Abstract
Objective
To evaluate time trends in mortality for hospitalized adults with systemic lupus erythematosus (SLE) compared to the general hospitalized population (GHP), and to identify factors associated with increased risk of death among hospitalized SLE patients.
Methods
We used the National (Nationwide) Inpatient Sample to estimate all-cause mortality for adults discharged from U.S. community hospitals between 2006 and 2016. Poisson regression models were used to estimate the risk of in-hospital death among all patients, including demographics, socioeconomic factors, comorbidity score, hospital region, SLE diagnosis and race/ethnicity as covariates.
Results
Among 340,467,049 hospitalizations analyzed, 1,903,279 had a discharge diagnosis of SLE. In adjusted analysis, the risk of inpatient death decreased among hospitalizations for patients with SLE from 2.2% to 1.5% (p-value<0.001) between 2006 and 2016. All of the decrease in SLE mortality occurred between 2006 and 2008; after 2008, mortality stabilized at a rate statistically similar to the GHP. Hospitalizations for Blacks, Hispanics, and Asian/Pacific Islanders with SLE were more likely to end in death compared to hospitalizations for either whites with SLE, or individuals of the same non-white race/ethnicity without SLE.
Conclusions
In the largest study of in-hospital SLE mortality published to date, we found significant improvements in mortality for U.S. hospitalized patients with SLE from 2006 until 2008, after which mortality stabilized at a level similar to that of the GHP. Our results also demonstrate a persistently high mortality burden among U.S. Blacks and Hispanics with SLE, and contribute new data revealing high mortality among Asian/Pacific Islanders with SLE.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease that can result in organ damage, frequent hospitalization, and premature death[1–4]. Advances in therapy in recent decades have altered rates of damage accrual, hospitalizations, and mortality for patients with SLE, with observational studies suggesting overall SLE survival has improved from less than 50% 5-year survival in the 1950s[5] to over 90% 10-year survival by 2000[6]. A recent analysis based on death certificate data in the U.S. noted a decrease in the SLE age standardized mortality rate between 1968 and 2013, but reported that the ratio of SLE to non-SLE mortality had increased 30% since 1968[7], which raises the question of whether SLE advances have lagged behind medical improvements for other conditions. Most other studies evaluating SLE mortality have been based on patient cohorts which may not represent the general population and capture a relatively small number of total deaths[1, 6, 8–10].
The most commonly cited reasons for SLE hospitalization both in the U.S. and internationally are SLE flare and infection[11–15]. SLE and connective tissue disorders had a 27% thirty-day hospital readmission rate in the U.S. in 2010, ranking it as the sixth most likely principal diagnosis associated with readmission[4]. It is known that the majority of deaths in the general population occur in the hospital, and that patients are more likely to be admitted to the hospital in the six months prior to their death[16]. Nevertheless, studies evaluating SLE in-hospital mortality are limited. The most recent multi-year national evaluation of mortality for U.S. hospitalized SLE patients is from data spanning 1998–2002[17]. The few studies describing SLE in-hospital mortality in the U.S. over the past 15 years suggest mortality may be decreasing over time[12,18]. To our knowledge, this research conducted over the past two decades also did not investigate U.S. in-hospital mortality risk differences by race or ethnicity.
Previous cohort and population-based studies of SLE mortality have consistently shown increased damage accrual and mortality among Black patients compared to whites[1, 7, 10, 19–21]. Although studies of Hispanic mortality rates have sometimes had conflicting results[1, 21–23], most studies suggest Hispanic ethnicity is associated with more active SLE and higher mortality. Population-based U.S. studies conducted in the 1970s and 1980s described mortality rates three to five times greater among Asian/Pacific Islanders compared to whites[24–25]. A study in the early 1990s evaluating 10,000 hospitalizations similarly showed a higher odds of mortality among hospitalized Asians compared to whites (OR 1.77, 95% CI 1.26–2.47)[26]. Other studies of Asian/Pacific Islander damage accrual and all-cause mortality in the U.S. and internationally that did not focus specifically on in-hospital mortality have had inconsistent results[1, 7, 23–28].
In this manuscript, we present results from the largest investigation of SLE hospital mortality to date. We evaluated mortality trends over the time span 2006–2016 for admissions with and without a diagnosis of SLE, and assessed risk factors we hypothesized would be associated with death. We also analyzed discrepancies in mortality risk by race/ethnicity, performing the first large-scale study in the past 20 years to evaluate in-hospital mortality for U.S. Asian/Pacific Islanders with SLE.
METHODS
Data source and population
We analyzed 2006–2016 data from the National Inpatient Sample and the Nationwide Inpatient Sample (NIS)ǂ, Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality[29–30]. In 2016, the NIS yielded annual national estimates for more than 35 million hospitalizations after weighting, representing over 97% of U.S. community hospital discharges (non-federal, general, and specialty hospitals, including public hospitals and academic medical centers). Details regarding the NIS sampling design and available data elements have been published extensively elsewhere[29–32]. In 2012, the NIS was redesigned from a sample of hospitals from which all discharges were retained, to a sample of discharges from all hospitals participating in HCUP[33]. The NIS is ideal for longitudinal analyses, and estimates over study years can be reliably calculated for the entire time of interest using discharge trend weights provided by HCUP for data prior to 2012[33–34]. The primary unit of analysis in the NIS is discharge record, and individual patients are not identifiable. Discharge diagnoses used included one primary and up to 30 secondary diagnosis codes as well as up to 15 procedure codes. International Classification of Diseases, Ninth Revision-Clinical Modification (ICD-9-CM) codes were used prior to October 2015 and ICD-10-CM codes were used thereafter. Given that this analysis uses publicly available data and contains no individual patient identifiers, it was exempt from UCSF Institutional Review Board approval.
Measures
The primary outcome in this study was all-cause mortality during hospitalization. SLE was captured in ICD-9-CM code 710.0 and ICD-10-CM codes M32.1×, M32.8, M32.9. Demographic characteristics for hospitalized individuals included age, sex, and race/ethnicity (white, Black, Asian/Pacific Islander, Hispanic, Native American, or other). Socioeconomic status was categorized based on quartiles of median household income for the patient’s ZIP Code. Primary expected payer was categorized as Medicare, Medicaid, private insurance, self-pay, or other (no charge, workers compensation, Title V and other government programs). Analyses were performed using the primary payer for each admission. Hospital census region was as defined by the U.S. Census Bureau, localizing hospitals to the Northeast, Midwest, East, or West. A Charlson Comorbidity Index score[35] was calculated with discharge diagnoses for each encounter using coding algorithms previously published[36] with minor modifications to include a small number of additional billing codes to ensure accurate index score calculations for U.S. discharges from 2006–2016 (see Supplementary Table).
Analysis
All analyses were designed and performed in adherence with the guidelines and recommendations outlined by the HCUP for appropriate use and interpretation of NIS data[29–30]. We applied discharge weights and trend weights provided by HCUP to account for the stratified sampling design of the NIS and to generate nationally representative estimates over multiple years[34]. The proportion of hospitalizations with SLE was compared between years using chi-squared tests. We compared the characteristics of hospitalized individuals with and without SLE using t-tests for continuous measures and chi-squared tests for categorical measures. We used Poisson regression with a log link and robust variance estimate to calculate the relative risk of in-hospital death associated with the following variables: demographics (age, sex, race/ethnicity), hospital region, comorbidity index score, socioeconomic factors (health insurance and income quartile for ZIP Code) and diagnosis of SLE. We fit a model to investigate possible interaction between SLE diagnosis (yes/no) and year of admission to evaluate trends in these hospitalizations over time. We also fit an expanded model to investigate possible interaction between SLE diagnosis (yes/no) and race/ethnicity. Finally, we restricted the original model to only include hospitalized SLE patients. Marginal predictions with 95% confidence intervals were calculated. The race/ethnicity variable had 13% missing values, while vital status, age, sex, primary expected payer, and income quartile by ZIP Code had between 0.06% and 2.3% missing values. In order to assess potential bias due to missing observations for these variables, we estimated regression results with and without imputation. Missing values were imputed using multiple imputation by chained equations (MICE)[37] using NIS hospital number, hospital stratum, discharge/trend weights, hospital region, SLE diagnosis, comorbidity index score, and year as predictors. The MICE method adheres to the recommendations outlined by HCUP for handling missing values[38]. We present results using imputation for missing observations. Restricting the analysis to complete case data results in no substantial change in study conclusions (results not presented). Data analysis was performed using Stata/MP 16.0 (StataCorp, College Station, TX)[39], and survey data analysis commands were used to account for the complex sampling design of the NIS. Multiple imputation was performed using the Stata mi package[40], and marginal estimates were obtained using the mimargins program[41].
RESULTS
After weighting, there were 1,903,279 adult hospitalizations with a discharge diagnosis of SLE during the studied time frame. The percent of hospitalizations containing a diagnosis code for SLE increased slightly between 2006 and 2016 from 0.5% (n=153,645) to 0.6% (n=173,749) (p-value <0.001). A description of the demographic and clinical characteristics for all hospitalized adults stratified by SLE diagnosis is provided in Table 1. Compared with the general hospitalized population, individuals with SLE were more likely to be young, female, Black or Hispanic race/ethnicity, reside in a lower income ZIP Code quartile, live in the South, and have a higher mean comorbidity index score.
Table 1.
Demographic and Clinical Characteristics of Adults, With and Without SLE, Discharged from US Community Hospitals between 2006 and 2016.
| Characteristic | SLE (n*=1,903,279) | No SLE (n*=338,563,769) | p valuea | |
|---|---|---|---|---|
| Mean Age, years (SE) | 51.4 (0.07) | 57.2 (0.08) | <0.001 | |
| Female, % | 89.0 | 59.4 | <0.001 | |
| Race, % | <0.001 | |||
| White | 53.0 | 68.9 | ||
| Black | 29.7 | 14.4 | ||
| Hispanic | 12.0 | 10.7 | ||
| Asian/Pacific Islander | 2.1 | 2.4 | ||
| Native American | 0.7 | 0.7 | ||
| Other | 2.6 | 3.0 | ||
| Primary Payer, % | <0.001 | |||
| Medicare | 47.0 | 45.5 | ||
| Private | 28.2 | 29.7 | ||
| Medicaid | 18.1 | 15.9 | ||
| Self-pay | 3.7 | 5.0 | ||
| Other | 3.0 | 3.8 | ||
| Income Quartile** | <0.001 | |||
| Quartile 1 (Low Income) | 33.7 | 29.6 | ||
| Quartile 2 | 24.8 | 26.0 | ||
| Quartile 3 | 22.6 | 23.7 | ||
| Quartile 4 | 18.9 | 20.7 | ||
| Hospital Region*** | <0.001 | |||
| Northeast | 17.7 | 19.5 | ||
| Midwest | 20.2 | 22.9 | ||
| South | 43.7 | 38.6 | ||
| West | 18.4 | 18.9 | ||
| Comorbidity Index, mean (SE) | 2.61 (0.005) | 1.54 (0.005) | <0.001 | |
National estimates were generated from raw counts using discharge and trend weights provided by HCUP. There were 69,526,462 observations for adults without SLE, and 390,360 observations for adults with SLE
Median household income in ZIP Code of residence, classified into quartiles
Hospital location by census region defined by U.S. Census Bureau
p value from t-test for continuous variables and Pearson’s chi-squared test for categorical measures
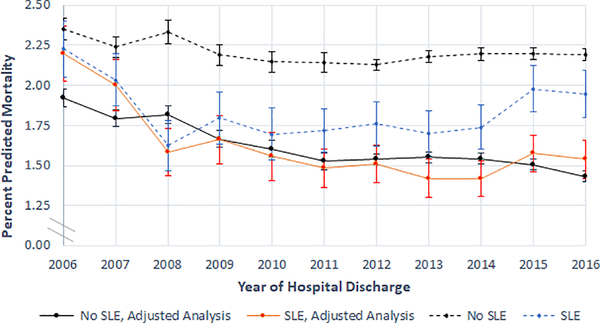
Next, we performed an analysis designed to assess time trends in hospital mortality. In unadjusted analysis, the proportion of hospitalizations for patients without SLE that ended in death slightly decreased from 2.35% (95% CI: 2.29%, 2.42%) in 2006 to 2.19% (95% CI: 2.16%, 2.23%) in 2016 (Figure 1). Among hospitalizations with a diagnosis code of SLE, in-hospital mortality decreased without statistical significance from 2.23% (95% CI: 2.05%, 2.40%) in 2006 to 1.95% (95% CI: 1.80%, 2.10%) in 2016. After including age, sex, race/ethnicity, income quartile for ZIP Code, and comorbidity index score as covariates in the regression, all cause yearly inpatient mortality decreased among admissions for individuals without SLE from 1.92% (95% CI: 1.87%, 1.98%) to 1.43% (95% CI: 1.40%, 1.46%) between 2006 and 2016. During the same time period, the risk of inpatient death decreased by 30% among hospitalizations for individuals with SLE, from 2.20% (95% CI: 2.03%, 2.37%) to 1.54% (95% CI: 1.42%, 1.66%). Nationally, there were an estimated 2,672 inpatient SLE deaths in U.S. community hospitals in 2016. This amounts to an estimated 1,147 fewer deaths in 2016 compared to what would have been expected under 2006 rates. All of the decrease in SLE in-hospital mortality occurred between 2006 and 2008, after which the mortality rate was statistically similar to that of the general hospitalized population and remained stable throughout the rest of the years analyzed.
Figure 1.
Predicted Mortality During Hospitalization Based on SLE Diagnosis, by Year of Hospitalization.
Adjusted analysis results show marginal predictions from Poisson regression model including age, sex, race/ethnicity, residence in a low income ZIP Code, comorbidity index score, primary payer, and an interaction term for SLE and time.
Vertical error bars represent 95% confidence intervals.
In an analysis designed to assess potential interactions between SLE diagnosis and race/ethnicity (Figure 2), we found that hospitalized Asians with SLE had a 43% higher risk of death compared to those without SLE (p-value<0.001), and a 79% higher risk of death compared to hospitalizations for whites with SLE (p-value<0.001). Hospitalizations for Black and Hispanic individuals with SLE were more likely to end in death compared to hospitalizations for people of the same racial/ethnic group without SLE (p-value<0.001), and compared to hospitalizations for whites with SLE (p-value<0.001). Conversely, hospitalizations among whites with SLE were less likely to end in death compared to those without SLE (p-value<0.001).
Figure 2.
Predicted In-Hospital Mortality (%) by Race and SLE Diagnosis, 2006–2016.
Marginal predictions and 95% confidence intervals from Poisson regression model including age, sex, race, income quartile by patient ZIP Code, comorbidity index score, primary payer, and an interaction term for race and SLE.
Asian/Pacific Islander abbreviated to Asian/PI in figure. Native American and Other race/ethnicity not included in the figure.
Finally, we performed an analysis designed to assess factors associated with death among hospitalizations with an SLE diagnosis. Asian/Pacific Islander race/ethnicity was associated with a 1.65 (95% CI: 1.42, 1.92) higher risk of death compared to whites (Table 2; all results from Poisson regression including covariates described above). The relative risk of in-hospital mortality was lower in the Midwest (RR 0.84; 95% CI 0.77, 0.92) compared to the Northeast. In comparison to SLE hospitalizations covered by Medicare, those not covered by insurance (self-pay) were associated with a higher risk of death (RR 1.36; 95% CI 1.18, 1.57). Older patients, men, and those with a higher comorbidity index score also had a higher risk of death during hospitalization.
Table 2.
Risk of In-Hospital Death for Adults with SLE Discharged from U.S. Community Hospitals between 2006 and 2016.
| Characteristic | RR for death (95% CI) | p value | |
|---|---|---|---|
| Age, per decade | 1.31 (1.28, 1.33) | <0.001 | |
| Female | 0.81 (0.76, 0.86) | <0.001 | |
| Race | <0.001 | ||
| White | ref | ||
| Black | 1.03 (0.97, 1.09) | ||
| Hispanic | 1.10 (1.02, 1.19) | ||
| Asian/Pacific Islander | 1.65 (1.42, 1.92) | ||
| Native American | 0.97 (0.71, 1.33) | ||
| Other | 1.36 (1.16, 1.60) | ||
| Primary Payer | <0.001 | ||
| Medicare | ref | ||
| Private | 0.99 (0.93, 1.06) | ||
| Medicaid | 1.06 (0.98, 1.15) | ||
| Self-pay | 1.36 (1.18, 1.57) | ||
| Other | 1.26 (1.08, 1.46) | ||
| Low Income* | 1.02 (0.97, 1.08) | 0.42 | |
| Hospital Region** | <0.001 | ||
| Northeast | ref | ||
| Midwest | 0.84 (0.77, 0.92) | ||
| South | 1.04 (0.96, 1.13) | ||
| West | 1.05 (0.96, 1.15) | ||
| Comorbidity Index | 1.32 (1.30, 1.33) | <0.001 |
Results from Poisson regression model controlling for survey year and including all covariates shown.
Analyses weighted to reflect the sampling design. RR=relative risk
Based on residence in a ZIP Code in the lowest quartile of median household income.
Hospital located in census region defined by U.S. Census Bureau.
DISCUSSION
We conducted the largest primary data analysis of SLE hospitalizations in the U.S. performed to date. Our results showed that in adjusted analysis, SLE hospitalizations were more likely than non-SLE hospitalizations to end in death in 2006 and 2007; the mortality rate for SLE hospitalizations decreased through 2008, after which mortality was statistically similar between the two groups. Our findings also demonstrate the continued high mortality burden of SLE among admitted Blacks, Hispanics and men and contribute data highlighting high mortality among Asian/Pacific Islanders.
Overall, our study and others conducted in prior years suggest improved in-hospital survival. An earlier publication describing a Californian cohort from 1991–1994 estimated that 5.1% of patients died during their admission[26]. In-hospital mortality was estimated at 3.1% using the NIS from 1998–2002[17]. This decreasing mortality rate parallels that found in Washington state where in-hospital mortality was estimated to decrease from 3.1% in 2003 to 1.3% by 2011[18]. Our results suggest that interventions to reduce all cause in-hospital mortality, such as programs to lessen hospital-acquired infections[42–43], and interventions primarily targeting patients with SLE, such as a broader array of immunosuppressive drugs, may have successfully improved in-hospital survival over one decade. The percent of SLE admissions only marginally increased in our study, suggesting that the findings cannot be explained by a change in admission thresholds. Furthermore, differences in coding practices over time would likely affect all hospitalized patients, highlighting once again the important strength of our study comparing SLE mortality to that of the general hospitalized population.
Despite significant advances in survival among individuals hospitalized with SLE, racial/ethnic minorities continue to have a disproportionately high risk of death. Our results demonstrate that Asians, Hispanics, and Blacks with SLE all have a higher adjusted risk of in-hospital death compared to hospitalized individuals of the same ethnic/racial group without SLE. Each of these SLE ethnic/racial minority groups also had a higher risk of in-hospital death compared to whites with SLE. We note the strikingly high mortality rate for Asians/Pacific Islanders discharged with SLE from U.S. community hospitals; these hospitalizations were associated with a 79% higher risk of death compared to hospitalizations for whites, and a 43% higher risk of death compared to hospitalizations for Asians/Pacific Islanders without SLE. Since our unit of analysis is hospital discharges, it could be possible that Asian/Pacific Islanders have unmeasured factors which make them less likely than other race/ethnicity groups to present to the hospital except when severely ill. This explanation however would not account for the clinically important difference in mortality we report between hospitalized SLE and non-SLE patients of Asian/Pacific Islander race/ethnicity, nor the results of smaller prior studies using individual patients as the unit of analysis which similarly reported high Asian mortality rates compared to whites[24–26]. Future studies should further investigate potential reasons for persistent inequalities in outcomes among these minority groups.
Male sex was also associated with a higher risk of SLE in-hospital mortality in our study. These findings are similar to prior analyses in hospitalized patients showing worse outcomes in men[17–18, 26]. Although SLE is more common in females, males may have faster disease progression and damage accrual[1, 44–45]. As expected, increasing age and higher comorbidity index score were associated with higher mortality among patients hospitalized with SLE. Self-pay status, which reflects a lack of insurance, was also associated with higher mortality. Future work could investigate whether self-pay SLE patients receive a different quality of inpatient care as a result of monetary constraints, whether likely limited primary preventive care may be driving differences in outcomes, or whether higher inpatient mortality per admission is a reflection of a higher threshold of illness severity to present for medical evaluation compared to individuals with insurance.
One potential limitation of this study is that we rely on ICD-9-CM and ICD-10-CM codes for SLE to be included in the discharge summary from the treating physician. Prior studies evaluating the accuracy of administrative data diagnoses using ICD-9-CM coding for SLE have suggested sensitivities of 67–98% and specificities of 72–90%[46–47]. The Charlson Comorbidity Index using the Quan coding algorithms has been validated as an acceptable predictor of in-hospital mortality (AUC 0.76–0.87)[36,48]. Another potential limitation of the study is the 13% missing race values in the NIS, which are known to not be missing completely at random. In order to decrease the risk for bias, we performed multiple imputation by chained equations according to the recommendations from HCUP[38]. Additionally, the NIS sampling scheme was redesigned in 2012. We have fully addressed this change in design using trend weights specifically developed by the NIS to allow for analysis over time[33–34]. There was no significant change in mortality among either SLE patients or the general adult population in our study between 2011–2012 when a change in mortality could potentially be expected to have been due to changes in survey design.
In summary, our results demonstrate a decrease in adjusted all-cause mortality among SLE hospitalizations in the U.S. to levels similar to that of the general hospitalized population. We show that mortality was relatively stable for both SLE and non-SLE admissions from 2008–2016, reflecting a shift from previous decades when inpatient mortality levels were decreasing over time. Despite advances in overall all-cause mortality among all patients with SLE, hospitalized ethnic and racial minorities including Asians/Pacific Islanders, Hispanics, and Blacks have a higher risk of inpatient death compared to both patients without SLE and to whites. Our study results demonstrate the importance of considering U.S. Asian/Pacific Islanders with SLE as at potentially heightened risk for poor outcomes, and therefore warranting special clinical attention and inclusion in future research studies. Comprehensive efforts addressing differences in disease severity, access to healthcare, and social determinants of health are likely necessary to narrow disparities in hospital mortality among men and racial/ethnic minorities with SLE.
Supplementary Material
Significance and Innovations.
To our knowledge, this is the largest ever performed primary analysis of hospitalizations for individuals with SLE, and the first large-scale population-based study in over 20 years to evaluate U.S. Asian/Pacific Islander in-hospital mortality among individuals with SLE.
In-hospital survival for individuals with SLE improved from 2006 through 2008, and then plateaued.
Hospitalizations for Asian/Pacific Islanders, Hispanics, and Blacks with SLE had a higher risk of ending in death compared to hospitalizations for whites.
Acknowledgments
Christine Anastasiou was supported by NIAMS of the National Institutes of Health under award 5T32AR007304-40 and the NIA of the National Institutes of Health under award 5T32AG049663-04. Milena Gianfrancesco is supported by NIH/NIAMS grant K01AR075085. Gabriela Schmajuk is supported by the Russell/Engleman Medical Research Center for Arthritis. Jinoos Yazdany is supported by the Robert L Kroc Chair in Rheumatic and Connective Tissue Diseases, NIH/NIAMS P30 AR070155, K24AR074534 and the Russell/Engleman Medical Research Center for Arthritis. Jinoos Yazdany reports grants from Pfizer, personal fees from Astra Zeneca and personal fees from Eli Lilly outside the submitted work.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The Nationwide Inpatient Sample was renamed to the National Inpatient Sample in 2012 at the time of the sampling redesign.
REFERENCES
- 1.Bruce IN, O’Keeffe AG, Farewell V, Hanly JG, Manzi S, Su L, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis 2015;74(9):1706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alarcón GS, McGwin G Jr, Bastian HM, Roseman J, Lisse J, Fessler BJ, et al. Systemic lupus erythematosus in three ethnic groups. VIII. Predictors of early mortality in the LUMINA cohort. Arthritis Rheum 2001;45(2):191–202. [DOI] [PubMed] [Google Scholar]
- 3.Yazdany J, Marafino BJ, Dean ML, Bardach NS, Duseja R, Ward MM, et al. Thirty-day Hospital Readmissions in Systemic Lupus Erythematosus: Predictors and Hospital and State-level Variation. Arthritis Rheumatol 2014;66(10): 2828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elixhauser A, Steiner C. Readmissions to U.S. Hospitals by Diagnosis, 2010: Statistical Brief #153. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Policy and Research. [Google Scholar]
- 5.Merrell M, Shulman LE. Determination of prognosis in chronic disease, illustrated by systemic lupus erythematosus. J Chron Dis 1955;1(1):12–32. [DOI] [PubMed] [Google Scholar]
- 6.Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 2003;82(5):299–308. [DOI] [PubMed] [Google Scholar]
- 7.Yen EY, Shaheen M, Woo JMP, Mercer N, Li N, McCurdy DK, et al. 46-Year Trends in Systemic Lupus Erythematosus Mortality in the United States, 1968 to 2013: A Nationwide Population-Based Study. Ann Intern Med 2017;167(11):777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uramoto KM, Michet CJ, Thumboo J, Sunku J, O’Fallon WM, Gabriel SE. Trends in the Incidence and Mortality of Systemic Lupus Erythematosus, 1950–1992. Arthritis Rheum 1999;42(1):46–50. [DOI] [PubMed] [Google Scholar]
- 9.Urowitz MB, Gladman DD, Tom BD, Ibañez D, Farewell VT. Changing patterns in mortality and disease outcomes for patients with systemic lupus erythematosus. J Rheumatol 2008;35(11):2152–58. [DOI] [PubMed] [Google Scholar]
- 10.Bernatsky S, Boivin JF, Joseph L, Manzi S, Ginzler E, Gladman DD, et al. Mortality in Systemic Lupus Erythematosus. Arthritis Rheum 2006;54(8):2550–57. [DOI] [PubMed] [Google Scholar]
- 11.Lee J, Dhillon N, Pope J. All-cause hospitalizations in systemic lupus erythematosus from a large Canadian referral centre. Rheumatology (Oxford) 2013;52(5):905–9. [DOI] [PubMed] [Google Scholar]
- 12.Anastasiou C, Dulai O, Baskaran A, Proudfoot J, Verhaegen S, Kalunian K. Immunosuppressant use and hospitalisations in adult patients with systemic lupus erythematosus admitted to a tertiary academic medical centre. Lupus Sci Med 2018;5(1):e000249. doi: 10.1136/lupus-2017-000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan K, Dekis A, Clarke AE, Pineau CA, Vinet E, Nashi E, et al. Hospitalizations in patients with systemic lupus erythematosus: updated analyses from 2006 to 2011. Arthritis Res Ther 2012;14(Suppl 3):A59. [Google Scholar]
- 14.Rosa GPD, Ortega MF, Teixeira A, Espinosa G, Cervera R. Causes and factors related to hospitalizations in patients with systemic lupus erythematosus: analysis of a 20-year period (1995–2015) from a single referral centre in Catalonia. Lupus 2019;28(9):1158–66. [DOI] [PubMed] [Google Scholar]
- 15.Gu K, Gladman DD, Su J, Urowitz MB. Hospitalizations in Patients with Systemic Lupus Erythematosus in an Academic Health Science Center. J Rheumatol 2017;44(8):1173–78. [DOI] [PubMed] [Google Scholar]
- 16.End of Life Care. Dartmouth Atlas Project. https://www.dartmouthatlas.org/interactive-apps/end-of-life-care/ (accessed 11 Oct 2019). [Google Scholar]
- 17.Krishnan E. Hospitalization and mortality of patients with systemic lupus erythematosus. J Rheumatol 2006;33(9):1770–74. [PubMed] [Google Scholar]
- 18.Goss LB, Ortiz JR, Okamura DM, Hayward K, Goss CH. Significant Reductions in Mortality in Hospitalized Patients with Systemic Lupus Erythematosus in Washington State from 2003 to 2011. PLoS ONE 10(6):e0128920. doi: 10.1371/journal.pone.0128920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasitanon N, Magder LS, Petri M. Predictors of survival in systemic lupus erythematosus. Medicine (Baltimore) 2006;85(3):147–56. [DOI] [PubMed] [Google Scholar]
- 20.Lim SS, Helmick CG, Bao G, Hootman J, Bayakly R, Gordon C, et al. Racial Disparities in Mortality Associated with Systemic Lupus Erythematosus — Fulton and DeKalb Counties, Georgia, 2002–2016. MMWR Morb Mortal Wkly Rep 2019;68(18):419–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alarcón GS, Friedman AW, Straaton KV, Moulds JM, Lisse J, Bastian HM, et al. Systemic lupus erythematosus in three ethnic groups: III. A comparison of characteristics early in the natural history of the LUMINA cohort. LUpus in MInority populations: NAture vs. Nurture. Lupus 1999;8(3):197–209. [DOI] [PubMed] [Google Scholar]
- 22.Walsh SJ, DeChello LM. Geographical variation in mortality from systemic lupus erythematosus in the United States. Lupus 2001;10(9):637–46. [DOI] [PubMed] [Google Scholar]
- 23.Gómez-Puerta JA, Barbhaiya M, Guan H, Feldman CH, Alarcón GS, Costenbader KH. Racial/Ethnic Variation In All-Cause Mortality Among United States Medicaid Recipients With Systemic Lupus Erythematosus: A Hispanic And Asian Paradox. Arthritis Rheumatol 2015;67(3):752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaslow RA. High rate of death caused by systemic lupus erythematosus among U. S. residents of Asian descent. Arthritis Rheum 1982;25(4):414–18. [DOI] [PubMed] [Google Scholar]
- 25.Serdula MK, Rhoads GG. Frequency of systemic lupus erythematosus in different ethnic groups in Hawaii. Arthritis Rheum 1979; 22:328–33. [DOI] [PubMed] [Google Scholar]
- 26.Ward MM. Hospital Experience and Mortality in Patients with Systemic Lupus Erythematosus. Arthritis Rheum 1999;42(5):891–98. [DOI] [PubMed] [Google Scholar]
- 27.Jakes RW, Bae S, Louthrenoo W, Mok CC, Navarra SV, Kwon N. Systematic review of the epidemiology of systemic lupus erythematosus in the Asia-Pacific region: prevalence, incidence, clinical features, and mortality. Arthritis Care Res (Hoboken) 2012;64(2):159–68. [DOI] [PubMed] [Google Scholar]
- 28.Lee YH, Choi SJ, Ji JD, Song GG. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus 2016;25(7):727–34. [DOI] [PubMed] [Google Scholar]
- 29.HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP) 2006–2011. Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/nisoverview.jsp (accessed 13 Oct 2019). [Google Scholar]
- 30.HCUP National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP) 2012–2016. Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/nisoverview.jsp (accessed 13 Oct 2019). [Google Scholar]
- 31.Rubin DS, Matsumoto MM, Moss HE, Joslin CE, Tung A, Roth S. Ischemic Optic Neuropathy in Cardiac Surgery: Incidence and Risk Factors in the United States from the National Inpatient Sample 1998 to 2013. Anesthesiology 2017;126(5):810–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burton BN, Jafari A, Asmerom B, Swisher MW, Gabriel RA, DeConde A. Inpatient Mortality After Endoscopic Sinus Surgery for Invasive Fungal Rhinosinusitis. Ann Otol Rhinol Laryngol 2019;128(4):300–08. [DOI] [PubMed] [Google Scholar]
- 33.Houchens RL, Ross DN, Elixhauser A, Jiang J. Nationwide Inpatient Sample Redesign Final Report. 2014. HCUP NIS Related Reports ONLINE. April 4 2014. U.S. Agency for Healthcare Research and Quality. https://www.hcup-us.ahrq.gov/db/nation/nis/reports/NISRedesignFinalReport040914.pdf (accessed 13 Oct 2019). [Google Scholar]
- 34.Trend Weights for 1993–2011 HCUP NIS Data. Healthcare Cost and Utilization Project (HCUP). 2015. Agency for Healthcare Research and Quality, Rockville, MD. https://www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp (accessed 13 Oct 2019). [Google Scholar]
- 35.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 36.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Med Care 2005;43(11):1130–39. [DOI] [PubMed] [Google Scholar]
- 37.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011;30(4):377–99. [DOI] [PubMed] [Google Scholar]
- 38.Houchens R. Missing Data Methods for the NIS and the SID. 2015. HCUP Methods Series Report # 2015–01 ONLINE. January 22, 2015. U.S. Agency for Healthcare Research and Quality. https://www.hcup-us.ahrq.gov/reports/methods/2015_01.pdf (accessed 13 Oct 2019). [Google Scholar]
- 39.StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC. [Google Scholar]
- 40.Stata Corporation. (2019). Stata Multiple Imputation Reference Manual Release 16. College Station, TX: Stata Press Publication. [Google Scholar]
- 41.Stata FAQ: How can I get margins for a multiply imputed survey logit model? UCLA: Statistical Consulting Group. https://stats.idre.ucla.edu/stata/faq/how-can-i-get-margins-for-a-multiply-imputed-survey-logit-model/ (accessed 19 Oct 2019). [Google Scholar]
- 42.Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of Simulation-Based Education to Reduce Catheter-Related Bloodstream Infections. Arch Intern Med 2009;169(15):1420–23. [DOI] [PubMed] [Google Scholar]
- 43.Weinstein RA, Milstone AM, Passaretti CL, Perl TM. Chlorhexidine: Expanding the Armamentarium for Infection Control and Prevention. Clin Infect Dis 2008:46(2):274–81. [DOI] [PubMed] [Google Scholar]
- 44.Andrade RM, Alarcón GS, Fernández M, Apte M, Vilá LM, Reveille JD, et al. Accelerated damage accrual among men with systemic lupus erythematosus: XLIV. Results from a multiethnic US cohort. Arthritis Rheum 2007;56(2):622–30. [DOI] [PubMed] [Google Scholar]
- 45.Schwartzman-Morris J, Putterman C. Gender Differences in the Pathogenesis and Outcome of Lupus and of Lupus Nephritis. Clin Dev Immunol 2012;2012:604892. doi: 10.1155/2012/604892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernatsky S, Linehan T, Hanly JG. The Accuracy of Administrative Data Diagnoses of Systemic Autoimmune Rheumatic Diseases. J Rheumatol 2011;38(8);1612–16. [DOI] [PubMed] [Google Scholar]
- 47.Lim SS, Jamal A, Bayakly R, Tong L, Drenkard C. Georgia Lupus Registry-accuracy of hospital discharge data in identifying systemic lupus erythematosus. Arthritis Rheum 2007;54(Suppl:S505). [Google Scholar]
- 48.Toson B, Harvey LA, Close JC. The ICD-10 Charlson Comorbidity Index predicted mortality but not resource utilization following hip fracture. J Clin Epidemiol 2015;68(1):44–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.