Summary
Candida albicans is a regular member of the intestinal microbiota in the majority of the human population. This underscores C. albicans’ adaptation to life in the intestine without inducing competitive interactions with other microbes or immune responses detrimental to its survival. However, specific conditions such as a dysbalanced microbiome, a suppression of the immune system, and an impaired intestinal barrier can predispose for invasive, mostly nosocomial C. albicans infections. Colonization of the intestine and translocation through the intestinal barrier are fundamental aspects of the processes preceding life-threatening systemic candidiasis. Insights into C. albicans’ commensal lifestyle and translocation can thus help us to understand how patients develop candidiasis and may provide leads for therapeutic strategies aimed at preventing infection. In this review, we discuss the commensal lifestyle of C. albicans in the intestine, the role of morphology for commensalism, the influence of diet, and the interactions with bacteria of the microbiota.
Keywords: Candida albicans, commensal, opportunistic pathogen, colonization, commensalism, pathogenicity nosocomial infections, candidiasis, intestinal microbiota, intestinal barrier, diet, antibiotics, morphology, invasion, translocation
Introduction
Invasive infections with Candida species are by far the most common cause of nosocomial fungal infections threatening patients of intensive care units (ICUs) and immunocompromised patients or patients with dysfunctional epithelial barriers [1,2]. Most human pathogenic fungi of the genus Candida are not ubiquitously present in the environment like, for example, Aspergillus, or Cryptococcus species, but associated with animals and humans. Systemic candidiasis of humans, therefore, originates from commensal body niches. In Westernized countries, most individuals carry Candida species as commensals in their intestinal microbiota [3,4] and patients show enrichment of Candida species in the majority of cases during prolonged stays in the ICU [5] or following transplantation [6]. Abdominal surgery or pathology to organs of the gastrointestinal (GI) tract is a risk factor for the development of intraabdominal candidiasis [7]. With regard to candidemia, or Candida bloodstream infections, Candida cells can be introduced directly into the bloodstream by contaminated central venous catheters. However, such infections are more commonly associated with C. parapsilosis, which, in contrast to other Candida species, colonizes the skin [8,9]. The evidence is overwhelming that C. albicans in the gastrointestinal (GI) tract is a major source of systemic candidiasis [6,8,10] and numerous experimental models have revealed that this fungus can enter the bloodstream by translocating through the intestinal barrier [11–13]. Therefore, it is essential to comprehend the pathogenesis of systemic candidiasis originating from the intestinal tract. Elucidating C. albicans’ role as a commensal, as well as a pathogen, can help us understand how this otherwise harmless commensal can undergo a shift towards an opportunistic pathogen. Further, it may aid to identify predisposing factors and to provide valuable insights for the development of strategies aimed at preventing candidiasis, an approach that will have a drastic impact on candidiasis-associated mortality [14].
C. albicans as a commensal of the gut
Even though a great deal is known about C. albicans pathogenicity [15], studies dealing with the commensal lifestyle of this fungus have only recently come into focus. [for further recent reviews see: 16,17–20].
As the GI of the majority of humans in Westernized societies is colonized by C. albicans [3,4] it can be concluded that this fungus has evolved to interact and compete with numerous bacterial cells and species that colonize this niche. However, as demonstrated in mouse models, the composition of the microbiota is critical for successful establishment, and some bacterial consortia can inhibit or prevent C. albicans colonization. In fact, C. albicans is not a normal colonizer of many mice strains and an intact murine bacterial microbiota can resist the ability of C. albicans to become established as a member of the community [21,22], whereas mice treated with antibiotics, in particular antibiotics targeting anaerobic bacteria [12,16,23], and germ-free mice [24] can be colonized by C. albicans yeast with ease. Colonization resistance can be accomplished by modifying the metabolite milieu in a way that diminishes fungal growth [25]. Additionally, commensal bacteria can stimulate the immune response, increasing the production of the antimicrobial peptide LL-37, which can contribute to colonization resistance [21]. On the other hand, colonization can be promoted by a specific diet (see below).
As C. albicans can colonize the majority of humans, colonization resistance is often considered only an aspect of adult murine models. It seems clear, however, that, in humans, the bacterial microbiome plays an essential role in controlling the level of C. albicans colonization since the use of broad-spectrum antibiotics is recognized as a major risk factor for candidemia [1,2]. Hematopoietic stem-cell transplant recipients treated with antibiotics targeting anaerobic bacteria show enrichment of Candida species in the intestinal fungal microbiome (the mycobiome) [6]. Similar enrichment of C. albicans (or C. glabrata) is observed in ICU patients treated with antibiotics leading to ultra-low-diversity microbiomes [5]. Overall these findings highlight a role for the microbiota in suppressing C. albicans overgrowth and pathogenicity in experimental models as well as patient cohorts. In contrast, C. albicans colonization itself can have an influence on the intestinal micro- and mycobiome [18] and can promote bacterial dysbiosis that promotes invasive infection [26]. Only recently has the role of the mycobiome come into focus [6,27–29], and it has been shown to play crucial roles in human diseases [30–32] (Reviewed in this issue by Hohl and colleagues).
Studies of direct interactions under laboratory conditions between bacterial species and C. albicans show that probiotic bacteria can antagonize the growth and pathogenicity of C. albicans. For example, a probiotic cocktail was able to reduce polymicrobial biofilm formation by C. albicans in combination with Escherichia coli and Serratia marcescens [33]. Bacterial metabolites such as fatty acids can inhibit C. albicans hyphal formation [34] and Lactobacillus rhamnosus reduces C. albicans overgrowth and prevents contact of the fungus with the intestinal barrier through shedding [35]. In models lacking bacteria, host epithelial cells provide some resistance to C. albicans [36]. However, C. albicans employs various strategies to successfully invade and translocate through the epithelial barrier [Reviewed in 37], unless it is genetically defective for these attributes [38]. The presence of only a single antagonistic bacterial species, e.g. L. rhamnosus, suppressed C. albicans to such an extent that reduced translocation through the epithelial barrier was observed in an in vitro intestine-on-chip model [39].
Influence of diet
The composition and the metabolism of our intestinal microbiota are highly influenced by diet. It is presumed that diet also influences C. albicans colonization of the gut. A Western diet, together with the use of antibiotics, has been posited as one of the reasons that the majority of the Western population is colonized by C. albicans [16]. Non-Western societies, for example, can exhibit much lower colonization rates as compared to many Western countries [18,40]. In murine models, a diet that does not support growth and effector functions of antagonistic bacteria like Lactobacillus species can be used to achieve colonization, which can lead to translocation under immunosuppressive treatment [11]. Of note, these diets consist of cornstarch, sucrose, and soybean oil, similar to a Western diet. Inclusion of fatty acid-rich coconut oil, however, reduces C. albicans colonization, even when other nutritional sources that support colonization are present [41]
Metabolic Adaptation to the GI tract
Niche-specific attributes, such as adaptation for effectively utilizing the nutrients that are present, make C. albicans highly efficient in colonizing the GI tract. Glucose levels in the GI tract, particularly the distal GI tract, are low and colonizers are thus obliged to utilize alternative carbon sources [42]. C. albicans has noted nutritional flexibility allowing it to adapt readily to the nutritional milieu that it encounters [43,44]. The organism is capable of simultaneously using different carbon sources and can continue to utilize lactate even when provided with glucose (in contrast to the baker’s yeast Saccharomyces cerevisiae). This nutritional flexibility is essential for colonization of the GI tract and a mutant unable to grow on less preferred carbon sources such as glycerol, citrate or lactate is out-competed by wild type cells in the GI tract [45].
As nutrient sources in the intestine are limited, tight regulation of nutrient acquisition pathways yields a fitness benefit by ensuring that genes are expressed only when absolutely necessary. N-acetyl glucosamine was postulated as a limiting nutrient that regulates nutrient sensing in the GI tract [46]. C. albicans undergoes “sugar-induced cell death” when grown with GlcNAc and no other nutrients but not when grown with glucose alone, consistent with the notion that GlcNAc is an important signal and a preferred carbon source [46].
Of note, nutrition sources affect stress resistance in C. albicans. A mutant lacking the transcription factor Rtg3 was found to exhibit hyper-susceptibility to cationic, oxidative and nitrosative stresses when grown in lactate but not glucose [42]; interestingly, Rtg3 is required for colonization of the murine GI tract [47] and plays a role in galactose [48] and sphingolipid metabolism [49].
The picture that emerges is that C. albicans is well adapted to growth without glucose. Remarkably, changing environmental conditions such as the availability of carbon sources (e.g. lactate instead of glucose) impacts fungal adaptation, which can influence colonization and pathogenicity [50]. For example, modification of cell wall architecture can either lead to immune evasion or hyper-activation of the host response [51] and altered cell wall morphology significantly modulates colonization in murine models [52].
Other key nutrients also affect the ability of C. albicans to colonize. For example, although the bioavailability of iron in the GI tract is higher than in tissue, Mamouei et al. showed that a mutant defective in iron permeases (ftr1Δ ftr2 Δ double null mutant) was outcompeted by wild type cells in a competitive GI colonization study [53] indicating that iron uptake is important in the gut. Further, C. albicans cells modify the GI tract metabolite milieu, for example, by producing the prostaglandin PGE2 from host-derived arachidonic acid. PGE2 production may change the environment of the GI tract, providing conditions that are more conducive for C. albicans colonization [54].
Further metabolites such as bile acids also have a significant impact on C. albicans’ behavior and, therefore, can influence colonization and/or infection. Secondary bile-acids such as lithocholic acid and deoxycholic acid have direct antifungal activity by limiting growth, filamentation, and adhesion to host cells [55], aspects that may contribute to the commensal lifestyle of C. albicans. However, antibiotic treatment prevents the conversion of primary into secondary bile acids by commensal bacteria. The consequent accumulation of primary bile acids such as taurocholic acid can increase C. albicans outgrowth [56], and stimulate filamentation [56,57].
In general, adaptation to gut metabolites and other specific conditions in the gut, such as hypoxia and diverse pH values, requires sophisticated regulatory networks, some of which may also contribute to pathogenicity [47,50,58].
Phenotypic adaptation to the GI tract
Invasive candidiasis is often associated with filamentous growth, which facilitates the invasion of host tissues [59].
Despite being essential for invasive disease, evidence is mounting that hyphae and expression of hyphal-associated proteins are detrimental for the commensal lifestyle of C. albicans [60–63]. Even though it was revealed that hyphal morphology is abundant during colonization of the intestine and is not associated solely with invasive disease, knockout of the hyphal-specific transcription factor Ume6 or the hyphal-associated factors Sap6 or Hyr1 is beneficial for colonisation [61]. In competition colonization experiments, strains adapted to the GI tract through long-term colonization easily outcompeted strains that were expanded in vitro or short-term colonizing strains [64], illustrating the flexibility to adapt to a commensal lifestyle. In fact, long-term experimental colonisation of the gut poses a selective pressure towards spontaneously mutated strains incapable of forming hyphae [60].
Since hyphae and their associated proteins seem detrimental to commensalism, it seems conceivable that the intestinal environment during homeostasis suppress filamentation. In addition to previously discussed bile acids that suppress filamentation, the hypoxic environment of the intestine has been postulated to play a role in supporting the restriction of hyphal morphogenesis detrimental to commensalism through modulation of Efg1 [65]. Furthermore, the expression of Efg1, which is a crucial determinant for commensalism, relies on the immune status of the host [62].
Finally, although hyphal formation does not seem to support GI colonization, the ability to form hypha is conserved among the vast majority of clinical isolates suggesting an evolutionary pressure to maintain this key attribute of C. albicans [60]. In this context, it should be noted that knockout of hyphal associated genes, which increases colonization fitness (above), results in a loss of filamentation in vitro, but not in vivo in the intestine [61].
C. albicans also forms a specialized cell type termed “GUT” cells during colonization [66]. GUT cells can be mimicked genetically and, during GI colonization, GUT-mimetic cells exhibit a transient decrease in competitive fitness versus WT followed by a transient enhancement [67]. GUT-mimetic cells thus have a competitive advantage once a proinflammatory host immune response against C. albicans has been initiated (>1 week of colonization). GUT-mimetic cells exhibit several differences from WT cells, including increased ability to adhere to the GI mucosa ex vivo, increased susceptibility to bile acids and altered respiratory metabolism. These differences may contribute to their altered fitness in the GI tract.
The role of different yeast morphotypes in the human host is further reviewed in [59]
The benefit of Candida albicans as a commensal for human health
From an evolutionary perspective, it is tempting to speculate that C. albicans may have some beneficial effects for human health since carriage of a potentially dangerous commensal might otherwise be subjected to negative natural selection. It is generally believed that C. albicans colonization may be crucial to educate our immune system. C. albicans colonization of the GI tract of mice, specifically with strains evolved to colonize the intestine, offer immunological protection against systemic infection [60]. As even Rag1−/− mice, lacking functional T and B cells could mount protective immunity [60], the intestinal colonization is believed to induce an innate immune memory called trained immunity. Yet intestinal C. albicans colonization is also instrumental in shaping the T-cell responses (Reviewed in this issue by Bacher and colleagues). Specifically, Th17 responses, which are essential in driving mucosal immunity, are polarized by C. albicans colonization and offer protection against systemic candidiasis [68]. Potentially, the evolutionary benefit of harboring C. albicans as a commensal lies not only in its ability to train the immune system against systemic candidiasis, but also to infections with other pathogens such as Staphylococcus aureus, [60,68], Aspergillus fumigatus, or Pseudomonas aeruginosa [60]. However, despite being able to protect against systemic infection, the Th17 polarization induced by intestinal C. albicans commensalism can aggravate airway inflammatory diseases [68,69].
Apart from educating our immune system, another evolutionary advantage of C. albicans colonization may be competition with other pathogens or an influence on the balance of the intestinal microbiota. In fact, targeting intestinal Candida spp. with antifungal drugs can result in a mycobiota dysbiosis that can drive the development of allergic airway disease [32] via activation of gut resident CX3CR1 mononuclear phagocytes [70]. The effect of C. albicans on Clostridium difficile infection (CDI) is an interesting example of an interaction of C. albicans with another opportunistic pathogen. C. albicans can support the survival of C. difficile under aerobic conditions, while C. difficile inhibits C. albicans filamentous growth [71], indicating a direct interaction between these two opportunistic pathogens. In murine models of CDI, administration of C. albicans immediately before challenge with C. difficile resulted in increased disease [72]. In contrast, administering C. albicans to mice and allowing the establishment of colonization and a return to homeostasis in the GI tract before C. difficile challenge, resulted in protection against lethal CDI [73]. These findings may reflect the fact that C. albicans colonization has anti-inflammatory effects under some conditions, but when inflammation is pre-existing, the introduction of C. albicans enhances inflammation [74]. The importance of the timing of colonization with C. albicans versus with C. difficile may contribute to differences observed in human studies; only some studies observed low levels of C. albicans colonization in CDI patients (reviewed in [75]). Another report has shown that C. albicans can reduce Pseudomonas aeruginosa pathogenicity [76].
Although C. albicans can overgrow in the intestine during antibiotic treatment as a result of the absence of antagonizing bacteria, C. albicans can actually promote increased bacterial diversity during recovery from antibiotic treatment [77]. C. albicans colonization following antibiotics supports recovery of Bacteroidetes, and promotes Enterococcus faecalis, but reduces lactobacilli in the long-term. Similarly, a high intestinal C. albicans burden before fecal microbiota transplantation (FMT) is also associated with increased bacterial diversity following FMT in ulcerative colitis patients [78]. Yet, C. albicans high levels of colonization post transplantation highlight negative impact outcome of FMT [79][78].
Overall this illustrates that C. albicans as a commensal is engaged in an intensive fungal-host-microbiota cross-talk that shapes immune responses and microbiota composition. Whether this cross-talk contributes to health or disease often is specific to conditions of each individual host.
Breaching the boundary: invasion of the epithelial barrier leading to systemic disease
As discussed above, a disturbed microbiota, a suppressed immune system, and/or a defective epithelial barrier can all promote C. albicans translocation through the epithelia barrier into the bloodstream. Uncovering the fungal and host aspects which influence epithelial barrier invasion and translocation may help to answer the question of why C. albicans originating from the gut can cause systemic disease under predisposing factors.
Dissection of C. albicans translocation revealed filamentous growth, damage capacity, and decreasing barrier integrity as crucial as translocation occurs via a transcellular pathway, associated with necrotic cell death [38]. Consequently, mutants incapable of adhesion, filamentation, or inflicting damage are severely impaired in translocation [38]. Of note, attenuated growth without impaired pathogenicity mechanisms does not necessarily reduce translocation. Further, filamentation per se is not sufficient for full translocation potential.
Similar to some intestinal bacteria, distinct intestinal epithelial cell types are important for C. albicans translocation. For example, intestinal epithelial models containing M-cells revealed that C. albicans preferentially interacts with and invades the intestinal barrier via M-cells [80]. As M-cells are part of the Peyers Patches, translocation via M-cells may also facilitate immune recognition. Other intestinal epithelial characteristics aid in resisting translocation. Mucins suppress C. albicans virulence [81]. Consequently, integration of mucus-producing goblet cells in intestinal epithelial infection models suppresses translocation [35]. During infection, intestinal epithelial cells respond by inducing NFκB signaling. Once activated, the NFκB signaling pathway induces resistance against C. albicans-induced cell damage, and its neutralization renders epithelial cells more susceptible to damage [36].
In the intestine, resident immune cells provide another layer of defense against C. albicans invasion and translocation. CX3CR1 positive phagocytes, for example, are highly efficient at recognizing fungi and initiating antifungal host defense [82].
Additional mechanisms that allow the intestinal epithelium to resist C. albicans invasion are reviewed in [17,37]. Importantly it remains to be elucidated how C. albicans initiates translocation. For example, does the fungus invade as a single hypha, or does it first form micro-colonies on the epithelium and then invade from the colony? Near-physiological modeling of C. albicans translocation using organ-on-chip technology [39] may be able to provide insights here.
Conclusion
The ability of C. albicans to colonize the human gut as a harmless commensal is also a vital aspect of its role as an opportunistic pathogen. Apart from other Candida species few pathogens exhibit similar strategies of causing infections in predisposed patients. A pressing question is whether or not in a healthy host C. albicans always tries to invade, but the absence of predisposing conditions does not permit invasion. Considering the currently available literature on C. albicans commensalism and pathogenicity summarized in this review (Fig. 1 and 2), several possibilities that drive the commensal-to-pathogenic shift can be considered. I) A commensal-to-pathogen shift is actively suppressed by the antagonistic bacterial microbiota and their metabolites, the intestinal barrier, and the host immune system. This model explains why C. albicans in simple model systems lacking these suppressing factors behaves like a vicious pathogen and invades and damages host cells. II) The combination of predisposing factors alters the chemical, physiological and nutritional intestinal environment to stimulate the commensal-to-pathogen shift, driving the fungus to engage the host actively. Deprivation of specific nutrients during dysbiosis could also be considered as a driving force of pathogenicity. III) The fungus actively supports a commensal growth mode and suppresses hyphal formation and thus invasion because it is detrimental in the gut as it causes inflammation leading to clearance. Studies of gene expression in C. albicans cells colonizing hosts of differing immune status support this possibility. Yet, hyphal formation must be beneficial at some stages of a commensal lifestyle or for survival in general. Possibly, “near-pathogenic” behavior is essential for commensalism and is tipped to “full pathogenicity” when predisposition occurs.
Fig. 1:
The key mechanisms preventing a commensal-to-pathogen shift of C. albicans.
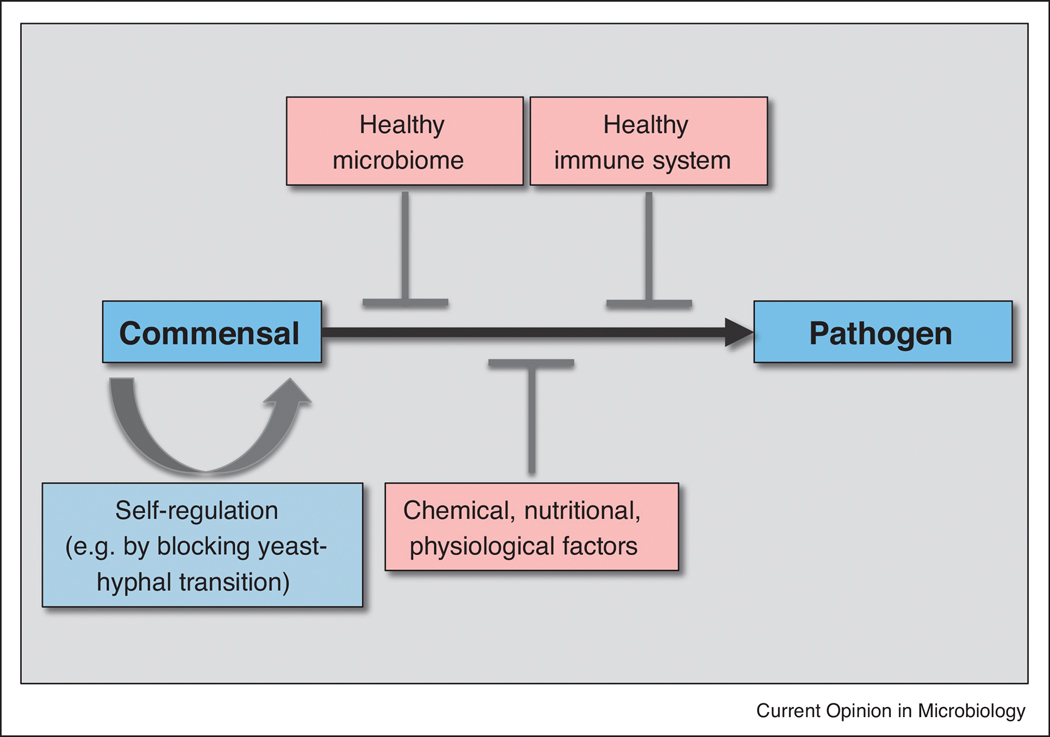
Fig. 2:

Mechanisms which contribute to a commensal versus pathogenic life style of C. albicans. A healthy diet feeds a healthy microbiome, which antagonizes colonization and hyphal formation of C. albicans via modulation of chemical, nutritional and physiological conditions. However, C. albicans cells also seem to self-regulate a commensal growth (via the “GUT” phenotype or transcriptional down-regulation of hyphal growth) and some gut niches may be colonized by hypha, which potentially show a near-pathogenic behaviour. Mucus production inhibits hyphal formation and keeps the fungus in distance from epithelial cells, which show resistance to occasionally invading hypha, supported by a healthy immune response.
In contrast, treatment with antibiotics and a Western diet can cause a dysbiosis of the microbiome and fungal overgrowth. This normally does not lead to fungal pathogenicity in the gut and the microbiome will return to a balanced state, for example after antibiotic therapy is terminated. However, overgrowth may favour the formation of invasive hypha and further predisposing conditions may lead to translocation through the epithelial barrier. This often includes a lack of epithelial fitness and proliferation as a result of cytostatic therapy or physical damage of the epithelial barrier. Further, a compromised status of the innate immune system can cause a failure of the final line of resistance against invasion into the bloodstream and dissemination to vital organs.
The complexity and multifactorial aspect of interactions between the fungus, host, and microbiota makes it difficult (at this stage of our knowledge) to comprehend the commensal-to-pathogen shift. Nevertheless, significant progress in understanding the two different lifestyles of C. albicans, commensalism and pathogenicity, has been made by a combination of experimental and clinical research and observations, and improved methodology to study microbial, metabolic, and immune interactions. Hopefully, this increased understanding can foster the development of strategies aimed at preventing candidiasis from an intestinal origin.
Acknowledgements
We acknowledge all contributions to the field of C. albicans commensalism and apologize to all colleagues whose work could not be cited due to space limitations. CAK was supported by NIH NIAID R01 AI118898 (to CAK.) MSG was supported by a Humboldt Research Fellowship for Postdoctoral Researchers by the Alexander von Humboldt-Foundation and a Research Grant 2019 from the European Society of Clinical Microbiology and Infectious Diseases (ESCMID; to M.S.G.). BH is supported by the European Union Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No 812969 (FunHoMic), the Deutsche Forschungsgemeinschaft (DFG) project Hu 532/20-1, project C1 within the Collaborative Research Centre (CRC)/Transregio 124 FungiNet and the Balance of the Microverse Cluster, the Leibniz Association Campus InfectoOptics SAS-2015-HKI-LWC and the Wellcome Trust (grant 215599/Z/19/Z).
Footnotes
CRediT author statement
Carol Kumamoto, Mark Gresnigt and Bernhard Hube:
- Conceptualization
- Writing
- Reviewing and Editing
Conflict of interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review have been highlighted as:
• of special interest
•• of outstanding interest
Zaborin, A. et al. Membership and behavior of ultra-low-diversity pathogen communities present in the gut of humans during prolonged critical illness. mBio 5, e01361–01314, doi:10.1128/mBio.0136114 (2014).
Special interest
A prolonged critical illness causes the emergence of ultra-low-diversity pathogen communities including Candida albicans or C. glabrata.
Fan, D. et al. Activation of HIF-1alpha and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat Med 21, 808–814, doi:10.1038/nm.3871 (2015).
Special interest
Careful study of colonization resistance by the intact community and the effects of antibiotic treatment.
Miramon, P. & Lorenz, M. C. A feast for Candida: Metabolic plasticity confers an edge for virulence. PLoS pathogens 13, e1006144, doi:10.1371/journal.ppat.1006144 (2017).
Special interest
Excellent review of nutrient utilization by Candida albicans.
Ramirez-Zavala, B. et al. The Snf1-activating kinase Sak1 is a key regulator of metabolic adaptation and in vivo fitness of Candida albicans. Mol Microbiol 104, 989–1007, doi:10.1111/mmi.13674 (2017).
Special interest
Demonstration of the importance of metabolic plasticity for in vivo fitness.
Kastora, S. L., Herrero-de-Dios, C., Avelar, G. M., Munro, C. A. & Brown, A. J. P. Sfp1 and Rtg3 reciprocally modulate carbon source-conditional stress adaptation in the pathogenic yeast Candida albicans. Molecular microbiology 105, 620–636, doi:10.1111/mmi.13722 (2017).
Special interest
Interesting role for Rtg3 in carbon source dependent stress resistance.
Witchley, J. N. et al. Candida albicans Morphogenesis Programs Control the Balance between Gut Commensalism and Invasive Infection. Cell Host Microbe 25, 432–443 e436, doi:10.1016/j.chom.2019.02.008 (2019).
Outstanding interest:
Hyphal-associated genes are detrimental for colonization fitness, but their knockout does not result in a loss of filamentation in the gut
Tso, G. H. W. et al. Experimental evolution of a fungal pathogen into a gut symbiont. Science 362, 589–595, doi:10.1126/science.aat0537 (2018).
Outstanding interest:
Colonization of the intestine poses a strong evolutionary pressure towards neutralizing hyphae formation, yet filamentation is evolutionary conserved in Candida albicans.
Prieto, D., Roman, E., Alonso-Monge, R. & Pla, J. Overexpression of the Transcriptional Regulator WOR1 Increases Susceptibility to Bile Salts and Adhesion to the Mouse Gut Mucosa in Candida albicans. Frontiers in cellular and infection microbiology 7, 389, doi:10.3389/fcimb.2017.00389 (2017).
Special interest
WOR1 overexpression results in GUT-like cells with multiple phenotypes that affect colonization.
Koh, A. Y., Kohler, J. R., Coggshall, K. T., Van Rooijen, N. & Pier, G. B. Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS Pathog 4, e35
Outstanding interest
Pioneering study dissecting the pathogenesis of systemic candidiasis from the gut in a mouse model
Iliev, I. D. et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science 336, 1314–1317, doi:10.1126/science.1221789 (2012).
Outstanding interest
Pioneering work showing the relevance of the intestinal mycobiome
- 1.Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ: Invasive candidiasis. Nat Rev Dis Primers 2018, 4:18026. [DOI] [PubMed] [Google Scholar]
- 2.Cesaro S, Tridello G, Blijlevens N, Ljungman P, Craddock C, Michallet M, Martin A, Snowden JA, Mohty M, Maertens J, et al. : Incidence, Risk Factors, and Long-term Outcome of Acute Leukemia Patients With Early Candidemia After Allogeneic Stem Cell Transplantation: A Study by the Acute Leukemia and Infectious Diseases Working Parties of European Society for Blood and Marrow Transplantation. Clin Infect Dis 2018, 67:564–572. [DOI] [PubMed] [Google Scholar]
- 3.Nash AK, Auchtung TA, Wong MC, Smith DP, Gesell JR, Ross MC, Stewart CJ, Metcalf GA, Muzny DM, Gibbs RA, et al. : The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 2017, 5:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallen-Adams HE, Suhr MJ: Fungi in the healthy human gastrointestinal tract. Virulence 2017, 8:352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaborin A, Smith D, Garfield K, Quensen J, Shakhsheer B, Kade M, Tirrell M, Tiedje J, Gilbert JA, Zaborina O, et al. : Membership and behavior of ultra-low-diversity pathogen communities present in the gut of humans during prolonged critical illness. mBio 2014, 5:e01361–01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhai B, Ola M, Rolling T, Tosini NL, Joshowitz S, Littmann ER, Amoretti LA, Fontana E, Wright RJ, Miranda E, et al. : High-resolution mycobiota analysis reveals dynamic intestinal translocation preceding invasive candidiasis. Nat Med 2020, 26:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vergidis P, Clancy CJ, Shields RK, Park SY, Wildfeuer BN, Simmons RL, Nguyen MH: Intra-Abdominal Candidiasis: The Importance of Early Source Control and Antifungal Treatment. PLoS One 2016, 11:e0153247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nucci M, Anaissie E: Revisiting the source of candidemia: skin or gut? Clin Infect Dis 2001, 33:1959–1967. [DOI] [PubMed] [Google Scholar]
- 9.van Asbeck EC, Clemons KV, Stevens DA: Candida parapsilosis: a review of its epidemiology, pathogenesis, clinical aspects, typing and antimicrobial susceptibility. Crit Rev Microbiol 2009, 35:283–309. [DOI] [PubMed] [Google Scholar]
- 10.Miranda LN, van der Heijden IM, Costa SF, Sousa AP, Sienra RA, Gobara S, Santos CR, Lobo RD, Pessoa VP Jr., Levin AS: Candida colonisation as a source for candidaemia. J Hosp Infect 2009, 72:9–16. [DOI] [PubMed] [Google Scholar]
- 11.Kadosh D, Najvar LK, Bocanegra R, Olivo M, Kirkpatrick WR, Wiederhold NP, Patterson TF: Effect of Antifungal Treatment in a Diet-Based Murine Model of Disseminated Candidiasis Acquired via the Gastrointestinal Tract. Antimicrob Agents Chemother 2016, 60:6703–6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koh AY, Kohler JR, Coggshall KT, Van Rooijen N, Pier GB: Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS Pathog 2008, 4:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vautier S, Drummond RA, Redelinghuys P, Murray GI, MacCallum DM, Brown GD: Dectin-1 is not required for controlling Candida albicans colonization of the gastrointestinal tract. Infect Immun 2012, 80:4216–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diekema DJ, Pfaller MA: Nosocomial candidemia: an ounce of prevention is better than a pound of cure. Infect Control Hosp Epidemiol 2004, 25:624–626. [DOI] [PubMed] [Google Scholar]
- 15.Mayer FL, Wilson D, Hube B: Candida albicans pathogenicity mechanisms. Virulence 2013, 4:119128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishra AA, Koh AY: Adaptation of Candida albicans during gastrointestinal tract colonization. Curr Clin Microbiol Rep 2018, 5:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prieto D, Correia I, Pla J, Roman E: Adaptation of Candida albicans to commensalism in the gut. Future Microbiol 2016, 11:567–583. [DOI] [PubMed] [Google Scholar]
- 18.Neville BA, d’Enfert C, Bougnoux ME: Candida albicans commensalism in the gastrointestinal tract. FEMS Yeast Res 2015, 15. [DOI] [PubMed] [Google Scholar]
- 19.Romo JA, Kumamoto CA: On Commensalism of Candida. J Fungi (Basel) 2020, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez JC: Candida albicans dwelling in the mammalian gut. Curr Opin Microbiol 2019, 52:41–46. [DOI] [PubMed] [Google Scholar]
- 21.Fan D, Coughlin LA, Neubauer MM, Kim J, Kim MS, Zhan X, Simms-Waldrip TR, Xie Y, Hooper LV, Koh AY: Activation of HIF-1alpha and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat Med 2015, 21:808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuo K, Haku A, Bi B, Takahashi H, Kamada N, Yaguchi T, Saijo S, Yoneyama M, Goto Y: Fecal microbiota transplantation prevents Candida albicans from colonizing the gastrointestinal tract. Microbiol Immunol 2019, 63:155–163. [DOI] [PubMed] [Google Scholar]
- 23.White SJ, Rosenbach A, Lephart P, Nguyen D, Benjamin A, Tzipori S, Whiteway M, Mecsas J, Kumamoto CA: Self-regulation of Candida albicans population size during GI colonization. PLoS Pathog 2007, 3:e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bohm L, Torsin S, Tint SH, Eckstein MT, Ludwig T, Perez JC: The yeast form of the fungus Candida albicans promotes persistence in the gut of gnotobiotic mice. PLoS Pathog 2017, 13:e1006699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia C, Tebbji F, Daigneault M, Liu NN, Kohler JR, Allen-Vercoe E, Sellam A: The Human Gut Microbial Metabolome Modulates Fungal Growth via the TOR Signaling Pathway. mSphere 2017, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertolini M, Ranjan A, Thompson A, Diaz PI, Sobue T, Maas K, Dongari-Bagtzoglou A: Candida albicans induces mucosal bacterial dysbiosis that promotes invasive infection. PLoS Pathog 2019, 15:e1007717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui L, Morris A, Ghedin E: The human mycobiome in health and disease. Genome Med 2013, 5:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lagunes L, Rello J: Invasive candidiasis: from mycobiome to infection, therapy, and prevention. Eur J Clin Microbiol Infect Dis 2016, 35:1221–1226. [DOI] [PubMed] [Google Scholar]
- 29.Underhill DM, Iliev ID: The mycobiota: interactions between commensal fungi and the host immune system. Nat Rev Immunol 2014, 14:405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, Brown J, Becker CA, Fleshner PR, Dubinsky M, et al. : Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science 2012, 336:1314–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iliev ID, Leonardi I: Fungal dysbiosis: immunity and interactions at mucosal barriers. Nat Rev Immunol 2017, 17:635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wheeler ML, Limon JJ, Bar AS, Leal CA, Gargus M, Tang J, Brown J, Funari VA, Wang HL, Crother TR, et al. : Immunological Consequences of Intestinal Fungal Dysbiosis. Cell Host Microbe 2016, 19:865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hager CL, Isham N, Schrom KP, Chandra J, McCormick T, Miyagi M, Ghannoum MA: Effects of a Novel Probiotic Combination on Pathogenic Bacterial-Fungal Polymicrobial Biofilms. mBio 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noverr MC, Huffnagle GB: Regulation of Candida albicans morphogenesis by fatty acid metabolites. Infect Immun 2004, 72:6206–6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graf K, Last A, Gratz R, Allert S, Linde S, Westermann M, Groger M, Mosig AS, Gresnigt MS, Hube B: Keeping Candida commensal: how lactobacilli antagonize pathogenicity of Candida albicans in an in vitro gut model. Dis Model Mech 2019, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bohringer M, Pohlers S, Schulze S, Albrecht-Eckardt D, Piegsa J, Weber M, Martin R, Hunniger K,Linde J, Guthke R, et al. : Candida albicans infection leads to barrier breakdown and a MAPK/NF-kappaB mediated stress response in the intestinal epithelial cell line C2BBe1. Cell Microbiol 2016, 18:889–904. [DOI] [PubMed] [Google Scholar]
- 37.Basmaciyan L, Bon F, Paradis T, Lapaquette P, Dalle F: “Candida Albicans Interactions With The Host: Crossing The Intestinal Epithelial Barrier”. Tissue Barriers 2019, 7:1612661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allert S, Forster TM, Svensson CM, Richardson JP, Pawlik T, Hebecker B, Rudolphi S, Juraschitz M, Schaller M, Blagojevic M, et al. : Candida albicans-Induced Epithelial Damage Mediates Translocation through Intestinal Barriers. MBio 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maurer M, Gresnigt MS, Last A, Wollny T, Berlinghof F, Pospich R, Cseresnyes Z, Medyukhina A,Graf K, Groger M, et al. : A three-dimensional immunocompetent intestine-on-chip model as in vitro platform for functional and microbial interaction studies. Biomaterials 2019, 220:119396. [DOI] [PubMed] [Google Scholar]
- 40.Angebault C, Djossou F, Abelanet S, Permal E, Ben Soltana M, Diancourt L, Bouchier C, Woerther PL, Catzeflis F, Andremont A, et al. : Candida albicans is not always the preferential yeast colonizing humans: a study in Wayampi Amerindians. J Infect Dis 2013, 208:1705–1716. [DOI] [PubMed] [Google Scholar]
- 41.Gunsalus KT, Tornberg-Belanger SN, Matthan NR, Lichtenstein AH, Kumamoto CA: Manipulation of Host Diet To Reduce Gastrointestinal Colonization by the Opportunistic Pathogen Candida albicans. mSphere 2016, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kastora SL, Herrero-de-Dios C, Avelar GM, Munro CA, Brown AJP: Sfp1 and Rtg3 reciprocally modulate carbon source-conditional stress adaptation in the pathogenic yeast Candida albicans. Mol Microbiol 2017, 105:620–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miramon P, Lorenz MC: A feast for Candida: Metabolic plasticity confers an edge for virulence. PLoS Pathog 2017, 13:e1006144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miramon P, Pountain AW, van Hoof A, Lorenz MC: The paralogous transcription factors Stp1 and Stp2 of Candida albicans have distinct functions in nutrient acquisition and host interaction. Infect Immun 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramirez-Zavala B, Mottola A, Haubenreisser J, Schneider S, Allert S, Brunke S, Ohlsen K, Hube B, Morschhauser J: The Snf1-activating kinase Sak1 is a key regulator of metabolic adaptation and in vivo fitness of Candida albicans. Mol Microbiol 2017, 104:989–1007. [DOI] [PubMed] [Google Scholar]
- 46.Du H, Guan G, Li X, Gulati M, Tao L, Cao C, Johnson AD, Nobile CJ, Huang G: N-Acetylglucosamine-Induced Cell Death in Candida albicans and Its Implications for Adaptive Mechanisms of Nutrient Sensing in Yeasts. mBio 2015, 6:e01376–01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perez JC, Kumamoto CA, Johnson AD: Candida albicans commensalism and pathogenicity are intertwined traits directed by a tightly knit transcriptional regulatory circuit. PLoS Biol 2013, 11:e1001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choudhury BI, Whiteway M: Evolutionary Transition of GAL Regulatory Circuit from Generalist to Specialist Function in Ascomycetes. Trends Microbiol 2018, 26:692–702. [DOI] [PubMed] [Google Scholar]
- 49.Moreno-Velasquez SD, Tint SH, Del Olmo Toledo V, Torsin S, De S, Perez JC: The Regulatory Proteins Rtg1/3 Govern Sphingolipid Homeostasis in the Human-Associated Yeast Candida albicans. Cell Rep 2020, 30:620–629 e626. [DOI] [PubMed] [Google Scholar]
- 50.Brown AJP, Gow NAR, Warris A, Brown GD: Memory in Fungal Pathogens Promotes Immune Evasion, Colonisation, and Infection. Trends Microbiol 2019, 27:219–230. [DOI] [PubMed] [Google Scholar]
- 51.Cottier F, Sherrington S, Cockerill S, Del Olmo Toledo V, Kissane S, Tournu H, Orsini L, Palmer GE, Perez JC, Hall RA: Remasking of Candida albicans beta-Glucan in Response to Environmental pH Is Regulated by Quorum Sensing. mBio 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sem X, Le GT, Tan AS, Tso G, Yurieva M, Liao WW, Lum J, Srinivasan KG, Poidinger M, Zolezzi F, et al. : beta-glucan Exposure on the Fungal Cell Wall Tightly Correlates with Competitive Fitness of Candida Species in the Mouse Gastrointestinal Tract. Front Cell Infect Microbiol 2016, 6:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mamouei Z, Zeng G, Wang YM, Wang Y: Candida albicans possess a highly versatile and dynamic high-affinity iron transport system important for its commensal-pathogenic lifestyle. Mol Microbiol 2017, 106:986–998. [DOI] [PubMed] [Google Scholar]
- 54.Tan TG, Lim YS, Tan A, Leong R, Pavelka N: Fungal Symbionts Produce Prostaglandin E2 to Promote Their Intestinal Colonization. Front Cell Infect Microbiol 2019, 9:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guinan J, Villa P, Thangamani S: Secondary bile acids inhibit Candida albicans growth and morphogenesis. Pathog Dis 2018, 76. [DOI] [PubMed] [Google Scholar]
- 56.Guinan J, Thangamani S: Antibiotic-induced alterations in taurocholic acid levels promote gastrointestinal colonization of Candida albicans. FEMS Microbiol Lett 2018, 365. [DOI] [PubMed] [Google Scholar]
- 57.Hsieh SH, Brunke S, Brock M: Encapsulation of Antifungals in Micelles Protects Candida albicans during Gall-Bladder Infection. Front Microbiol 2017, 8:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Znaidi S, van Wijlick L, Hernandez-Cervantes A, Sertour N, Desseyn JL, Vincent F, Atanassova R, Gouyer V, Munro CA, Bachellier-Bassi S, et al. : Systematic gene overexpression in Candida albicans identifies a regulator of early adaptation to the mammalian gut. Cell Microbiol 2018, 20:e12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noble SM, Gianetti BA, Witchley JN: Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat Rev Microbiol 2017, 15:96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tso GHW, Reales-Calderon JA, Tan ASM, Sem X, Le GTT, Tan TG, Lai GC, Srinivasan KG, Yurieva M, Liao W, et al. : Experimental evolution of a fungal pathogen into a gut symbiont. Science 2018, 362:589–595. [DOI] [PubMed] [Google Scholar]
- 61.Witchley JN, Penumetcha P, Abon NV, Woolford CA, Mitchell AP, Noble SM: Candida albicans Morphogenesis Programs Control the Balance between Gut Commensalism and Invasive Infection. Cell Host Microbe 2019, 25:432–443 e436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pierce JV, Kumamoto CA: Variation in Candida albicans EFG1 expression enables host-dependent changes in colonizing fungal populations. MBio 2012, 3:e00117–00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Desai JV, Lionakis MS: Setting Up Home: Fungal Rules of Commensalism in the Mammalian Gut. Cell Host Microbe 2019, 25:347–349. [DOI] [PubMed] [Google Scholar]
- 64.Prieto D, Pla J: Distinct stages during colonization of the mouse gastrointestinal tract by Candida albicans. Front Microbiol 2015, 6:792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Desai PR, van Wijlick L, Kurtz D, Juchimiuk M, Ernst JF: Hypoxia and Temperature Regulated Morphogenesis in Candida albicans. PLoS Genet 2015, 11:e1005447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pande K, Chen C, Noble SM: Passage through the mammalian gut triggers a phenotypic switch that promotes Candida albicans commensalism. Nat Genet 2013, 45:1088–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prieto D, Roman E, Alonso-Monge R, Pla J: Overexpression of the Transcriptional Regulator WOR1 Increases Susceptibility to Bile Salts and Adhesion to the Mouse Gut Mucosa in Candida albicans. Front Cell Infect Microbiol 2017, 7:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shao TY, Ang WXG, Jiang TT, Huang FS, Andersen H, Kinder JM, Pham G, Burg AR, Ruff B, Gonzalez T, et al. : Commensal Candida albicans Positively Calibrates Systemic Th17 Immunological Responses. Cell Host Microbe 2019, 25:404–417 e406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bacher P, Hohnstein T, Beerbaum E, Rocker M, Blango MG, Kaufmann S, Rohmel J, Eschenhagen P, Grehn C, Seidel K, et al. : Human Anti-fungal Th17 Immunity and Pathology Rely on Cross-Reactivity against Candida albicans. Cell 2019, 176:1340–1355 e1315. [DOI] [PubMed] [Google Scholar]
- 70.Li X, Leonardi I, Semon A, Doron I, Gao IH, Putzel GG, Kim Y, Kabata H, Artis D, Fiers WD, et al. : Response to Fungal Dysbiosis by Gut-Resident CX3CR1(+) Mononuclear Phagocytes Aggravates Allergic Airway Disease. Cell Host Microbe 2018, 24:847–856 e844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Leeuwen PT, van der Peet JM, Bikker FJ, Hoogenkamp MA, Oliveira Paiva AM, Kostidis S, Mayboroda OA, Smits WK, Krom BP: Interspecies Interactions between Clostridium difficile and Candida albicans. mSphere 2016, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Panpetch W, Somboonna N, Palasuk M, Hiengrach P, Finkelman M, Tumwasorn S, Leelahavanichkul A: Oral Candida administration in a Clostridium difficile mouse model worsens disease severity but is attenuated by Bifidobacterium. PLoS One 2019, 14:e0210798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Markey L, Shaban L, Green ER, Lemon KP, Mecsas J, Kumamoto CA: Pre-colonization with the commensal fungus Candida albicans reduces murine susceptibility to Clostridium difficile infection. Gut Microbes 2018, 9:497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumamoto CA: Inflammation and gastrointestinal Candida colonization. Curr Opin Microbiol 2011, 14:386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stewart D, Romo JA, Lamendella R, Kumamoto CA: The role of fungi in C. difficile infection: An underappreciated transkingdom interaction. Fungal Genet Biol 2019, 129:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lopez-Medina E, Fan D, Coughlin LA, Ho EX, Lamont IL, Reimmann C, Hooper LV, Koh AY: Candida albicans Inhibits Pseudomonas aeruginosa Virulence through Suppression of Pyochelin and Pyoverdine Biosynthesis. PLoS Pathog 2015, 11:e1005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mason KL, Erb Downward JR, Mason KD, Falkowski NR, Eaton KA, Kao JY, Young VB, Huffnagle GB: Candida albicans and bacterial microbiota interactions in the cecum during recolonization following broad-spectrum antibiotic therapy. Infect Immun 2012, 80:3371–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leonardi I, Paramsothy S, Doron I, Semon A, Kaakoush NO, Clemente JC, Faith JJ, Borody TJ, Mitchell HM, Colombel JF, et al. : Fungal Trans-kingdom Dynamics Linked to Responsiveness to Fecal Microbiota Transplantation (FMT) Therapy in Ulcerative Colitis. Cell Host Microbe 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zuo T, Wong SH, Cheung CP, Lam K, Lui R, Cheung K, Zhang F, Tang W, Ching JYL, Wu JCY, et al. : Gut fungal dysbiosis correlates with reduced efficacy of fecal microbiota transplantation in Clostridium difficile infection. Nat Commun 2018, 9:3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Albac S, Schmitz A, Lopez-Alayon C, d’Enfert C, Sautour M, Ducreux A, Labruere-Chazal C, Laue M, Holland G, Bonnin A, et al. : Candida albicans is able to use M cells as a portal of entry across the intestinal barrier in vitro. Cell Microbiol 2016, 18:195–210. [DOI] [PubMed] [Google Scholar]
- 81.Kavanaugh NL, Zhang AQ, Nobile CJ, Johnson AD, Ribbeck K: Mucins suppress virulence traits of Candida albicans. mBio 2014, 5:e01911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leonardi I, Li X, Semon A, Li D, Doron I, Putzel G, Bar A, Prieto D, Rescigno M, McGovern DPB, et al. : CX3CR1(+) mononuclear phagocytes control immunity to intestinal fungi. Science 2018, 359:232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]