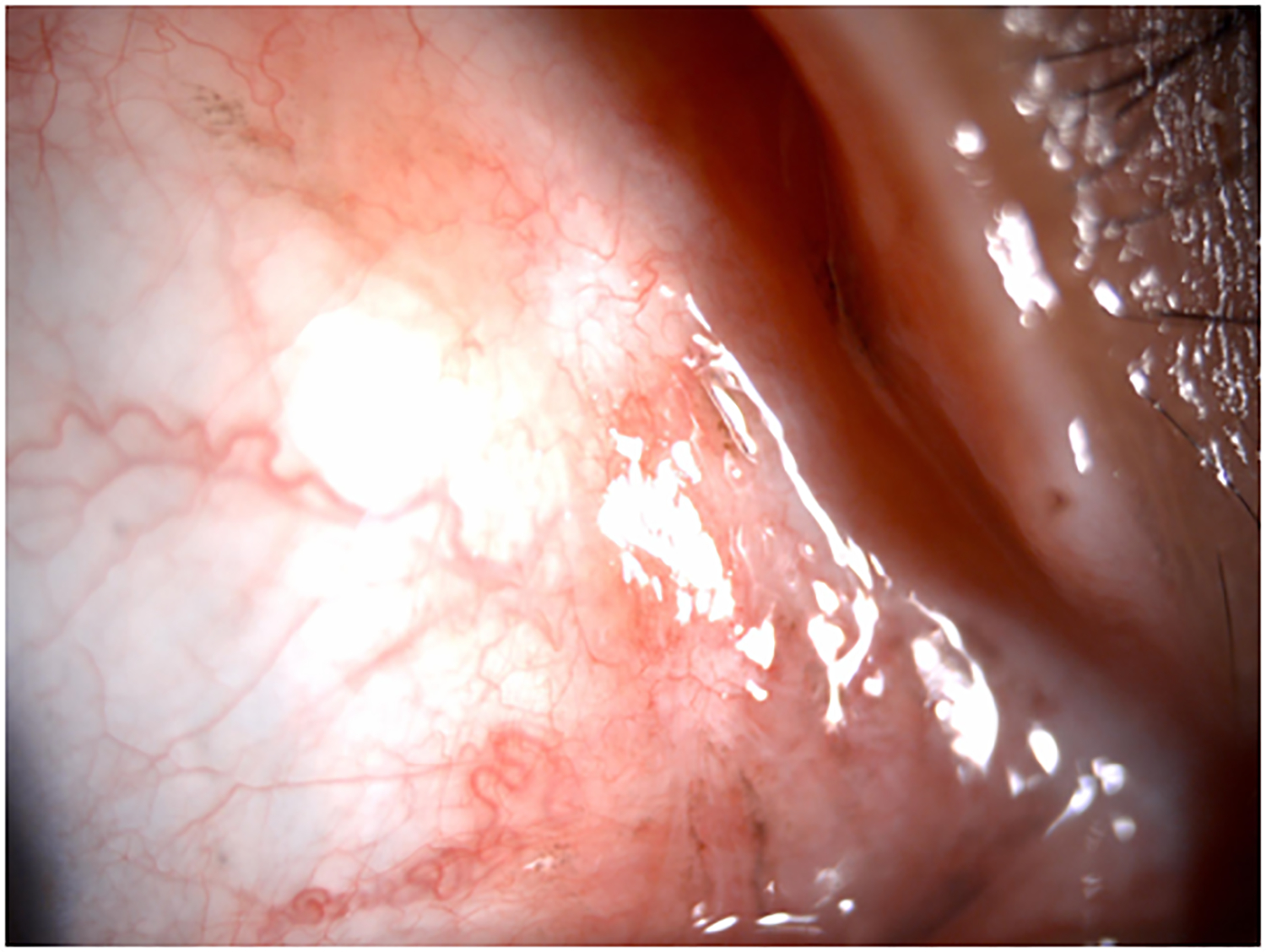
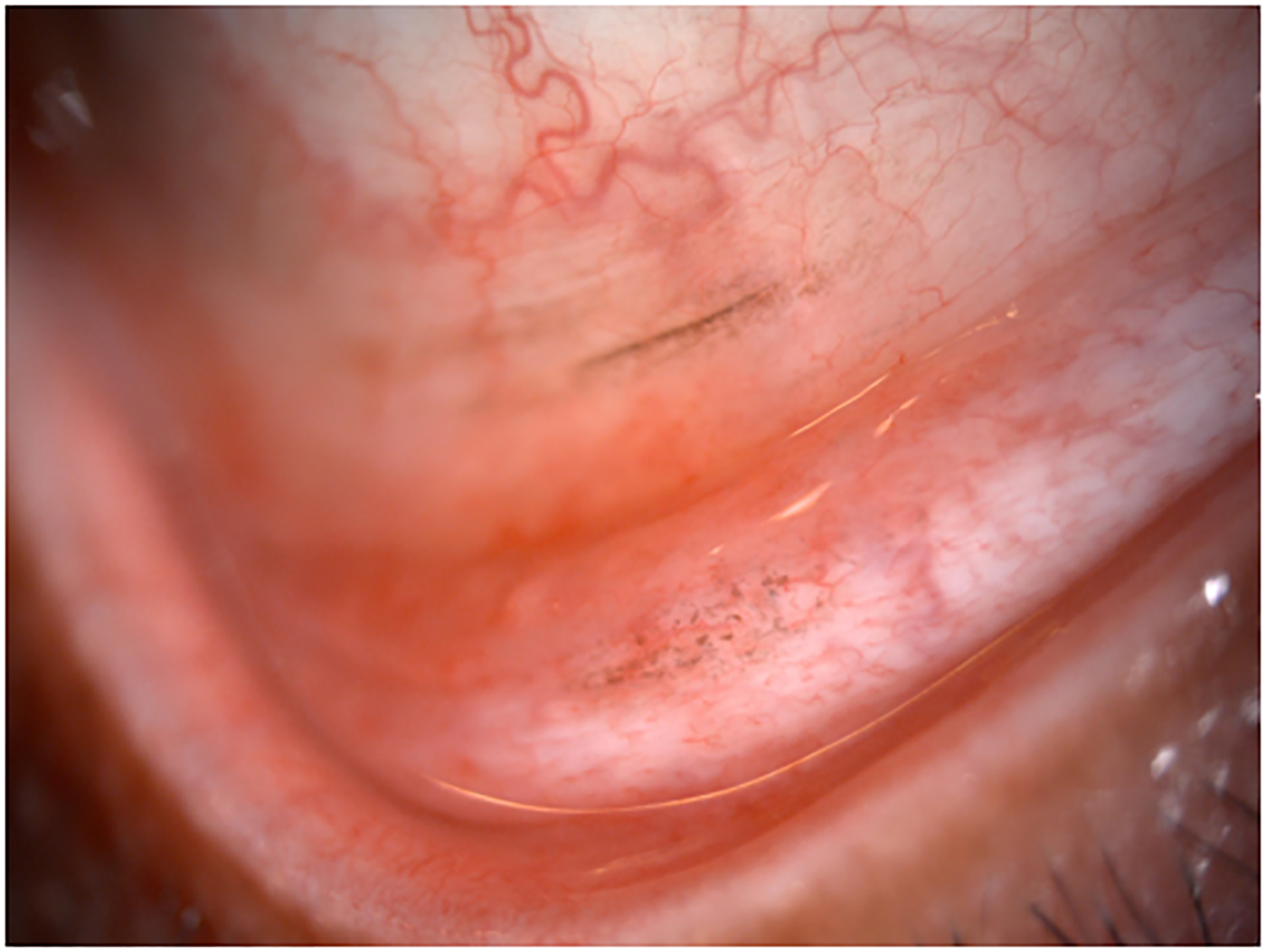
Clinical findings in Patient 1 with extensive conjunctival melanoma before and after treatment with immune checkpoint blockade. (A,B)Before treatment, primary acquired melanosis and wide areas of pigmentation were present involving the bulbar, forniceal, and tarsal conjunctiva as well as the upper punctum, semilunar fold, and caruncle. (C,D)There was modest response after 4 cycles of pembrolizumab. (E,F) After 8 months (10 cycles) of pembrolizumab, there was marked improvement of all sites of pigmentation. After 12 months of therapy with pembrolizumab, there was near-total resolution of all pigmented lesions (G,H). Multiple biopsies of all residual conjunctival pigmentation showed pigmented macrophages and no melanoma cells.
By the end of the fourth cycle of pembrolizumab, modest reduction in pigmentation was noted on the clinical examination (Figure 2 C,D). At this point, topical mitomycin C (0.04%) drops were added in an effort to expedite the response. Mitomycin C was delivered in 2-week cycles consisting of mitomycin C (0.02 %) 4 times a day for 4 days followed by 10 days of no treatment. The pigmentation did not improve further after 4 cycles of mitomycin C, and it was stopped. After 8 months (10 cycles) of pembrolizumab, the patient showed marked improvement of all sites of pigmentation in the right eye (Figure 2 E,F)
Pembrolizumab therapy was stopped after 12 months (15 cycles) because only very faint conjunctival pigmentation remained (Figure 2 G,H). Biopsy of these lesions revealed conjunctival tissue with a variably dense lymphohistiocytic inflammatory infiltrate with scattered pigmented macrophages (Figure 1E–H). Immunohistochemical studies with antibodies against Sox-10 did not demonstrate persistent melanoma. Thus, the patient experienced a complete histologic response.
As of this writing, the patient has been followed for 12 months without any treatment and remains disease free. She experienced no significant side effects from immunotherapy other than mild generalized cutaneous pruritus.
Patient 2
A 66-year-old man presented with a large pigmented upper fornix mass (Figure 3A). He gave a history of recurrent red eye that was attributed to subconjunctival hemorrhage, prior to noting pigmented patches appearing in the superior bulbar conjunctiva. The mass had been present for approximately three months prior to presentation.
Figure 3.
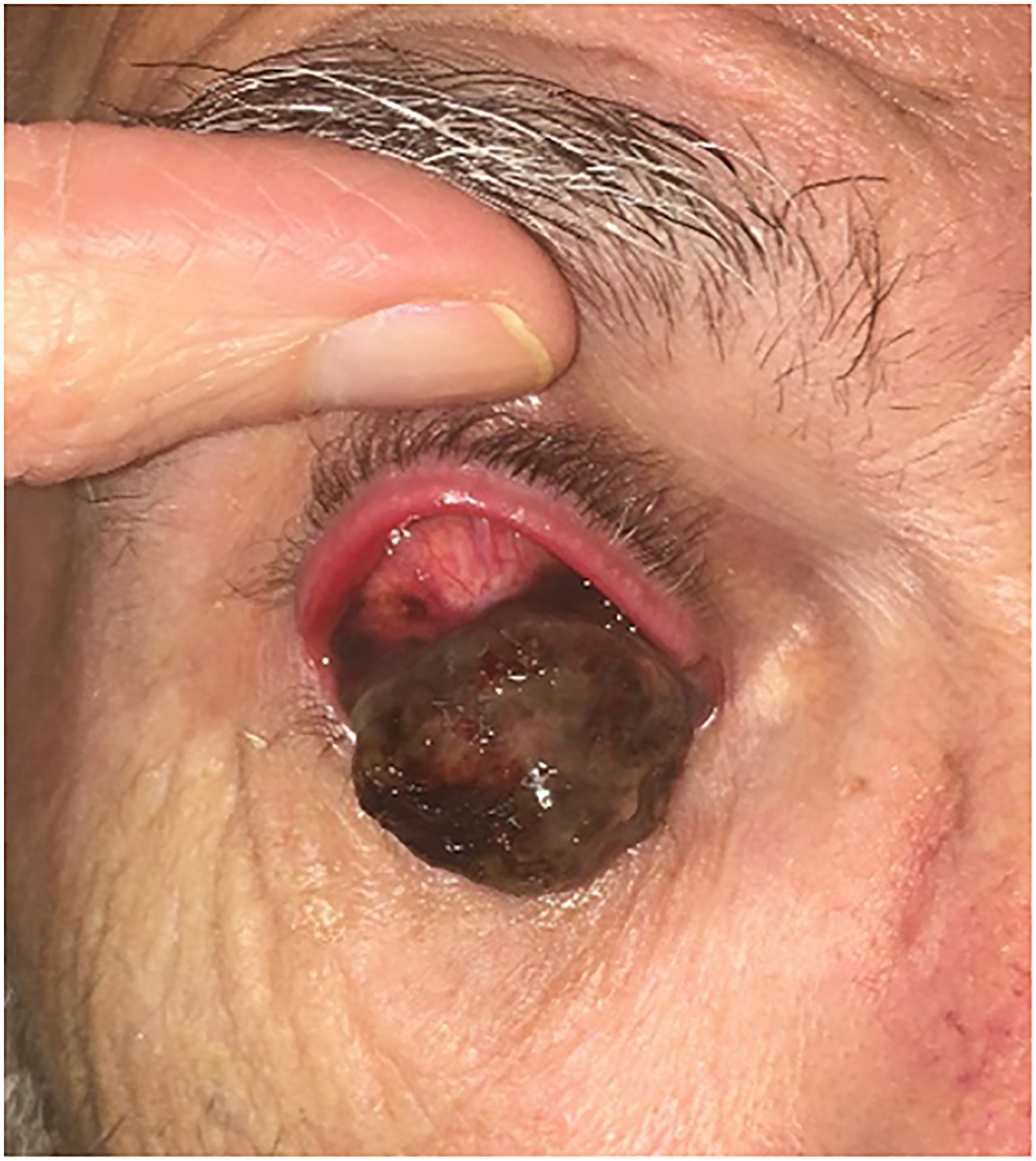

Clinical findings in Patient 2 with a massive invasive conjunctival melanoma involving the upper bulbar conjunctiva before and after treatment with immune checkpoint blockade. A) Before treatment, the patient had very bulky disease involving the superior bulbar conjunctiva and upper eyelid tarsal plate B) After 6 cycles of therapy with ipilimumab and nivolumab combination therapy he experienced remarkable response with only a very trace amount of pigment remaining on the upper tarsal plate as seen in this external photograph.
Biopsies taken of the conjunctival mass demonstrated invasive conjunctival melanoma adjacent to areas of primary acquired melanosis with severe atypia. Mapping biopsies indicated the presence of melanosis with severe atypia in the inferior fornix as well. Computed-tomography (CT) imaging of the orbits demonstrated the posterior extent of the mass to be anterior to the insertion of the rectus muscles. Staging whole-body PET scan demonstrated metastatic deposits within the lung and liver. These findings were typical of metastatic melanoma thus a specific biopsy of the lung and liver metastasis was not done at the discretion of the treating oncologist. The AJCC criteria per 8th edition Manual at this presentation was T3aN0M1.
The patient was started on combination immune checkpoint inhibitor therapy with ipilimumab and nivolumab for his locally advanced and metastatic conjunctival melanoma. Over a treatment course of 5 months (6 cycles of therapy) with ipilimumab and nivolumab he demonstrated dramatic resolution of the subtarsal mass (Figure 3B). His systemic immunotherapy was complicated by pituitary failure which was treated with replacement hydrocortisone and thyroxine. The distant metastatic sites in lung and liver also responded nicely to immunotherapy based on the follow-up CT imaging.
At the patient’s most recent visit, 9 months after the initial presentation, the best corrected vision in this eye remained 20/20 with no diplopia and a full range of extraocular motility. Continued observation of the residual very small amount of pigmentation on the tarsal plate was advised.
Discussion
Here, we present two patients with locally advanced conjunctival melanoma who had impressive local responses to immune checkpoint inhibitor therapy that was administered in an attempt to avoid orbital exenteration. Pembrolizumab was selected for Patient 1 because pembrolizumab is approved for treatment of metastatic cutaneous melanoma; cutaneous and conjunctival melanoma share molecular and genetic characteristics; and pembrolizumab has previously shown some efficacy in a few patients with metastatic conjunctival melanoma.14,15,17 In the second case treated in the United Kingdom the patient presented with locally advanced conjunctival melanoma with eyelid and anterior orbital extension and also with distant metastatic sites. His surgical options for the orbital area were initially very limited and only palliative orbital exenteration could be offered. However the dramatic response to immunotherapy using a combination of ipilimumab and nivolumab led to a significant response of his ocular tumor shrinking it to a resectable size with minimal eye-preserving surgery vs. observation as an option. The choice of which immune checkpoint inhibitor or combination to use in our patients was based on the treating oncologist’s choice and availability of drugs at the treating centers in the United States and the United Kingdom, respectively.
In a review of the literature, we found few reports of immune checkpoint inhibitor therapy for treatment of conjunctival melanoma.13–18 Kini et al reported efficacy of pembrolizumab against locally recurrent conjunctival melanoma; the patient in their report was treated with pembrolizumab for 6 months before re-excision and had reached 1 year of remission at the time of writing.13 Sagiv et al, in 2018, reported on 5 patients with metastatic conjunctival melanoma who were successfully treated with anti-PD-1 immunotherapy (both pembrolizumab and nivolumab) at our center.14 Our center’s positive experience with the use of immunotherapy in these 5 patients with metastatic conjunctival melanoma encouraged us to try this same strategy as an eye-preserving treatment in the patients described in this report. PD1 and PDL-1 expression in conjunctival melanoma has been reported by several groups.19
Admittedly, pembrolizumab for treatment of locally advanced conjunctival melanoma as was the case in Patient 1 is an off-label use of anti-PD-1 therapy; however, given the significant functional, cosmetic, and quality-of-life consequences of orbital exenteration, which was the only surgical alternative for our patient, we feel that the use of anti-PD-1 immunotherapy for this patient was justified. The biopsy specimens examined showed a pathologic complete response in the sections examined, and the patient remains without evidence of disease at this writing, 12months after discontinuation of anti-PD-1 therapy. She will be followed carefully for possible future local recurrences, but even if she were to have a recurrence, less morbid surgery than orbital exenteration or a rechallenge with the same immunotherapy would likely be possible assuming early detection of small foci of recurrent melanoma on the conjunctival surface. In Patient 2 who presented with locally advanced and metastatic conjunctival melanoma the dramatic clinical response to combination therapy with nivolumab and ipilimumab allowed with preservation of ocular function and likely prolonged survival.
The common side effects of immunotherapy are a skin rash, colitis, pruritus, autoimmune hepatitis, pneumonitis, and endocrinopathies such as hypophysitis and thyroid abnormalities. Orbita and adnexal toxicity are as follows: inflammatory myositis and extraocular muscle enlargement, uveitis, episcleritis and conjunctivitis. Sever toxicities are more common with ipilimumab (10–15%) compared with nivolumab or pembrolizumab (5%).20,21
Supplementary Material
Conflicts of Interest and Source of Funding:
Dr. Glitza received research funding from Merck. The other authors have no conflicts of interest to disclose. The authors received no funding specifically for this work.
References
- 1.Kastelan S, Fverovic A, Beketic O, Salopek Rabatić J, Kasun B, Bakija I. Conjunctival melanoma—epidemiological trends and features. Pathol Oncol Res 2018; 24:787–796. [DOI] [PubMed] [Google Scholar]
- 2.Dalvin LA, Salomao DR, Patel SV. Population-based incidence of conjunctival tumors in Olmsted County, Minnesota. Br J Ophthalmol 2018; 102:1728–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu GP, Hu DN, McCormick S, Finger PT. Conjunctival melanoma: is it increasing in the United States? Am J Ophthalmol 2003; 135:800–806. [DOI] [PubMed] [Google Scholar]
- 4.Savar A, Esmaeli B, Ho H, Liu S, Prieto VG. Conjunctival melanoma: local-regional control rates, and impact of high-risk histologic features. J Cutan Pathol 2011; 38:18–24. [DOI] [PubMed] [Google Scholar]
- 5.Esmaeli B, Rubin ML, Xu S, Goepfert RP, Curry JL, Prieto VG, et al. Greater tumor thickness, ulceration, and positive sentinel lymph node are associated with worse prognosis in patients with conjunctival melanoma: implications for future AJCC classifications. Am J Surg Pathol 2019. August 14 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Shields CL, Kaliki S, Al-Dahmash SA, Lally SE, Shields JA. American Joint Committee on Cancer (AJCC) clinical classification predicts conjunctival melanoma outcomes. Ophthal Plast Reconstr Surg 2012; 28:313–323. [DOI] [PubMed] [Google Scholar]
- 7.Vora GK, Demirci H, Marr B, Mruthyunjaya P. Advances in the management of conjunctival melanoma. Surv Ophthalmol 2017; 62:26–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brouwer NJ, Marinkovic M, vanDuinen SG, Bleeker JC, Jager MJ, Luyten GPM. Treatment of conjunctival melanoma in a Dutch referral center. Br J Ophthalmol 2018; 102:1277–1282. [DOI] [PubMed] [Google Scholar]
- 9.Bonanno A, Esmaeli B, Fingeret MC, Nelson DV, Weber RS. Social challenges of cancer patients with orbitofacial disfigurement. Ophthal Plast Reconstr Surg 2010; 26:18–22. [DOI] [PubMed] [Google Scholar]
- 10.US-FDA. FDA labeling information - YERVOY. FDA website; Online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125377s074lbl.pdf. 2015. [Google Scholar]
- 11.US-FDA. FDA labeling information - KEYTRUDA. FDA website; Online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125514s015lbl.pdf. 2017. [Google Scholar]
- 12.US-FDA. FDA labeling information - OPDIVO. FDA website; Online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125554s024lbl.pdf. 2017. [Google Scholar]
- 13.Kini A, Fu R, Compton C, Miller DM, Ramasubramanian A. Pembrolizumab for recurrent conjunctival melanoma. JAMA Ophthalmol 2017; 135:891–892. [DOI] [PubMed] [Google Scholar]
- 14.Sagiv O, Thakar SD, Kandl TJ, Ford J, Sniegowski MC, Hwu WJ, et al. Immunotherapy with programmed cell death 1 inhibitors for 5 patients with conjunctival melanoma. JAMA Ophthalmol 2018; 136:1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esmaeli B, Sagiv O. Targeted biological drugs and immune check point inhibitors for locally advanced or metastatic cancers of the conjunctiva, eyelid, and orbit. Int Ophthalmol Clin 2019; 59:13–26. [DOI] [PubMed] [Google Scholar]
- 16.Pinto Torres S, André T, Gouveia E, Costa L, Passos MJ. Systemic treatment of metastatic conjunctival melanoma. Case Rep Oncol Med 2017; 2017:4623964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford J, Thuro BA, Thakar S, Hwu W-J, Richani K, Esmaeli B. Immune checkpoint inhibitors for treatment of metastatic melanoma of the orbit and ocular adnexa. Ophthalmic Plast Reconstr Surg 2017; 33:e82–e85. [DOI] [PubMed] [Google Scholar]
- 18.Chang M, Lally SE, Dalvin LA, et al. Conjunctival melanoma with orbital invasion and liver metastasis managed with systemic immune checkpoint inhibitor therapy. Indian J Ophthalmol. 2019;67:2071–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao J, Brouwer NJ, Richards KE, et al. PD-L1/PD-1 expression and tumor-infiltrating lymphocytes in conjunctival melanoma Jinfeng Oncotarget, 2017; 8:54722–54734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esmaeli B, Sagiv O. Targeted Biological Drugs and Immune Check Point Inhibitors for Locally Advanced or Metastatic Cancers of the Conjunctiva, Eyelid, and Orbit. Int Ophthalmol Clin 59(2):13–26, 2019. [DOI] [PubMed] [Google Scholar]
- 21.Sagiv O, Kandl TJ, Thakar SD, et al. Extraocular Muscle Enlargement and Thyroid Eye Disease-like Orbital Inflammation Associated with Immune Checkpoint Inhibitor Therapy in Cancer Patients. Ophthalmic Plast Reconstr Surg 35(1):50–52, Jan-Feb, 1/2019. e-Pub 6/2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


