Abstract
The immune system is capable of generating robust antibody responses to foreign antigens during infection and vaccination, while simultaneously limiting antibodies to self-antigens. T follicular regulatory (Tfr) cells are a subset of follicular T cell with specialized roles in regulating humoral immunity. Although Tfr cells have been studied for the past 10 years, their roles have remained elusive. In this review we discuss the current understanding of Tfr cell functions in autoimmunity and how Tfr cells simultaneously control foreign and autoantigen specific antibody responses. We highlight new tools that enable in-depth study of Tfr cells in vivo and recent data suggesting an important role for Tfr cells in limiting participation of autoreactive B cells in germinal centers.
Keywords: T follicular regulatory cells, humoral immunity
Introduction
Antibody responses to most foreign antigens result from interactions between T follicular helper (Tfh) cells and B cells in the B cell follicle and germinal centers (GCs)1. Tfh cells promote class switch recombination, somatic hypermutation and affinity maturation of B cells which enhance the effector functions of antibodies. After interaction with Tfh cells, B cells either differentiate into memory B cells which respond after antigen re-exposure, or into plasma cells that produce high affinity antibody2. Dysregulation of Tfh cells can result in autoreactive antibodies and systemic autoimmune disease. The immune system therefore must ensure Tfh and B cell responses are appropriate to eliminate foreign pathogens while simultaneously preventing autoimmune disease. Although central tolerance can limit most autoreactive T and B cells from making it into the periphery, it does not prevent this completely. Additional peripheral immunoregulatory mechanisms are required to ensure antibody responses are appropriate in terms of specificity, strength and duration. Mechanisms that control antibody responses after antigenic exposure are still being elucidated.
Tfr cells are a specialized T regulatory (Treg) cell subset that can gain access to B cell follicles and regulate Tfh-mediated B cell responses after antigenic exposure3, 4, 5, 6, 7, 8. Although other pathways likely contribute, Tfr cells are thought to be an important mechanism that regulates antibody responses following antigen exposure. Tfr cells have a unique transcriptional program compared to Treg cells in which the transcription factor FoxP3 coopts a Tfh cell transcriptional program to elicit Tfr cell identity and suppressive function6, 9. Tfr cells regulate B cell responses through a number of mechanisms, including CTLA-4-mediated inhibition of B cells and inhibition of proinflammatory cytokine production by Tfh cells10, 11, 12 Although some mechanisms of Tfr cell differentiation and function have been elucidated, the in vivo functions of Tfr cells are an active area of investigation. Early adoptive transfer work suggested an important role for Tfr cells in controlling antibody responses. Recently, studies using an inducible Tfr-deleter mouse demonstrate that Tfr cells regulate early, but not late, GC responses to limit autoreactive and antigen-specific antibody responses13. However, non-inducible models of Bcl6 deficiency in Treg cells have suggested either subtle, or positive, roles for Tfr cells in controlling antibody responses14, 15, 16. These divergent results have led to ambiguity in the roles of Tfr cells in vivo. In this review we discuss the current understanding of the in vivo functions of Tfr cells in settings of autoimmunity and suggest a new paradigm to rectify inconsistencies and elucidate the complex immunoregulatory roles of Tfr cells.
Tfr Ceil Identity
Brief History of Tfr cells
Tfr cells were discovered in 2011 when three groups simultaneously identified a population of FoxP3+ Treg cells that expressed CXCR5 and migrated to B cell follicles5, 6, 7 Although precise functions of Tfr cells were not fully elucidated at that time due to a lack of specific tools, Tfr cells were identified as a distinct population based on phenotype, positioning and/or transcriptional program. Since these first reports, the precise definition of a Tfr cell has been somewhat inconsistent. Currently Tfr cells are defined as FoxP3+ CD4+ T cells that express at least some levels of the chemokine receptor CXCR5, have a distinct transcriptional program consistent with a Tfr cell, can gain access to B cell follicles, and are capable of modulating B cell responses. The most common way to identify Tfr cells is through flow cytometric analysis of CXCR5 expression on FoxP3+ cell populations. However, inconsistent gating strategies and the difficulty of measuring CXCR5 expression have led to variability in Tfr cell identification in the literature, leading to confusion. In addition, costaining with a second receptor or a transcription factor (PD-1, ICOS or Bcl6) has added another dimension of variability, since some of these strategies weigh expression of PD-1, ICOS or Bcl6 more than CXCR5. Since Tfr cell function is likely most potent in B cell follicles, immunofluorescence of FoxP3+ cells in the B cell follicle also has been used as a secondary approach to identify Tfr cells. In addition, some previous studies, including ours, have assessed the presence of FoxP3+ cells directly in GCs to identify Tfr cells. However, these strategies were based on the assumption that Tfr cells predominantly controlled antibody responses within GCs, an assumption that is not quite accurate, as discussed below. Tfr cells likely also control responses at the T-B border, but delineating differences between Treg and Tfr cells at the T-B border can be extremely difficult using immunofluorescence approaches.
Direct comparisons of Tfr cells to Tfh cells also have contributed to inconsistencies in Tfr cell identification and function. Although Tfh and Tfr cells share similar expression of a number of cell surface receptors and transcription factors, these cells are quite distinct. In particular, gating strategies for Tfh cells are not always valid for Tfr cells. For instance, some studies that simultaneously analyze Tfh and Tfr cells use typical Tfh gating strategies of CXCR5highBcl6high cells and then subdivide these cells based on FoxP3 to delineate Tfh and Tfr cells. This approach assumes that the only difference between Tfh and Tfr cells is FoxP3 expression; however, this is an oversimplification. Tfr cells typically have lower expression of CXCR5 and Bcl6, compared to Tfh cells9, 17, 18, 19 Therefore, using typical Tfh gating strategies may “gate out” and fail to identify some Tfr cell populations. As a result, Tfr cells are likely present but missing when such gating strategies are used, leading to misinterpretation of the presence of Tfr cells as rare in some settings. In addition, the tissue microenvironment can impact expression of receptors used to identify Tfh and Tfr cells. For example, we have shown that Tfh (and Tfr) cells express higher levels of PD-1 in the spleen versus skin draining lymph nodes, whereas the opposite is true for ICOS9. Thus, caution must be used in overinterpreting data based on Tfh cell gating strategies. We recommend a flow cytometric gating strategy to identify Tfr cells where CXCR5+ FoxP3+ cells are identified using strict staining controls. Gating strategies should be validated with functional assays and/or transcriptional analyses, when available. If costimulatory and/or transcription factors are used for costaining, multiple costaining strategies should be employed. Gating strategies for human Tfr cells are more complex than in murine systems because of variable immunological histories between patients, less definitive identification of Treg cells using FoxP3 staining, and the limited availability of healthy lymphoid tissue. Nevertheless, the phenotype of Tfr cells in humans seems to match murine Tfr cells. It is possible that human Tfr cells may express lower levels of PD-1 and Bcl6 than murine Tfr cells, but it is not possible to make direct comparisons19. More definitive studies of human Tfr cells, including transcriptomic analysis in a number of tissues, need to be performed.
Differentiation of Tfr cells
Tfr cells differentiate from natural Treg precursors upon interaction with dendritic cells, following similar cues as Tfh cells, although with slightly delayed kinetics7, 18. While some Tfr cells may originate from induced Treg populations, this is infrequent even in the most permissive circumstances20. Tfr cell differentiation begins with dendritic cell interactions, which initiate the Tfr cell program18. Circulating blood Tfr cell development requires priming by DCs but these circulating Tfr cells do not require interactions with B cells for their generation9, 18. Within the B cell follicle Tfr cells likely receive full effector differentiation signals through interactions with B cells, strengthening the Tfr cell transcriptional program and facilitating suppression, although this has yet to be proven experimentally6, 21, 22. A recently published study found that Tfh (and fibroblastic reticular cells) cells induce Tfr cell generation through secretion of sclerostin domaincontaining protein 1 (SOSTDC1) which inhibits the β-catenin pathway in Tfr cells23. The Tfr cell transcriptional program encodes both Tfr cell identity and functionality. Although Tfr cells are fairly stable, we recently found that a small percentage of Tfr cells (~10-20%) can lose FoxP3 and become “ex-Tfr” cells with diminished suppressive capacity9. Importantly, without fate mapping alleles, these ex-Tfr cells would be contained in typical Tfh cell gating strategies.
Interestingly, the transcription factor FoxP3 is necessary, but not sufficient, for the Tfr cell transcriptional program. Tfr cells do not have a distinct lineage transcription factor, but instead rely on multiple transcription factors. In a recent study we showed that loss of FoxP3 results in an altered transcriptional program and increased features of Tfh cells9. When FoxP3 expression was enforced in Tfh cells, a Tfr-like cell phenotype and suppressive potential resulted. These studies suggest that FoxP3 alters the Tfh transcriptional program to induce Tfr cell suppressive function and transcriptional identity. This is similar to tissue Treg cell subsets in which FoxP3 may alter tissue-specific programs to facilitate unique features of suppression24. For instance, adipose tissue Treg cells require FoxP3 to alter tissue-specific programs within visceral fat 25. The overlap of transcriptional programming between tissue Treg and Tfr cells further suggests that the B cell follicle is similar to a peripheral tissue regarding how microenvironment may direct and strengthen Treg transcriptional identity26. The role of ex-Tfr cells in autoimmune disease has yet to be established, but the altered functionality of these cells suggests a putative role in altered autoantibody production.
Strategies to Study Tfr ceils
Models to Study Tfr cells
The lack of specific tools for studying Tfr cells has impeded our understanding of the role of Tfr cells in controlling antibody responses. In vitro suppression assays have been developed to study Tfr cell functions. In these assays Tfr cells are cultured with Tfh and B cells. These in vitro assays have demonstrated that Tfr cells regulate class switch recombination, antibody secretion and somatic hypermutation of B cells9, 13, 17, 27, and shown that Tfr cells alter metabolic reprogramming and suppress IL-21 production by Tfh cells. The ability of Tfr cells to suppress IL-21 production by Tfh is critical for Tfr cell function, as IL-21 can inhibit Tfr cells12, 28, 29
Although these studies have been useful for mechanistic interrogation of B, Tfh and Tfr communication, they do not recapitulate all components of the B cell follicle. Early in vivo studies used adoptive transfer of Tfr cells into lymphopenic mice to show that Tfr cells suppress Tfh-mediated B cell responses including antibody secretion, GC and plasma cells17, 7, 10. However, such transfer approaches have limitations because they do not reconstitute the entire Tfr cell compartment, Tfr cells are lost over time, and de novo Tfr cells are unable to form. Therefore, only short-term Tfr cell functionality can be assessed by these adoptive transfer assays. Additional strategies using mixed bone marrow chimeras of Sh2d1a/SAP deficient and Foxp3-DTR cells have achieved attenuated Tfr cell frequencies, resulting in enhanced germinal center B cell responses, but not antigen-specific antibody6. Although these strategies achieved diminished Tfr cell frequencies, the complexities of performing these chimeras have limited their broad use.
Newer strategies have been employed to study the immunoregulatory roles of Tfr cells in intact mice in vivo. One approach utilized mice in which Bcl6 was selectively deleted in Treg cells with a FoxP3Cre system14, 15, 16, 30, 31, 32 The rationale behind this strategy was that since Bcl6 is required for follicular T cell populations, loss of Bcl6 would result in elimination of all Tfr cells from birth. However, although there is an essential requirement for Bcl6 in Tfh cell development, the absolute requirement for Bcl6 in Tfr cells has been suggested, but not tested rigorously5, 6. The Bcl6-Treg cell deletion approach strongly attenuates CXCR5+PD1+ Treg cells, but CXCR5+PD1-Treg cells are less affected and may remain in some settings15, 16, 30, 31, 24 Since PD-1high Tfr cells are likely the most activated Tfr cells, and Bcl6 seems to be enriched in Tfr cells in GCs, the Bcl6-Treg Tfr cell deficiency model may only attenuate highly activated, or “GC”, Tfr cells. In addition, recent studies in which CXCR5 was deleted in an inducible manner from Treg cells using a crelox system suggest that CXCR5 may not be absolutely required for Tfr cell positioning33. Since Bcl6 controls optimal CXCR5 expression, Bcl6 deficiency may result in Tfr cells without CXCR5 expression (and these Tfr cells are therefore lost in flow cytometric gating strategies), but nevertheless may gain access to B cell follicles to suppress B cell responses. Moreover, Bcl6 is expressed (at least at the transcriptional level) in other effector Treg subsets and may have important roles in these Treg subsets, complicating interpretation of these data9, 24, 34 Studies using the Bcl6-Treg Tfr cell deletion approach have suggested Tfr cells may have minor or positive roles in regulating foreign antigen-specific antibody responses15, 16, 30, 31, 32 Lack of a strong phenotype was also found when the CXCR5 gene was eliminated from Treg cells33. These findings have raised questions about whether Tfr cells have a less dominant and more complex role in regulating humoral immune response than initially thought.
To overcome limitations of existing tools to perturb Tfr cells, a Tfr-DTR mouse was developed to delete Tfr cells and assess Tfr cell function in vivo. This mouse uses a dual genedriven system (requiring FoxP3 and CXCR5 expression) to induce diphtheria toxin receptor (DTR) on the surface of Tfr cells, making them susceptible to deletion by diphtheria toxin administration. Importantly, this system allows temporal deletion studies. These studies are distinct from CXCR5 floxed FoxP3-creERT2 studies because the CXCR5 floxed FoxP3-creERT2 strategy only eliminates the CXCR5 gene from Tfr cells so CXCR5 cannot be expressed on the surface of Tfr cells, whereas the Tfr-DTR strategy eliminates the Tfr cells all together. Studies with the Tfr-DTR mouse demonstrated that Tfr cells potently regulate antibody responses elicited by a foreign antigen vaccine before GC formation, but have less of a role after GC formation13. Interestingly, although vaccine-specific antibody was increased when Tfr cells were deleted, the antibody was of lower affinity. Therefore, although Tfr cells are thought to broadly “inhibit” B cell responses, the end result of Tfr cell suppression may be more or less protective antibody responses, depending upon whether low amounts of high affinity antibody, or large amounts of low affinity antibody, can mediate protection from a particular pathogen.
Tfr cells can regulate antibody isotypes beyond IgG. We co-discovered a population of IL-13 producing Tfh cells, Tfh13 cells, which have important roles in mediating IgE responses13, 35. In the Tfr-DTR model, Tfr cells potently suppressed Tfh13 cell mediated IgE responses as well as allergen-specific IgE in vivo to limit allergic airway disease. Recent studies in the Bcl6-Treg model of Tfr deficiency have also demonstrated a role for Tfr cells in controlling allergen specific IgE and lung inflammation36. In humans, Tfr cell frequencies have been suggested to contribute to allergic rhinitis, and allergen immunotherapy may correct this defect to limit disease37. However, Tfr cells may have a stimulatory role in food allergy through production of IL-1038. This broad role of Tfr cells in regulating antibody isotypes has implications for a variety of protective and pathogenic antibody responses.
Roles of Tfr cells in Autoimmunity
Since the discovery of Tfr cells, a number of groups have assessed Tfr cell frequencies in human autoimmune diseases. Although initial studies suggested that the frequency of Tfr cells inversely correlates with autoimmune disease, more recent studies with improved gating strategies have demonstrated more complex relationships and inconsistencies in findings4, 21. In settings of systemic lupus erythematosus (SLE) recent studies have shown inverse, positive, or no correlation of Tfr cells (or the Tfr:Tfh ratio) with disease severity39, 40, 41. In primary Sjogren’s syndrome, a reduced Tfr:Tfh ratio positively correlates with disease as well as tertiary lymphoid structures in tissues42, 43. In rheumatoid arthritis circulating Tfr cells have been found to have either positive or negative correlations with disease44, 45, 46, 47, 48. Increased Tfr frequencies (or Tfr:Tfh ratios) negatively correlate with disease in multiple sclerosis, autoimmune hepatitis, and ulcerative colitis49, 50, 51. Inconsistencies in findings regarding alterations in Tfr cells are likely due to variability in gating strategies, complexities in treatment of patients, and the relatively late stages of analysis. We hypothesize that Tfr cells have key functional roles in mitigating autoimmune disease, but in clinical settings where the disease has progressed substantially, Tfr cells may be indicators of enhanced inflammation and not suppressive state. For this reason, elucidating the roles of Tfr cells in autoimmunity in pre-clinical models is essential.
Studies using both the Bcl6-Treg knockout and the Tfr-DTR models suggest Tfr cells control autoreactive antibody responses. Bcl6-Treg deficiency resulted in substantial increases in autoreactive antibodies to salivary glands, kidney antigens and dsDNA, but these occurred only after 30 weeks in spontaneous settings15. Antinuclear antibodies (ANA) were not increased in Bcl6-Treg deficient mice in the basal state in another study, but were increased 30 days after influenza infection compared to controls32. In a lupus-like pristane injection model Bcl6-Treg deficient mice had small increases in anti-dsDNA IgA, but not IgG, 4 months after injection16. Also using the Bcl6-Treg knockout model, experimental Sjogren’s syndrome was exacerbated, but only after 5 weeks after initiation15. Therefore, in the Bcl6-Treg deficiency model autoantibodies develop relatively slowly in unmanipulated mice, but are accelerated in settings of induced inflammation.
In the Tfr-DTR model autoreactive IgG was found as early as 21 days after Tfr cell deletion, suggesting that Tfr cells consistently control autoreactive responses13. However, it is important to note that these mice had been vaccinated with a foreign antigen and strong adjuvant. The autoreactive IgG reacted with a diverse array of autoantigens found in many different diseases. Interestingly, there also were substantial amounts of autoreactive IgE in the vaccinated Tfr-DTR mice. Although autoreactive IgE has been suggested to have roles in SLE nephritis, the function of autoreactive IgE after Tfr cell deletion is unclear52, 53. When Tfr cells were deleted after GC formation in Tfr-DTR mice, although the vaccine-specific IgG was not altered, total IgG, IgA and IgE was increased, suggesting that Tfr cells may have roles in controlling autoreactive antibodies after GC formation. These studies illustrate the key function for Tfr cells in restraining autoreactive antibody responses. Although the temporal roles for Tfr cells in controlling autoreactive antibody responses are not yet clear, in vitro functional assays suggest Tfr cells can suppress initial activation of autoreactive B cells13.
Integrated paradigm for regulation of antibody responses by Tfr ceils
Based on current data, a new paradigm of how Tfr cells function is emerging (Figure 1). Outside of germinal centers in the B cell follicle (for instance, early during vaccination, infection or allergen exposure, before GCs form), Tfr cells prevent autoreactive B cells from being activated by Tfh, most likely by attenuating cytokine production (e.g. IL-21 and IL-4) and/or costimulation. At the same time, Tfr cells prevent GCs elicited by foreign antigen (vaccine, microbe or allergen) from forming by inhibiting cytokine production by Tfh cells (such as IL-21 and IL-4) and dampening B cell metabolic flux. However, in some settings B and Tfh cells can overcome this suppression due to lack of local Tfr cells, high affinity BCR driving high signals, high metabolic flux, unusual concentration of Tfh cells, and/or other mechanisms. In settings where Tfh and B cells are able to escape Tfr cell suppression, GCs start to form. Within developing GCs, Tfr cells may be present but are suppressed by IL-21 and other cytokines produced by Tfh cells. The high concentration of cytokines simultaneously activates GC B cells to expand and enhances their stimulatory capacity (through CD80/86, antigen presentation, e.g.). This creates a stimulatory positive feedback loop, allowing more cytokine-producing Tfh cells to become activated and more GC B cell activation/expansion. The stimulatory positive feedback loop also suppresses Tfir cells, facilitating more Tfh and GC B cell responses. Within GCs, Tfir cells are present (although at lower concentrations) but have difficulty suppressing GC B cells responding to a foreign antigen vaccine or pathogen, but can still suppress autoreactive B cells that may wander into GCs and become activated by high levels of cytokines present there. Tfr cells may be able to suppress autoreactive B cells in GCs because cytokines alone cannot overcome Tfr cell suppression. Cytokines in combination with strong BCR signaling and close interaction with antigen-specific Tfh cell may be needed to overcome Tfr cell suppression. If this is the case, Tfr cells are the “guardians” of the GC and ensure antibody responses are appropriate and targeted. Although it has not yet been demonstrated, Tfr cells may have additional roles in existing GCs beyond restraining autoreactive B cells. For instance, Tfr cells may participate in altering the breadth of antibody responses, in GC resolution after antigen exposure ends, or by preventing hyperproliferation of effector cells.
Figure 1. Integrated paradigm for regulation of antibody responses by Tfr cells.
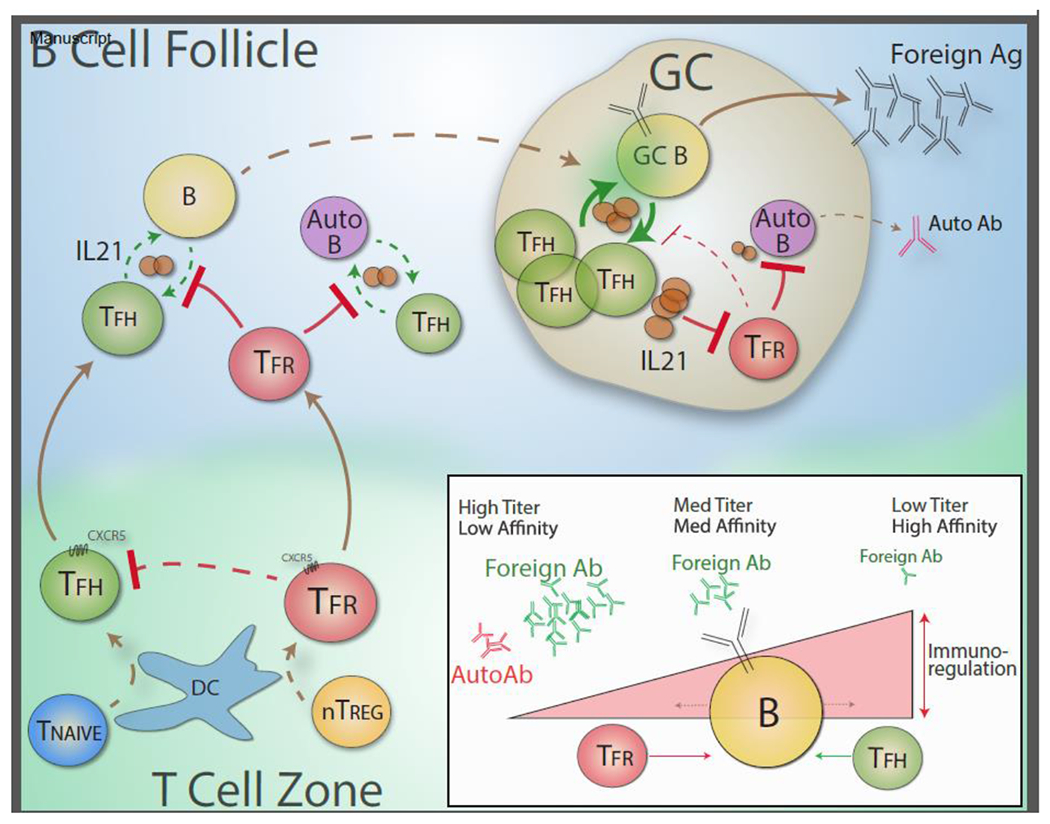
Tfr cell differentiation is initiated by DCs upon interaction with nTreg cells. The Tfr transcriptional program forms, including expression of CXCR5 which, in part, directs Tfr cells to the B cell follicle. While crossing the T cell zone Tfr cells may inhibit Tfh cell differentiation. In the B cell follicle Tfr cells interact with B cells which strengthens the Tfr phenotype, enhancing and strengthening the Tfr transcriptional program. In the B cell follicle, Tfr cells can attenuate foreign vaccine-specific Tfh cell cytokine production and B cell responses, thereby preventing initial GC formation. At the same time, Tfr cells prevent autoreactive B cells from being activated through similar mechanisms. If Tfh and B cells are able to escape suppression in the B cell follicle and form a GC, Tfh and B cells in GCs become activated which induces heightened IL-21 production by Tfh cells and enhanced costimulation/antigenic signals from B cells. IL-21 production by Tfh cells inhibits Tfr cells, preventing Tfr cell expansion. The lower concentration of Tfr cells results in more substantial Tfh and GC B cell interactions and expansion, resulting in more IL-21 and more suppressed Tfr cells, and leading to a positive feedback loop in which Tfr cells are no longer able to suppress foreign antigen-specific GC B cells. In situations where autoreactive B cells stray into GCs, Tfr cells may be able to suppress autoreactive GC B cells because of lower BCR signaling in these cells and/or less substantial Tfh cognate recognition, even in the presence of high IL-21 levels. (inset) Tfr cells control activation thresholds in B cells. In settings of high Tfr cells, small amounts of foreign antigen-specific antibody can be made, but it will be high affinity since only B cells with the highest affinity BCR can escape Tfr cell suppression. In settings with intermediate levels of Tfr cells, moderate amounts of antibody with moderate affinity can be made because Tfr cells impose a moderate activation threshold allowing foreign-antigen specific antibody to be made but preventing autoreactive B cells from being activated. In settings of low Tfr cells, high amounts of low affinity foreign-specific antibody can be made since signaling thresholds to overcome Tfr suppression are extremely low and even low affinity BCRs can overcome suppression. In this setting, even autoreactive antibody can be made even with relatively low affinity BCRs and lack of Tfh help.
Conclusions
Tfr cells appear to have critical roles in controlling both foreign antigen-specific and autoreactive B cells. In order to modulate Tfr cells to enhance vaccine-specific responses and promote protective antibody responses to pathogens, methods are needed to uncouple Tfr cell suppression of foreign antigen-specific and autoreactive antibody responses. Such approaches could make possible selective targeting of Tfr cells to enhance vaccine-specific responses. Likewise, if Tfr cells can be augmented to limit autoreactive antibody while still allowing responses to vaccines or pathogens, this may provide new strategies for treating autoimmune diseases. The tools developed by the field will be instrumental in enabling a deeper understanding of Tfr cells in health and disease, and their therapeutic potential.
Highlights.
Tfr cells regulate humoral immunity, thereby controlling antibody responses
Roles of Tfr cells have been unclear due to lack of specific tools to study them
We propose a paradigm for how Tfr cells control foreign and autoantibody responses
Acknowledgements
This work was supported by National Institutes of Health grant P0156299 to P.T.S. and A.H.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest
The authors declare no competing financial interests.
Conflict
Arlene Sharpe and Peter Sage do not have conflicts of interest related to this manuscript.
References
We apologize to our colleagues for not being able to cite all relevant work due to space constraints.
- 1.Victora GD & Nussenzweig MC Germinal centers. Annu Rev Immunol 30, 429–457 (2012). [DOI] [PubMed] [Google Scholar]
- 2.McHeyzer-Williams M, Okitsu S, Wang N & McHeyzer-Williams L Molecular programming of B cell memory. Nat Rev Immunol 12, 24–34 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sage PT & Sharpe AH T follicular regulatory cells in the regulation of B cell responses. Trends Immunol 36, 410–418 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sage PT & Sharpe AH T follicular regulatory cells. Immunol Rev 271, 246–259 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Chung Y et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med 17, 983–988 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linterman MA et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med 17, 975–982 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wollenberg I et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol 187, 4553–4560 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Wu H, Xie MM, Liu H & Dent AL Stat3 Is Important for Follicular Regulatory T Cell Differentiation. PLoS One 11, e0155040 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou S et al. FoxP3 and Ezh2 regulate Tfr cell suppressive function and transcriptional program. J Exp Med 216, 605–620 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; *In this study we assessed the Tfr cell transcriptional program in various tissues, identified a population of ex-Tfr cells, and assessed the roles of FoxP3 and Ezh2 in suppression.
- 10.Sage PT, Paterson AM, Lovitch SB & Sharpe AH The coinhibitory receptor ctla-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity 41, 1026–1039 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wing JB, Ise W, Kurosaki T & Sakaguchi S Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity 41, 1013–1025 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Sage PT et al. Suppression by TFR cells leads to durable and selective inhibition of B cell effector function. Nature immunology 17, 1436–1446 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clement RL et al. Follicular regulatory T cells control humoral and allergic immunity by restraining early B cell responses. Nature immunology 20, 1360–1371 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; **In this study we created an inducible Tfr-DTR mouse model and showed that Tfr cells control vaccine-elicited antibody and B cell memory before GC formation, as well as control autoreactive B cells. We also co-discovered Tfh13 cells and showed that Tfr cells regulate Tfh13-mediated IgE responses to control allergic airway disease.
- 14.Laidlaw BJ et al. Interleukin-10 from CD4(+) follicular regulatory T cells promotes the germinal center response. Sci Immunol 2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu W et al. Deficiency in T follicular regulatory cells promotes autoimmunity. J Exp Med 215, 815–825 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** In this study, the authors use a Bcl6-Treg model of Tfr deficiency and show worsening of experimental Sjogren's syndrome.
- 16.Wu H et al. Follicular regulatory T cells repress cytokine production by follicular helper T cells and optimize IgG responses in mice. Eur J Immunol 46, 1152–1161 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; *In this study, the authors develop a Bcl6-Treg model of Tfr cell deficiency but find only minor roles for Tfr cells in controlling foreign antigen specific antibody, and small increases in autoreactive antibodies in induced lupus-like disease.
- 17.Sage PT, Francisco LM, Carman CV & Sharpe AH The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nature immunology 14, 152–161 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sage PT, Alvarez D, Godec J, von Andrian UH & Sharpe AH Circulating T follicular regulatory and helper cells have memory-like properties. J Clin Invest 124, 5191–5204 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sayin I et al. Spatial distribution and function of T follicular regulatory cells in human lymph nodes. J Exp Med 215, 1531–1542 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aloulou M et al. Follicular regulatory T cells can be specific for the immunizing antigen and derive from naive T cells. Nature communications 7, 10579 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng J, Wei Y, Fonseca VR, Graca L & Yu D T follicular helper cells and T follicular regulatory cells in rheumatic diseases. Nature reviews. Rheumatology 15, 475–490 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Wing JB, Lim EL & Sakaguchi S Control of foreign Ag-specific Ab responses by Treg and Tfr. Immunol Rev (2020). [DOI] [PubMed] [Google Scholar]
- 23.Wu X et al. SOSTDC1-producing follicular helper T cells promote regulatory follicular T cell differentiation. Science 369, 984–988 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Panduro M, Benoist C & Mathis D Tissue Tregs. Annu Rev Immunol 34, 609–633 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C et al. TCR Transgenic Mice Reveal Stepwise, Multi-site Acquisition of the Distinctive Fat-Treg Phenotype. Cell 174, 285–299 e212 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zemmour D et al. Single-cell gene expression reveals a landscape of regulatory T cell phenotypes shaped by the TCR. Nature immunology 19, 291–301 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sage PT & Sharpe AH In Vitro Assay to Sensitively Measure TFR Suppressive Capacity and TFH Stimulation of B Cell Responses. Methods Mol Biol 1291, 151–160 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Jandl C et al. IL-21 restricts T follicular regulatory T cell proliferation through Bcl-6 mediated inhibition of responsiveness to IL-2. Nature communications 8, 14647 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding Y et al. Interleukin-21 promotes germinal center reaction by skewing the follicular regulatory T cell to follicular helper T cell balance in autoimmune BXD2 mice. Arthritis & rheumatology 66, 2601–2612 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie MM et al. Follicular regulatory T cells inhibit the development of granzyme B-expressing follicular helper T cells. JCI Insight 4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie MM et al. Roles of T Follicular Helper Cells and T Follicular Regulatory Cells in Autoantibody Production in IL-2-Deficient Mice. Immunohorizons 3, 306–316 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Botta D et al. Dynamic regulation of T follicular regulatory cell responses by interleukin 2 during influenza infection. Nature immunology 18, 1249–1260 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanderleyden I et al. Follicular Regulatory T Cells Can Access the Germinal Center Independently of CXCR5. Cell reports 30, 611–619 e614 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y et al. Bcl6 Preserves the Suppressive Function of Regulatory T Cells During Tumorigenesis. Frontiers in immunology 11, 806 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gowthaman U et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science 365 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koh B et al. Bcl6 and Blimp1 reciprocally regulate ST2(+) Treg-cell development in the context of allergic airway inflammation. The Journal of allergy and clinical immunology (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; *In this study the authors use the Bcl6-Treg model to show that Tfr cells regulate allergic airway inflammation.
- 37.Yao Y et al. Allergen immunotherapy improves defective follicular regulatory T cells in patients with allergic rhinitis. The Journal of allergy and clinical immunology (2019). [DOI] [PubMed] [Google Scholar]; * In this study, the authors find that defects in Tfr cells are associated with allergic rhinitis in patients. Allergen immunotherapy normalizes Tfr cell defects and alleviates disease suggesting function of Tfr cells in human allergy.
- 38.Xie MM et al. T follicular regulatory cells and IL-10 promote food antigen-specific IgE. J Clin Invest (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu B et al. The ratio of circulating follicular T helper cell to follicular T regulatory cell is correlated with disease activity in systemic lupus erythematosus. Clinical immunology 183, 46–53 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu C et al. Increased circulating CD4(+)CXCR5(+)FoxP3(+) follicular regulatory T cells correlated with severity of systemic lupus erythematosus patients. Int Immunopharmacol 56, 261–268 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Jacquemin C et al. OX40L/OX40 axis impairs follicular and natural Treg function in human SLE. JCI Insight 3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fonseca VR et al. Blood T Follicular Regulatory Cells / T Follicular Helper Cells ratio Marks Ectopic Lymphoid Structure Formation and PD-1(+) ICOS(+) T Follicular Helper Cells Indicate Disease Activity in Primary Sjogren’s Syndrome. Arthritis & rheumatology (2018). [DOI] [PubMed] [Google Scholar]; * In this study, the authors use rigorous flow cytometric analysis to show that Tfr cells may be a biomarker of disease as well as ectopic lymphoid structures.
- 43.Kim JW et al. Circulating CCR7(lo)PD-1(hi) Follicular Helper T Cells Indicate Disease Activity and Glandular Inflammation in Patients with Primary Sjogren’s Syndrome. Immune Netw 19, e26 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu C et al. Increased Circulating Follicular Treg Cells Are Associated With Lower Levels of Autoantibodies in Patients With Rheumatoid Arthritis in Stable Remission. Arthritis & rheumatology 70, 711–721 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Wang X et al. Imbalance of circulating Tfr/Tfh ratio in patients with rheumatoid arthritis. Clin Exp Med 19, 55–64 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Niu Q et al. Enhanced IL-6/phosphorylated STAT3 signaling is related to the imbalance of circulating T follicular helper/T follicular regulatory cells in patients with rheumatoid arthritis. Arthritis research & therapy 20, 200 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romao VC, Fonseca JE, Agua-Doce A & Graca L T Follicular Regulatory Cells Are Decreased in Patients With Established Treated Rheumatoid Arthritis With Active Disease: Comment on the Article by Liu et al. Arthritis & rheumatology 70, 1893–1895 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Cao G et al. An imbalance between blood CD4(+)CXCR5(+)Foxp3(+) Tfr cells and CD4(+)CXCR5(+)Tfh cells may contribute to the immunopathogenesis of rheumatoid arthritis. Mol Immunol 125, 1–8 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Puthenparampil M et al. Peripheral imbalanced TFH/TFR ratio correlates with intrathecal IgG synthesis in multiple sclerosis at clinical onset. Mult Scler 25, 918–926 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Liang M et al. Dysregulated TFR and TFH cells correlate with B-cell differentiation and antibody production in autoimmune hepatitis. J Cell Mol Med 24, 3948–3957 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Long Y et al. The Imbalance of Circulating Follicular Helper T Cells and Follicular Regulatory T Cells Is Associated With Disease Activity in Patients With Ulcerative Colitis. Frontiers in immunology 11, 104 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dema B et al. Immunoglobulin E plays an immunoregulatory role in lupus. J Exp Med 211, 2159–2168 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dema B et al. Autoreactive IgE is prevalent in systemic lupus erythematosus and is associated with increased disease activity and nephritis. PLoS One 9, e90424 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


