Abstract
Purpose
Cyclin-dependent kinase 4/6 (CDK4/6) inhibitors have improved progression free survival for metastatic, estrogen receptor positive (ER+) breast cancers, but their role in the non-metastatic setting remains unclear. We sought to understand the effects of CDK4/6 inhibition (CDK4/6i) and radiation (RT) in multiple preclinical breast cancer models.
Methods
Transcriptomic and proteomic analyses were used to identify significantly altered pathways after CDK4/6i. Clonogenic assays were used to quantify the RT enhancement ratio (rER). DNA damage was quantified using γH2AX staining and the neutral comet assay. DNA repair was assessed using RAD51 foci formation and non-homologous end joining (NHEJ) reporter assays. Orthotopic xenografts were used to assess the efficacy of combination therapy.
Results
Palbociclib significantly radiosensitized multiple ER+ cell lines at low nanomolar, sub IC50 concentrations (rER: 1.21 – 1.52) and led to a decrease in the surviving fraction of cells at 2 Gy (p < 0.001). Similar results were observed in ribociclib- (rER: 1.08 – 1.68) and abemaciclib-treated (rER: 1.19 – 2.05) cells. Combination treatment decreased RAD51 foci formation (p < 0.001), leading to a suppression of HR activity, but did not affect NHEJ efficiency (p > 0.05). Immortalized breast epithelial cells and cells with acquired resistance to CDK4/6i did not demonstrate radiosensitization (rER: 0.94 – 1.11) or changes in RAD51 foci. In xenograft models, concurrent palbociclib and RT led to a significant decrease in tumor growth.
Conclusions
These studies provide preclinical rationale to test CDK4/6i + RT in women with locally-advanced ER+ breast cancer at high risk for locoregional recurrence.
Keywords: breast cancer, CDK4/6, radiation sensitivity, ER+ breast cancer
Introduction
The treatment of breast cancer is guided, in part, by the presence or absence of hormone receptors including the estrogen receptor (ER). Nearly 75% of new breast cancer diagnoses will be classified as estrogen receptor positive (ER+) disease(1). For these patients, precision medicine strategies that target the ER using selective estrogen receptor modulators (SERMs), selective estrogen receptor degraders (SERDs), and aromatase inhibitors (AIs) that block ER signaling have resulted in significant improvements in recurrence-free and overall survival rates(1). While many women with ER+ metastatic breast cancer initially respond to endocrine therapy, nearly all will become refractory to endocrine therapy(1). Treatment options in the metastatic setting are expanding, and the recent introduction of cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors into the clinic has significantly improved outcomes for these patients(2,3).
In contrast to anti-estrogen therapies, CDK4/6 inhibitors work by targeting the cell cycle(4). When cells are actively proliferating, the levels of cyclin proteins rise and fall in a series of predetermined, cyclic patterns. The activation of specific cyclins at fixed points in the cell cycle is crucial for proper regulation of cyclin-dependent kinases (CDKs). CDKs are serine/threonine kinases that act as master regulators of the cell cycle; they phosphorylate downstream target proteins necessary to proceed through cell cycle “checkpoints” designed to control abnormal proliferation(4). For example, cyclin D1 forms a complex with CDK4/6 and leads to the phosphorylation of many downstream targets – including the inactivation of the retinoblastoma (RB1) tumor suppressor(4). The ability of cancer cells to evade growth suppressors, one of the hallmarks of cancer, has long been appreciated in many cancer types based on the dysregulation of cyclins and cyclin-dependent kinases (CDKs)(4).
With the development of selective CDK4/6 inhibitors including palbociclib, the ability to selectively target this cell cycle dysregulation in metastatic, ER+ breast cancer became possible. There are currently 3 FDA-approved CDK4/6 inhibitors: palbociclib (PD0332991)(5), ribociclib (LEE011)(6) and abemaciclib (LY2835219)(7). All three are orally bioavailable ATP-competitive inhibitors of CDK4 and CDK6. Early preclinical studies suggested that ER+ breast cancer cell lines are more sensitive to the antiproliferative effects of specific CDK4/6 inhibitors compared to other breast cancer subtypes, like triple negative breast cancer, where alterations in RB1 are more frequent(5). This differential response, later validated by others, provided the rationale to restrict early clinical trials to ER+ breast cancers(3). Based on several practice changing clinical trials(2,3,8,9), CDK4/6 inhibitors are now standard-of-care for women diagnosed with metastatic ER+ breast cancer in combination with hormone therapies such as letrozole or tamoxifen.
For patients with metastatic, ER+ breast cancer, CDK4/6 inhibitors have improved progression free survival, but acquired resistance to these drugs remains a critical clinical issue(4). While the exact mechanism(s) of therapy resistance remain unclear, recent data suggest changes in phosphorylation of RB1(5,10,11), and changes in cyclin/CDK expression(12–16), may contribute to drug resistance; however, currently there is no known consensus pathway of resistance. CDK4/6 inhibitors have also demonstrated the ability to slow progression in many types of cancers as well as the potential to synergize with other agents for more durable responses.
Although use of CDK4/6 inhibitors is currently limited to the metastatic setting, there are ongoing efforts to evaluate the efficacy of CDK4/6 inhibitors in the upfront setting for women with locally advanced or high-risk ER+ disease(17). This resistance may become a more critical issue as these inhibitors make their way into the clinical management of patients with locally advanced, non-metastatic disease where cure remains the therapeutic goal. Thus, there is a critical unmet need to identify strategies to improve the local efficacy of CDK4/6 inhibitor therapy in patients with ER+ breast cancer.
Radiation (RT) therapy remains a mainstay in the treatment of women with locally advanced ER+ breast cancer(18). Despite its ubiquitous use, combination studies testing the use of RT in combination with CDK4/6 inhibition are lacking. It is well established that pharmacological CDK4/6 inhibition interferes with cell cycle regulation(4), but recent studies have also shown that single agent palbociclib can also affect regulation of the DNA damage response pathways(19,20). However, our understanding of this interaction and the resulting effects of CDK4/6 inhibitor therapy and RT is incomplete. Therefore, we sought to determine whether combining CDK4/6 inhibitors with RT would prove to be more effective than either treatment alone in multiple models of ER+ breast cancer, and to evaluate the physiological significance of this phenomenon in vivo.
Materials and Methods
Cell Culture
All cell lines were obtained from ATCC and cultured at 37°C and 5% CO2 at subconfluent densities. Cell culture media and additives can be found in Supplemental Methods. Parental cell lines (MCF-7, T47D, CAMA-1, ZR-75–1) are ER+ breast cancer cells that are sensitive to both estrogen supplementation and hormone therapies. CDK4/6 inhibitor-resistant cell lines were developed through serial passaging with dose-escalation of either palbociclib, ribociclib, or abemaciclib every 2–3 weeks. Cells were selected for approximately three months (Figure 1D) and resistant pools were continuously cultured in 1μM CDK4/6 inhibitor. Before use in assays, drug was removed for at least 24 hours and cells were plated in drug-free media. The identity of the cell lines was confirmed by STR profiling and mycoplasma testing was done monthly (Lonza #LT07–318).
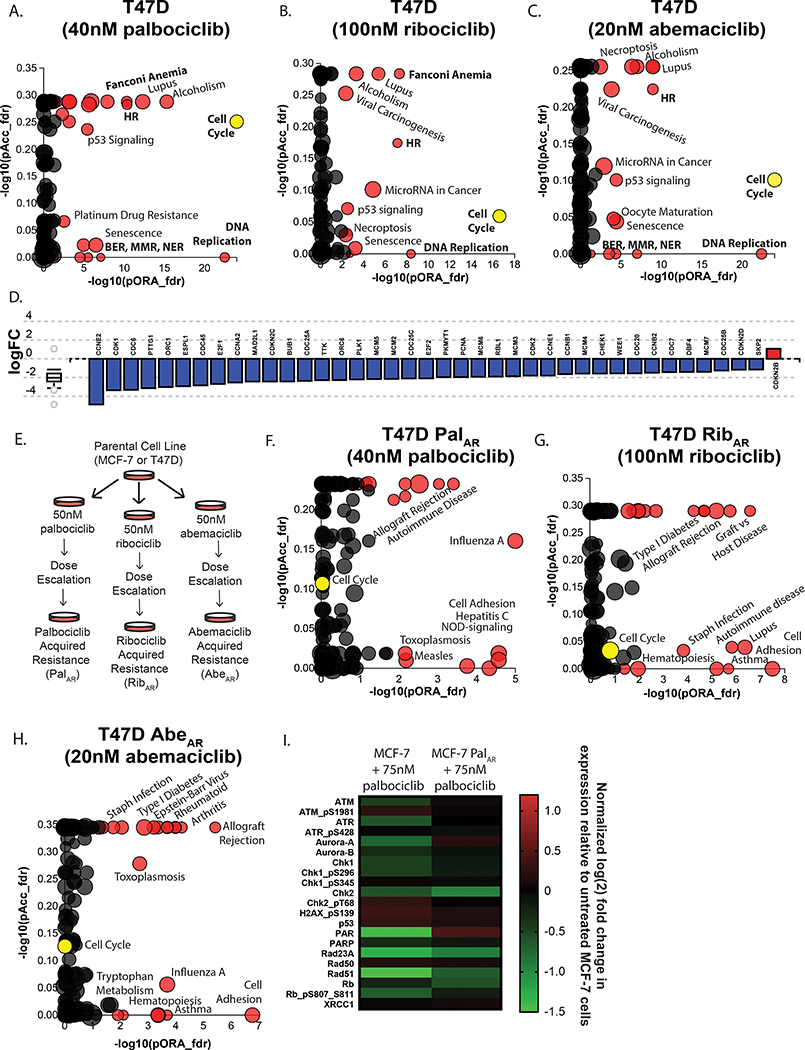
Figure 1: Multi-omic analysis of ER+ breast cancer cells after CDK4/6 inhibition.
T47D cells were treated with 40nM palbociclib (A), 100nM ribociclib (B), or 20nM abemaciclib (C) (the IC50 value of each drug) for 16 hours to assess transcriptomic changes at the pathway level. The x-axis measures the p-value obtained using the classical over-representation analysis (pORA). The y-axis represents the p-value obtained from total perturbation accumulation (pAcc) in the pathway. Each dot represents a pathway. The size of each dot denotes the number of genes on the pathway. Genes related to the cell cycle are globally suppressed in T47D cells treated with palbociclib (D). CDK4/6 inhibitor-resistant MCF-7 and T47D cell lines were generated using long term selection (E). Differentially expressed pathways in CDK4/6 inhibitor-resistant T47D PalAR (F), RibAR (G), or AbeAR (H) cells treated with a CDK4/6 inhibitor for 16 hours compared to parental T47D cells treated with the corresponding CDK4/6 inhibitor. (I) Reverse phase protein lysate array (RPPA) data from MCF-7 and MCF-7 PalAR cells treated with 75nM palbociclib for 16 hours. Protein and phosphoprotein log2 expression values were normalized for total protein loading and shown as the fold change in expression over untreated MCF-7 cells. All p-values and pathway plots were FDR corrected, where red dots indicate significantly differentially expressed pathways and (unlabeled) grey pathways are not significantly changed. The yellow dot in panels A-F is used to denote the cell cycle.
Drugs
All drugs were solubilized in 100% DMSO for a stock concentration of 10mM for use in all cell culture assays. Palbociclib (Sigma #PZ0199), ribociclib (Med Chem Express #HY-15777A), abemaciclib (Med Chem Express #HY-16297A), staurosporin (Sigma #S6942), NU7441 (Selleck #S2638), and AZD7762 (Sigma #SML0350) were all purchased commercially.
Clonogenic Survival assay
Cells were seeded at single cell density in 6 well plates and allowed to adhere overnight. The following morning, cells were pretreated with drug for one hour (except where indicated otherwise) and radiated. Colony counts were used to determine toxicity, the surviving fraction of cells at 2 Gy (SF 2 Gy), and the radiation enhancement ratio (rER) for each treatment condition. Clonogenic data were fit to a linear-quadratic model and enhancement ratios were calculated as the ratio of the area under the curve (AUC) from control cells / experimental conditions.
Immunoblotting
Cell lysates were prepared with RIPA buffer (Thermo Fisher #89901) containing commercially available phosphatase and protease inhibitor tablets (Sigma #PHOSS-RO, #CO-RO). Protein lysates were sonicated and reduced with 2% β-Mercaptoethanol and 4x Nu-Page buffer (Life Technologies #NP0007). Immunoreactivity was detected using the following antibodies: anti-RAD51 (Millipore ABE257 1:1000, pKu80 (Invitrogen #38118, 1:1000), Ku80 (CST #2180S, 1:1000), γH2AX (Milipore #05–636, 1:1000), cleaved PARP (CST #5625S, 1:1000), PARP1 (CST # 9542S, 1:1000), anti-β-Actin-HRP (CST #12262S, 1:50,000).
Irradiation
Irradiation was carried out as described previously in the University of Michigan Experimental Irradiation Core(21,22). Briefly, a Philips RT250 (Kimtron Medical), which is calibrated to meet the standards of the National Institute of Standards and Technology (NIST), was used at a dose rate of approximately 2 Gy/min for both in vitro and in vivo irradiation experiments.
Immunofluorescence
Immunofluorescence was performed as described previously(21,22). Antibody and dilution information can be found in the Supplemental Methods.
NHEJ Reporter and qPCR
NHEJ reporter assays were performed as described previously(21,22). Briefly, a linearized GFP reporter plasmid was transfected into cells and plasmid DNA was isolated to perform comparative qPCR (ΔΔCt) using GFP and internal control primers. All Ct values were normalized to untreated control cells. Additional information can be found in Supplemental Methods.
Xenograft Studies
MCF-7 cells (n = 4 × 106) were injected bilaterally into the mammary fat pads of 8–10 week old CB17-SCID female mice in 50% Matrigel (Thermo #CB-40234). Estrogen pellets (Innovative Research of America, #SE-121) were implanted subcutaneously in the nape of the neck on the day of tumor injection and removed after visible tumor formation. When tumors reached approximately 80mm3, mice were randomized into four groups (14–16 tumors per group): vehicle (Sodium L-Lactate, 50mmol/L pH 4.0, Sigma #L-7022), palbociclib only, RT only, or combination treatment. Mice in the palbociclib only or combination groups were treated with 25mg/kg palbociclib by oral gavage for 6 days. Mice receiving RT only received fractions of 2 Gy for five days. Mice in the combination group started palbociclib treatment one day before RT, but drug in all groups was discontinued after the last RT fraction. Tumor growth was measured 1–3 times a week and tumor volume was calculated using the equation V=(L*W2)*π/6. All xenograft experiments and procedures were done with the approval of the Institutional Animal Care & Use Committee (IACUC) at the University of Michigan.
Transcriptomic Analysis
RNA was isolated using QIAzol and the RNeasy mini kit (Qiagen #74104) and sent to the University of Michigan Advanced Genomics Core. For transcriptomic analyses, expression values were calculated using a robust multi-array average (RMA)(23) to convert probe values into log2 expression values for each gene which were then fit using linear models(24). The standard error (SE) for each gene was standardized across all arrays used for a median SE of 1. All p-values were corrected for a false discovery rate. Analyses were done using the oligo and limma packages of Bioconductor in R at the University of Michigan Bioinformatics Core. Data from this study is publicly available through GEO (GSE155570).
Proteomic Analysis
Protein for reverse phase protein array (RPPA) was extracted from cells and standardized to 1.5 μg/μL in RIPA buffer. Cell lysate was reduced with β-mercaptoethanol and 4x SDS and sent to the Functional Proteomics RPPA Core Facility at M.D. Anderson Cancer Center for analysis(25). Briefly, serial dilutions of each sample were prepared and used to capture the linear antibody/antigen reaction for accurate data analysis using validated antibodies. In addition, 48 unique cell lysates were printed on each slide and served as controls to develop Replicate-Based-Normalization used for quality control for data generation and analysis and RPPA data merging across different slides. Subsequent algorithms of spatial correction, quality control of antibody probing, protein loading correction, replicate-based-normalization, and quality of antibody batches were performed with each run and an automated program for RPPA Pipeline processing was used. Each sample was quantitated and run in triplicate for each condition.
Statistical Analysis
The SF-2 Gy values and the NHEJ reporter data were compared to control cells using a one-way ANOVA with Dunnett’s Test. A t-test was used to compare RT and combination groups in the immunofluorescence experiments, and p-values were corrected for multiple comparisons. All in vitro experiments were completed as the average of at least three independent experiments and pooled for statistical analysis. Xenograft tumor size and mouse weights were compared using a two-way ANOVA, and survival curves were compared using the log-rank (Mantel-Cox) test. Fractional tumor volume (FTV) was calculated in a manner consistent with previous studies(22,26).
Results
Single-agent CDK4/6 inhibition leads to a suppression of cell cycle and DNA damage response pathways
To determine the effects of CDK4/6 inhibition in breast cancer, we performed proliferation assays and calculated the IC50 values of palbociclib, ribociclib, and abemaciclib in the estrogen-dependent, ER+ breast cancer cell lines MCF-7, T47D, (Supplemental Fig. 1), CAMA-1, and ZR-75–1 (Supplemental Fig. 2). In order to understand the biological changes that are induced by short term CDK4/6 inhibition, we analyzed transcriptomic changes of T47D cells treated with 40nM palbociclib for 16 hours (Fig. 1A). Overrepresentation (pORA) and total pathway accumulation (pAcc) were computed using iPathway (Advaita) to find pathways that were significantly differentially expressed.
As expected, the cell cycle pathway was differentially expressed between vehicle-treated and palbociclib-treated T47D cells (p = 9.283 × 10−5) and expression of RB1, a canonical target of CDK4/6, was significantly decreased compared to control cells (p = 2.014 × 10−4). Surprisingly, pathway analysis identified DNA damage response as the pathway most significantly overrepresented after palbociclib treatment. These significantly altered pathways (with FDR-corrected p values) include DNA replication (p = 6.198×10−23), Mismatch repair (p = 3.209×10−7), Base excision repair (p = 1.249×10−5), Fanconi anemia (p = 1.585×10−5), nucleotide excision repair (p = 4.935 × 10−5), and homologous recombination (p = 8.874 × 10−5). Cell cycle downregulation also led to the global suppression of cell cycle genes (Fig. 1D) including Cyclin E2 (CCNE2), the transcription factor E2F (E2F1), and RB1 (RB1). T47D cells treated with either 100nM ribociclib (Fig. 1B) or 20nM abemaciclib (Fig. 1C) demonstrated similar pathway changes in cell cycle and DNA damage response pathways. MCF-7 cells treated with low concentrations of CDK4/6 inhibition for 16 hours (Supplemental Fig. 3) showed less robust changes in gene expression overall and no significant changes in DNA damage response pathways, but showed some change in cell cycle response along with significant changes in interleukin and chemokine signaling.
To understand how these pathway changes might be altered after the development of CDK4/6 inhibitor resistance, we developed models of acquired resistance (Fig. 1E) to palbociclib (PalAR) ribociclib, (RibAR) and abemaciclib (AbeAR) in MCF-7 and T47D cells. After selection, CDK4/6 inhibitor resistant MCF-7 and T47D cells demonstrated a 10–100 fold greater resistance to CDK4/6 inhibition as evident by a significant shift in the dose-response curves (Supplemental Fig. 1). CDK4/6 inhibitor resistant cell lines also developed cross resistance to all three CDK4/6 inhibitors, suggesting commonality in resistance mechanisms (Supplemental Fig. 4). In contrast to the changes observed after CDK4/6 inhibition in CDK4/6 inhibitor-sensitive parental cell lines, short term treatment of palbociclib-resistant T47D PalAR cells with 40nM palbociclib (Fig. 1F) predominately led to changes in pathways involved in adhesion, cytokine signaling, and immune regulation, which has been demonstrated by others(19). T47D RibAR (Fig. 1G) and T47D AbeAR (Fig. 1H) cells and CDK4/6 inhibitor-resistant MCF-7 cells (Supplemental Fig. 3D–F) also showed minimal changes in cell cycle and DNA damage response pathways, suggesting that CDK4/6 inhibitor-resistant cell lines are not as susceptible to manipulations of DNA repair and DNA damage as their wild-type counterparts.
In order to confirm these observed transcriptomic changes, we used reverse phase protein array to quantify changes in protein and phosphoprotein expression in MCF-7 and MCF-7 PalAR cells 16 hours after treatment with 75nM palbociclib. Along with expected changes in cell cycle proteins – including decreased pRB1 in MCF-7 parental cells – we saw a decrease in the expression of a significant number of proteins and phospho-proteins involved in the DNA damage response (Fig. 1I). CDK4/6 inhibition with palbociclib did not cause significant suppression of these proteins in MCF-7 PalAR cells treated with 75nM palbociclib, suggesting that CDK4/6 inhibitor-resistant cells are no longer susceptible to CDK4/6 inhibitor-mediated suppression of DNA repair.
CDK4/6 inhibition radiosensitizes CDK4/6 inhibitor-naïve ER+ breast cancer cell lines
Because CDK4 and CDK6 act at the G1/S checkpoint, it has been hypothesized that the use of CDK4/6 inhibitors would be radioprotective by arresting cells in the G1 phase; cells are typically most sensitive to RT in G2/M. Our data demonstrating significant changes in DNA damage response proteins and phosphoproteins suggested that CDK4/6 inhibitors may be directly impacting these pathways independent of the cell cycle effects and may potentiate the effects of DNA damaging agents (including ionizing RT). In order to assess the ability of CDK4/6 inhibition to influence the radiosensitivity of ER+ breast cancer cell lines, we performed clonogenic cell survival assays. In these assays, ER+ breast cancer cells were pretreated with low-concentration CDK4/6 inhibition one hour prior to RT to minimize potential confounding effects of cell cycle reassortment.
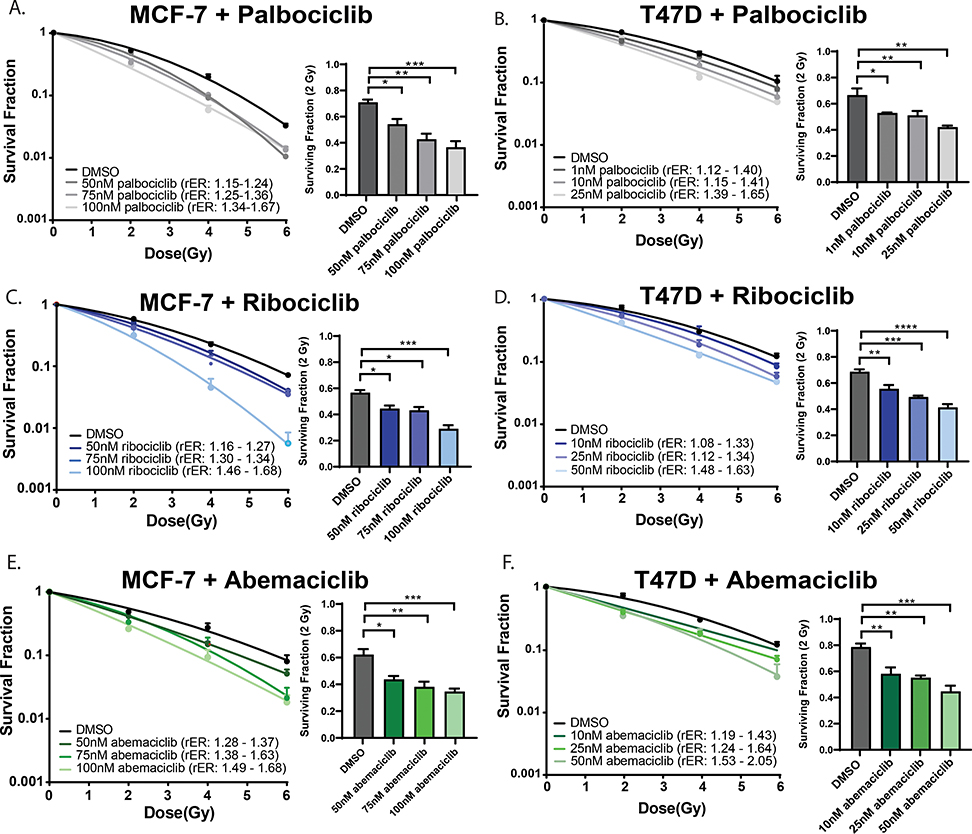
We demonstrate that escalating doses of palbociclib produced a dose-dependent increase in radiosensitization in MCF-7 cells (rER 1.15 – 1.67) at concentrations at or below the IC50 value (Fig. 2A). The radiation enhancement ratio (rER) of clinically approved radiosensitizing agents such as cisplatin(27) is ~1.2, suggesting that this radiosensitization is clinically meaningful(27,28). Similar results were observed in T47D (rER: 1.12 – 1.65, Fig. 2B), CAMA-1 (rER: 1.14 – 1.42, Supplemental Fig. 2D), and ZR-75–1 cells (rER: 1.12 – 1.43, Supplemental Fig. 2F) when treated with sub-IC50 concentrations of palbociclib. Radiosensitization occurred in MCF-7 and T47D cells to a similar degree with ribociclib (Fig. 2C&D) and abemaciclib (Fig. 2E&F), suggesting that all three CDK4/6 inhibitors led to comparable levels of radiosensitization in vitro. Furthermore, all three drugs showed modest single agent toxicity (Supplemental Table 1), predominately at concentrations closer to the IC50 value. However, at these concentrations, CDK4/6 inhibition did not significantly radiosensitize the transformed, mammary epithelial cell line MCF-10A (Supplemental Fig 5), suggesting that the combination treatment would be unlikely to cause significant toxicity to normal breast tissue when treated with RT (Supplemental Table 3).
Figure 2: Concurrent pharmacological inhibition of CDK4/6 in ER+ breast cancer cell lines confers radiosensitivity.
Clonogenic survival assays were performed in MCF-7 (A) and T47D (B) cells with 1-hour pretreatment of the CDK4/6 inhibitor palbociclib at 1–100nM palbociclib. Clonogenics were also performed with ribociclib (C,D) and abemaciclib (E,F) and the surviving fraction of cells was calculated for each treatment condition at 2 Gy. Radiation enhancement ratios (rER) are shown. (p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***).
Because CDK4/6 inhibitors change the cell cycle distribution of exponentially growing ER+ breast cancer cells, we sought to understand how cell cycle changes may play a role in the radiosensitization. To that end, we performed propidium iodide-based cell cycle analysis in T47D and MCF-7 cells (Supplemental Fig. 6A,C) to determine the timecourse of G1 arrest in our cell lines. In T47D cells, G1 arrest did not occur at 1 or 6 hours after CDK4/6 inhibitor treatment, but cells were significantly arrested by 16 hours and remained arrested at 24 and 48 hours. MCF-7 cells did not arrest after 1 hour of drug treatment - equivalent to our one hour pretreatment in other assays – but did demonstrate cell cycle arrest at 48 hours even at these low concentrations. In addition, we performed clonogenic assays in MCF-7 cells with varied CDK4/6 inhibitor pretreatment times before radiation. In spite of these differences in cell cycle accumulation, pretreatment with 50–100nM palbociclib for either 6 or 24 hours led to nearly equivalent levels of radiosensitization in MCF-7 cells (rER 1.29–1.58 at 6 hrs, 1.13–1.71 at 24 hrs; Supplemental Fig. 6E&F, Supplemental Table 4), suggesting that radiosensitization is cell cycle independent and that radiosensitization occurs even in cells arresting at the G1 checkpoint.
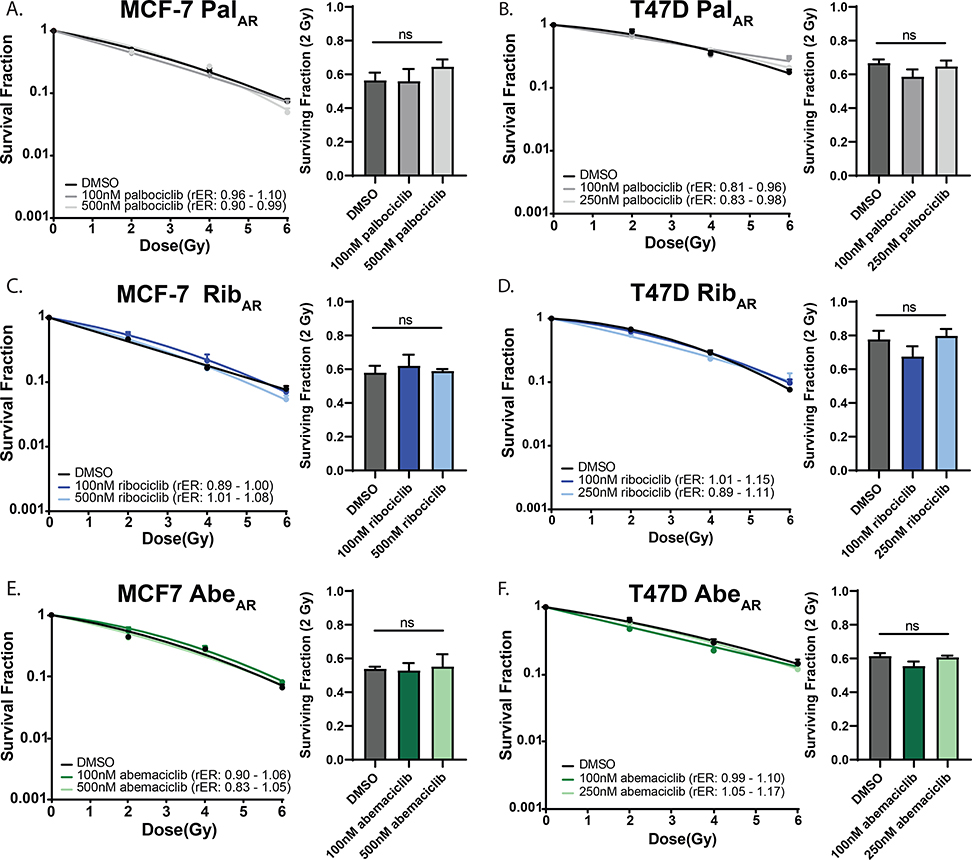
Given that CDK4/6 inhibitor-resistant cell lines respond differently to short-term CDK4/6 inhibition compared to their parental cell lines, we were interested in understanding whether CDK4/6 inhibitor resistance would play a role in the response of ER+ breast cancer cells to ionizing radiation. As expected, cell cycle analysis demonstrated that CDK4/6 inhibitor-resistant MCF-7 and T47D cells did not arrest at G1 after treatment with palbociclib, ribociclib, or abemaciclib (Supplemental Fig. 6B&D) even after 48 hours. Much higher concentrations of all 3 drugs (100–500nM) also failed to radiosensitize CDK4/6 inhibitor-resistant MCF-7 (Fig 3A,C,E) and T47D cells (Fig 3B,D,F) in clonogenic cell survival assays. Because these cells were selected for acquired resistance to CDK4/6 inhibitor monotherapy, single agent toxicity (Supplemental Table 2) was minimal, as expected.
Figure 3: CDK4/6 inhibition does not radiosensitize CDK4/6 inhibitor-resistant cell lines.
After three months of selection in CDK4/6 inhibitor-containing media, clonogenic survival assays were repeated in MCF-7 PalAR (A) and T47D PalAR cells (B). Ribociclib- (C,D) and abemaciclib-resistant (E,F) cell lines were also unaffected by combination therapy. Radiation enhancement ratios (rER) are shown. Data are graphed as the mean ± SEM.
Short term CDK4/6 inhibition leads to a decrease in homologous recombination efficiency
Radiosensitization can occur through a variety of mechanisms, including changes in the efficiency of the DNA damage response, cell cycle reassortment, changes in oxygenation, and upregulation of other cellular response pathways such as apoptosis or senescence. Given that we observed cellular changes in multiple DNA damage response pathways at the transcriptomic and proteomic/phosphoproteomic level after short term CDK4/6 inhibition, we wanted to understand whether CDK4/6 inhibition affects specific DNA damage response pathways, including the two major double-strand break repair pathways of homologous recombination (HR) and non-homologous end joining (NHEJ).
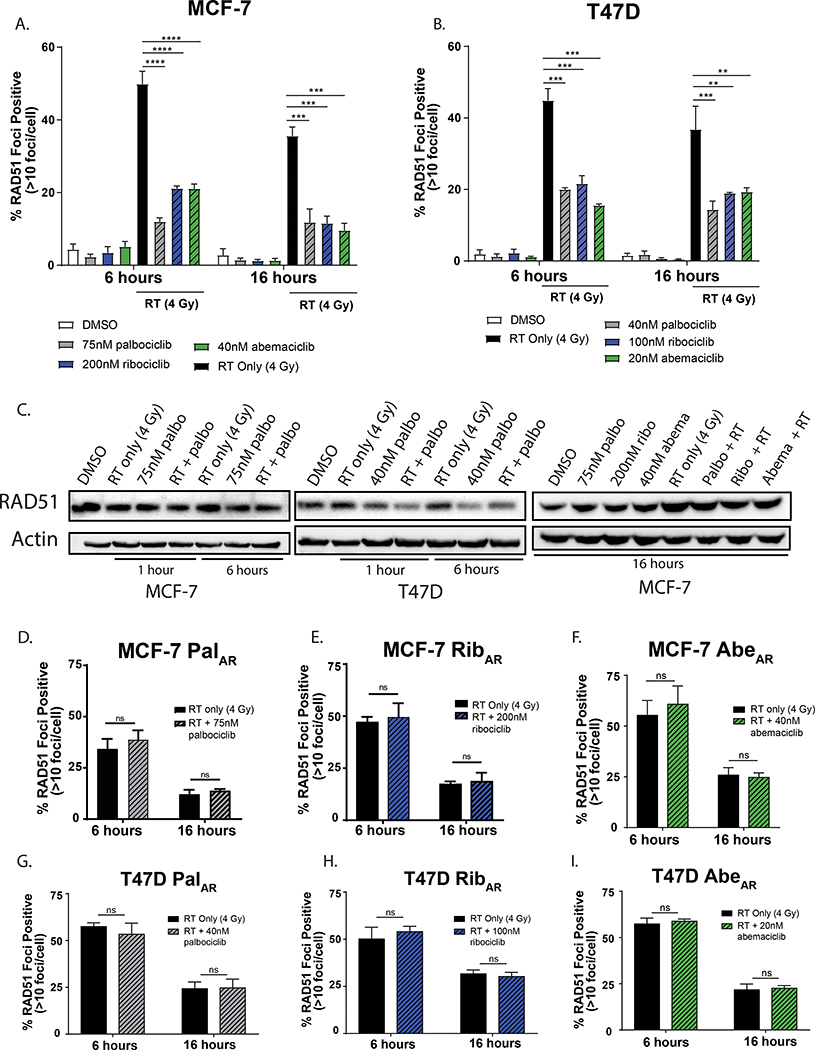
To assess HR-mediated effects, we performed RAD51 foci formation assays. RAD51 is a recombinase responsible for protecting single stranded DNA at the site of DNA strand breaks and initiating the catalysis of HR-mediated DNA repair. RAD51 foci are indicative of active HR and can be quantified using immunofluorescence microscopy to assess HR competency. In MCF-7 (Fig. 4A) and T47D (Fig 4B) cells, RT (4 Gy) led to an increase in RAD51 foci at 6 and 16 hours following RT. In contrast, a 1-hour pretreatment with either palbociclib, ribociclib, or abemaciclib led to a significant decrease in RAD51 foci at 6 and 16 hours post-RT compared to RT alone. The inability of ER+ breast cancer cells to respond to and repair double stranded breaks using HR cannot solely be attributed to an absence of RAD51 protein (Fig. 4C), though there was a slight decrease in total RAD51 protein levels in the palbociclib- and combination-treated groups in T47D cells at these time points. In contrast to CDK4/6 inhibitor-sensitive cell lines, CDK4/6 inhibitor-resistant MCF7AR (Fig. 4D–F) and T47DAR cells (Fig. 4G–I) did not demonstrate changes in RAD51 foci formation at either 6 or 16 hours. CDK4/6 inhibitor resistant cell lines still formed RAD51 foci and retained the ability to perform HR, but the addition of a CDK4/6 inhibitor did not further suppress the repair capacity of these cells. Representative foci are shown in both CDK4/6 inhibitor-sensitive (Supplemental Fig 7A&B) and CDK4/6 inhibitor-resistant (Supplemental Fig. 7C&D) cells.
Figure 4: CDK4/6 inhibition suppresses HR in CDK4/6 inhibitor-sensitive breast cancer cells.
After a 1-hour pretreatment ± CDK4/6 inhibition (colored bars), MCF-7 (A) and T47D (B) cells were treated ± 4 Gy RT (black / patterned bars) and slides were stained for immunofluorescent RAD51 foci 6- and 16-hours after radiation. Total RAD51 protein levels were assessed by western blot (C) at 1, 6, or 16 hours following radiation, with a one hour drug treatment prior to RT (4 Gy). CDK4/6 inhibitor-resistant cell lines were also used to assess RAD51 foci formation by immunofluorescence (D-I). Data points represent the average ± SEM for three independent experiments. (p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***).
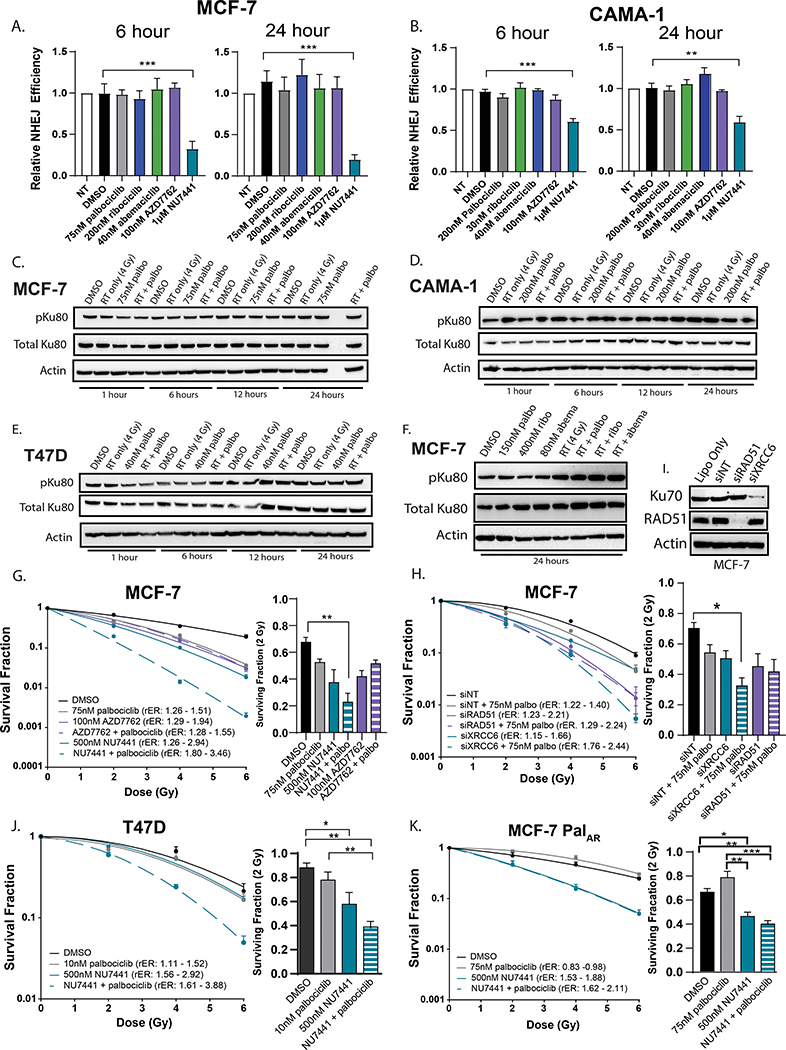
CDK4/6 inhibition does not suppress NHEJ repair
To understand how CDK4/6 inhibition may affect NHEJ efficiency, we used a transient GFP reporter system(22) to assess NHEJ proficiency in MCF-7 (Fig. 5A) and CAMA-1 (Fig. 5B) cells. In this system, CDK4/6 inhibitor monotherapy did not affect NHEJ efficiency in either cell line compared to vehicle controls. As a control, treatment with 1μM NU7441 (a DNAPK inhibitor) significantly decreases NHEJ activity in both MCF-7 and CAMA-1 cells, but the CHK1/2 inhibitor AZD7762 does not affect NHEJ repair efficiency. Furthermore, when combined with RT, we observed stable or increased expression of the NHEJ protein mediator pKu80 in MCF-7 (Fig. 5C), CAMA-1 (Fig. 5D), and T47D cells (Fig. 5E) suggesting that NHEJ repair was not inhibited and may be activated in response to decreases in HR. In addition, at concentrations that are double the IC50 values of each CDK4/6 inhibitor in MCF-7 cells, pKu80 expression was significantly higher in cells treated with the combination of drug and RT (Fig. 5F).
Figure 5: CDK4/6 inhibition does not suppress NHEJ efficiency in ER+ breast cancer cells.
A GFP reporter system was used to assess relative NHEJ efficiency in MCF-7 (A) and CAMA1 (B) cells at 6- and 24-hours following CDK4/6 inhibitor treatment. DNAPK inhibition (1μM NU7441) was used as a positive control and CHK1/2 inhibition (100nM AZD7762) was used as a negative control in this assay. Western blots (C-E) were used to assess pKu80 and Ku80 expression at 1, 6, 12, and 24 hours after RT (4 Gy) and a 1-hour drug pretreatment. Western blots were also used to assess pKu80/Ku80 expression at 24 hours after treatment of MCF-7 cells with higher concentrations of CDK4/6 inhibition (F). Clonogenics were performed in MCF-7 (G), T47D (J), and MCF-7 PalAR cells (K) with palbociclib in combination with RT and either NU7441 (teal) and/or AZD7762 (purple). MCF-7 cells were also used for clonogenics with siXRCC6 and siRAD51 (H) with or without 75nM palbociclib. Protein was harvested 48 hours after transfection (the timepoint at which clonogenics are radiated) to demonstrate successful RAD51 and Ku70 protein knockdown (I). Data points represent the average ± SEM (p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***).
If CDK4/6 inhibition suppresses the ability of ER+ breast cancer cells to undergo HR, NHEJ becomes the predominant form of dsDNA break repair. Thus, we hypothesized that combining CDK4/6 inhibition with pharmacological or genetic inhibition of proteins in the NHEJ pathway would be synergistic. In both MCF-7 and T47D cells (Fig. 5G,5J), palbociclib in combination with 500nM NU7441 leads to extremely significant levels of radiosensitization (rER: 1.80 – 3.46) compared to either compound alone. However, in MCF-7 cells, pharmacologic CHK1/2 inhibition is not synergistic with CDK4/6 inhibition – consistent with the hypothesis that CDK4/6 inhibitors act redundantly to suppress HR repair. Similar results were obtained using genetic knockdown of Ku70 (XRCC6) and RAD51 (RAD51) in MCF-7 cells (Fig. 5H,I). Although the ability of single agent DNAPK inhibition to radiosensitize MCF-7 cells is retained in MCF-7 PalAR cells (Fig 5K), the addition of palbociclib does not lead to additional or synergistic levels of radiosensitization.
These studies demonstrate that CDK4/6 inhibition impairs the ability of cells to undergo HR and may shunt dsDNA break repair through the NHEJ pathway. In our models, neither immunofluorescent γH2AX foci (Supplemental Fig. 8A,B,E,F) or γH2AX total protein (Supplemental Fig. 8C&D) were significantly different between cells treated with RT alone (2 Gy) or the combination of RT and palbociclib, suggesting that combination treatment did not significantly affect the persistence of dsDNA damage in the cell. In our proteomic analysis using RPPA we did not see any changes in γH2AX (pS139) expression in our MCF-7 cells treated with 75nM palbociclib (Figure 1I). To further confirm this, we performed the neutral COMET assay in MCF-7 cells to detect changes in dsDNA breaks (Supplemental Fig. 8 G&H). Although RT (4 Gy) caused an increase in dsDNA breaks at both 6 and 16 hours after RT, CDK4/6 inhibition did not potentiate a delay in dsDNA break repair. Thus, the ability of cells to repair dsDNA breaks in breast cancer cells treated with CDK4/6 inhibition and RT may be limited to low-fidelity NHEJ repair.
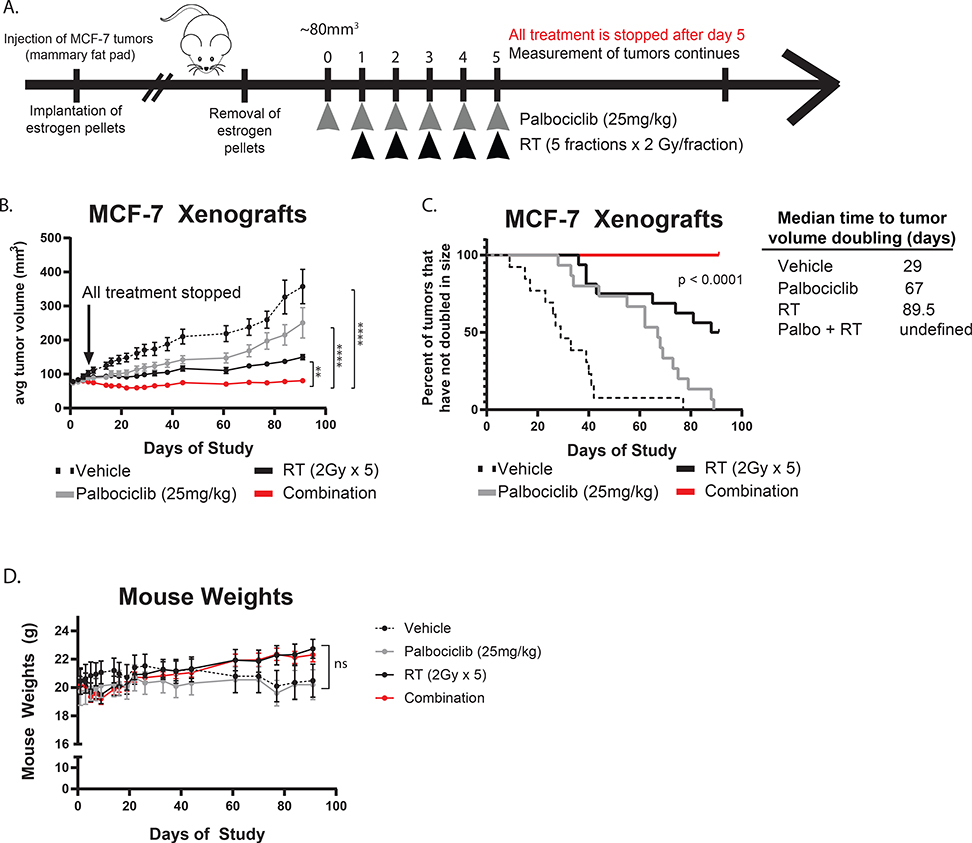
CDK4/6 inhibition radiosensitizes ER+ breast cancer cells in vivo
To understand if CDK4/6 inhibition leads to clinically relevant levels of radiosensitization in vivo, we generated orthotopic xenograft models with the MCF-7 cells (Fig. 6A). In the combination group, palbociclib treatment was started one day before fractionated RT and was discontinued after the last fraction in order to measure the radiosensitizing effects of CDK4/6 inhibition with palbociclib independent from its single agent efficacy. Treatment with the combination of palbociclib and RT significantly suppressed tumor growth (p < 0.01, Fig. 6B) and prolonged time to tumor doubling (p < 0.0001, Fig. 6C) compared to mice treated with RT or palbociclib alone. These treatments did not lead to any visible toxicities or significant changes in body weights of mice (Fig. 6D) throughout the duration of the study, suggesting that the therapy was generally well-tolerated. In addition, we calculated the expected and observed fractional tumor volume (FTV)(26) for each treatment condition (Supplemental Table 6) and our results suggest that combination treatment with palbociclib and RT has synergistic (expected/observed ratio > 1) rather than additive effects.
Figure 6: CDK4/6 inhibition radiosensitizes ER+ breast cancer cells in vivo.
MCF-7 cells were used to generate orthotopic xenograft tumors in the mammary fat pads of CB17-SCID mice. Once tumors reached 80mm3, mice were treated with either vehicle, palbociclib alone (25mg/kg), RT alone (5 × 2 Gy), or the combination and all treatment was stopped on the 5th day as indicated (A). Tumor size (B) was measured throughout the duration of the study and used to calculate the time to tumor doubling (C). Mouse weights (D) were recorded on the same days that tumor measurements were taken. (p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***).
Discussion
In this study, we demonstrate that short term treatment of ER+ breast cancer cell lines with the CDK4/6 inhibitors palbociclib, ribociclib, and abemaciclib led to alterations in many cellular pathways, including suppression of cell cycle signaling and changes in the DNA damage response (Fig. 1). In ER+ breast cancer cells that are sensitive to CDK4/6 inhibitor monotherapy, the combination of CDK4/6 inhibition and ionizing RT led to significant radiosensitization with each of the three clinically approved CDK4/6 inhibitors (Fig. 2). This radiosensitizing ability, however, was lost in ER+ breast cancer cells with acquired resistance to CDK4/6 inhibition and was not observed in normal breast epithelial cells (Fig. 3 and Supplemental Fig. 5). Mechanistically, the radiosensitization observed in CDK4/6 inhibitor sensitive models was mediated by impaired homologous recombination that shunted dsDNA break repair towards error-prone NHEJ (Fig. 5). In contrast, both HR and NHEJ repair remained intact in CDK4/6 inhibitor-resistant cell lines (Fig. 4&5). In xenograft models of ER+ breast cancer, CDK4/6 inhibition led to tumor radiosensitization (Fig. 6). Taken together, these results demonstrate that the combination of CDK4/6 inhibition and RT is a potentially effective strategy for the radiosensitization of ER+ breast cancer that is lost in cells that have become resistant to CDK4/6 inhibitor monotherapy. Our data also suggest that concurrent administration of CDK4/6 inhibition with RT (instead of adjuvant therapy) may be a more effective strategy to decrease the rates of disease recurrence in patients with ER+ breast cancer at high risk of locoregional recurrence and that this strategy warrants clinical investigation.
In contrast to the conventional use of CDK4/6 inhibitors in the metastatic setting, our work challenges the standard treatment paradigm and highlights the therapeutic potential of using CDK4/6 inhibitors in combination with ionizing RT to radiosensitize CDK4/6 inhibitor-naïve ER+ breast cancers. In contrast to studies that seek to overcome CDK4/6 inhibitor resistance or propose therapeutic alternatives for CDK4/6 inhibitor-resistant tumors, our approach in combining CDK4/6 inhibitors and RT is a novel therapeutic strategy that can be utilized prior to the development of drug resistance; thus, this approach has the potential to cure women prior to the development of metastatic disease. Finally, in our study, all three clinically approved CDK4/6 inhibitors demonstrated the ability to radiosensitize ER+ breast cancer cell lines at similar concentrations, suggesting that this effect could be achieved in patients regardless of the specific CDK4/6 inhibitor chosen for therapy.
In current practice, RT is only given in combination with CDK4/6 inhibitors for palliative management in patients with metastatic disease. While there have been a few studies that report additional skin and GI toxicities for these patients(29,30), recent analyses report that combination therapy in the palliative setting has been generally well-tolerated(31–34). Our data in normal breast epithelial cells suggested that CDK4/6 inhibition did not potentiate radiation effects and therefore should be well tolerated by the normal breast tissue when translated clinically.
It is important to note that our in vivo murine studies were designed to test the effect of low dose CDK4/6 inhibition (25mg/kg) as a radiosensitizing strategy, rather than the efficacy of continued combination therapy at standard, optimal doses (50–100mg/kg). Because drug was not continued after completion of fractionated RT, we would expect that long term CDK4/6 inhibition after completion of RT would lead to even further reduction in overall tumor burden. Importantly, our data suggest that much lower doses of CDK4/6 inhibition can confer radiosensitivity, and one potential translational strategy would be to use these low doses in combination with RT which would potentially limit the frequency of systemic toxicities that lead to the discontinuation of therapy. Alternatively, future studies will assess if monotherapy doses of CDK4/6 inhibition lead to an even greater degree of radiosensitization with an acceptably low toxicity profile. These two competing strategies are currently being considered in the planned phase I/II clinical trials testing this combination treatment.
While this work may be beneficial for ER+ breast cancer patients, our study may also provide valuable mechanistic insights that could be applied to other cancers where CDK4/6 inhibitors are being studied preclinically. Indeed, whether CDK4/6 inhibitor-mediated radiosensitization is clinically effective in other subtypes of breast cancer (basal-like, HER2 enriched, etc.) remains an unanswered question, as well as if this is effective in other histologies, including invasive lobular or inflammatory breast cancer. Previous studies have, however, demonstrated that CDK4/6 inhibition may radiosensitize head and neck squamous cell carcinomas(35,36), glioblastomas(37,38), and colorectal and lung cancer cell lines(39). In line with findings in head and neck squamous cell carcinoma cell lines(35,36), impaired HR efficiency might be important for CDK4/6 inhibitor-mediated radiosensitization in multiple cancer types.
Along the same lines, other preclinical studies performed in pancreatic cancer(40) and triple negative breast cancer(20,41) cell lines have suggested that CDK4/6 inhibition impairs homologous recombination efficiency after administration of cytotoxic chemotherapies or RT(42). This will be an important clinical consideration for future studies with CDK4/6 inhibitors as radiosensitizing agents, as cytotoxic chemotherapies are routinely used to treat patients with ER+ breast cancer in the neoadjuvant setting. However, in contrast to studies in lung and colorectal cancer cell lines that suggest that radiosensitization is p53-dependent(39), our data showed that CDK4/6 inhibitor-mediated radiosensitization occurs in both p53 wild type (MCF-7, ZR-75–1) and p53 mutant (T47D, CAMA-1) models. However, all of our models express the tumor suppressor RB1 which has recently been shown to directly promote HR in breast cancer cell lines(43).
There are some limitations to this study that need to be considered. Although we focused on CDK4/6 inhibition and the DNA damage response, other mechanisms of radiosensitization may play a minor role in this phenotype. CDK4/6 inhibitor monotherapy has been shown to increase apoptosis in triple negative breast cancer cell lines(44–46), but in our models we did not see an increase in apoptosis with CDK4/6 inhibition or combination treatment (Supplemental Fig. 9). There are conflicting reports about the effect of CDK4/6 inhibition on senescence in breast and other cancer types(47–52), and further studies could address any potential contributions of senescence to the radiosensitization phenotype that we see in ER+ breast cancer models. We also did not explore mechanisms of single strand break repair, such as mismatch repair, base excision repair, or nucleotide excision repair, but our transcriptomic data (Fig. 1) suggested that these pathways could play a minor role in radiosensitization. Finally, confirmatory animal studies demonstrating radiosensitization in CDK4/6 inhibitor-treatment naïve PDX models and lack of radiosensitization in CDK4/6 inhibitor-resistant PDXs (from women whose disease progressed on CDK4/6 inhibitor therapy) are needed. These studies were underway when the COVID-19 pandemic arose and are still planned when circumstances allow.
It is possible that other CDK inhibitors may be able to radiosensitize ER+ breast cancer cells. Flavopiridol, a nonspecific CDK inhibitor, has been shown to radiosensitize cancer cell lines(53,54) and to potentiate cell death after cytotoxic therapy(55), though it is has an unacceptable safety profile that has prevented its clinical developent(56). Furthermore, studies of CDK12/13 in triple negative breast cancer have demonstrated changes in radiation sensitivity based on direct interaction with transcriptional machinery and changes in polyadenylation(57), and support the idea that radiosensitization in ER+ breast cancers may be achieved with inhibition of other cyclin-dependent kinases as well. Finally, there is recent evidence to suggest that hormone therapy and CDK4/6 inhibitor resistance may lead to alterations in genes like AKT1 and AURKA that are involved in DNA repair(58), which may have important clinical implications for patients receiving both types of therapy.
In conclusion, our results suggest that CDK4/6 inhibitor therapy would be effective in decreasing tumor growth in ER+ breast cancer patients by radiosensitizing tumor cells during fractionated RT. Our data also suggests that the development of CDK4/6 inhibitor resistance with one drug leads to cross-resistance with the others in its class, consistent with what others have shown, which is an important clinical consideration as clinicians start to use CDK4/6 inhibitors in the adjuvant setting with RT. We also found that CDK4/6 inhibitor-mediated radiosensitization can be used as a therapeutic strategy in the absence (or prior to) the initiation of hormone therapies, but future studies will seek to understand the interaction between CDK4/6 inhibition and RT with concurrent endocrine therapy. A complete understanding of the mechanism of CDK4/6 inhibitor-mediated radiosensitization will provide further insight into future treatment protocols and strategies to more effectively treat patients with ER+ breast cancers.
Supplementary Material
Statement of Translational Relevance.
Although CDK4/6 inhibitors are currently indicated for patients with metastatic, ER+ breast cancer, their utility in the non-metastatic setting is still being established. Our understanding of the interaction between CDK4/6 inhibitors and the ionizing radiation given as part of the standard of care is lacking, and the utility of this approach in women at high risk for locoregional failure is unknown. In this manuscript, we demonstrate using multiple non-overlapping in vitro and in vivo models that combination therapy with RT and each of the three clinically approved CDK4/6 inhibitors is more effective at decreasing cell proliferation and tumor growth when compared to either RT or CDK4/6 inhibition alone. Further, this sensitization is due, at least in part, through the suppression of homologous recombination (HR)-mediated DNA repair. In contrast, preclinical models with acquired resistance to CDK4/6 inhibition do not demonstrate radiosensitization or suppression of HR. These data suggest that combination CDK4/6 inhibition and RT represent a novel indication for CDK4/6 inhibitors and a clinically feasible strategy for the radiosensitization of ER+ breast cancers that warrants clinical exploration.
Acknowledgments
AP is supported by T32-GM007767, F31-CA254138, and the University of Michigan Center for the Education of Women (CEW+) Irma M. Wyman Fund, AM is supported by T32-GM007315 and T32-GM113900, and BC is supported by T32-CA140044. AP, AM, and BC are all supported by Rackham Graduate School Research Grants.
The authors would also like to thank the Breast Cancer Research Foundation (N02600 to LP, N003173 to JR) and the University of Michigan Rogel Cancer Center (P30CA046592 and G023324). Finally, the authors greatly appreciate the contributions of the University of Michigan core facilities (Flow Cytometry, Advanced Genomics, Bioinformatics), and the M.D. Anderson Functional Proteomics RPPA Core Facility (NCI #CA16672) for support and technical assistance with the experiments in this manuscript.
Footnotes
The authors declare no potential conflicts of interest.
Disclosure of Potential Conflicts of Interest: The authors have no relevant conflicts of interest.
References
- 1.(EBCTCG) EBCTCG. Effects of Chemotherapy and Hormonal Therapy for Early Breast Cancer on Recurrence and 15-year Survival: An Overview of the Randomised Trials. Lancet (London, England) 2005;365(9472) doi 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Turner NC, Slamon DJ, Ro J, Bondarenko I, Im S-A, Masuda N, et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N Engl J Med 2018. doi NJ201811153792008. [DOI] [PubMed] [Google Scholar]
- 3.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015;16(1):25–35 doi 10.1016/s1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 4.Finn RS, Aleshin A, Slamon DJ. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res 2016;18(1):17 doi 10.1186/s13058-015-0661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 2009;11(5):R77 doi 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tripathy D, Bardia A, Sellers WR. Ribociclib (LEE011): Mechanism of Action and Clinical Impact of This Selective Cyclin-Dependent Kinase 4/6 Inhibitor in Various Solid Tumors. Clin Cancer Res 2017;23(13):3251–62 doi 10.1158/1078-0432.ccr-16-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickler MN, Tolaney SM, Rugo HS, Cortes J, Dieras V, Patt D, et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR(+)/HER2(−) Metastatic Breast Cancer. Clin Cancer Res 2017;23(17):5218–24 doi 10.1158/1078-0432.ccr-17-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George W, Sledge J, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2− Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol 2017. doi 10.1200/JCO.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 9.OS J, K P, GS S, P C, CL A, DA C, et al. Ribociclib Plus Letrozole Versus Letrozole Alone in Patients With De Novo HR+, HER2- Advanced Breast Cancer in the Randomized MONALEESA-2 Trial. Breast cancer research and treatment 2018;168(1) doi 10.1007/s10549-017-4518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Condorelli R, Spring L, O’Shaughnessy J, Lacroix L, Bailleux C, Scott V, et al. Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann Oncol 2018;29(3):640–5 doi 10.1093/annonc/mdx784. [DOI] [PubMed] [Google Scholar]
- 11.Arnedos M, Bayar MA, Cheaib B, Scott V, Bouakka I, Valent A, et al. Modulation of Rb phosphorylation and antiproliferative response to palbociclib: the preoperative-palbociclib (POP) randomized clinical trial. Ann Oncol 2018;29(8):1755–62 doi 10.1093/annonc/mdy202. [DOI] [PubMed] [Google Scholar]
- 12.Herrera-Abreu MT, Palafox M, Asghar U, Rivas MA, Cutts RJ, Garcia-Murillas I, et al. Early Adaptation and Acquired Resistance to CDK4/6 Inhibition in Estrogen Receptor-Positive Breast Cancer. Cancer Res 2016;76(8):2301–13 doi 10.1158/0008-5472.can-15-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asghar US, Barr AR, Cutts R, Beaney M, Babina I, Sampath D, et al. Single-Cell Dynamics Determines Response to CDK4/6 Inhibition in Triple-Negative Breast Cancer. Clin Cancer Res 2017;23(18):5561–72 doi 10.1158/1078-0432.ccr-17-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dean JL, Thangavel C, McClendon AK, Reed CA, Knudsen ES. Therapeutic CDK4/6 inhibition in breast cancer: key mechanisms of response and failure. Oncogene 2010;29(28):4018–32 doi 10.1038/onc.2010.154. [DOI] [PubMed] [Google Scholar]
- 15.Gong X, Litchfield LM, Webster Y, Chio LC, Wong SS, Stewart TR, et al. Genomic Aberrations that Activate D-type Cyclins Are Associated with Enhanced Sensitivity to the CDK4 and CDK6 Inhibitor Abemaciclib. Cancer Cell 2017;32(6):761–76.e6 doi 10.1016/j.ccell.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Patel P, Tsiperson V, Gottesman SRS, Somma J, Blain SW. Dual Inhibition of CDK4 and CDK2 via Targeting p27 Tyrosine Phosphorylation Induces a Potent and Durable Response in Breast Cancer Cells. Mol Cancer Res 2018;16(3):361–77 doi 10.1158/1541-7786.mcr-17-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohammed AA, Rashied H, Elsayed FM. CDK4/6 inhibitors in advanced breast cancer, what is beyond? Oncol Rev 2019;13(2) doi 10.4081/oncol.2019.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378(9804):1707–16 doi 10.1016/s0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kettner NM, Vijayaraghavan S, Durak MG, Bui T, Kohansal M, Ha MJ, et al. Combined Inhibition of STAT3 and DNA Repair in Palbociclib-Resistant ER-Positive Breast Cancer. Clin Cancer Res 2019;25(13):3996–4013 doi 10.1158/1078-0432.ccr-18-3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dean JL, McClendon AK, Knudsen ES. Modification of the DNA damage response by therapeutic CDK4/6 inhibition. J Biol Chem 2012;287(34):29075–87 doi 10.1074/jbc.M112.365494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michmerhuizen AR, Pesch AM, Moubadder L, Chandler BC, Wilder-Romans K, Cameron M, et al. PARP1 Inhibition Radiosensitizes Models of Inflammatory Breast Cancer to Ionizing Radiation. Mol Cancer Ther 2019. doi 10.1158/1535-7163.mct-19-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandler B, Moubadder L, Ritter C, Liu M, Cameron M, Wilder-Romans K, et al. TTK inhibition radiosensitizes basal-like breast cancer through impaired homologous recombination. Journal of Clinical Investigation 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003;4(2):249–64 doi 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 24.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 2004;3:Article3 doi 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 25.Spurrier B, Ramalingam S, Nishizuka S. Reverse-phase protein lysate microarrays for cell signaling analysis. Nat Protoc 2008;3(11):1796–808 doi 10.1038/nprot.2008.179. [DOI] [PubMed] [Google Scholar]
- 26.Yokoyama Y, Dhanabal M, Griffioen AW, Sukhatme VP, Ramakrishnan S. Synergy between angiostatin and endostatin: inhibition of ovarian cancer growth. Cancer Res 2000;60(8):2190–6. [PubMed] [Google Scholar]
- 27.Skov K, Macphail S. Interaction of platinum drugs with clinically relevant x-ray doses in mammalian cells: a comparison of cisplatin, carboplatin, iproplatin, and tetraplatin. International Journal of Radiation Oncology* Biology* Physics 1991;20(2):221–5. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Yang H, Gu K, Chen J, Rui M, Jiang G-L. In vitro and in vivo study of a nanoliposomal cisplatin as a radiosensitizer. International journal of nanomedicine 2011;6:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawamoto T, Shikama N, Sasai K. Severe acute radiation-induced enterocolitis after combined palbociclib and palliative radiotherapy treatment. Radiother Oncol 2019;131:240–1 doi 10.1016/j.radonc.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Messer JA, Ekinci E, Patel TA, Teh BS. Enhanced dermatologic toxicity following concurrent treatment with palbociclib and radiation therapy: A case report. Rep Pract Oncol Radiother. Volume 24 Netherlands2019. p 276–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ippolito E, Greco C, Silipigni S, Dell’Aquila E, Petrianni GM, Tonini G, et al. Concurrent radiotherapy with palbociclib or ribociclib for metastatic breast cancer patients: Preliminary assessment of toxicity. Breast 2019;46:70–4 doi 10.1016/j.breast.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Figura NB, Potluri TK, Mohammadi H, Oliver DE, Arrington JA, Robinson TJ, et al. CDK 4/6 inhibitors and stereotactic radiation in the management of hormone receptor positive breast cancer brain metastases. J Neurooncol 2019;144(3):583–9 doi 10.1007/s11060-019-03260-6. [DOI] [PubMed] [Google Scholar]
- 33.Chowdhary M, Sen N, Chowdhary A, Usha L, Cobleigh MA, Wang D, et al. Safety and Efficacy of Palbociclib and Radiation Therapy in Patients With Metastatic Breast Cancer: Initial Results of a Novel Combination. Adv Radiat Oncol 2019;4(3):453–7 doi 10.1016/j.adro.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meattini I, Desideri I, Scotti V, Simontacchi G, Livi L. Ribociclib plus letrozole and concomitant palliative radiotherapy for metastatic breast cancer. Breast 2018;42:1–2 doi 10.1016/j.breast.2018.08.096. [DOI] [PubMed] [Google Scholar]
- 35.Gottgens EL, Bussink J, Leszczynska KB, Peters H, Span PN, Hammond EM. Inhibition of CDK4/CDK6 Enhances Radiosensitivity of HPV Negative Head and Neck Squamous Cell Carcinomas. Int J Radiat Oncol Biol Phys 2019;105(3):548–58 doi 10.1016/j.ijrobp.2019.06.2531. [DOI] [PubMed] [Google Scholar]
- 36.Tai TS, Lin PM, Wu CF, Hung SK, Huang CI, Wang CC, et al. CDK4/6 Inhibitor LEE011 Is a Potential Radiation-sensitizer in Head and Neck Squamous Cell Carcinoma: An In Vitro Study. Anticancer Res 2019;39(2):713–20 doi 10.21873/anticanres.13167. [DOI] [PubMed] [Google Scholar]
- 37.Hashizume R, Zhang A, Mueller S, Prados MD, Lulla RR, Goldman S, et al. Inhibition of DNA damage repair by the CDK4/6 inhibitor palbociclib delays irradiated intracranial atypical teratoid rhabdoid tumor and glioblastoma xenograft regrowth. Neuro Oncol 2016;18(11):1519–28 doi 10.1093/neuonc/now106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whittaker S, Madani D, Joshi S, Chung SA, Johns T, Day B, et al. Combination of palbociclib and radiotherapy for glioblastoma. Cell Death Discov 2017;3:17033 doi 10.1038/cddiscovery.2017.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tao Z, Le Blanc JM, Wang C, Zhan T, Zhuang H, Wang P, et al. Coadministration of Trametinib and Palbociclib Radiosensitizes KRAS-Mutant Non-Small Cell Lung Cancers In Vitro and In Vivo. Clin Cancer Res 2016;22(1):122–33 doi 10.1158/1078-0432.ccr-15-0589. [DOI] [PubMed] [Google Scholar]
- 40.Salvador-Barbero B, Alvarez-Fernandez M, Zapatero-Solana E, El Bakkali A, Menendez MDC, Lopez-Casas PP, et al. CDK4/6 Inhibitors Impair Recovery from Cytotoxic Chemotherapy in Pancreatic Adenocarcinoma. Cancer Cell 2020;37(3):340–53.e6 doi 10.1016/j.ccell.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Choi C, Park S, Cho WK, Choi DH. Cyclin D1 is Associated with Radiosensitivity of Triple-Negative Breast Cancer Cells to Proton Beam Irradiation. Int J Mol Sci 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts PJ, Kumarasamy V, Witkiewicz AK, Knudsen ES. Chemotherapy and CDK4/6 Inhibitors: Unexpected Bedfellows. Molecular Cancer Therapeutics 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vélez-Cruz R, Manickavinayaham S, Biswas AK, Clary RW, Premkumar T, Cole F, et al. RB localizes to DNA double-strand breaks and promotes DNA end resection and homologous recombination through the recruitment of BRG1. Genes Dev 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li T, Xiong Y, Wang Q, Chen F, Zeng Y, Yu X, et al. Ribociclib (LEE011) suppresses cell proliferation and induces apoptosis of MDA-MB-231 by inhibiting CDK4/6-cyclin D-Rb-E2F pathway. Artif Cells Nanomed Biotechnol 2019;47(1):4001–11 doi 10.1080/21691401.2019.1670670. [DOI] [PubMed] [Google Scholar]
- 45.Gao Y, Shen J, Choy E, Mankin H, Hornicek F, Duan Z. Inhibition of CDK4 sensitizes multidrug resistant ovarian cancer cells to paclitaxel by increasing apoptosiss. Cell Oncol (Dordr) 2017;40(3):209–18 doi 10.1007/s13402-017-0316-x. [DOI] [PubMed] [Google Scholar]
- 46.Xiong Y, Li T, Assani G, Ling H, Zhou Q, Zeng Y, et al. Ribociclib, a selective cyclin D kinase 4/6 inhibitor, inhibits proliferation and induces apoptosis of human cervical cancer in vitro and in vivo. Biomed Pharmacother 2019;112:108602 doi 10.1016/j.biopha.2019.108602. [DOI] [PubMed] [Google Scholar]
- 47.Goel S, Wang Q, Watt AC, Tolaney SM, Dillon DA, Li W, et al. Overcoming Therapeutic Resistance in HER2-Positive Breast Cancers with CDK4/6 Inhibitors. Cancer Cell 2016;29(3):255–69 doi 10.1016/j.ccell.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bollard J, Miguela V, Galarreta MRd, Venkatesh A, Bian CB, Roberto MP, et al. Palbociclib (PD-0332991), a selective CDK4/6 inhibitor, restricts tumour growth in preclinical models of hepatocellular carcinoma. 2017. doi 10.1136/gutjnl-2016-312268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valenzuela CA, Vargas L, Martinez V, Bravo S, Brown NE. Palbociclib-induced autophagy and senescence in gastric cancer cells. Exp Cell Res 2017;360(2):390–6 doi 10.1016/j.yexcr.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 50.Min A, Kim JE, Kim YJ, Lim JM, Kim S, Kim JW, et al. Cyclin E overexpression confers resistance to the CDK4/6 specific inhibitor palbociclib in gastric cancer cells. Cancer Lett 2018;430:123–32 doi 10.1016/j.canlet.2018.04.037. [DOI] [PubMed] [Google Scholar]
- 51.Kishino E, Ogata R, Saitoh W, Koike Y, Ohta Y, Kanomata N, et al. Anti-cell growth and anti-cancer stem cell activity of the CDK4/6 inhibitor palbociclib in breast cancer cells. Breast Cancer 2019. doi 10.1007/s12282-019-01035-5. [DOI] [PubMed] [Google Scholar]
- 52.Morris-Hanon O, Marazita MC, Romorini L, Isaja L, Fernandez-Espinosa DD, Sevlever GE, et al. Palbociclib Effectively Halts Proliferation but Fails to Induce Senescence in Patient-Derived Glioma Stem Cells. Mol Neurobiol 2019;56(11):7810–21 doi 10.1007/s12035-019-1633-z. [DOI] [PubMed] [Google Scholar]
- 53.Raju U, Nakata E, Mason KA, Ang KK, Milas L. Flavopiridol, a cyclin-dependent kinase inhibitor, enhances radiosensitivity of ovarian carcinoma cells. Cancer Res 2003;63(12):3263–7. [PubMed] [Google Scholar]
- 54.Camphausen K, Brady KJ, Burgan WE, Cerra MA, Russell JS, Bull EE, et al. Flavopiridol enhances human tumor cell radiosensitivity and prolongs expression of gammaH2AX foci. Mol Cancer Ther 2004;3(4):409–16. [PubMed] [Google Scholar]
- 55.Motwani M, Rizzo C, Sirotnak F, She Y, Schwartz GK. Flavopiridol Enhances the Effect of Docetaxel in Vitro and in Vivo in Human Gastric Cancer Cells 1 2003. [PubMed] [Google Scholar]
- 56.Tan AR, Yang X, Berman A, Zhai S, Sparreboom A, Parr AL, et al. Phase I trial of the cyclin-dependent kinase inhibitor flavopiridol in combination with docetaxel in patients with metastatic breast cancer. Clin Cancer Res 2004;10(15):5038–47 doi 10.1158/1078-0432.ccr-04-0025. [DOI] [PubMed] [Google Scholar]
- 57.Quereda V, Bayle S, Vena F, Frydman SM, Monastyrskyi A, Roush WR, et al. Therapeutic Targeting of CDK12/CDK13 in Triple-Negative Breast Cancer. Cancer Cell 2019;36(5):545–58.e7 doi 10.1016/j.ccell.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 58.Wander SA, Cohen O, Gong X, Johnson GN, Buendia-Buendia JE, Lloyd MR, et al. The Genomic Landscape of Intrinsic and Acquired Resistance to Cyclin-Dependent Kinase 4/6 Inhibitors in Patients with Hormone Receptor–Positive Metastatic Breast Cancer. Cancer Discovery 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.