Abstract
Brain derived neurotrophic factor (BDNF) is produced in skeletal muscle as a myokine that plays a role in muscle metabolism. However, how metabolic changes affect skeletal muscle BDNF expression and release remains to be fully understood. Amino acid restrictions such as methionine restriction (MR) are considered as an alternative fasting approach. Here we reported that in C2C12 myotubes, MR enhanced BDNF release, which was measured using ELISA, RT-qPCR, cell immunostaining, and Western blot. Inhibition of protein transport pathway blocked the MR enhanced BDNF release, confirming that MR-induced BDNF release involved classic protein secretory pathway. MR increased L-lactate product in media, suggesting that MR promoted glycolysis. Treatment with 2-deoxy glucose (2-DG) attenuated lactate production as well as BDNF release, suggesting that glycolysis is involved in the enhanced BDNF release induced by MR. Moreover, treatment with L-Lactate, the end-product of glycolysis, enhanced BDNF gene expression and release in control cells in a dose dependent manner, suggesting lactate produced by glycolysis may mediate the enhanced BDNF release by MR. Overall, the results of this study suggest that MR promotes BDNF secretion from C2C12 myotubes at least partially via enhancing glycolysis and lactate production.
Keywords: brain derived neurotrophic factor, myokine, C2C12, methionine restriction, lactate
Introduction
Emerging evidence indicates that skeletal muscles possess endocrine function in addition to their roles in body locomotion and posture. Numerous peptides and proteins have been identified to be released by skeletal muscles. These muscle derived peptides and proteins are termed myokines [1, 2]. Myokines include many classic cytokines, chemokines, and growth factors. These myokines function in autocrine, paracrine, and endocrine manners to elicit a broad range of effects on skeletal muscle and other tissues and organs[3].
Brain derived neurotrophic factor (BDNF) belongs to the neurotrophic factor family that plays a critical role in neuron growth, neuronal network development, and synaptic plasticity and function[4] [5-8]. Emerging evidence indicates that BDNF is also expressed and released by skeletal muscle as a myokine[9]. It was shown that muscle-derived BDNF is involved in regulation of metabolism and energy homeostasis[9-11], and muscle regeneration[12]. On the other hand, however, how metabolic alterations regulate BDNF expression and secretion in skeletal muscle and underlying mechanisms remain to be fully understood. Exercise[10, 13] and fasting[14] upregulated skeletal muscle BDNF expression, suggesting that BDNF expression and release from skeletal muscle may be altered by the changes in metabolism and energy homeostasis. Methionine restriction (MR) is considered as an alternative fasting approach that exhibits various beneficial effects[15, 16]. In this study, we tested whether MR affects BDNF expression and secretion using differentiated C2C12 myotubes as an in vitro model.
Method
Culture and treatments of C2C12 cells
C2C12 mouse myoblast cells were cultured in growing media (GM) that was dulbecco’s modified eagle’s medium (DMEM, ThermoFisher-Gibco) containing 10% fetal bovine serum and 1% penicillin-streptomycin. Upon full confluence, cells were switched into differentiating media (DM) that was DMEM supplemented with 2% horse serum and 1% penicillin-streptomycin. Following 3 to 4 days of differentiation, the media was replaced with fresh, serum-free DMEM (control) or DMEM without methionine (MR, ThermoFisher-Gibco). For additional treatments, the test substances were diluted and added into the control or MR media at desired concentrations as described in figure legends. Following incubation for 8 to 16 hours, the media and cells were collected for assigned assays.
Measurement of BDNF expression and release
BDNF released in media was measured using BDNF ELISA kit (R&D Systems) by following the manufacturer’s instruction.
BDNF in cells was detected using Western blot or immunostaining. For Western blot, cells were lysed in RIPA buffer containing protease inhibitor cocktail (Santa Cruz). The samples were subjected to standard PAGE, membrane transfer, and immunoblotting as described previously[17, 18]. To increase the retention of small protein BDNF on the nitrocellulose membrane, the membranes were fixed with 50% methanol[19] prior to immunoblotting. Primary antibodies for BDNF (Abcam 108319), and beta actin (Santa Cruz, sc-47778) were used.
For cell immunostaining, cells were fixed with prechilled ethanol/methanol mixture for 10 min, washed, blocked with 1.5% BSA at room temperature for 1 hour, and then incubated with a primary antibody against BDNF (abcam, 1:100) at room temperature for 2 hours or at 4 °C overnight. Following washes, cells were incubated with an appropriate fluorescence conjugated secondary antibody (ThermoFisher). The images were taken using a fluorescent microscope (Nikon Eclipse e200) and a CCD camera (Leica K5).
Realtime quantitative RT-PCR
Total RNA extraction, DNase digestion, and RNA purification were performed using Zymo Direct-zol RNA Microprep kit (Zymo Research, R2061) following the manufacturer’s instruction. The resultant total RNA from C2C12 cells with designated treatments were used for one-step RT-qPCR using a Promega GoTaq One-Step RT-qPCR kit (Promega, A6020). The realtime PCR was performed on StepOnePlus System (Applied Biosystems). Primers for mouse BDNF and GAPDH were purchased from Integrated DNA Technologies (IDT). One-step RT-qPCR program was as following: RT reaction at 37°C for 15 min; RTase inactivation at 95°C for 10 min; and 40 PCR cycles of 95°C for 10 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. The PCR results were analyzed with the ABI SDS RQ Manager 1.2 software (Applied Biosystems), using ΔΔCT values as relative gene expression[20]. The fold changes of BDNF gene expression in the treated groups relative to the control group were determined.
L-lactate assay
L-lactate in the collected media was measured using commercial kit (L-Lactate kit, Cayman Chemicals 700510).
Data analysis and statistics
Data of ELISA and lactate assays of experimental groups were quantified as ratios of the mean value of the corresponding control group. Data were presented as mean ± standard deviation. Comparisons were performed using One-way or two-way ANOVA followed by post hoc student t test. P value less than 0.05 was considered as statistically significant.
Results
1, MR enhanced BDNF release in C2C12 myotubes:
When differentiated C2C12 myotubes were incubated in control or MR media for 8 to 16 hours, BDNF level in culture media was significantly increased in the MR group as compared with the control group (Fig. 1A), indicating that MR enhances BDNF release. The realtime qRT-PCR result confirmed that mRNA level of BDNF was significantly upregulated in MR group as compared with the control (Fig. 1B). Immunostaining revealed that MR markedly increased the BDNF distribution in cell plasma membrane (Fig. 1C), further suggesting the enhanced release of BDNF. Consistently, Western blot showed increased pro- and mature-BDNF protein bands in cell lysates in MR cells (Fig. 1D). These results suggest that MR upregulates BDNF expression and release in C2C12 cells.
Fig. 1,
MR enhanced BDNF release: A: BDNF ELISA with media of cells in control and MR media for 8 to 16 hours. n=8. B: Relative BDNF gene expression by RT-qPCR in cells in control or MR media for 8 hours. n=5. C: BDNF immunostaining cells in control and MR media for 8 hours. The bar is 200 μM. The bottom images were the enlarged views of the boxed areas in the top images. Arrows point to the enhanced BDNF distribution in plasma membrane. D: Western blot: showing pro- and mature-BDNF bands and beta actin in cell lysate from MR and control cells.
2, MR-induced BDNF release was blocked by inhibition of protein secretory pathway:
To test whether BDNF release induced by MR is via protein secretion processes, we used a cocktail of Brefeldin A and Monensin to inhibit protein secretory transport from endoplasmic reticulum (ER) to Golgi network. The cocktail treatment for 16 hours markedly blocked the release of BDNF (Fig. 2A). BDNF immunostaining further revealed that inhibition of protein secretory pathway resulted in BDNF accumulation in the area of endoplasmic reticulum, with stronger staining in MR cells (Fig. 2B). These results provide additional evidence that MR enhances BDNF expression and secretion.
Fig. 2,
Inhibition of protein secretory pathway blocked BDNF release. A: ELISA assay of BDNF in media of control and MR with or without protein transport inhibitor cocktail (1:500 dilution) for 16 hours. n=4. B: BDNF immunostaining of cells in control or MR media with or without protein secretion inhibitor cocktail for 16 hours. The bars are 100 μM. Arrows indicate the accumulation of BDNF in ER area.
3, Enhanced glycolysis is involved in MR-induced BDNF release:
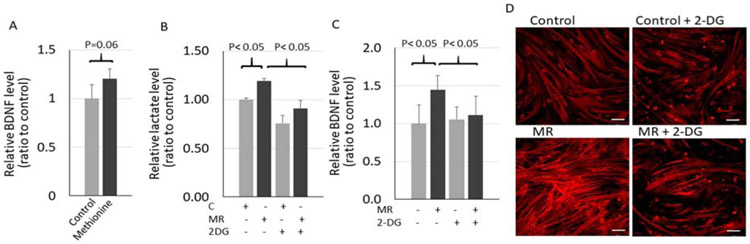
To determine the mechanism by which MR promotes BDNF release, we first tested whether methionine inhibits BDNF release. Interestingly, instead of an inhibitory effect, addition of methionine exhibited a stimulatory effect on BDNF release (Fig. 3A). This result suggests that methionine restriction enhancement of BDNF release is unlikely due to removal of methionine inhibitory effect. We then observed that MR increased the level of L-lactate in the media (Fig. 3B). L-Lactate is an end-product of glycolysis. It was reported that MR enhances glycolysis in endothelial cells[16]. We then tested whether enhanced glycolysis is involved in BDNF release. Indeed, we observed that treatment with 2-deoxy glucose (2-DG), a well-known glycolysis blocker, diminished enhanced L-lactate (Fig. 3B) and BDNF release (Fig. 3C and D) in MR cells. These results suggest that MR enhanced glycolysis, which promoted BDNF secretion.
Fig. 3,
Glycolysis is involved in MR-enhanced BDNF release. A: ELISA of BDNF level in media of cells in control media with or without addition of L-methionine (50ug/ml) for 8 hours. B: L-lactate level in media of control and MR cells with or without 2-DG (1 mM) for 8 hours. n=4. C: ELISA of BDNF in media of control and MR with or without 2-DG for 8 hours. n=4. D: BDNF immunostaining in control or MR cells with or without 2-DG for 8 hours. The bars are 200 μM.
4, L-Lactate stimulated BDNF release in C2C12 cells:
L-Lactate is an end-product of glycolysis. It was evident that lactate mediates exercise induced BDNF upregulation in neuronal cells[21,22]. We then tested whether lactate is a mediator that directly stimulates BDNF release from C2C12 cells. Indeed, treatment with L-lactate at 5 mM and 10 mM significantly enhanced BDNF release in control cells in a dose-dependent manner (Fig. 4A). The realtime qRT-PCR result confirmed that mRNA level of BDNF was significantly upregulated in L-lactate treated cells as compared with the control cells (Fig. 4B). Increased BDNF in cells treated with L-lactate was shown by cell immunostaining (Fig. 4C). However, the treatment with the same doses of lactate were not able to further enhance BDNF release from MR cells (Fig. 4D), suggesting the endogenous lactate may mask the effect of exogenous lactate.
Fig. 4,
Lactate stimulated BDNF release: A: ELISA of BDNF in media of control cells with L-lactate at 0, 5 and 10 mM for 8 hours. n=4. B: Relative BDNF gene expression measured by RT-qPCR from cells treated with control or L-lactate for 8 hours. n=5. C: Immunostaining of BDNF in cells in control media with or without L-lactate for 8 hours. The bars are 200 μM. D: ELISA of BDNF in media of MR cells with or without lactate. n=4.
Discussion
The results of this study provide novel insight into the regulation of BDNF secretion from C2C12 myotubes. Our data shows that methionine restriction promotes BDNF gene expression and release. In addition to the increased BDNF level in the media, we were also able to show that MR increased mRNA level and BDNF distribution in plasma membrane in cell immune staining. Moreover, we observed that inhibition of the protein secretory pathway blocked BDNF secretion and caused a greater accumulation of BDNF in ER area. Classical protein secretion pathway includes protein transport from ER to Golgi network where proteins move to the cell surface to cross plasma membrane via various mechanism[23]. These results demonstrate that MR enhances BDNF release via a conventional secretory pathway.
Methionine is an essential amino acid. We found that methionine did not inhibit BDNF release in C2C12 cells. Instead, addition of methionine into media enhanced BDNF release, suggesting that MR promotion of BDNF release is unlikely due to the removal of methionine as an inhibitory factor. We found that MR increased lactate product, which is an end-product of glycolysis. Blocking of glycolysis by 2-DG reduced lactate production and BDNF release. Furthermore, lactate treatment directly stimulated BDNF release. These results strongly suggest that MR enhances glycolysis and that lactate, as the end-product of glycolysis, mediates the enhanced BDNF release by MR.
It has been recognized that lactate is not only a metabolite but a signaling molecule as well[24]. Our data suggests that lactate may play a role in regulation of skeletal muscle secretory function during metabolic and energy stresses. For example, lactate may be involved in exercise upregulation of skeletal muscle BDNF expression. During endurance exercise, accumulation of lactate in skeletal muscle could promote BDNF release from working muscle. This could be of functional significance because increased myokine BDNF enhances mitochondrial oxidation[14] to maintain energy homeostasis and muscle function during endurance exercise.
The role of BDNF in skeletal muscle metabolism has been highlighted by recent studies. Delezie, et al reported that skeletal muscle specific knockout of BDNF caused a shift of muscle fiber type from type IIb glycolytic fibers to type IIx oxidative fibers, suggesting BDNF is an essential factor in maintenance of glycolytic metabolism in skeletal muscle[25]. Our data shows that glycolysis is indeed involved in MR-enhanced BDNF secretion, which is consistent with the notion that BDNF is involved in glycolytic metabolism in skeletal muscle. On the other hand, Yang, et al. reported that BDNF is a fasting-induced myokine that promotes mitochondrial oxidation[14]. These seemingly contradictory results indicate the complex role of BDNF in skeletal muscle metabolism that requires further investigation. It would be interesting and important to determine whether regulation of BDNF expression and secretion in skeletal muscle is fiber type specific and whether BDNF mediates the cross talk between different muscle fibers.
While this study has its limitation by its in vitro nature, it provides a strong rationale for further investigation of MR and lactate treatment as approaches to enhance myokine BDNF release in animal models. BDNF has protective functions against aging related neuronal diseases[26] and depression[27, 28]. In addition to its autocrine and paracrine function, systemic roles of skeletal muscle derived BDNF has long been expected but remains elusive. Skeletal muscle accounts for about 40 % of total mass in the human body[29] and thus myokine BDNF as a source of systemic BDNF is plausible. One of our goals is to identify means to effectively enhance myokine BDNF as a regional and systemic protective factor to prevent and mitigate injuries, degenerations, and dysfunctions in skeletal muscle, vascular system, and nervous system.
Supplementary Material
Highlight.
This study reports that methionine restriction (MR) enhanced the secretion of brain derived neurotrophic factor (BDNF) from differentiated C2C12 myotubes;
Increased glycolysis was involved in the MR enhancement of BDNF secretion from the myotubes;
The glycolysis end-product lactate mediated the MR enhancement of BDNF secretion form C2C12 myotubes;
These results help to understand regulations of BDNF secretion from skeletal muscle.
Acknowledgments
Funding: This study is supported by an NIH grant No. R01HL147105 and the Faculty Research Bridge Funds of Division of Basic Biomedical Sciences of University of South Dakota Sanford School of Medicine (to YL).
Footnotes
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Pedersen BK and Febbraio MA, Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol, 2012. 8(8): p. 457–65. [DOI] [PubMed] [Google Scholar]
- 2.Iizuka K, Machida T, and Hirafuji M, Skeletal muscle is an endocrine organ. J Pharmacol Sci, 2014. 125(2): p. 125–31. [DOI] [PubMed] [Google Scholar]
- 3.Giudice J and Taylor JM, Muscle as a paracrine and endocrine organ. Curr Opin Pharmacol, 2017. 34: p. 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barde YA, Edgar D, and Thoenen H, Purification of a new neurotrophic factor from mammalian brain. EMBO J, 1982. 1(5): p. 549–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lohof AM, Ip NY, and Poo MM, Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature, 1993. 363(6427): p. 350–3. [DOI] [PubMed] [Google Scholar]
- 6.Nagahara AH and Tuszynski MH, Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov, 2011. 10(3): p. 209–19. [DOI] [PubMed] [Google Scholar]
- 7.Levine ES, et al. , Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci U S A, 1995. 92(17): p. 8074–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kossel AH, et al. , A caged Ab reveals an immediate/instructive effect of BDNF during hippocampal synaptic potentiation. Proc Natl Acad Sci U S A, 2001. 98(25): p. 14702–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedersen BK, et al. , Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp Physiol, 2009. 94(12): p. 1153–60. [DOI] [PubMed] [Google Scholar]
- 10.Matthews VB, et al. , Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia, 2009. 52(7): p. 1409–18. [DOI] [PubMed] [Google Scholar]
- 11.Chan CB, et al. , Activation of muscular TrkB by its small molecular agonist 7,8-dihydroxyflavone sex-dependently regulates energy metabolism in diet-induced obese mice. Chem Biol, 2015. 22(3): p. 355–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clow C and Jasmin BJ, Brain-derived neurotrophic factor regulates satellite cell differentiation and skeltal muscle regeneration. Mol Biol Cell, 2010. 21(13): p. 2182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez-Pinilla F, et al. , Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur J Neurosci, 2001. 13(6): p. 1078–84. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, et al. , Muscle-generated BDNF is a sexually dimorphic myokine that controls metabolic flexibility. Sci Signal, 2019. 12(594). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das A, et al. , Impairment of an Endothelial NAD(+)-H2S Signaling Network Is a Reversible Cause of Vascular Aging. Cell, 2018. 173(1): p. 74–89 e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longchamp A, et al. , Amino Acid Restriction Triggers Angiogenesis via GCN2/ATF4 Regulation of VEGF and H2S Production. Cell, 2018. 173(1): p. 117–129 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao H, et al. , UCHL1 regulates muscle fibers and mTORC1 activity in skeletal muscle. Life Sci, 2019. 233:p. 116699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao H, Hartnett S, and Li Y, Ubiquitin C-Terminal Hydrolase L1 regulates myoblast proliferation and differentiation. Biochem Biophys Res Commun, 2017. 492(1): p. 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, et al. , A fixation method for the optimisation of western blotting. Sci Rep, 2019. 9(1): p. 6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ and Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods, 2001. 25(4): p. 402–8. [DOI] [PubMed] [Google Scholar]
- 21.Muller P, et al. , Lactate and BDNF: Key Mediators of Exercise Induced Neuroplasticity? J Clin Med, 2020. 9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiffer T, et al. , Lactate infusion at rest increases BDNF blood concentration in humans. Neurosci Lett, 2011. 488(3): p. 234–7. [DOI] [PubMed] [Google Scholar]
- 23.Stow JL and Murray RZ, Intracellular trafficking and secretion of inflammatory cytokines. Cytokine Growth Factor Rev, 2013. 24(3): p. 227–39. [DOI] [PubMed] [Google Scholar]
- 24.Magistretti PJ and Allaman I, Lactate in the brain: from metabolic end-product to signalling molecule. Nat Rev Neurosci, 2018. 19(4): p. 235–249. [DOI] [PubMed] [Google Scholar]
- 25.Delezie J, et al. , BDNF is a mediator of glycolytic fiber-type specification in mouse skeletal muscle. Proc Natl Acad Sci U S A, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Budni J, et al. , The involvement of BDNF, NGF and GDNF in aging and Alzheimer's disease. Aging Dis, 2015. 6(5): p. 331–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adachi M, et al. , Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol Psychiatry, 2008. 63(7): p. 642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Autry AE and Monteggia LM, Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev, 2012. 64(2): p. 238–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zurlo F, et al. , Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest, 1990. 86(5): p. 1423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.