Abstract
Objective:
We conducted a prospective cohort study to examine the risk of incident mild cognitive impairment (MCI) as predicted by baseline neuropsychiatric symptoms (NPS) and brain regional glucose metabolic dysfunction.
Methods:
1363 cognitively unimpaired (CU) individuals (52.8% males) aged ≥ 50 years were followed for a median of 4.8 years to the outcome of incident MCI. NPS were assessed using Beck Depression and Anxiety Inventories (BDI-II, BAI) and Neuropsychiatric Inventory Questionnaire. Glucose hypometabolism was measured by FDG-PET and defined as SUVR ≤ 1.47 in regions typically affected in Alzheimer’s disease. Cox proportional hazards models were adjusted for age, sex, education and APOE ε4 status.
Results:
Participants with regional glucose hypometabolism and depression (BDI-II ≥ 13) had a more than threefold increased risk of incident MCI (HR [95% CI], 3.66 [1.75, 7.65], p < 0.001, X2 = 11.83, df = 1) as compared to the reference group (normal regional glucose metabolism and no depression), and the risk was also significantly elevated (7.21 [3.54, 14.7], p < 0.001, X2 = 29.68, df = 1) for participants with glucose hypometabolism and anxiety (BAI ≥ 10). Having glucose hypometabolism and ≥ 1 NPS (3.74 [2.40, 5.82], p < 0.001, X2 = 34.13, df = 1) or ≥ 2 NPS (3.89 [2.20, 6.86], p < 0.001, X2 = 21.92, df = 1) increased the risk of incident MCI by more than three times, and having ≥ 3 NPS increased the risk by more than four times (4.12 [2.03, 8.37], p < 0.001, X2 = 15.39, df = 1).
Conclusion:
Combined presence of NPS with regional glucose hypometabolism is associated with an increased risk of incident MCI, with FDG-PET appearing to be a stronger driving force of cognitive decline than NPS.
Keywords: Neuropsychiatric symptoms, FDG-PET, mild cognitive impairment
Introduction
Presence of neuropsychiatric symptoms (NPS) and abnormalities in brain regional glucose metabolism as measured by fluorodeoxyglucose positron emission tomography (FDG-PET) are independent risk factors for cognitive impairment. Research has shown that brain glucose metabolism declines with increasing age (1), that individuals with mild cognitive impairment (MCI) and dementia due to Alzheimer’s disease (AD) have reduced FDG uptake (2–4), and that FDG-PET abnormalities are associated with an increased risk of cognitive decline (5–7). Brain glucose hypometabolism as measured by FDG-PET is thus considered a biomarker of neurodegeneration in AD and may be indicative of synaptic dysfunction or neuronal injury (8). Furthermore, older adults with cognitive impairment are at risk of having or developing NPS (9, 10); and presence of NPS is associated with higher risk of cognitive decline (11–13).
Because NPS and cognitive impairment are both manifestations of similar brain pathologies, it is important to investigate pathways linking NPS with AD neuroimaging biomarkers and cognitive decline. We and others have reported cross-sectional associations between an abnormal FDG-PET and NPS among cognitively unimpaired (CU) elderly persons (14–16). However, little is known about the associations between regional brain glucose hypometabolism and NPS in predicting cognitive decline.
Therefore, the aim of the current study was to examine whether a combined presence of regional glucose metabolic dysfunction, as measured by FDG-PET, and NPS is associated with an increased risk of incident MCI among older adults that were CU at baseline, and whether this risk is greater than having either risk factor alone. In addition to using data from the Neuropsychiatric Inventory Questionnaire (NPI-Q) (17), we also included data from the Beck Depression lnventory-ll (BDI-II) (18) and Beck Anxiety Inventory (BAI) (19) which measure depressive and anxiety symptoms with higher specificity than the NPI-Q. We hypothesized that community-dwelling individuals with regional brain glucose hypometabolism and presence of NPS would have a higher risk of developing incident MCI than participants without altered brain glucose metabolism and presence of NPS.
Methods and Materials
Design and Sample
We conducted a prospective cohort study derived from the Mayo Clinic Study of Aging (MCSA), a longitudinal, population-based study of cognitive aging and MCI in Olmsted County, Minnesota. Details of the study procedures have been reported elsewhere (20). We assembled a cohort of 4956 CU participants aged ≥ 50 years who underwent cognitive evaluation and NPS assessment at baseline. 3593 persons were excluded for various reasons, thus the final cohort consisted of 1363 participants (Figure 1). Included participants differed significantly (p<0.05) from those excluded with regards to sex (included: 52.8% males, excluded: 48.7% males), age (mean [SD]; included: 70.4 [9.9], excluded: 73.9 [10.0]), years of education (mean [SD]; included: 14.9 [2.6], excluded: 14.2 [2.8]) and Charlson comorbidity index (mean [SD]; included: 2.7 [2.8], excluded: 3.2 [3.2]). FDG-PET imaging was conducted after the baseline cognitive evaluation and NPS assessment at a median of 2.8 months [interquartile range, IQR; 2.2, 3.3], Participants were followed for a median of 4.8 years [IQR; 2.8, 6.2] to the outcome of incident MCI. The MCSA study protocols have been approved by the IRB of the Mayo Clinic and Olmsted Medical Center in Rochester, Minnesota. All participants provided written informed consent.
Figure 1.
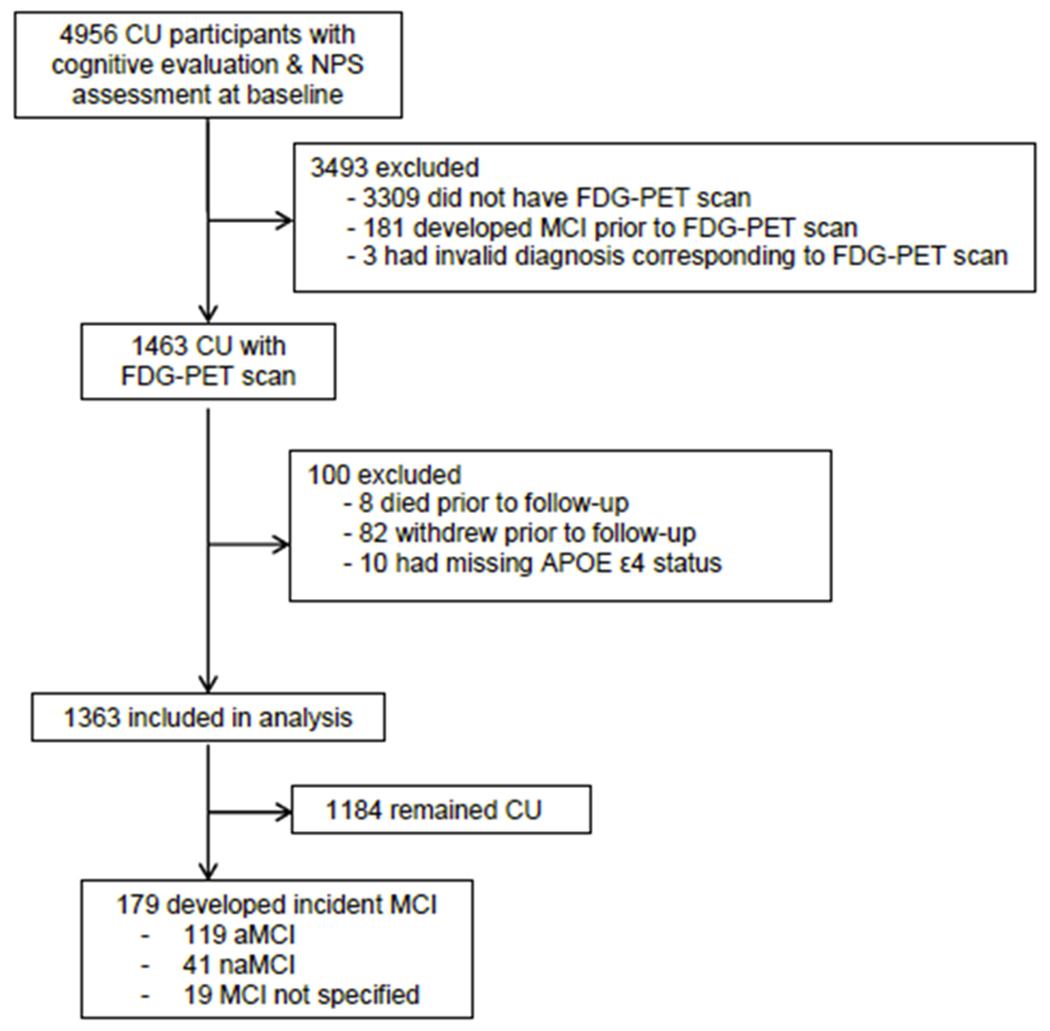
Study flowchart
Abbreviations: CU = cognitively unimpaired; NPS = neuropsychiatric symptoms; MCI = mild cognitive impairment.
Neurocognitive Evaluation
Participants underwent a face-to-face evaluation including a neurological examination, a study coordinator visit, and neuropsychological testing (20). Briefly, the neurological evaluation comprised a neurological history review, administration of the Short Test of Mental Status (21), and a neurological examination. The study coordinator visit included the Clinical Dementia Rating Scale (CDR) (22). Neuropsychological testing was administered by a psychometrist to assess performance in four cognitive domains: memory (delayed recall trials from Auditory Verbal Learning Test (23), Wechsler Memory Scale-Revised (24), Logical Memory and Visual Reproduction subtests); language (Boston Naming Test (25), category fluency (26)); visuospatial skills (Wechsler Adult Intelligence Scale-Revised (27), Picture Completion and Block Design subtests); and attention/executive function (Trail-Making Test Part B (28), Wechsler Adult Intelligent Scale-Revised (27), Digit Symbol Substitution subtest). An expert consensus panel of physicians, study coordinators and neuropsychologists reviewed the results and determined whether a participant was CU or had MCI. Individuals were classified as CU based on normative data developed in this community. For MCI, the revised Mayo Clinic criteria (29) were used: (1) cognitive concern expressed by a physician, informant, participant, or nurse; (2) impairment in one or more cognitive domains (executive functions, memory, language, or visuospatial skills); (3) essentially normal functional activities; and (4) absence of dementia. Participants with MCI had a CDR score of 0 or 0.5; however, the final diagnosis was based on all available data.
Neuropsychiatric Assessment
We measured NPS using the NPI-Q (17) which is designed to assess presence or absence of 12 emotional behaviors (i.e. agitation, delusion, hallucination, depression, anxiety, euphoria, apathy, disinhibition, irritability, aberrant motor behavior, sleep, and eating/appetite). It was administered as a structured interview to an informant by a research nurse or study coordinator. Participants also completed the BDI-II (18) and BAI (19). Both inventories are validated and consist of 21 items that measure common symptoms of depression (such as feeling guilty or loss of interest) over the last two weeks; and symptoms of anxiety (such as nervousness or fear of losing control) over the last week. The severity of each symptom is rated on a Likert scale ranging from 0-3, with a total score ranging from 0-63. We used a BDI-II cutoff-score of ≥ 13 to indicate clinical depression, and a BAI cutoff-score of ≥ 10 to indicate clinical anxiety.
FDG-PET Acquisition
366-399 MBq of 18fluorodeoxyglucose was injected intravenously to study participants followed by an uptake period of 30 minutes. During this time, participants were left undisturbed in a darkened room and instructed to rest quietly without activity with their eyes open. Participants were then imaged with their eyes open for an 8-minute image acquisition consisting of four 2-minute dynamic frames. Also, a CT image was obtained for attenuation correction. Quantitative image analysis for FDG-PET was performed using our in-house fully automated image processing pipeline (30). Statistics on image voxel values were extracted from automatically labeled cortical regions of interest (ROIs) using an atlas (31) modified in-house. There were 19 regions of interest after combining the left and right regions from the atlas. The meta-region of interest consisted of bilateral angular gyrus, posterior cingulate/ precuneus, and inferior temporal cortical regions from both hemispheres and was identified as AD signature ROI elsewhere (7, 32). The ratio of this AD signature ROI and the pons as well as the cerebellar vermis is referred to as standardized uptake value ratio (SUVR). These two regions of references were chosen because they have preserved glucose metabolism in AD (33). Participants were classified as having glucose hypometabolism based on SUVR of ≤ 1.47 (34).
Assessment of Confounders
In addition to traditional confounders (age, sex, and education), we also adjusted our analyses for APOE ε4 genotype, medical comorbidity, and antidepressant medication. Medical comorbidity was assessed using the weighted Charlson Index (35). Antidepressant medication included use of selective serotonin reuptake inhibitor (SSRI), serotonin-norepinephrine reuptake inhibitor (SNRI), citalopram, tetracyclic, or tricyclic antidepressant medication. For determination of APOE ε4 genotype, blood was drawn from participants after they had provided written informed consent. DNA was amplified by means of polymerase chain reaction, and the presence of an APOE ε4 allele was determined using standard methods (36).
Statistical Analysis
For better readability throughout the manuscript, we use the term “FDG-PET+” to refer to participants with regional glucose hypometabolism, and “FDG-PET−” to refer to participants with normal glucose metabolism. We first examined the association between regional glucose hypometabolism and incident MCI, as well as between each indvidual NPS and incident MCI. We calculated hazard ratios (HR) and 95% confidence intervals (95% CI) using Cox proportional hazards models with age as the time scale, and adjusted for sex, education and APOE ε4 genotype. P-values for all HR were based on Wald Chi-squared (X2) tests with one degree of freedom (df). We also ran the models for each individual NPS with an additional adjustment for FDG-PET status. Second, we examined the association between combined presence of glucose hypometabolism and NPS with the risk of incident MCI. We calculated HR and 95% CI with age as the time scale, and after adjusting for sex, education and APOE ε4 genotype status (Model 1); we also additionally adjusted for medical comorbidity and antidepressant medication (Model 2). Once again, p-values for all HR were based on Wald Chi-squared (X2) tests with one df. Furthermore, we calculated multiplicative interactions. In our models, we compared four groups: FDG-PET− / NPS− (normal regional glucose metabolism / no NPS; defined as reference group), FDG-PET+ / NPS− (regional glucose hypometabolism / no NPS), FDG-PET− / NPS+ (normal regional glucose metabolism / NPS), and FDG-PET+ / NPS+ (regional glucose hypometabolism / NPS). We conducted this analysis for seven NPS as assessed by the NPI-Q (depression, apathy, anxiety, agitation, irritability, appetite, nighttime behavior); and for depression and anxiety as assessed by the BDI-II (cutoff-score ≥ 13) and the BAI (cutoff-score ≥ 10), respectively. Finally, we created groups based on normal regional glucose metabolism vs. hypometabolism and number of NPS (any 1 NPS, any ≥ 2 NPS, any ≥ 3 NPS) as well as combinations of NPS. Due to a low count of participants with NPS as assessed by NPI-Q, we only looked at the six most frequent combinations of co-occuring NPS in our sample (i.e. depression and irritability; depression and anxiety; depression and apathy; anxiety and irritability; depression and nighttime behavior, and apathy and irritability). The proportional hazards assumption was checked for all models by looking at Schoenfeld residuals; the assumption was met for all models. Statistical testing was performed at the conventional 2-tailed alpha level of 0.05. All analyses were performed using SAS System, version 9.4 software (SAS Institute, Cary, North Carolina).
Results
Demographics
Of 1363 participants (52.8% males) who were CU at baseline, 1012 (74.2%) were FDG-PET−, and 351 (25.8%) were FDG-PET+. 377 (27.7%) participants were APOE ε4 carriers. After a median follow-up of 4.8 years [IQR; 2.8, 6.2], 179 participants (13.1%) developed incident MCI (119 amnestic MCI, 41 non-amnestic MCI, and 19 MCI not specified). FDG-PET+ and FDG-PET− participants differed in terms of sex, age, education, Charlson index, antidepressant medication, and some of the NPI-Q variables (i.e. depression, apathy, nighttime behavior, and having ≥ 1, ≥ 2 or ≥ 3 NPS). The characteristics of the study sample at baseline are displayed in Table 1.
Table 1.
Characteristics of the study cohort at baseline
| Variable | FDG-PET+ (N=351) N (%) | FDG-PET− (N=1012) N (%) | Total (N=1363) N (%) | p |
|---|---|---|---|---|
| Males | 223 (63.5) | 496 (49.0) | 719 (52.8) | <0.0011 |
| Follow-up (years), median [IQR] | 4.5 [2.5, 6.4] | 4.8 [3.5, 6.1] | 4.8 [2.8, 6.2] | 0.132 |
| Age (years), median [IQR] | 76.5 [71.0, 81.9] | 68.8 [61.4, 75.7] | 71.1 [63.2, 77.7] | <0.0012 |
| Education (years), median [IQR] | 14 [12, 16] | 15 [13, 17] | 15 [13, 17] | <0.012 |
| > 12 years | 246 (70.1) | 784 (77.5) | 1030 (75.6) | <0.011 |
| APOEε4 carriers | 106 (30.2) | 271 (26.8) | 377 (27.7) | 0.221 |
| Charlson index, median [IQR] | 3.0 [1.0, 6.0] | 2.0 [1.0, 3.0] | 2.0 [1.0, 4.0] | <0.0012 |
| Antidepressant medications | 57 (16.2) | 219 (21.6) | 276 (20.2) | 0.031 |
| Racial background, Whitesa | 136 (99.3) | 539 (97.8) | 675 (98.1) | 0.891 |
| BDI-II ≥ 13 (clinical depression)b | 23 (6.6) | 49 (4.9) | 72 (5.3) | 0.221 |
| BAI ≥ 10 (clinical anxiety)c | 19 (5.4) | 50 (5.0) | 69 (5.1) | 0.731 |
| NPI-Q itemsd | ||||
| Depression | 43 (12.8) | 78 (8.5) | 121 (9.7) | 0.021 |
| Apathy | 17 (5.1) | 25 (2.7) | 42 (3.4) | 0.041 |
| Anxiety | 19 (5.7) | 35 (3.8) | 54 (4.3) | 0.161 |
| Agitation | 7 (2.1) | 12 (1.3) | 19 (1.5) | 0.321 |
| Irritability | 28 (8.3) | 57 (6.2) | 85 (6.8) | 0.191 |
| Appetite change | 12 (3.6) | 26 (2.8) | 38 (3.0) | 0.501 |
| Motor disturbance | 4 (1.2) | 5 (0.5) | 9(0.7) | 0.231 |
| Nighttime behavior | 24 (7.9) | 37 (4.5) | 61 (5.4) | 0.021 |
| Disinhibition | 4 (1.2) | 4 (0.4) | 8 (0.6) | 0.141 |
| Euphoria | 2 (0.6) | 1 (0.1) | 3 (0.2) | 0.121 |
| Delusions | 0 (0.0) | 1 (0.1) | 1 (0.1) | 0.541 |
| Hallucinations | 1 (0.3) | 0 (0.0) | 1 (0.1) | 0.101 |
| ≥ 1 NPS | 88 (26.2) | 166 (18.1) | 254 (20.3) | <0.011 |
| ≥ 2 NPS | 38 (11.3) | 65 (7.1) | 103 (8.2) | 0.021 |
| ≥ 3 NPS | 19 (5.7) | 25 (2.7) | 44 (3.5) | 0.011 |
| Depression + Irritability | 13 (3.9) | 23 (2.5) | 36 (2.9) | 0.201 |
| Depression + Anxiety | 11 (3.3) | 16 (1.7) | 27 (2.2) | 0.1 o1 |
| Depression + Apathy | 10 (3.0) | 15 (1.6) | 25 (2.0) | 0.131 |
| Anxiety + Irritability | 9 (2.7) | 13 (1.4) | 22 (1.8) | 0.131 |
| Depression + Nighttime behavior | 9 (2.7) | 13 (1.4) | 22 (1.8) | 0.131 |
| Apathy + Irritability | 6 (1.8) | 14 (1.5) | 20 (1.6) | 0.751 |
Abbreviations: FDG-PET+ = Participants with regional brain glucose hypometabolism; FDG-PET− = Participants with normal regional brain glucose metabolism; IQR = Interquartile range; BDI = Beck Depression Inventory; BAI = Beck Anxiety Inventory; NPI-Q = Neuropsychiatric Inventory Questionnaire; p indicates difference between FDG-PET+ and FDG-PET− participants
= p derived from Chi squared test;
= p derived from Kruskal Wallis test; degree of freedom (df) = 1.
Information on racial background is missing on 675 participants.
BDI-II information is missing on 6 participants.
BAI information is missing on 3 participants.
NPI-Q information missing on 112 participants for all variables except nighttime behavior. For nighttime behavior, information on 236 participants is missing.
Associations between Glucose Metabolism and Incident MCI; and between NPS and Incident MCI
Being FDG-PET+ was associated with an increased risk of incident MCI after adjusting for age (as time scale), sex, education, and APOE ε4 status (HR [95% CI], 2.35 [1.74, 3.18], p < 0.001, X2 = 30.70, df = 1).
Having anxiety (BAI ≥ 10) was associated with an increased risk of incident MCI (2.40 [1.33, 4.33], p = 0.004, X2 = 8.41, df = 1), whereas having depression (BDI-II ≥ 13) was not associated with the risk of incident MCI (1.51 [0.86, 2.65], p = 0.15, X2 = 2.10, df = 1). Participants with presence of depression (1.97 [1.26, 3.06], p = 0.003, X2 = 8.97, df =1), apathy (4.29 [2.57, 7.16], p < 0.001, X2 = 30.94, df = 1), appetite change (2.46 [1.33, 4.55], p = 0.004, X2 = 8.20, df = 1), or nighttime behavior (2.36 [1.47, 3.78], p < 0.001, X2 = 12.78, df = 1) as assessed by the NPI-Q had an increased risk of incident MCI. Having ≥ 1 NPS (1.93 [1.40, 2.66], p < 0.001, X2 = 16.24, df = 1), ≥ 2 NPS (2.22 [1.43, 3.43], p < 0.001, X2 = 12.81, df = 1) and ≥ 3 NPS (2.94 [1.69, 5.12], p < 0.001, X2 = 14.60, df = 1) was associated with an increased risk of incident MCI. Furthermore, having comorbid depression and apathy (5.99 [3.11, 11.53], p < 0.001, X2 = 28.67, df = 1), and comorbid apathy and irritability (3.18 [1.40, 7.24], p = 0.006, X2 = 7.60, df = 1) was associated with an increased risk of incident MCI after adjusting for age (as time scale), sex, education, and APOE ε4 status.
When we additionally adjusted for FDG-PET, all observed associations remained significant: Having anxiety (BAI ≥ 10) was associated with an increased risk of incident MCI (2.29 [1.27, 4.14], p = 0.006, X2 = 7.53, df = 1), whereas having depression (BDI-II ≥ 13) was not associated with the risk of incident MCI (1.52 [0.87, 2.65], p = 0.14, X2 = 2.17, df = 1). Participants with presence of depression (1.63 [1.04, 2.55], p = 0.034, X2 = 4.51, df = 1), apathy (3.64 [2.18, 6.10], p < 0.001, X2 = 24.16, df = 1), appetite change (2.82 [1.52, 5.22], p = 0.001, X2 = 10.81, df = 1), or nighttime behavior (2.04 [1.26, 3.28], p = 0.004, X2 = 8.55, df = 1) as assessed by the NPI-Q had an increased risk of incident MCI. Having ≥1 NPS (1.73 [1.25, 2.39], p < 0.001, X2 = 10.99, df = 1), ≥2 NPS (1.88 [1.21,2.91], p = 0.005, X2 = 7.86, df = 1) and ≥ 3 NPS (2.26 [1.29, 3.97], p = 0.004, X2 = 8.12, df = 1) was associated with an increased risk of incident MCI. Furthermore, having comorbid depression and apathy (5.45 [2.82, 10.53], p < 0.001, X2 = 25.48, df = 1), and comorbid apathy and irritability (2.81 [1.23, 6.41], p = 0.014, X2 = 6.04, df = 1) was associated with an increased risk of incident MCI.
Associations between Glucose Metabolism and NPS (combined) with Incident MCI
After adjusting for age (as time scale), sex, education, and APOE ε4 status, FDG-PET+ participants who had clinical depression (3.66 [1.75, 7.65], p < 0.001, X2 = 11.83, df = 1) or clinical anxiety (7.21 [3.54, 14.7], p < 0.001, X2 = 29.68, df = 1) were at an increased risk of incident MCI as compared to the reference group (FDG-PET− and no depression or anxiety, respectively; Table 2). Similarly, FDG-PET+ participants with presence of depression (3.47 [1.97, 6.12], p <0.001, X2 = 18.47, df = 1), apathy (5.73 [2.72, 12.1], p <0.001, X2 = 21.08, df = 1), anxiety (2.86 [1.31, 6.25], p = 0.008, X2 = 6.98, df = 1), agitation (3.77 [1.17, 12.2], p = 0.0026, X2 = 4.94, df = 1), appetite change (10.5 [4.76, 23.1], p< 0.001, X2 = 34.05, df= 1), nighttime behavior (3.72 [1.87, 7.41], p < 0.001, X2 = 14.03, df = 1), or any psychotic NPS (3.32 [1.03, 10.7], p = 0.045, X2 = 4.03, df = 1) as measured by the NPI-Q had an increased risk of incident MCI as compared to the reference group. When we additionally adjusted the models for medical comorbidity and antidepressant medication, only the associations between FDG-PET+ and agitation (2.77 [0.84, 9.12], p = 0.09, X2 = 2.82, df = 1), as well as between FDG-PET+ and any psychotic NPS (2.86 [0.88, 9.27], p = 0.08, X2 = 3.08, df = 1) with the risk of incident MCI no longer remained significant (Table 2). However, as shown in table 2, only the multiplicative interactions for the models including apathy and nighttime behavior were significant.
Table 2.
Association between regional brain glucose metabolism and NPS in predicting the risk of incident MCI
| Stratum | No. at Risk | No. of Events | Model 1 | Model 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p | Multipl. Interaction p | df | X2 | HR (95%CI) | p | Multipl. Interaction p | df | X2 | |||
| FDG-PET− / BDI-II Depression− | 958 | 77 | 1.00 (ref. group) | 0.88 | 1.00 (ref. group) | 0.95 | ||||||
| FDG-PET+ / BDI-II Depression − | 327 | 86 | 2.32 (1.69, 3.18) | <0.001 | 1 | 26.96 | 2.17 (1.57, 2.99) | <0.001 | 1 | 22.18 | ||
| FDG-PET− / BDI-II Depression+ | 49 | 6 | 1.45 (0.62, 3.38) | 0.39 | 1 | 0.73 | 1.43 (0.61,3.35) | 0.41 | 1 | 0.68 | ||
| FDG-PET+ / BDI-II Depression+ | 23 | 8 | 3.66 (1.75, 7.65) | <0.001 | 1 | 11.83 | 3.21 (1.52, 6.80) | 0.002 | 1 | 9.30 | ||
| FDG-PET− / BAI Anxiety− | 960 | 80 | 1.00 (ref. group) | 0.15 | 1.00 (ref. group) | 0.17 | ||||||
| FDG-PET+ / BAI Anxiety− | 331 | 85 | 2.20 (1.61,3.01) | <0.001 | 1 | 24.20 | 2.04 (1.48, 2.80) | <0.001 | 1 | 19.19 | ||
| FDG-PET− / BAI Anxiety+ | 50 | 3 | 1.22 (0.38, 3.88) | 0.74 | 1 | 0.11 | 1.25 (0.39, 3.99) | 0.71 | 1 | 0.14 | ||
| FDG-PET+ / BAI Anxiety+ | 19 | 9 | 7.21 (3.54, 14.7) | <0.001 | 1 | 29.68 | 6.61 (3.23, 13.5) | <0.001 | 1 | 26.79 | ||
| FDG-PET− / Depression− | 837 | 71 | 1.00 (ref. group) | 0.49 | 1.00 (ref. group) | 0.45 | ||||||
| FDG-PET+ / Depression− | 293 | 78 | 2.39 (1.72, 3.32) | <0.001 | 1 | 27.19 | 2.24 (1.61, 3.12) | <0.001 | 1 | 22.67 | ||
| FDG-PET− / Depression+ | 78 | 8 | 2.02 (0.96, 4.25) | 0.06 | 1 | 3.48 | 2.01 (0.96, 4.24) | 0.07 | 1 | 3.40 | ||
| FDG-PET+ / Depression+ | 43 | 16 | 3.47 (1.97, 6.12) | <0.001 | 1 | 18.47 | 3.13 (1.76, 5.56) | <0.001 | 1 | 15.21 | ||
| FDG-PET− / Apathy− | 890 | 70 | 1.00 (ref. group) | 0.024 | 1.00 (ref. group) | 0.030 | ||||||
| FDG-PET+ / Apathy− | 319 | 85 | 2.53 (1.83, 3.50) | <0.001 | 1 | 31.76 | 2.37 (1.71, 3.29) | <0.001 | 1 | 26.87 | ||
| FDG-PET− /Apathy+ | 25 | 9 | 7.28 (3.58, 14.8) | <0.001 | 1 | 29.95 | 6.85 (3.36, 14.0) | <0.001 | 1 | 28.08 | ||
| FDG-PET+ / Apathy+ | 17 | 9 | 5.73 (2.72, 12.1) | <0.001 | 1 | 21.08 | 5.26 (2.48, 11.1) | <0.001 | 1 | 18.81 | ||
| FDG-PET− / Anxiety− | 880 | 76 | 1.00 (ref. group) | 0.85 | 1.00 (ref. group) | 0.83 | ||||||
| FDG-PET+ / Anxiety− | 317 | 87 | 2.40 (1.75, 3.28) | <0.001 | 1 | 29.47 | 2.24 (1.63, 3.09) | <0.001 | 1 | 24.64 | ||
| FDG-PET− / Anxiety+ | 35 | 3 | 1.37 (0.43, 4.38) | 0.59 | 1 | 0.29 | 1.35 (0.42, 4.31) | 0.61 | 1 | 0.26 | ||
| FDG-PET+ / Anxiety+ | 19 | 7 | 2.86 (1.31,6.25) | 0.008 | 1 | 6.98 | 2.60 (1.19, 5.68) | 0.017 | 1 | 5.70 | ||
| FDG-PET− / Agitation− | 903 | 77 | 1.00 (ref. group) | 0.87 | 1.00 (ref. group) | 0.65 | ||||||
| FDG-PET+ / Agitation− | 329 | 91 | 2.41 (1.76, 3.29) | <0.001 | 1 | 30.53 | 2.26 (1.65, 3.10) | <0.001 | 1 | 25.93 | ||
| FDG-PET− / Agitation+ | 12 | 2 | 1.83 (0.44, 7.53) | 0.40 | 1 | 0.70 | 1.86 (0.45, 7.64) | 0.39 | 1 | 0.75 | ||
| FDG-PET+ / Agitation+ | 7 | 3 | 3.77 (1.17, 12.2) | 0.026 | 1 | 4.94 | 2.77 (0.84, 9.12) | 0.09 | 1 | 2.82 | ||
| FDG-PET− / irritability− | 858 | 73 | 1.00 (ref. group) | 0.17 | 1.00 (ref. group) | 0.19 | ||||||
| FDG-PET+ / irritability− | 308 | 86 | 2.54 (1.85, 3.50) | <0.001 | 1 | 32.94 | 2.38 (1.72, 3.28) | <0.001 | 1 | 27.75 | ||
| FDG-PET− / Irritability+ | 57 | 6 | 1.57 (0.68, 3.64) | 0.29 | 1 | 1.12 | 1.46 (0.63, 3.39) | 0.37 | 1 | 0.79 | ||
| FDG-PET+ / Irritability+ | 28 | 8 | 1.81 (0.83, 3.95) | 0.14 | 1 | 2.20 | 1.63 (0.74, 3.57) | 0.23 | 1 | 1.47 | ||
| FDG-PET− / Appetite− | 889 | 75 | 1.00 (ref. group) | 0.13 | 1.00 (ref. group) | 0.23 | ||||||
| FDG-PET+ / Appetite− | 324 | 87 | 2.31 (1.68, 3.17) | <0.001 | 1 | 26.54 | 2.18 (1.58, 3.00) | <0.001 | 1 | 22.59 | ||
| FDG-PET− / Appetite+ | 26 | 4 | 1.67 (0.60, 4.58) | 0.32 | 1 | 0.97 | 1.74 (0.63, 4.80) | 0.28 | 1 | 1.16 | ||
| FDG-PET+ / Appetite+ | 12 | 7 | 10.5 (4.76, 23.1) | <0.001 | 1 | 34.05 | 8.41 (3.75, 18.9) | <0.001 | 1 | 26.63 | ||
| FDG-PET− / Nightt. behavior− | 788 | 54 | 1.00 (ref. group) | 0.018 | 1.00 (ref. group) | 0.017 | ||||||
| FDG-PET+ / Nightt. behavior− | 278 | 73 | 2.93 (2.04, 4.21) | <0.001 | 1 | 34.08 | 2.79 (1.94, 4.03) | <0.001 | 1 | 30.30 | ||
| FDG-PET− / Nightt. behavior+ | 37 | 11 | 3.93 (2.04, 7.58) | <0.001 | 1 | 16.63 | 3.89 (2.01,7.54) | <0.001 | 1 | 16.20 | ||
| FDG-PET+ / Nightt. behavior+ | 24 | 10 | 3.72 (1.87, 7.41) | <0.001 | 1 | 14.03 | 3.44 (1.73, 6.87) | <0.001 | 1 | 12.32 | ||
| FDG-PET− / psychot. NPS− | 910 | 77 | 1.00 (ref. group) | 0.34 | 1.00 (ref. group) | 0.33 | ||||||
| FDG-PET+ / psychot. NPS− | 330 | 91 | 2.43 (1.78, 3.32) | <0.001 | 1 | 31.23 | 2.28 (1.66, 3.13) | <0.001 | 1 | 26.25 | ||
| FDG-PET− / psychot. NPS+ | 5 | 2 | 3.36 (0.81, 14.0) | 0.10 | 1 | 2.78 | 3.12 (0.74, 13.1) | 0.12 | 1 | 2.42 | ||
| FDG-PET+ / psychot. NPS+ | 6 | 3 | 3.32 (1.03, 10.7) | 0.045 | 1 | 4.03 | 2.86 (0.88, 9.27) | 0.08 | 1 | 3.08 | ||
Abbreviations: FDG-PET+ = Participants with regional brain glucose hypometabolism; FDG-PET− = Participants with normal regional brain glucose metabolism; HR = Hazard ratio; 95% CI = 95% confidence interval; p indicates significance; df = degree of freedom; X2 = Wald Chi-squared; ref. group = Reference group; BDI = Beck Depression Inventory; BAI = Beck Anxiety Inventory. Results based on Cox proportional hazards models. Wald Chi-squared test was used to test for multiplicative interaction (df = 1). Model 1 adjusted for age (as the time scale), sex, education, and APOE ε4 status. Model 2 additionally adjusted for medical comorbidity and antidepressant medication intake.
Being FDG-PET+ and having ≥ 1 NPS (3.74 [2.40, 5.82], p < 0.001, X2 = 34.13, df = 1) or ≥ 2 NPS (3.89 [2.20, 6.86], p < 0.001, X2 = 21.92, df = 1) increased the risk of new onset of MCI by more than three times, and having ≥ 3 NPS by more than four times (4.12 [2.03, 8.37], p < 0.001, X2 = 15.39, df = 1) relative to the reference group. Additional adjustment for medical comorbidity and antidepressant medication did not alter the results (Table 3). The tests for multiplicative interactions were non significant.
Table 3.
Association between regional brain glucose metabolism and number of NPS in predicting the risk of incident MCI
| Stratum | No. at Risk | No. of Events | Model 1 | Model 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p | Multipl. Interaction p | df | X2 | HR (95%CI) | p | Multipl. Interaction p | df | X2 | |||
| FDG-PET− / ≥ 1 NPS− | 749 | 55 | 1.00 (ref. group) | 0.25 | 1.00 (ref. group) | 0.29 | ||||||
| FDG-PET+ / ≥ 1 NPS− | 248 | 61 | 2.55 (1.76, 3.70) | <0.001 | 1 | 24.40 | 2.35 (1.62, 3.43) | <0.001 | 1 | 19.97 | ||
| FDG-PET− / ≥ 1 NPS+ | 166 | 24 | 2.15 (1.33, 3.48) | 0.002 | 1 | 9.77 | 2.15 (1.33, 3.48) | 0.002 | 1 | 9.70 | ||
| FDG-PET+ / ≥ 1 NPS+ | 88 | 33 | 3.74 (2.40, 5.82) | <0.001 | 1 | 34.13 | 3.58 (2.30, 5.57) | <0.001 | 1 | 31.73 | ||
| FDG-PET− / ≥ 2 NPS− | 850 | 70 | 1.00 (ref. group) | 0.33 | 1.00 (ref. group) | 0.29 | ||||||
| FDG-PET+ / ≥2 NPS− | 298 | 78 | 2.42 (1.74, 3.36) | <0.001 | 1 | 27.70 | 2.27 (1.63, 3.16) | <0.001 | 1 | 23.38 | ||
| FDG-PET− / ≥ 2 NPS+ | 65 | 9 | 2.50 (1.24, 5.04) | 0.011 | 1 | 6.53 | 2.48 (1.23, 5.01) | 0.012 | 1 | 6.38 | ||
| FDG-PET+ / ≥ 2 NPS+ | 38 | 16 | 3.89 (2.20, 6.86) | <0.001 | 1 | 21.92 | 3.47 (1.96, 6.16) | <0.001 | 1 | 18.17 | ||
| FDG-PET− / ≥ 3 NPS− | 890 | 74 | 1.00 (ref. group) | 0.08 | 1.00 (ref. group) | 0.08 | ||||||
| FDG-PET+ / ≥3 NPS− | 317 | 84 | 2.42 (1.76, 3.33) | <0.001 | 1 | 29.56 | 2.28 (1.65, 3.15) | <0.001 | 1 | 25.19 | ||
| FDG-PET− / ≥ 3 NPS+ | 25 | 5 | 4.75 (1.89, 11.9) | <0.001 | 1 | 11.03 | 4.23 (1.68, 10.6) | 0.002 | 1 | 9.41 | ||
| FDG-PET+ / ≥ 3 NPS+ | 19 | 10 | 4.12 (2.03, 8.37) | <0.001 | 1 | 15.39 | 3.47 (1.70, 7.10) | <0.001 | 1 | 11.61 | ||
Abbreviations: FDG-PET+ = Participants with regional brain glucose hypometabolism; FDG-PET− = Participants with normal regional brain glucose metabolism; HR = Hazard ratio; 95% CI = 95% confidence interval; p indicates significance; df = degree of freedom; X2 = Wald Chi-squared; ref. group = Reference group. Results based on Cox proportional hazards models. Wald Chi-squared test was used to test for multiplicative interaction (df = 1). Model 1 adjusted for age (as the time scale), sex, education, and APOE ε4 status. Model 2 additionally adjusted for medical comorbidity and antidepressant medication intake.
Furthermore, being FDG-PET+ and having combined presence of depression and apathy (9.95 [3.90, 25.3], p < 0.001, X2 = 23.17, df = 1) or apathy and irritability (4.98 [1.54, 16.0], p = 0.007, X2 = 7.23, df = 1) was associated with an increased risk of incident MCI. Additional adjustment for medical comorbidity and antidepressant medication did not alter the results (Table 4). There was a significant multiplicative interaction for the model on combined presence of depression and irritability.
Table 4.
Association between regional brain glucose metabolism and combination of NPS in predicting the risk of incident MCI
| Stratum | No. at Risk | No. of Events | Model 1 | Model 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p | Multipl. Interaction p | df | X2 | HR (95%CI) | p | Multipl. Interaction p | df | X2 | |||
| FDG-PET− / Depr. & Irritab.− | 892 | 76 | 1.00 (ref. group) | 0.039 | 1.00 (ref. group) | 0.033 | ||||||
| FDG-PET+ / Depr. & Irritab.− | 323 | 90 | 2.50 (1.83, 3.42) | <0.001 | 1 | 33.23 | 2.36 (1.72, 3.23) | <0.001 | 1 | 28.39 | ||
| FDG-PET− / Depr. & Irritab.+ | 23 | 3 | 3.68 (1.14, 11.9) | 0.029 | 1 | 4.74 | 3.38 (1.04, 10.9) | 0.042 | 1 | 4.13 | ||
| FDG-PET+ / Depr. & Irritab.+ | 13 | 4 | 1.63 (0.51, 5.20) | 0.41 | 1 | 0.68 | 1.33 (0.41,4.28) | 0.63 | 1 | 0.23 | ||
| FDG-PET− / Depr. & Anxiety− | 899 | 77 | 1.00 (ref. group) | 0.36 | 1.00 (ref. group) | 0.40 | ||||||
| FDG-PET+ / Depr. & Anxiety− | 325 | 90 | 2.42 (1.77, 3.31) | <0.001 | 1 | 30.92 | 2.27 (1.66, 3.12) | <0.001 | 1 | 25.95 | ||
| FDG-PET− / Depr. & Anxiety+ | 16 | 2 | 2.50 (0.61, 10.3) | 0.20 | 1 | 1.61 | 2.17 (0.53, 8.96) | 0.28 | 1 | 1.15 | ||
| FDG-PET+ / Depr. & Anxiety+ | 11 | 4 | 2.68 (0.97, 7.40) | 0.06 | 1 | 3.62 | 2.33 (0.84, 6.44) | 0.10 | 1 | 2.67 | ||
| FDG-PET− / Depr. & Apathy− | 900 | 74 | 1.00 (ref. group) | 0.32 | 1.00 (ref. group) | 0.25 | ||||||
| FDG-PET+ / Depr. & Apathy− | 326 | 88 | 2.44 (1.78, 3.34) | <0.001 | 1 | 30.59 | 2.30 (1.67, 3.17) | <0.001 | 1 | 26.09 | ||
| FDG-PET− / Depr. & Apathy+ | 15 | 5 | 7.96 (3.16, 20.0) | <0.001 | 1 | 19.35 | 6.98 (2.75, 17.7) | <0.001 | 1 | 16.77 | ||
| FDG-PET+ / Depr. & Apathy+ | 10 | 6 | 9.95 (3.90, 25.3) | <0.001 | 1 | 23.17 | 7.41 (2.81, 19.5) | <0.001 | 1 | 16.40 | ||
| FDG-PET− / Anxiety & Irritab.− | 902 | 78 | 1.00 (ref. group) | 0.53 | 1.00 (ref. group) | 0.55 | ||||||
| FDG-PET+ / Anxiety & Irritab.− | 327 | 91 | 2.44 (1.79, 3.33) | <0.001 | 1 | 31.65 | 2.29 (1.67, 3.13) | <0.001 | 1 | 26.61 | ||
| FDG-PET− / Anxiety & Irritab.+ | 13 | 1 | 1.57 (0.22, 11.4) | 0.65 | 1 | 0.20 | 1.38 (0.19, 10.0) | 0.75 | 1 | 0.10 | ||
| FDG-PET+ / Anxiety & Irritab.+ | 9 | 3 | 1.84 (0.57, 5.88) | 0.30 | 1 | 1.05 | 1.57 (0.49, 5.02) | 0.45 | 1 | 0.57 | ||
| FDG-PET− / Depr. & Nightt. beh.− | 891 | 74 | 1.00 (ref. group) | 0.07 | 1.00 (ref. group) | 0.06 | ||||||
| FDG-PET+ / Depr. & Nightt. beh.− | 322 | 89 | 2.47 (1.80, 3.38) | <0.001 | 1 | 31.31 | 2.33 (1.70, 3.21) | <0.001 | 1 | 27.04 | ||
| FDG-PET− / Depr. & Nightt. beh.+ | 13 | 3 | 4.46 (1.38, 14.4) | 0.013 | 1 | 6.24 | 4.00 (1.23, 13.0) | 0.021 | 1 | 5.34 | ||
| FDG-PET+ / Depr. & Nightt. beh.+ | 9 | 2 | 1.99 (0.48, 8.20) | 0.34 | 1 | 0.90 | 1.63 (0.39, 6.81) | 0.50 | 1 | 0.45 | ||
| FDG-PET− / Apathy & Irritab.− | 901 | 76 | 1.00 (ref. group) | 0.36 | 1.00 (ref. group) | 0.33 | ||||||
| FDG-PET+ / Apathy & Irritab.− | 330 | 90 | 2.43 (1.78, 3.32) | <0.001 | 1 | 31.06 | 2.28 (1.67, 3.13) | <0.001 | 1 | 26.23 | ||
| FDG-PET− /Apathy & Irritab.+ | 14 | 3 | 4.40 (1.37, 14.2) | 0.013 | 1 | 6.16 | 3.93 (1.22, 12.7) | 0.022 | 1 | 5.24 | ||
| FDG-PET+ / Apathy & Irritab.+ | 6 | 4 | 4.98 (1.54, 16.0) | 0.007 | 1 | 7.23 | 3.95 (1.21, 12.9) | 0.023 | 1 | 5.20 | ||
Abbreviations: FDG-PET+ = Participants with regional brain glucose hypometabolism; FDG-PET− = Participants with normal regional brain glucose metabolism; HR = Hazard ratio; 95% CI = 95% confidence interval; p indicates significance; df = degree of freedom; X2 = Wald Chi-squared; ref. group = Reference group. Results based on Cox proportional hazards models. Wald Chi-squared test was used to test for multiplicative interaction (df = 1). Model 1 adjusted for age (as the time scale), sex, education, and APOE ε4 status. Model 2 additionally adjusted for medical comorbidity and antidepressant medication intake.
Discussion
Here we report that participants with regional brain glucose hypometabolism and presence of NPS (i.e. depression, anxiety, apathy, agitation, appetite change, nighttime behavior, and any psychotic NPS) have an increased risk of incident MCI as compared to participants with normal glucose metabolism and absence of the NPS. Of note, FDG-PET+ participants with depression and anxiety had an increased MCI risk regardless of whether depression and anxiety were assessed through the informant-based NPI-Q (measuring presence vs. absence) or self-reported BDI-II and BAI (measuring severity of symptoms).
Overall, a combined presence of regional brain glucose hypometabolism and NPS resulted in higher point estimates for the risk of incident MCI than either brain glucose hypometabolism or NPS alone. For example, FDG-PET+ participants with clinical anxiety had a 7-fold increased risk of developing incident MCI; whereas being FDG-PET+ was associated with a 2-fold increased risk, and having clinical anxiety was also associated with a 2-fold increased MCI risk.
However, the results also indicate that brain glucose hypometabolism may be a stronger predictor of incident MCI than NPS. For example, being FDG-PET+ and having anxiety as assessed by the NPI-Q was associated with a 2.9-fold increased risk of developing MCI, whereas the point estimate was 2.4 for FDG-PET+ in the absence of anxiety, and 1.4 for FDG-PET− in the presence of anxiety. In line with this, for the majority of models presented in Table 2, the HR are significantly different from the reference group for FDG-PET+ participants regardless of NPS, but not for those who are FDG-PET− and have NPS. Interestingly, we did not observe increased hazards of progression to MCI even in FDG-PET− participants with clinical depression (BDI-II ≥ 13) or anxiety (BAI ≥ 10) and as compared to the reference group (FDG-PET−/ NPS−). Furthermore, only apathy or nighttime behavior in FDG-PET− participants were significantly associated with an increased risk of incident MCI. These results show that clinical depression and anxiety or self-reported NPS (with the exception of apathy and nighttime behavior) in the presence, but not absence, of neurodegeneration are associated with an increased risk of developing incident MCI. This may suggest that in the absence of neurodegeneration, older adults with NPS are able to compensate better for cognitive decline.
At the same time, it may also be informative to look at the 95% CI when examining whether NPS increase the prognostic value of FDG-PET in predicting MCI. Indeed, taking clinical anxiety as an example, we observed that the lower bound of the Cl for FDG-PET+ and anxiety is higher than the upper bound for FDG-PET+ and no anxiety. Thus, it may be possible that having anxiety in addition to being FDG-PET+ does, to some extent, add to an increased risk of MCI. In few cases, the combination of both risk factors was associated with a lower risk of developing MCI than having either risk factor alone. For example, FDG-PET+ participants in the absence of apathy had a HR of 2.53 and FDG-PET− participants in the presence of apathy had a HR of 7.28; whereas, the HR for FDG-PET+ and apathy was only 5.73. Of note, all three groups had a significantly increased MCI risk compared to the reference group and the p-value for this model was significant when we tested for multiplicative interaction. However, overall, only few models were significant when we tested for multiplicative interactions. More research is thus needed to examine whether the effects are over and above what can be explained from simply adding the effects of regional brain glucose hypometabolism and NPS in predicting incident MCI.
We also observed a dose-response pattern, i.e. the risk of incident MCI among persons with regional brain glucose hypometabolism and NPS increased with a higher number of NPS. Finally, as different NPS are known to co-occur, we found that FDG-PET+ participants with an overlap of depression and apathy, as well as apathy and irritability had an increased risk of developing incident MCI. However, the number of participants was low for some of the groups; thus, these results should be considered as preliminary until confirmed by other studies.
Our study suggests that combining information about brain regional glucose metabolism and NPS may be meaningful in predicting the risk of incident MCI. Previously, we and others have examined the associations between baseline NPS and the risk of incident MCI (37–40). In addition, few studies have reported associations between baseline NPS and changes in brain metabolic dysfunction (8), as well as between FDG-PET and changes in apathy (15). However, to date, there is paucity of research on the association between NPS and an altered FDG-PET in the context of cognitive aging. Few studies have examined the cross-sectional associations between brain glucose metabolism and NPS in CU older persons (14, 16), and in individuals with MCI (41) and dementia (42–44). Finally, a meta-analysis revealed associations between major depressive disorder and altered brain metabolism in various ROI such as insula, limbic system, basal ganglia, thalamus, and cerebellum (45). Our study expands on the existing body of research by showing that an impaired glucose metabolism in regions typically affected in AD, coupled with informant- or self-reported NPS is associated with an increased risk of incident MCI, and regional brain glucose hypometabolism appears to be a stronger driving force for cognitive decline than NPS among CU older adults. More research is needed to explore potential mechanisms that may underlie the association between altered brain glucose metabolism and NPS in driving cognitive decline. It is also paramount to untangle the time sequence of events that lead to the outcome of incident MCI, i.e. whether neuropathological changes or NPS occur first.
The strengths of our study include the large scale, population-based cohort study, a relatively long follow-up time, and the rigorous analysis with adjustment for traditional confounders as well as medical comorbidity and antidepressant medication. Furthermore, we assessed depression and anxiety using both informant-based and self-reported instruments. Limitations pertain to the observational nature of our study which does not allow for drawing conclusions about the cause-effect relationship between predictors (i.e. FDG-PET and NPS) and outcomes of interest (i.e. incident MCI). Also, some strata in our models have few events and small overall totals as indicated by relatively wide CIs (e.g. apathy and agitation). This may have led to inflated (higher) point estimates, and due to this limitation we were also not able to conduct analyses stratified by MCI type. Moreover, we were not able to consider all NPI-Q-assessed NPS in our analyses due to low numbers, particularly for psychotic NPS such as hallucinations or euphoria. However, this is expected given that our study sample consisted of community-dwelling persons. We also did not adjust for multiple comparisons. If we assume a Bonferroni correction for Table 2, the alpha significance level would be 0.00167 (i.e., 0.05/30 since we have a total of 10 models with 3 comparisons per model). Thus, when applying this correction, only the associations between FDG-PET abnormality and anxiety, agitation and any psychotic NPS with the risk of incident MCI would no longer remain significant. Furthermore, our sample is relatively highly educated and 99% of study participants are of Caucasian decent. However, it has been shown that data from Olmsted County are generalizable to the U.S. population (46). Nevertheless, there still remains a lack of generalizability to minorities. For instance, minority-specific stress has been shown to be associated with cognitive decline; thus, our results may not be all that generalizable to non-White race/ ethnicities. Finally, for this analysis, we used a dichotomous approach to classify participants as either having altered or preserved regional brain glucose metabolism (SUVR ≤ 1.47 vs. ≥ 1.48), and future research using continuous variables is needed.
This research provides evidence that combined presence of NPS and glucose hypometabolism in brain regions typically affected in AD are associated with an increased risk of incident MCI. Underlying AD pathology as indicated by an altered FDG-PET appears to be a stronger driving force of cognitive decline than NPS among CU older individuals. More longitudinal research is needed to examine whether NPS may increase the prognostic value of regional brain glucose hypometabolism for predicting incident MCI.
Highlights.
1). What is the primary question addressed by this study?
The primary research question is whether a combined presence of regional glucose metabolic dysfunction as measured by FDG-PET, and neuropsychiatric symptoms (NPS) is associated with an increased risk of incident mild cognitive impairment (MCI) among older adults, and whether this risk is greater than having either risk factor alone.
2). What is the main finding of this study?
Combined presence of NPS with regional glucose hypometabolism is associated with an increased risk of incident MCI among older adults who were cognitively unimpaired at baseline. An altered FDG-PET appears to be a stronger driving force of cognitive decline than NPS.
3). What is the meaning of the finding?
Both neurodegeneration and neuropsychiatric symptoms contribute to an increased risk of incident MCI among older adults who are at risk for cognitive decline and should thus be assessed by clinicians.
Acknowledgments
Funding and Conflict of Interest
Support for this research was provided by NIH grants: National Institute on Aging (R01 AG057708; U01 AG006786; P50AG016574; R01 AG034676; R01 AG011378; R01 AG041851), National Institute of Mental Health (K01 MH068351), and National Institute of Neurological Disorders and Stroke (R01 NS097495). This project was also supported by the Robert Wood Johnson Foundation, the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program, the GHR Foundation, the Mayo Foundation for Medical Education and Research, Project LQ1605 from the National Program of Sustainability II (MEYS CR), the Edli Foundation and the Arizona Alzheimer’s Consortium.
M.V. receives research funding from the NIH, Roche and Biogen. V.J.L. serves on scientific advisory boards for Bayer Schering Pharma, Piramal Life Sciences, and Merck Research, and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, and the NIH. P.V. receives funding from the NIH. M.M.Mi. has consulted for Eli Lilly and Lysosomal Therapeutics, Inc. and receives unrestricted research grants from Biogen, and Lundbeck, and research funding from the NIH and the Department of Defense. M.M.Ma. receives research funding from the NIH. W.K.K. receives research funding from the Department of Defense, the NIH, Astra Zeneca, Biogen, and Roche. C.R.J. serves on scientific advisory board for Eli Lilly, and IDSMB for Roche. But, he receives no compensation from any commercial entity. He receives research support from the NIH and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation. D.S.K. serves on a Data Safety Monitoring Board for the DIAN study. He is an investigator in clinical trials sponsored by Lilly Pharmaceuticals, Biogen, and the Alzheimer’s Treatment and Research Institute at USC, and receives research support from the NIH. R.C.P. is a consultant for Roche, Biogen, Merck, Eli Lilly, and Genentech. He receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, 2003) and research support from the NIH. Y.E.G. receives funding from the NIH and Roche, and served on Lundbeck Advisory Board. J.K.R, J.A.S., G.B.S., and T.J.C. report no disclosures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Knopman DS, Jack CR Jr., Wiste HJ, et al. : 18F-fluorodeoxyglucose positron emission tomography, aging, and apolipoprotein E genotype in cognitively normal persons. Neurobiol Aging 2014; 35:2096–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minoshima S, Giordani B, Berent S, et al. : Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol 1997; 42:85–94 [DOI] [PubMed] [Google Scholar]
- 3.Herholz K, Salmon E, Perani D, et al. : Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage 2002; 17:302–316 [DOI] [PubMed] [Google Scholar]
- 4.Jagust W, Gitcho A, Sun F, et al. : Brain imaging evidence of preclinical Alzheimer’s disease in normal aging. Ann Neurol 2006; 59:673–681 [DOI] [PubMed] [Google Scholar]
- 5.Alexander GE, Chen K, Pietrini P, et al. : Longitudinal PET Evaluation of Cerebral Metabolic Decline in Dementia: A Potential Outcome Measure in Alzheimer’s Disease Treatment Studies. Am J Psychiatry 2002; 159:738–745 [DOI] [PubMed] [Google Scholar]
- 6.Drzezga A, Lautenschlager N, Siebner H, et al. : Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol Imaging 2003; 30:1104–1113 [DOI] [PubMed] [Google Scholar]
- 7.Landau SM, Harvey D, Madison CM, et al. : Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging 2011; 32:1207–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng KP, Pascoal TA, Mathotaarachchi S, et al. : Neuropsychiatric symptoms predict hypometabolism in preclinical Alzheimer disease. Neurology 2017; 88:1814–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyketsos CG, Lopez O, Jones B, et al. : Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the Cardiovascular Health Study. Jama 2002; 288:1475–1483 [DOI] [PubMed] [Google Scholar]
- 10.Geda YE, Roberts RO, Knopman DS, et al. : Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry 2008; 65:1193–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg PB, Mielke MM, Appleby BS, et al. : The association of neuropsychiatric symptoms in MCI with incident dementia and Alzheimer disease. Am J Geriatr Psychiatry 2013; 21:685–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forrester SN, Gallo JJ, Smith GS, et al. : Patterns of Neuropsychiatric Symptoms in Mild Cognitive Impairment and Risk of Dementia. Am J Geriatr Psychiatry 2016; 24:117–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pink A, Stokin GB, Bartley MM, et al. : Neuropsychiatric symptoms, APOE epsilon4, and the risk of incident dementia: a population-based study. Neurology 2015; 84:935–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donovan NJ, Hsu DC, Dagley AS, et al. : Depressive Symptoms and Biomarkers of Alzheimer’s Disease in Cognitively Normal Older Adults. J Alzheimers Dis 2015; 46:63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gatchel JR, Donovan NJ, Locascio JJ, et al. : Regional 18F-Fluorodeoxyglucose Hypometabolism is Associated with Higher Apathy Scores Over Time in Early Alzheimer Disease. Am J Geriatr Psychiatry 2017; 25:683–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krell-Roesch J, Ruider H, Lowe VJ, et al. : FDG-PET and Neuropsychiatric Symptoms among Cognitively Normal Elderly Persons: The Mayo Clinic Study of Aging. J Alzheimers Dis 2016; 53:1609–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufer DI, Cummings JL, Ketchel P, et al. : Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci 2000; 12:233–239 [DOI] [PubMed] [Google Scholar]
- 18.Beck AT, Steer RA,Brown GK: BDI-II, Beck Depression Inventory: Manual, 2nd San Antonio, TX; Boston, MA, Psychological Corp.; Harcourt Brace, 1996 [Google Scholar]
- 19.Beck AT,Steer RA: BAI, Beck anxiety inventory: Manual, San Antonio, Psychological Corp. : Harcourt Brace Jovanovich, 1990 [Google Scholar]
- 20.Roberts RO, Geda YE, Knopman DS, et al. : The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008; 30:58–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kokmen E, Smith GE, Petersen RC, et al. : The Short Test of Mental Status: Correlations With Standardized Psychometric Testing. Archives of Neurology 1991; 48:725–728 [DOI] [PubMed] [Google Scholar]
- 22.Morris JC: The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43:2412–2414 [DOI] [PubMed] [Google Scholar]
- 23.Rey A: L’examen clinique en psychologie, Paris, Presses Universitaires de France, 1964 [Google Scholar]
- 24.Wechsler D: Wechsler Memory Scale-Revised, New York, The Psychological Corporation, 1987 [Google Scholar]
- 25.Kaplan E, Goodglass H,Weintraub S: Boston Naming Test, 2nd Philadelphia, Lippincott Williams & Wilkins, 2001 [Google Scholar]
- 26.Lucas JA, Ivnik RJ, Smith GE, et al. : Mayo’s Older Americans Normative Studies: category fluency norms. J Clin Exp Neuropsychol 1998; 20:194–200 [DOI] [PubMed] [Google Scholar]
- 27.Wechsler D: Wechsler Adult Intelligence Scale-Revised, New York, Psychological Corporation, 1981 [Google Scholar]
- 28.Reitan RM: Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills 1958; 8:271–276 [Google Scholar]
- 29.Petersen RC: Mild cognitive impairment as a diagnostic entity. J Intern Med 2004; 256:183–194 [DOI] [PubMed] [Google Scholar]
- 30.Jack CR Jr., Lowe VJ, Senjem ML, et al. : 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain 2008; 131:665–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. : Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15:273–289 [DOI] [PubMed] [Google Scholar]
- 32.Jagust WJ, Bandy D, Chen K, et al. : The Alzheimer’s Disease Neuroimaging Initiative positron emission tomography core. Alzheimers Dement 2010; 6:221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minoshima S, Frey KA, Foster NL, et al. : Preserved pontine glucose metabolism in Alzheimer disease: a reference region for functional brain image (PET) analysis. J Comput Assist Tomogr 1995; 19:541–547 [DOI] [PubMed] [Google Scholar]
- 34.Jack CR Jr., Wiste HJ, Weigand SD, et al. : Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement 2017; 13:205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charlson ME, Pompei P, Ales KL, et al. : A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383 [DOI] [PubMed] [Google Scholar]
- 36.Hixson JE,Vernier DT: Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res 1990; 31:545–548 [PubMed] [Google Scholar]
- 37.Geda YE, Roberts RO, Mielke MM, et al. : Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry 2014; 171:572–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richard E, Reitz C, Honig LH, et al. : Late-life depression, mild cognitive impairment, and dementia. JAMA Neurol 2013; 70:374–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steenland K, Karnes C, Seals R, et al. : Late-life depression as a risk factor for mild cognitive impairment or Alzheimer’s disease in 30 US Alzheimer’s disease centers. J Alzheimers Dis 2012; 31:265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson RS, Schneider JA, Boyle PA, et al. : Chronic distress and incidence of mild cognitive impairment. Neurology 2007; 68:2085–2092 [DOI] [PubMed] [Google Scholar]
- 41.Delrieu J, Desmidt T, Camus V, et al. : Apathy as a feature of prodromal Alzheimer’s disease: an FDG-PET ADNI study. Int J Geriatr Psychiatry 2015; 30:470–477 [DOI] [PubMed] [Google Scholar]
- 42.Lee DY, Choo IH, Jhoo JH, et al. : Frontal dysfunction underlies depressive syndrome in Alzheimer disease: a FDG-PET study. Am J Geriatr Psychiatry 2006; 14:625–628 [DOI] [PubMed] [Google Scholar]
- 43.Holthoff VA, Beuthien-Baumann B, Kalbe E, et al. : Regional cerebral metabolism in early Alzheimer’s disease with clinically significant apathy or depression. Biol Psychiatry 2005; 57:412–421 [DOI] [PubMed] [Google Scholar]
- 44.Weissberger GH, Melrose RJ, Narvaez TA, et al. : (18)F-Fluorodeoxyglucose Positron Emission Tomography Cortical Metabolic Activity Associated with Distinct Agitation Behaviors in Alzheimer Disease. Am J Geriatr Psychiatry 2017; 25:569–579 [DOI] [PubMed] [Google Scholar]
- 45.Su L, Cai Y, Xu Y, et al. : Cerebral metabolism in major depressive disorder: a voxel-based meta-analysis of positron emission tomography studies. BMC Psychiatry 2014; 14:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.St Sauver JL, Grossardt BR, Leibson CL, et al. : Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clinic proceedings 2012; 87:151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]


