Abstract
Background/Objectives
To estimate associations between lower urinary tract symptoms (LUTS) and phenotypic frailty in older men.
Design
Cross-sectional study.
Setting
Community-dwelling men recruited 2000–2002 from 6 U.S. academic centers for the Osteoporotic Fractures in Men (MrOS) Study.
Participants
5,979 men age 65 years or older.
Measurements
The independent variable was LUTS severity (none/mild, moderate, severe) assessed with the American Urologic Association Symptom Index (AUASI). Participants were categorized as frail, intermediate stage, or robust using an adapted Cardiovascular Health Study index (components: low lean mass, weakness, exhaustion, slowness, and low physical activity). Associations were estimated with odds ratios and 95% confidence intervals (CI) from multivariable multinomial logistic regression models adjusted for potential confounders including age, other demographics, health-related behaviors, and comorbidities.
Results
The prevalence of frailty was 7%, 11%, and 18% among men with none/mild, moderate, and severe LUTS, respectively. Moderate and severe LUTS, overall and by storage and voiding subscores, were associated with higher odds of both intermediate stage and frailty in all models. After adjustment for confounders, the odds of frailty was 1.41 times higher among men with moderate LUTS (95% CI 1.14, 1.74) and 2.51 times higher among men with severe LUTS (95% CI 1.76, 3.55), compared to none/mild LUTS. Severe LUTS was associated with a greater odds of individual frailty components exhaustion and low physical activity.
Conclusion
The prevalence of phenotypic frailty is higher among older community-dwelling men with moderate or severe LUTS compared to those with mild or no LUTS. The positive association between LUTS severity and frailty among older men appears independent of age and known frailty risk factors. Although the temporal direction of this association and the utility of LUTS or frailty interventions in this population remain unclear, the high co-occurrence of these conditions could lead to earlier identification of frailty when clinically appropriate.
Keywords: Lower Urinary Tract Symptoms, Urinary Incontinence, Frailty, Geriatric Syndrome (1–5 MESH)
INTRODUCTION
More than half of men will develop lower urinary tract symptoms (LUTS) by age 70, leading to reduced health-related quality of life.4 LUTS are a complex syndrome of overlapping and chronic symptoms that occur during urine storage (urgency, frequency, nocturia, incontinence) or voiding (weak or intermittent stream, hesitancy, straining, incomplete bladder emptying).5 LUTS in older men are often solely attributed to an enlarged prostate causing bladder outlet obstruction and widely treated with medications that are effective at reducing obstruction but only minimally improve symptoms.6,7 However, the pathophysiology of LUTS in older men remains poorly understood, which has limited our ability to effectively treat this condition. Meanwhile, there is an increasing appreciation for the potential harms of current LUTS medications, including falls, depression and suicidality, delayed prostate cancer diagnosis, and dementia.8–11 This lack of progress for a condition that affects a majority of men in the 7th and 8th decade of life has motivated a renewed effort to identify novel age-related risk factors and consequences of LUTS.
LUTS in older men share many similarities with geriatric syndromes (e.g. delirium, depression, dementia, falls); they are multifactorial with overlapping urologic and non-urologic causes and have both physical and psychological manifestations.12 Although both LUTS and frailty are associated with older age13 and increased risk of falls14, it remains unknown if LUTS are independently associated with frailty. LUTS is also a heterogenous syndrome and associations with frailty may differ by storage or voiding symptoms, which would inform future mechanistic investigations and interventions. To our knowledge only a few small studies with important limitations have evaluated the association between LUTS and frailty in older men.1–3 A better understanding of how LUTS are related to frailty will inform whether these two age-related conditions are likely to co-occur among men of similar age and risk factor profile.
To address this gap in knowledge, we evaluated the association of LUTS severity, overall and by storage and voiding subscores, with phenotypic frailty in a large cohort of older, community-dwelling men. We hypothesized that severe LUTS would be associated with a higher prevalence of frailty.
METHODS
Participants
The Osteoporotic Fractures in Men (MrOS) study is a large, multicenter cohort study of 5,994 community-dwelling men age 65 years or older as previously described.15,16 Briefly, this cohort was designed to collect comprehensive data to study older men’s health, with a particular focus on falls, fractures, and genitourinary diseases. Men were recruited from March 2000 to April 2002 from six academic medical centers in Birmingham, Alabama, Minneapolis, Minnesota, Palo Alto, California, Pittsburgh, Pennsylvania, Portland, Oregon, and San Diego, California. All participants gave written informed consent, and Institutional Review Boards at each participating institution approved the study.
LUTS Assessment
LUTS were assessed using the validated and widely used 7-item American Urologic Association Symptom Index (AUASI)5, including individual items on urinary frequency, urgency, intermittency, straining, weak urinary stream, incomplete bladder emptying, and nocturia. Responses to each item are on an ordinal scale with values ranging from 0 to 5, with 0 representing no symptoms and 5 representing the highest symptom burden (e.g., frequency of difficulty postponing urination reported as “Almost Always”); total scores range from 0 to 35. AUASI also has clinically relevant categories of 0 to 7 (none/mild), 8 to 19 (moderate), and 20 to 35 (severe).17 In addition to the total score, we calculated AUASI subscores separately for storage symptoms (urgency, frequency, nocturia) and for voiding symptoms (incomplete emptying, intermittency, weak stream, straining), consistent with the literature.18 For example, to evaluate the storage symptom of urgency men were asked “Over the past month, how often have you found it difficult to postpone urination?” and to evaluate the voiding symptom of incomplete emptying men were asked “Over the past month, how often have you had a sensation of not emptying your bladder completely after you finish urinating?” Response options included “Not at all”, “Less than 1 time in 5”, “Less than half the time”, “About half the time”, “More than half the time”, or “Almost always”. Because subscores do not have established thresholds, we categorized them into approximately equal thirds (tertiles) for analysis.
Other Measurements
Age, race, education, marital status, smoking status, alcohol consumption, instrumental activities of daily living (IADL) were assessed via self-administered questionnaires.15 Comprehensive prescription medication use was coded from labels on pill packets and canisters brought in by the participant and medications to treat LUTS (α-antagonist, 5α-reductase, or anti-cholinergic) were identified using the Iowa Drug Information System (IDIS).19. Multimorbidity was defined as the cumulative number of the following self-reported physician-diagnosed chronic medical conditions as in prior studies: stroke, Parkinson’s disease, myocardial infarction, angina, chronic obstructive pulmonary disease, heart failure, diabetes mellitus, osteoporosis, osteoarthritis, hyperthyroidism or hypothyroidism.20 Cognitive function was assessed using the Teng Modified Mini-Mental State Examination (3MS).21 All participants completed the Medical Outcomes Study Short Form (SF-12), and the mental health component score was used to screen participants for psychological distress.22 Urinary incontinence was assessed by asking “Over the past month, how often did you drip or leak urine?” Men were asked if a doctor had told them they “have or had an enlarged prostate (benign prostatic hyperplasia)” and if so, they were asked if they received “Surgery”, “Prescription medications”, or “Other” treatments for this condition, which was used to define self-reported benign prostatic hyperplasia surgery.
Frailty Phenotype Component Measurements
For determining frailty status, physical activity was assessed using the Physical Activity Scale for the Elderly (PASE).23 Tests of physical function included grip strength (using a hand-held Jamar dynamometer) and walk speed (time in seconds to walk 6 meters at usual pace expressed as m/sec). Body weight and height measurements were used to calculate a standard body mass index (BMI). Appendicular skeletal muscle mass, as the measure of lean mass, as well as body fat were determined using dual-energy x-ray absorptiometry (DXA; Hologic QDR4500W scanners, Hologic Inc., Bedford, MA) using standard protocols.
Assessment of the Frailty Phenotype
We used the framework of phenotypic frailty proposed by Fried et al24,25 with data collected in the Cardiovascular Health Study and adapted for the MrOS cohort26. The following frailty phenotype components were assessed at baseline:
Low lean mass (as a surrogate for shrinking), identified by an appendicular lean mass (adjusted for height and total body fat) in the lowest quintile;
Weakness, identified by a grip strength in the lowest quintile stratified by BMI (quartiles);
Exhaustion, identified by an answer of “a little or none” to the question “How much of the time during the past four weeks did you have a lot of energy?” from the SF-12;
Slowness, identified by a walk speed in the lowest quintile stratified by standing height (median); and
Low physical activity, level as identified by a PASE score in the lowest quintile.
Men with ≥3 components were considered frail, those with 1 or 2 components were considered in an intermediate stage, and those with none of the above components were considered robust. If there were missing data on 1 or 2 of the frailty components, the frailty phenotype index was rescaled to 5 based on the average number of non-missing components present.
Statistical Analysis
For this study, the primary analyses were conducted among men with complete data. The primary independent variables were LUTS severity based on AUASI score (total, storage subscore, and voiding subscore) and the primary dependent variable was frailty status based on the frailty phenotype. We excluded men who did not complete the AUASI (n=4), were missing >2 frailty components (n=2), or missing covariates (n=12).
We first compared distributions of established frailty and LUTS risk factors across LUTS categories using Chi-square tests for categorical variables and Kruskal Wallis test for continuous variables. Because the dependent variable, frailty, is categorical with three levels (robust, intermediate stage, frail), multinomial logistic regression with a robust variance estimator was used to calculate multinomial logit estimates. The two logits were then exponentiated to produce one odds ratio (OR) and 95% confidence interval (CI) for intermediate stage relative to robust and another for frail relative to robust. We then created separate multinomial models using AUASI storage and voiding subscore categories as the independent variables. To determine if any observed associations were due to specific frailty phenotype components, we also used multivariable logistic regression to model associations of LUTS severity with individual frailty components as a series of binary outcomes (low lean mass, weakness, exhaustion, slowness, and low physical activity) in relation to LUTS severity. Because men can have severe storage LUTS with minimal voiding LUTS and vice versa, we conducted a sensitivity analysis with men categorized into 1 of 9 mutually exclusive groups resulting from cross-classification of their storage and voiding subscore categories.
To identify and control for confounding factors, we applied a change in estimate criteria27 and then applied a forward and backward stepwise selection procedure to eliminate weak confounding variables. First, we created a base model containing terms for LUTS severity, age, and study site. In advance of the analysis, we specified four pre-planned groups of potential confounders: other demographics (education, race, marital status), lifestyle (usual alcohol consumption, cigarette smoking), cardiovascular comorbidities (self-reported history of myocardial infarction, angina, health failure, hypertension), and other medical comorbidities (self-reported history of chronic obstructive pulmonary disease, diabetes mellitus, prostate cancer [51% treated with surgery, 29% with radiation only, 14% with hormones only, and 6% were not treated]). We then successively added groups of variables to the base model and each time calculated the % change in the OR compared to the base model alone, with a change of at least 10% used to indicate important confounding.28 This process eliminated the groups of other demographics (<2% change) and lifestyle factors (<4% change) from further consideration because they did not meet the 10% change in estimate criterion. Next, we included all remaining potential confounders in a multivariable multinomial logistic regression model with stepwise selection (to remove variables that were weakly or not associated with frailty, a priori significance thresholds of ≥0.20 for variable removal and ≤0.15 for variable retention were used28). We repeated this process for the storage and voiding subscores to ensure that all potential confounders of the association between AUASI overall or subtypes and frailty were included. The final multivariable model included age (continuous), study site, self-reported history of myocardial infarction or angina (yes/no), heart failure (yes/no), chronic obstructive pulmonary disease (yes/no), diabetes mellitus (yes/no), and hypertension (yes/no). Finally, we considered that the variables psychological distress (SF-12 mental health component score ≤5022), multimorbidity (0, 1, 2, or ≥3 chronic medical conditions), or any LUTS medication use (yes/no) could be potential intermediate factors or confounders. Therefore, in sensitivity analyses we added these variables to the final multivariable model to determine if their inclusion attenuated the adjusted OR. We did not include BMI in our models because the components of total body mass (lean body mass plus fat mass) and height are included in the low lean mass frailty phenotype component, and inclusion of BMI in these models would therefore constitute over-adjustment.
To evaluate for an interaction with age, we visually inspected the unadjusted prevalence of frailty across categories of LUTS severity plotted separately for men above and below the median age (73 years) and performed an omnibus chi-square test. Because there was no evidence for effect modification by age (P for interaction=0.51), results are reported for the entire analytic sample. We next conducted a sensitivity analysis excluding 56 men missing any frailty components. We also conducted sensitivity analyses by restricting the analytic sample to men without urinary incontinence (at least weekly) or without neurologic comorbidities (Teng 3MS <80, self-reported stroke, or Parkinson’s disease). P <0.05 was considered statistically significant. All analyses were performed using STATA version 15.1 (StataCorp LLC, College Station, TX).
RESULTS
In this cohort of community-dwelling older men, 54% reported none/mild LUTS (AUASI <8), 39% reported moderate LUTS (AUASI 8–19) and 7% reported severe LUTS (AUASI ≥20). Baseline demographic and health-related characteristics of the 5979 men with complete data are reported in Table 1. Compared to men with none/mild LUTS, men with severe LUTS were on average older, less likely to report excellent general health status, less likely to report never smoking, and more likely to have both individual comorbidities as well as multimorbidity and at least one IADL impairment. α-antagonist use was reported by 35% of men with severe LUTS, whereas 5α-reductase inhibitors or anti-cholinergic use was rare (≤5%). Approximately 1 in 7 men reported a history of surgery for benign prostatic hyperplasia.
Table 1.
Baseline characteristics of 5979 men enrolled in MrOS, by Lower Urinary Tract Symptom (LUTS) Severity.
| Variable | None/Mild (AUASI 0–7) |
Moderate (AUASI 8–19) |
Severe (AUASI 20–35) |
P-value* |
|---|---|---|---|---|
| Sample size, n (%) | 3230 (54) | 2351 (39) | 398 (7) | |
| Demographics | ||||
| Age, years, mean ± SD | 73 ± 6 | 74 ± 6 | 75 ± 6 | <0.001 |
| College education, n (%) | 1707 (53) | 1275 (54) | 198 (50) | 0.22 |
| Married, n (%) | 2652 (82) | 1950 (83) | 316 (79) | 0.22 |
| Nonwhite race, n (%) | 339 (11) | 222 (9) | 46 (12) | 0.28 |
| Biometrics, mean ± SD | ||||
| Body mass index (BMI), kg/m2 | 27.3 ± 3.7 | 27.4 ± 4.0 | 27.7 ± 3.9 | 0.24 |
| Appendicular skeletal lean mass, kg | 24.3 ± 3.5 | 24.1 ± 3.5 | 24.3 ± 3.6 | 0.03 |
| Walking speed, m/s | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.1 ± 0.3 | <0.001 |
| Grip strength, kg | 42 ± 8 | 41 ± 9 | 40 ± 9 | <0.001 |
| Questionnaires | ||||
| General Health Status, n (%) | <0.001 | |||
| Excellent | 1218 (38) | 711 (30) | 87 (22) | |
| Good | 1686 (52) | 1214 (52) | 205 (52) | |
| Fair | 292 (9) | 378 (16) | 89 (22) | |
| Poor or Very Poor | 34 (1) | 46 (2) | 17 (4) | |
| Feeling full of energy†, n (%) | 3070 (95) | 2096 (89) | 308 (77) | <0.001 |
| PASE score, mean ± SD | 151 ± 69 | 141 ± 66 | 134 ± 69 | <0.001 |
| ≥1 IADL limitation, n (%) | 489 (15) | 582 (25) | 151 (38) | <0.001 |
| Health Behaviors | ||||
| Smoking, n (%) | 0.004 | |||
| Never | 1245 (39) | 861 (37) | 137 (34) | |
| Past | 1853 (57) | 1430 (61) | 248 (62) | |
| Current | 132 (4) | 60 (3) | 13 (3) | |
| Drinking, n (%) | 0.35 | |||
| <1 drink/week | 1528 (47) | 1120 (48) | 210 (53) | |
| 1–13 drinks/week | 1537 (48) | 1117 (48) | 170 (43) | |
| ≥14 drinks/week | 165 (5) | 114 (5) | 18 (5) | |
| Health Conditions, N (%) | ||||
| Myocardial Infarction or Angina | 589 (18) | 573 (24) | 121 (30) | <0.001 |
| Heart Failure | 141 (4) | 143 (6) | 30 (8) | 0.002 |
| Hypertension | 1312 (41) | 1064 (45) | 199 (50) | <0.001 |
| Stroke or Parkinson’s Disease | 174 (5) | 169 (7) | 45 (11) | <0.001 |
| Diabetes | 343 (11) | 251 (11) | 58 (15) | 0.05 |
| Chronic Obstructive Pulmonary Disease | 277 (9) | 292 (12) | 68 (17) | <0.001 |
| Prostate Cancer | 389 (12) | 264 (11) | 52 (13) | 0.47 |
| Physiological Distress‡ | 416 (13) | 463 (20) | 94 (24) | <0.001 |
| Cognitive Impairment§ | 83 (3) | 66 (3) | 17 (4) | 0.15 |
| Multimorbidity¶ | <0.001 | |||
| 0 chronic conditions | 1582 (49) | 957 (41) | 124 (31) | |
| 1 chronic condition | 1005 (31) | 745 (32) | 384 (16) | |
| 2 chronic conditions | 415 (13) | 384 (16) | 81 (20) | |
| ≥3 chronic conditions | 228 (7) | 265 (11) | 65 (16) | |
| LUTS Treatments, n (%) | ||||
| α-antagonist | 286 (9) | 504 (22) | 135 (35) | <0.001 |
| 5α-reductase inhibitor | 60 (2) | 94 (4) | 19 (5) | <0.001 |
| Anti-cholinergic | 17 (1) | 41 (2) | 16 (4) | <0.001 |
| Self-reported BPH Surgery | 469 (15) | 321 (14) | 56 (14) | 0.66 |
AUASI American Urologic Association Symptom Index; n sample size; SD standard deviation; IADL instrumental activities of daily living; BPH benign prostatic hyperplasia
P values calculated using Chi-square tests for categorical variables and Kruskal Wallis test for continuous variables.
Patients who reported feeling like they “have a lot of energy” at least some of the time.
Short Form-12 Mental Health Component Summary ≤50
Teng 3MS <80
Cumulative number of the following chronic medical conditions: stroke, Parkinson’s disease, myocardial infarction, angina, chronic obstructive pulmonary disease, congestive heart failure, diabetes mellitus, osteoporosis, osteoarthritis, hyperthyroidism or hypothyroidism.
The unadjusted prevalence of men classified as robust, intermediate stage, or frail is displayed in Figure 1, stratified by LUTS category. Frailty prevalence was 7% among men with none/mild LUTS, 11% among men with moderate LUTS, and 18% among men with severe LUTS (Figure 1). The prevalence of intermediate stage was also modestly higher with worse LUTS severity whereas the prevalence of robust was lowest among men with severe LUTS.
Figure 1.
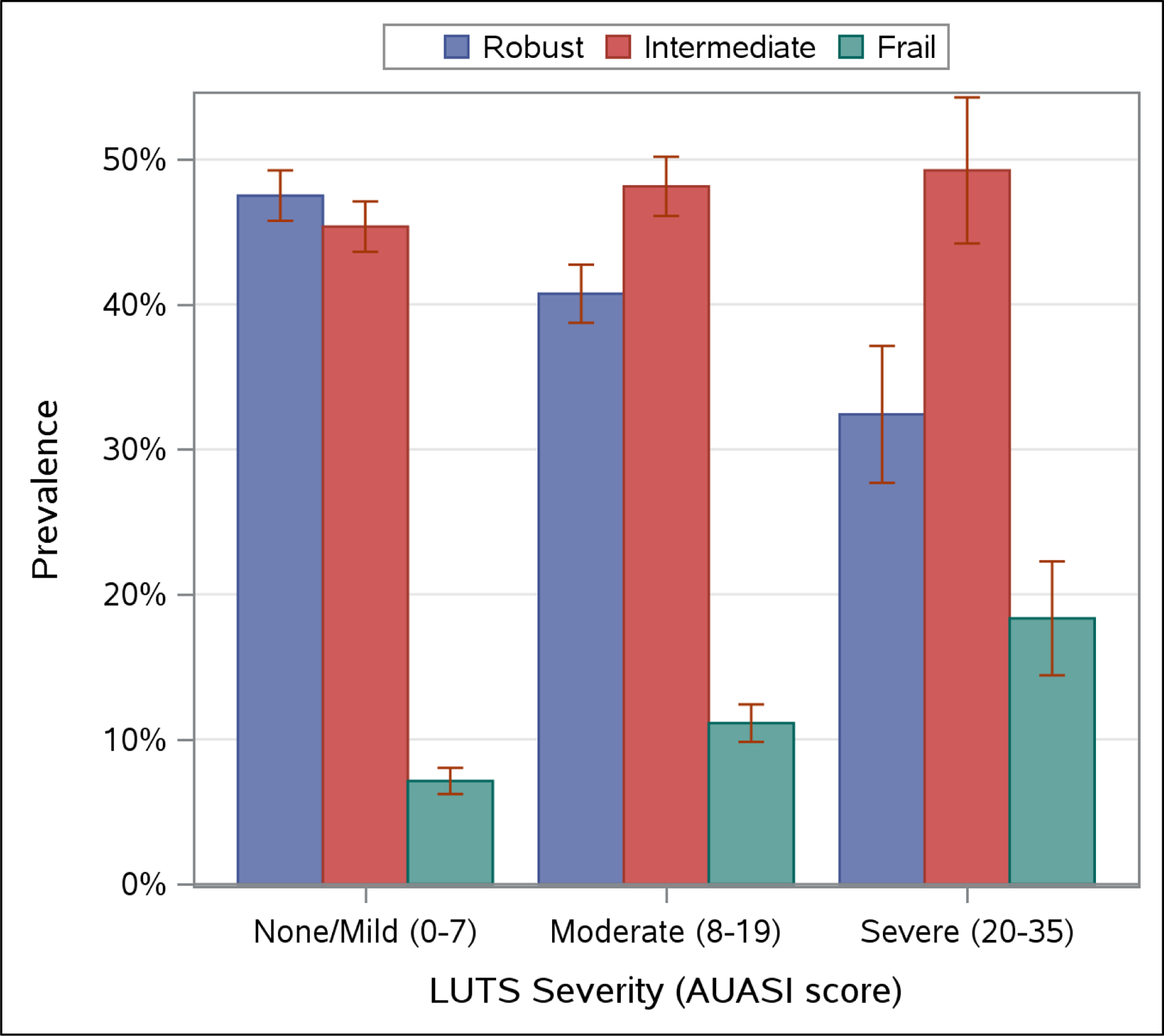
Unadjusted prevalence of frailty status, by Lower Urinary Tract Symptom (LUTS) severity*.
AUASI American Urologic Association Symptom Index; LUTS Lower Urinary Tract Symptoms
* AUASI score range is 0 to 35 and is used to categorize LUTS severity using validated thresholds of 0–7 for none/mild (n=3230), 8–19 for moderate (n=2351), and 20–35 for severe (n=398) LUTS.
Moderate and severe LUTS were associated with greater odds of both intermediate stage and frailty in minimal and fully adjusted models (Table 2). After adjusting for age and study site, the odds of frailty was 1.55 times higher for men with moderate LUTS (95% CI 1.26, 1.90) and 3.07 times higher for men with severe LUTS (95% CI 2.19, 4.31), compared to men with none/mild LUTS. In the final multivariable model, the odds of frailty was 1.41 times higher for men with moderate LUTS (95% CI 1.14, 1.74) and 2.51 times higher for men with severe LUTS (95% CI 1.76, 3.55), compared to men with none/mild LUTS. Moderate and severe LUTS were also modestly associated with higher odds of intermediate stage compared to none/mild LUTS. When storage and voiding subscores were examined separately, higher storage subscore was associated with higher odds of both intermediate stage and frailty. Higher voiding subscore was associated with higher odds of frailty, but not intermediate stage. When storage and voiding subscores using the cross-classified groups were fit in the model (Supplemental Table 1), men with the highest storage and voiding subscores had the highest odds of frailty compared to men with the lowest storage and voiding subscore. High storage subscore and low voiding subscore was also associated with higher odds of frailty whereas low subscore and high voiding subscore was not.
Table 2.
Association of American Urologic Association Symptom Index total score, storage subscore, and voiding subscore with frailty phenotype classification among 5979 men enrolled in MrOS.
| Multinomial Odds Ratio (95% Confidence Interval) | ||||
|---|---|---|---|---|
| Age and Site-Adjusted Model | Multivariable Model† | |||
| Intermediate vs Robust | Frail vs Robust | Intermediate vs Robust | Frail vs Robust | |
| Overall LUTS Severity (AUASI total score*) | ||||
| None/Mild (0–7) | 1.00 | 1.00 | 1.00 | 1.00 |
| Moderate (8–19) | 1.15 (1.02, 1.29) | 1.55 (1.26, 1.90) | 1.11 (0.99, 1.25) | 1.41 (1.14, 1.74) |
| Severe (20–35) | 1.46 (1.14, 1.85) | 3.07 (2.19, 4.31) | 1.35 (1.06, 1.73) | 2.51 (1.76, 3.55) |
| AUASI storage subscore* | ||||
| 0–3 | 1.00 | 1.00 | 1.00 | 1.00 |
| 4–6 | 1.07 (0.94, 1.22) | 1.25 (0.98, 1.59) | 1.06 (0.93, 1.20) | 1.17 (0.92, 1.50) |
| 7–15 | 1.32 (1.15, 1.52) | 2.39 (1.89, 3.02) | 1.25 (1.08, 1.44) | 2.02 (1.59, 2.57) |
| AUASI voiding subscore* | ||||
| 0–1 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2–4 | 1.04 (0.91, 1.20) | 1.10 (0.85, 1.42) | 1.02 (0.89, 1.17) | 1.05 (0.81, 1.37) |
| 5–20 | 1.12 (0.99, 1.28) | 1.71 (1.37, 2.14) | 1.08 (0.95, 1.23) | 1.53 (1.22, 1.93) |
AUASI American Urologic Association Symptom Index; LUTS Lower Urinary Tract Symptoms
AUASI score range is 0 to 35 and equals the sum of 2 validated subscores based on symptom type: storage (urgency, frequency, nocturia) and voiding (intermittency, weak stream, straining, incomplete emptying). Higher score indicates more frequent symptoms. AUASI total score categorized using validated thresholds for none/mild (0–7), moderate (8–19), and severe (20–35) LUTS and AUASI subscores categorized into approximately equal thirds (tertiles) of the subscore distribution.
Multivariable multinomial regression model adjusted for study site, age, and self-reported history of cardiovascular disease (myocardial infarction or angina), diabetes, congestive heart failure, chronic obstructive pulmonary disease, or hypertension.
Table 3 reports multivariable-adjusted associations of AUASI total score and subscores with individual frailty phenotype components (low lean mass, weakness, exhaustion, slowness, and low physical activity). Severe LUTS was most strongly associated with higher odds of exhaustion (OR = 4.37, 95% CI 3.25, 5.86) and, to a lesser degree, low physical activity (OR = 1.41, 95% 1.10, 1.80), with a similar pattern observed for storage and voiding subscores when examined separately. Higher storage subscore was also independently associated with higher odds of weakness and slowness. AUASI scores and subscores were not associated with shrinkage as manifested by low lean mass.
Table 3.
Multivariable-adjusted association of American Urologic Association Symptom Index total score, storage subscore, and voiding subscore with frailty phenotype components.
| Odds Ratio (95% Confidence Interval) | |||||
|---|---|---|---|---|---|
| Low Lean Mass | Weakness | Exhaustion | Slowness | Low Physical Activity | |
| Overall LUTS Severity (AUASI total score*) | |||||
| None/Mild (0–7) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Moderate (8–19) | 0.98 (0.85, 1.12) | 1.16 (1.02, 1.33) | 2.02 (1.64, 2.50) | 1.12 (0.97, 1.29) | 1.19 (1.04, 1.37) |
| Severe (20–35) | 1.05 (0.80, 1.36) | 1.19 (0.93, 1.52) | 4.37 (3.25, 5.86) | 1.23 (0.95, 1.60) | 1.41 (1.10, 1.80) |
| AUASI storage subscore* | |||||
| 0–3 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 4–6 | 1.03 (0.88, 1.20) | 1.03 (0.89, 1.20) | 1.53 (1.20, 1.97) | 1.09 (0.93, 1.28) | 1.07 (0.92, 1.26) |
| 7–15 | 0.98 (0.83, 1.16) | 1.35 (1.16, 1.58) | 2.74 (2.17, 3.47) | 1.32 (1.11, 1.55) | 1.31 (1.12, 1.54) |
| AUASI voiding subscore* | |||||
| 0–1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2–4 | 0.87 (0.73, 1.03) | 1.07 (0.92, 1.26) | 1.38 (1.06, 1.82) | 1.01 (0.85, 1.20) | 1.04 (0.88, 1.23) |
| 5–20 | 1.05 (0.90, 1.22) | 1.05 (0.91, 1.22) | 2.59 (2.06, 3.26) | 0.99 (0.85, 1.16) | 1.22 (1.05, 1.42) |
AUASI American Urologic Association Symptom Index; LUTS Lower Urinary Tract Symptoms
AUASI score range is 0 to 35 and equals the sum of 2 validated subscores based on symptom type: storage (urgency, frequency, nocturia) and voiding (intermittency, weak stream, straining, incomplete emptying). Higher score indicates more frequent symptoms. AUASI total score categorized using validated thresholds for none/mild (0–7), moderate (8–19), and severe (20–35) LUTS and AUASI subscores categorized into approximately equal thirds (tertiles) of the subscore distribution.
Multivariable logistic regression model adjusted for study site, age, and self-reported history of cardiovascular disease (myocardial infarction or angina), diabetes, congestive heart failure, chronic obstructive pulmonary disease, or hypertension.
In sensitivity analyses, odds of frailty compared to robust were unchanged when restricted to men without urinary incontinence (moderate vs none/mild LUTS OR = 1.29, 95% CI 1.02, 1.63; severe vs none/mild LUTS: OR = 2.97, 95% CI 1.90, 4.65) or without neurologic comorbidities (moderate vs none/mild LUTS OR = 1.37, 95% CI 1.09, 1.73; severe vs none/mild LUTS: OR = 2.58, 95% CI 1.75, 3.80). Excluding men missing any frailty components and further adjustment for psychological distress, multimorbidity, and any LUTS medication use also did not change the results (data not shown).
DISCUSSION
In this large, multicenter cross-sectional study of community-dwelling older men, we observed that moderate and severe LUTS were associated with higher odds of phenotypic frailty. Storage (urgency, frequency, nocturia, incontinence) and voiding (weak or intermittent stream, hesitancy, straining, incomplete bladder emptying) LUTS were both associated with higher odds of frailty. When individual frailty components were examined as separate outcomes, associations were strongest for exhaustion and, to a lesser degree, low physical activity. The results of this study provide evidence that LUTS and phenotypic frailty frequently co-occur, independent of older age, urinary incontinence, or multimorbidity, and suggest that it may be clinically useful to evaluate older men with LUTS for frailty.
Our results are consistent with the few small prior studies that evaluated the relationship between LUTS and frailty. Among 492 community-dwelling older men enrolled in the Aging Study of PyeongChang Rural Area, the prevalence of phenotypic frailty was presented descriptively as 7% among men with none/mild LUTS, 16% among men with moderate LUTS, and 43% among men with severe LUTS.1 Among 710 Japanese men and women age 60 years or older enrolled in the Iwaki Health Promotion Project, three frailty measures were examined in relation to LUTS in a cross-sectional analysis. The frailty discriminant score was positively associated with LUTS (adjusted OR = 2.13, 95% CI 1.48, 3.06) and all frailty measures were associated with higher odds of nocturia (≥2 voids/night), however frailty phenotype and modified frailty index were not significantly associated with LUTS.2 Our study significantly improves upon the existing evidence with rigorous adjustment for confounding and a large sample size of community-dwelling older American men which minimizes selection bias and allows for more detailed investigations of associations with LUTS subtypes and individual frailty criteria.
Other studies have examined LUTS and measures that could serve as a proxy for frailty. Prior studies have also demonstrated an association between slower gait speed, a component of the frailty phenotype, and overactive bladder, a type of storage LUTS. Suskind et al. reported that among 201 patients referred to an academic urology clinic, the Timed Up and Go Test ≥15 seconds was independently associated with overactive bladder (OR = 3.0, 95% CI 2.0, 4.8).29 Consistent with our findings, 32% of patients seeking treatment for storage LUTS due to overactive bladder were frail according to a slow Timed Up and Go Test compared to 11% of patients without overactive bladder. Similarly, among 350 community-dwelling Japanese adults age 75 years or older enrolled in the Sukagama Study, each standard deviation of slower gait speed was associated with higher odds of storage LUTS (overactive bladder: adjusted OR = 1.47, 95% CI 1.11, 1.95; urinary urgency: adjusted OR = 1.35, 95% CI 1.04, 1.74; and urgency urinary incontinence: adjusted OR = 1.40, 95% CI 1.06, 1.84), but muscle mass based on bioelectrical impedance and grip strength corrected for BMI were not.3
The temporal direction and mechanisms of an association between LUTS and frailty remain unknown. Frailty could contribute to LUTS by changing behaviors that are known to reduce the risk of LUTS or LUTS progression, such as regular physical activity30–32, or by causing comorbidities that are known to precede LUTS and may contribute to poor energy or exhaustion, such as poor sleep quality33 and depression34,35. In addition to bidirectional associations with the factors above, LUTS could contribute to frailty via interference with employment, willingness to leave the house or travel, social connections, and hobbies,36,37 all of which are protective factors against increased social isolation, sedentary lifestyle, and frailty as men age. Older men with LUTS are also at increased risk of falls and fall-related injuries which can lead to frailty directly or indirectly via fear of falling and decreased physical activity.14,38 Through unknown mechanisms, older men with LUTS and high frailty index scores are more likely to have excess nocturnal urine production compared to men with lower frailty index scores which can worsen nocturnal storage LUTS.39 Lastly, the cumulative deficit model, a conceptual model of frailty that was not assessed in our study, defines frailty as the accumulation of health and functional problems, including LUTS or lower urinary tract dysfunction, which cumulatively (rather than individually) contribute to increased risk of morbidity and mortality.25
It is also unknown if LUTS and frailty are a consequence of shared pathophysiology. According to Inouye et al., what unifies LUTS, and particularly urinary incontinence, with other geriatric syndromes are the mutual risk factors of older age, cognitive impairment, functional impairment, and impaired mobility.40 These risk factors may represent shared pathophysiological pathways that lead to an inability to adapt or cope with stressors, such as mild or previously manageable LUTS. Common metabolic derangements that increase with age, including insulin resistance and41 inflammatory markers42, as well as conditions that lead to accelerated aging, including obesity43 and impaired mobility44, are also associated with increased risk or progression of LUTS. A shared mechanism contributing to increased biological age and/or decreased reserve and resilience to stressors could manifest as LUTS or phenotypic frailty or both conditions simultaneously. Thus, it remains unknown if associations between LUTS and frailty are observed because they contribute to each other or they are caused by a shared mechanism.
The clinical implications of a relationship between frailty and LUTS remains speculative at this time, but there are several possibilities. Although effective interventions to directly treat frailty do not currently exist, significant efforts have been made to integrate frailty assessments into clinical practice and the high co-occurrence of these conditions suggests that it may be clinically useful to evaluate older men with LUTS for frailty and other geriatric syndromes. Urinary incontinence is a well-established geriatric syndrome and should trigger a comprehensive geriatric examination45, but older man presenting with LUTS may also benefit from earlier screening for falls, polypharmacy, and mobility impairments. Similarly, older men with frailty suffer from a higher burden of LUTS despite their lack of representation in most LUTS intervention trials. While LUTS in older men is predominantly managed with medication, frail older men are more likely to suffer from adverse drug reactions46 which could be caused by poorly targeted LUTS medications in addition to directly contributing to polypharmacy. Lastly, if a causal relationship is demonstrated, effective LUTS treatments (particularly those targeting systemic rather than urogenital-specific mechanisms) could be included in multicomponent interventions to treat frailty or specific frailty phenotype components, such as exhaustion.
We recognize several limitations to our study. There are multiple valid definitions and conceptual models of frailty but we only tested associations with phenotypic frailty. Individual frailty phenotype criterion, such as low lean body mass estimated with DXA, are surrogates of the target frailty component (e.g., shrinkage based on recent weight loss), although this frailty phenotype is consistent with the Cardiovascular Health Study criterion24,25 and is consistently associated with adverse outcomes in older adults.26,47 To be eligible for the study, men must have been able to walk without assistance and must have lived in the community; therefore, the prevalence of frailty is likely lower than the true prevalence in the general population. Therefore, the results of this study may not be generalizable to institutionalized, less-healthy, or more racially diverse older men. Analyses using individual components of LUTS or frailty indices must be interpreted with caution since they are less well validated than the index as a whole and may be more susceptible to type I error. Lastly, unmeasured confounding is always possible with observational data and could lead to falsely positive associations if an unmeasured variable is positively associated with both the exposure and the outcome. For example, men with lower income are more likely to be frail and have LUTS, however we suspect residual confounding by income is explained by demographic or clinical variables that we included in our multivariable model.
In conclusion, the prevalence of phenotypic frailty is higher among older community-dwelling men with moderate or severe LUTS compared to those with mild or no LUTS. The positive association between LUTS severity and frailty among older men appears independent of age and other known frailty risk factors.
Supplementary Material
ACKNOWLEDGMENTS
Conflict of Interest: The authors have no conflicts.
Author Contributions: S. Bauer: conception and design, acquisition of data, analysis and interpretation of data, drafting and revising the article, final approval of the version to be published
R. Scherzer: analysis and interpretation of data, revising the article for important intellectual content, final approval of the version to be published
A. Suskind: analysis and interpretation of data, revising the article for important intellectual content, final approval of the version to be published
P. Cawthon: conception and design, acquisition of data, analysis and interpretation of data, revising the article for important intellectual content, final approval of the version to be published
K. Ensrud: analysis and interpretation of data, revising the article for important intellectual content, final approval of the version to be published
W. Ricke: analysis and interpretation of data, revising the article for important intellectual content, final approval of the version to be published
K. Covinsky: analysis and interpretation of data, revising the article for important intellectual content, final approval of the version to be published
L. Marshall: conception and design, acquisition of data, analysis and interpretation of data, revising the article for important intellectual content, final approval of the version to be published
Sponsor’s Role: conception and design, acquisition of data, analysis and interpretation of data, drafting and revising the article, final approval of the version to be published
Source of Funding: Dr. Bauer is supported by grant 1K12DK111028 from the National Institute of Diabetes, Digestive, and Kidney Disorders. The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and the NIH Roadmap for Medical Research provided support under grant numbers U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01 AG027810, UL1 TR000128, and U54 104310.
Footnotes
Publicly available data: http://mrosdata.sfcc-cpmc.net
Conflict of Interest: The authors have no conflicts.
This study was presented orally via teleconference at the Claude D Pepper Older Americans Independence Centers (OAIC) Annual Meeting on April 3, 2020 and was accepted for the Presidential Poster session at the 2020 American Geriatric Society Meeting, May 7, 2020, but was not presented due to the coronavirus pandemic.
REFERENCES
- 1.Jang IY, Lee CK, Jung HW, et al. Urologic symptoms and burden of frailty and geriatric conditions in older men: the Aging Study of PyeongChang Rural Area. Clin Interv Aging. 2018;13:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soma O, Hatakeyama S, Imai A, et al. Relationship between frailty and lower urinary tract symptoms among community-dwelling adults. Lower urinary tract symptoms. 2019. [DOI] [PubMed] [Google Scholar]
- 3.Omae K, Yamamoto Y, Kurita N, et al. Gait speed and overactive bladder in the healthy community-dwelling super elderly: The Sukagawa Study. Neurourology and urodynamics. 2019;38(8):2324–2332. [DOI] [PubMed] [Google Scholar]
- 4.Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in america project: benign prostatic hyperplasia. The Journal of urology. 2008;179(5 Suppl):S75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barry MJ, Fowler FJ Jr., O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. The Journal of urology. 1992;148(5):1549–1557; discussion 1564. [DOI] [PubMed] [Google Scholar]
- 6.Yuan JQ, Mao C, Wong SY, et al. Comparative Effectiveness and Safety of Monodrug Therapies for Lower Urinary Tract Symptoms Associated With Benign Prostatic Hyperplasia: A Network Meta-analysis. Medicine. 2015;94(27):e974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abrams P, Chapple C, Khoury S, Roehrborn C, de la Rosette J. Evaluation and treatment of lower urinary tract symptoms in older men. The Journal of urology. 2013;189(1 Suppl):S93–s101. [DOI] [PubMed] [Google Scholar]
- 8.Welk B, McArthur E, Ordon M, Anderson KK, Hayward J, Dixon S. Association of Suicidality and Depression With 5alpha-Reductase Inhibitors. JAMA internal medicine. 2017;177(5):683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic Drug Exposure and the Risk of Dementia: A Nested Case-Control Study. JAMA internal medicine. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkar RR, Parsons JK, Bryant AK, et al. Association of Treatment With 5alpha-Reductase Inhibitors With Time to Diagnosis and Mortality in Prostate Cancer. JAMA internal medicine. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welk B, McArthur E, Fraser LA, et al. The risk of fall and fracture with the initiation of a prostate-selective alpha antagonist: a population based cohort study. BMJ (Clinical research ed). 2015;351:h5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lepor H Pathophysiology of lower urinary tract symptoms in the aging male population. Reviews in urology. 2005;7 Suppl 7:S3–s11. [PMC free article] [PubMed] [Google Scholar]
- 13.Platz EA, Joshu CE, Mondul AM, Peskoe SB, Willett WC, Giovannucci E. Incidence and progression of lower urinary tract symptoms in a large prospective cohort of United States men. The Journal of urology. 2012;188(2):496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noguchi N, Chan L, Cumming RG, Blyth FM, Naganathan V. A systematic review of the association between lower urinary tract symptoms and falls, injuries, and fractures in community-dwelling older men. The aging male : the official journal of the International Society for the Study of the Aging Male. 2016;19(3):168–174. [DOI] [PubMed] [Google Scholar]
- 15.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemporary clinical trials. 2005;26(5):569–585. [DOI] [PubMed] [Google Scholar]
- 16.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemporary clinical trials. 2005;26(5):557–568. [DOI] [PubMed] [Google Scholar]
- 17.McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. The Journal of urology. 2011;185(5):1793–1803. [DOI] [PubMed] [Google Scholar]
- 18.Barry MJ, Williford WO, Fowler FJ Jr., Jones KM, Lepor H. Filling and voiding symptoms in the American Urological Association symptom index: the value of their distinction in a Veterans Affairs randomized trial of medical therapy in men with a clinical diagnosis of benign prostatic hyperplasia. The Journal of urology. 2000;164(5):1559–1564. [PubMed] [Google Scholar]
- 19.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10(4):405–411. [DOI] [PubMed] [Google Scholar]
- 20.Cauley JA, Cawthon PM, Peters KE, et al. Risk Factors for Hip Fracture in Older Men: The Osteoporotic Fractures in Men Study (MrOS). J Bone Miner Res. 2016;31(10):1810–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. The Journal of clinical psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 22.Gill SC, Butterworth P, Rodgers B, Mackinnon A. Validity of the mental health component scale of the 12-item Short-Form Health Survey (MCS-12) as measure of common mental disorders in the general population. Psychiatry research. 2007;152(1):63–71. [DOI] [PubMed] [Google Scholar]
- 23.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. Journal of clinical epidemiology. 1993;46(2):153–162. [DOI] [PubMed] [Google Scholar]
- 24.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. The journals of gerontology Series A, Biological sciences and medical sciences. 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 25.Walston J, Bandeen-Roche K, Buta B, et al. Moving Frailty Toward Clinical Practice: NIA Intramural Frailty Science Symposium Summary. Journal of the American Geriatrics Society. 2019;67(8):1559–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cawthon PM, Marshall LM, Michael Y, et al. Frailty in older men: prevalence, progression, and relationship with mortality. Journal of the American Geriatrics Society. 2007;55(8):1216–1223. [DOI] [PubMed] [Google Scholar]
- 27.Greenland S, Daniel R, Pearce N. Outcome modelling strategies in epidemiology: traditional methods and basic alternatives. Int J Epidemiol. 2016;45(2):565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenland S, Pearce N. Statistical foundations for model-based adjustments. Annu Rev Public Health. 2015;36:89–108. [DOI] [PubMed] [Google Scholar]
- 29.Suskind AM, Quanstrom K, Zhao S, et al. Overactive Bladder Is Strongly Associated With Frailty in Older Individuals. Urology. 2017;106:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maserejian NN, Kupelian V, Miyasato G, McVary KT, McKinlay JB. Are physical activity, smoking and alcohol consumption associated with lower urinary tract symptoms in men or women? Results from a population based observational study. The Journal of urology. 2012;188(2):490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolin KY, Grubb RL 3rd, Pakpahan R, et al. Physical activity and benign prostatic hyperplasia-related outcomes and nocturia. Medicine and science in sports and exercise. 2015;47(3):581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raheem OA, Parsons JK. Associations of obesity, physical activity and diet with benign prostatic hyperplasia and lower urinary tract symptoms. Current opinion in urology. 2014;24(1):10–14. [DOI] [PubMed] [Google Scholar]
- 33.Branche BL, Howard LE, Moreira DM, et al. Sleep problems are associated with development and progression of lower urinary tract symptoms (LUTS): Results from REDUCE. The Journal of urology. 2017. [DOI] [PubMed] [Google Scholar]
- 34.Hakkinen JT, Shiri R, Koskimaki J, Tammela TL, Auvinen A, Hakama M. Depressive symptoms increase the incidence of nocturia: Tampere Aging Male Urologic Study (TAMUS). The Journal of urology. 2008;179(5):1897–1901. [DOI] [PubMed] [Google Scholar]
- 35.Huang CL, Wu MP, Ho CH, Wang JJ. The bidirectional relationship between anxiety, depression, and lower urinary track symptoms: A nationwide population-based cohort study. Journal of psychosomatic research. 2017;100:77–82. [DOI] [PubMed] [Google Scholar]
- 36.Coyne KS, Sexton CC, Irwin DE, Kopp ZS, Kelleher CJ, Milsom I. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU international. 2008;101(11):1388–1395. [DOI] [PubMed] [Google Scholar]
- 37.Suen LKP, Cheng HL, Yeung SKW, et al. Qualitative insights into the experiences of living with moderate-to-severe lower urinary tract symptoms among community-dwelling ageing males. PloS one. 2017;12(10):e0187085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gibson W, Hunter KF, Camicioli R, et al. The association between lower urinary tract symptoms and falls: Forming a theoretical model for a research agenda. Neurourology and urodynamics. 2018;37(1):501–509. [DOI] [PubMed] [Google Scholar]
- 39.Monaghan TF, Wagg AS, Bliwise DL, et al. Association between nocturia and frailty among elderly males in a veterans administration population. Aging clinical and experimental research. 2019. [DOI] [PubMed] [Google Scholar]
- 40.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. Journal of the American Geriatrics Society. 2007;55(5):780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russo GI, Castelli T, Urzi D, et al. Emerging links between non-neurogenic lower urinary tract symptoms secondary to benign prostatic obstruction, metabolic syndrome and its components: A systematic review. International journal of urology : official journal of the Japanese Urological Association. 2015;22(11):982–990. [DOI] [PubMed] [Google Scholar]
- 42.Siddiqui NY, Helfand BT, Andreev VP, et al. Biomarkers implicated in lower urinary tract symptoms: systematic review and pathway analyses. The Journal of urology. 2019:101097ju0000000000000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parsons JK, Sarma AV, McVary K, Wei JT. Obesity and benign prostatic hyperplasia: clinical connections, emerging etiological paradigms and future directions. The Journal of urology. 2013;189(1 Suppl):S102–106. [DOI] [PubMed] [Google Scholar]
- 44.Marshall LM, Holton KF, Parsons JK, Lapidus JA, Ramsey K, Barrett-Connor E. Lifestyle and health factors associated with progressing and remitting trajectories of untreated lower urinary tract symptoms among elderly men. Prostate cancer and prostatic diseases. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elsawy B, Higgins KE. The geriatric assessment. Am Fam Physician. 2011;83(1):48–56. [PubMed] [Google Scholar]
- 46.Cullinan S, O’Mahony D, O’Sullivan D, Byrne S. Use of a frailty index to identify potentially inappropriate prescribing and adverse drug reaction risks in older patients. Age and ageing. 2016;45(1):115–120. [DOI] [PubMed] [Google Scholar]
- 47.Ensrud KE, Ewing SK, Cawthon PM, et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. Journal of the American Geriatrics Society. 2009;57(3):492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


