Abstract
Ischemic heart disease is the most common type of heart disease, responsible for roughly 10 million deaths worldwide annually. While standard clinical interventions have resulted in improved patient outcomes, access to small diameter vessels required for cardiovascular interventions, and long-term patient mortality rates associated with eventual heart failure, remain critical challenges. In this current opinion piece we discuss novel methodologies for the advancement of vascular grafts, cardiac patches, and injectable drug delivery depot technologies as they relate to treatment of ischemic heart disease, including bilayered conduits, acellular bioactive extracellular matrix (ECM) scaffolds, and protease-responsive hydrogel delivery platforms. We address the motivation for innovation and current limitations in the field of engineered biomaterials for myocardial ischemia therapeutics and interventions.
Keywords: Biomaterials, Vascular Grafts, Cardiac Scaffold, Hydrogels, Ischemic Heart Disease
Introduction
Cardiovascular disease is the leading cause of death worldwide, claiming the lives of 17.5 million people each year globally and accounting for annual direct costs of $213 billion in the United States [1]. The most common type of cardiovascular disease is ischemic heart disease (IHD), a condition that claims the lives of roughly 10 million people globally each year and currently affects over 18.2 million Americans [2]. Furthermore, the prevalence of ischemic heart disease is expected to rise by 46% by 2030 and subsequently double the associated annual healthcare expenditures [2]. While current clinical interventions, such as percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) improve patient outcomes, many patients ultimately succumb to heart failure due to inability to reverse or prevent downstream left ventricular remodeling [3]. Moreover, up to 37% of patients requiring CABG procedures are limited in eligibility due to prior surgeries and patient’s medical conditions, which limit the ability to harvest autologous small diameter vessels [4]. Additionally, long-term outcomes associated with both autologous and synthetic graft options are limited due to thrombosis [5]. Finally, standard clinical interventions such as PCI and CABG mainly restore macrovascular reperfusion following myocardial infarction (MI) and fail to address microvascular perfusion deficits contributing to eventual heart failure [3]. Thus, there is a critical need to improve upon vascular grafts required for CABG procedures and address persistent microvascular perfusion deficits following MI and subsequent cardiovascular intervention. In this current opinion piece, we will discuss several biomaterial approaches seeking to address critical challenges relating to engineered vascular grafts, cardiac scaffolds, and sustained local delivery of therapeutics using injectable hydrogel depots (Figure 1).
Figure 1:
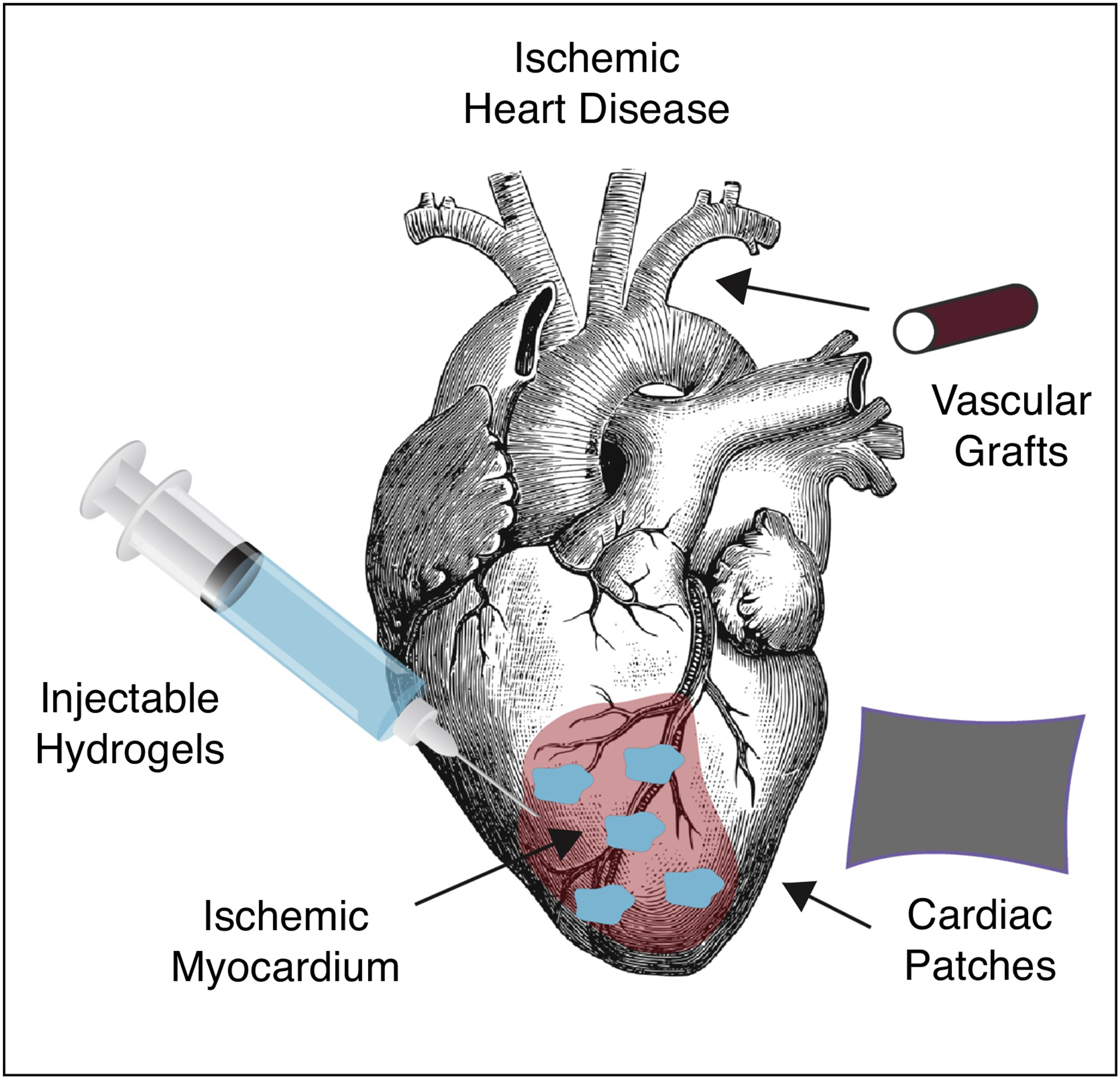
Schematic overview of specific topics covered in this current opinion article.
Vascular Grafts
Vascular grafts are conduits that can support blood flow, withstand the pressures exerted by blood flow, and, ideally, have the capability to grow, remodel, and self-repair in vivo [6,7]. The first engineered vascular graft was proposed by Bell and colleagues in the 1980’s [8]; however, it took nearly 20 years for engineered vascular grafts to be implanted in humans [9]. The first engineered vascular graft implanted in humans was generated using autologous cells isolated from explanted peripheral vein and seeded in a polymer scaffold composed of polycaprolactone-polylactic acid copolymer reinforced with woven polyglycolic acid [9]. Following this invention, a human trial was initiated to evaluate similar grafts using the same biodegradable scaffold seeded with mononuclear cells harvested from autologous bone marrow in patients with single ventricle physiology [10]. Although late-term results demonstrated feasibility of this technology in the application of extracardiac total cavopulmonary circulation, graft stenosis was noted to be the primary mode of failure [11]. Additionally, other similar polymer-based grafts were unable to demonstrate adequate mechanical strength to allow for implantation in the arterial system [12,13]. This limitation was attributed to the synthetic or chemically modified components of the scaffold interfering with natural extracellular matrix protein assembly.
L’Heureux and colleagues generated engineered vascular conduits using fibroblasts, smooth muscle cells (SMC), and endothelial cells without the use of any synthetic materials [14]. The authors used sheet-based tissue engineering and demonstrated the ability of the engineered vessels to withstand supraphysiological burst strength constructed exclusively from human and human-derived constituents [15]. Promising pre-clinical results led to a clinical trial that demonstrated satisfactory early-term results in patients on dialysis [15–17]. Another approach, which laid the foundation for other clinical trials and “off-the-shelf” vascular grafts, involved the generation of decellularized grafts for both engineered and autologous conduits [18,19]. Specifically, rapidly degradable polyglycolic acid tubular scaffolds have been used for cell seeding and graft maturation in a bioreactor [19]. At the end of the culture period, the resultant structure is decellularized, leaving only the secreted collagenous matrix produced by seeded cells. Although these vascular grafts were not immunogenic, these studies exhibited early graft failure due to thrombosis and required prolonged production times of up to 10 weeks in culture [16,19].
To address these limitations, innovative approaches have more recently been investigated. Von Bornstädt and coworkers described a methodology utilizing cell sheets to generate a bilayered conduit. The conduit was strengthened by an FDA-approved biodegradable tissue glue and perfused with endothelial cells (Figure 2G). The graft was able to reach maturity with supraphysiological burst strength in just 2 weeks and demonstrated excellent early term patency in a rodent hindlimb ischemia model (Figure 2A–F) [20]. Electrospinning techniques have also been utilized to develop nanofibrous vascular scaffolds with promising biomechanical properties and structural integrity [21–24]. This approach exhibited exciting results 6 months after transplantation in a sheep carotid arterial interposition model [25]. Furthermore, with the recent advancements in 3D live cell bioprinting, progress has been made in generating vascular conduits by harnessing the versatility of 3D printing technology. Marga et al. used a scaffold-free approach to generate a tubular structure by using contiguously arranged cellular aggregates as printer cartridges [26]. While promising results and innovative technologies have been demonstrated, suggesting the need for further exploration, shorter production times for mature, autologous conduits remains a critical challenge for high risk patients. Specifically, patients with severe, unstable coronary artery diseases may require surgical treatment within CABG within several days of symptom onset, necessitating the need for rapidly available, robust conduits. Significant efforts are still required to readily scale-up engineered vascular grafts to satisfy intra-cardiac needs and enhance the production process for future commercialization.
Figure 2: Structural maturation of the engineered vascular graft in vitro and in vivo.
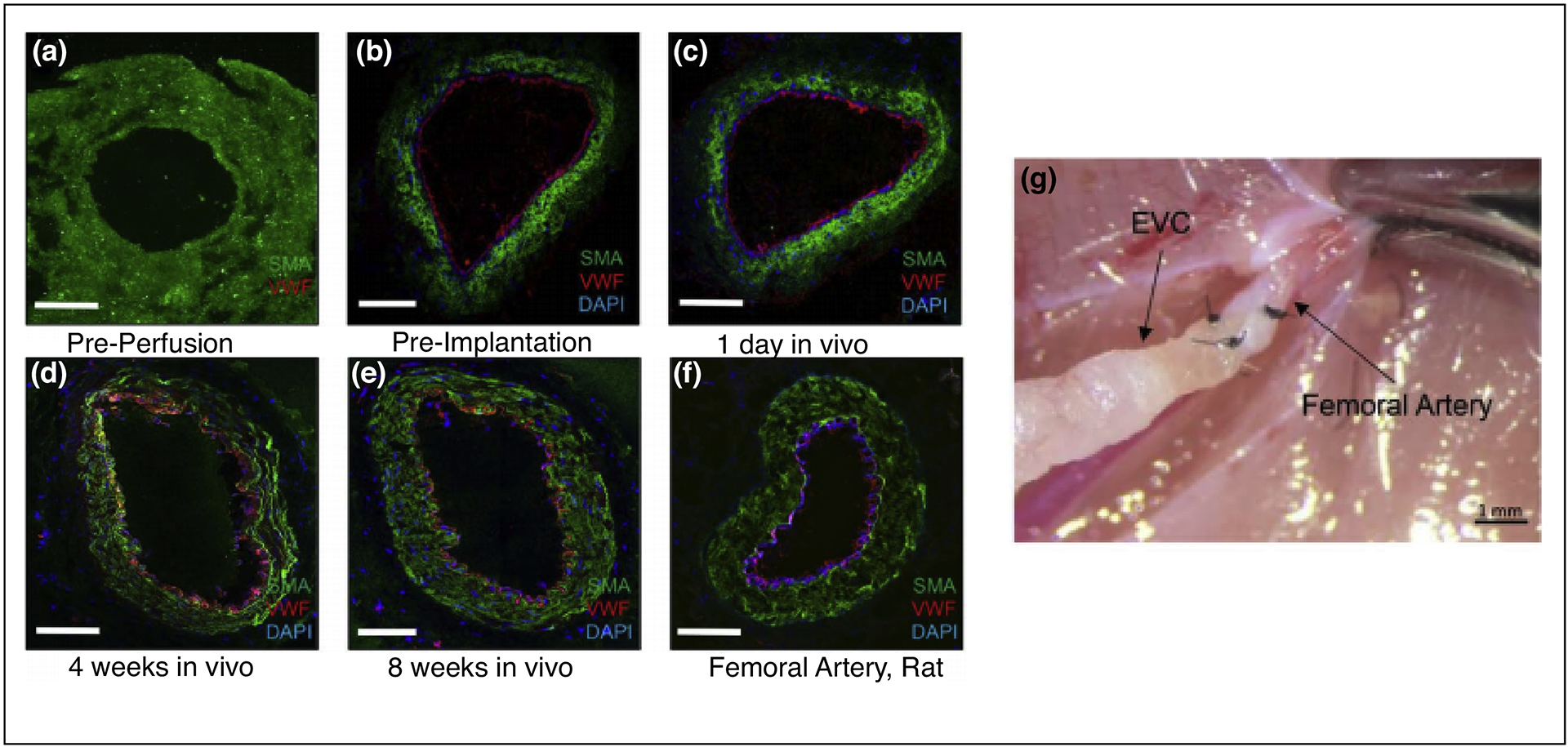
A–F. Engineered vascular conduit (EVC) immediately before in vitro perfusion, immediately before implantation in vivo, and after 1 day, 4 weeks, and 8 weeks after implantation in vivo compared with a native femoral artery of a nude rat. Human smooth muscle actin antibody, green fluorescent protein; von Willebrand factor antibody, Texas Red; 4’, 6-diamidino-2-phenylindole, blue; confocal microscopy, ×20. G. EVCs were anastomosed to the femoral artery as an interposition graft in end-to-end fashion (10–0 nylon suture, 5 stitches). Reproduced with edits and permissions from Wolters Kluwer Health, Inc.
Cardiac Scaffolds
Cardiac scaffolds (or cardiac patches) are in vitro engineered constructs that can provide mechanical support to promote endogenous repair and regeneration of the ischemic tissue, or otherwise act as a vehicle to deliver therapeutic cargo to the ischemic tissue [27]. Many of these types of scaffolds have the potential to maintain the cellular microenvironment, support cellular differentiation and organization, and prevent anoikis [28]. Cardiac scaffolds can be built upon natural or synthetic biomaterials, or created scaffold-free via cellular self-assembly, to produce functional myocardial tissue [29,30]. Researchers have shown that collagen and chitosan based cardiac patches alone, without cellular or molecular cargo, prevent negative myocardial remodeling and induce angiogenesis throughout the infarcted region of the heart [31,32]. Expanding upon cardiac patch delivery alone, therapeutic cargo such as proteins, stem cells, cytokines, and growth factors can be seeded onto the cardiac patch and further enhance the therapeutic benefit of this approach [33].
In particular, cell-loaded cardiac scaffolds composed of a variety of different cell types have been widely investigated due to the ability of these scaffolds to address limitations in scaffold thickness and the potential to be more readily vascularized by the host circulatory system [34]. Common cell sources for these applications include skeletal myoblasts, bone marrow-derived cells, mesenchymal stem cells, embryonic stem cells, induced pluripotent stem cells, induced pluripotent stem cell derived cardiomyocytes, and cardiac stem cells [35]. In 2008, Chachques et al. reported the results of the first clinical trial investigating a cardiac patch (MAGNUM) where a bone marrow stem cell (BMSC)-seeded collagen scaffold was implanted intramyocardially during CABG surgery in patients with post-ischemic injuries. Patients who received the cell-seeded cardiac scaffold exhibited an increase in scar thickness and improvement in left-ventricular end-diastolic volume compared to the cell-only group demonstrating promise for the therapeutic benefit of a cell-loaded cardiac patch [36]. Ten years later, Larghero et al. published the results of a clinical study investigating a fibrin patch laden with embryonic stem cell-derived cardiac progenitor cells. These cardiac patches were administered to six patients with severe ischemic left ventricular dysfunction during CABG surgery. After a median 18-month follow-up, investigators determined that the patches demonstrated promising safety outcomes, but warranted further efficacy studies with a more adequately powered study [37].
Preclinical and clinical studies investigating cell-laden cardiac scaffolds have shown promise and warrant further investigation due to the demonstrated therapeutic benefits of paracrine signaling and direct interaction with injured cardiomyocytes [38]. However, it is important to note the inconsistent results associated with cell-laden therapeutics for cardiac applications [39]. While clinical trials investigating cell-based therapeutics have demonstrated the potential safety of a cellular approach, functional improvements have been modest and associated with uncertain clinical significance [40–44]. Several limitations may be contributing to the observed inconsistencies in efficacy, including fragile cellular cargo, limited long-term stability, extensive production times and costs, and the presence of undifferentiated cells contributing to uncontrolled cell growth or tumorgenicity following transplantation [45]. These limitations can potentially be addressed with further exploration into acellular approaches [45]. Previous work investigating acellular cardiac scaffolds have demonstrated significant cardiogenesis, vasculogenesis, and promising functional recovery in post-ischemic myocardial tissue in both small and large preclinical models of myocardial ischemia [46]. Furthermore, these acellular scaffold studies encouraged a first-in-man pilot study, investigating feasibility, infarct size, cardiac function, and treatment related-adverse events resulting from the implantation of an acellular bioactive ECM scaffold implanted at the time of CABG surgery (ClinicalTrials.gov ID: NCT02887768).
More recently, exciting work published by Huang et al. demonstrated an off-the-shelf artificial cardiac patch composed of a decellularized porcine myocardial extracellular matrix and synthetic cardiac stromal cells. This fully artificial cardiac patch (artCP) retained potency following long-term cryopreservation of 28 days, improved cardiac function, reduced infarct size, and increased angiogenesis when applied to rodent and porcine myocardium following myocardial infarction (Figure 3) [47]. Moreover, the artCP demonstrated a negligible immune response offering an additional advantage over other cardiac patch approaches due to the use of synthetic cardiac stromal cells. Previous reports have shown that the use of allogenic cells risk immunogenicity and the use of autologous cells can be expensive and time-consuming for an independent batch per patient [45,47]. However, while the artCP utilized a synthetic cell source and resulted in no observed immune response, the use of a decellularized porcine myocardial extracellular matrix scaffold in the artCP is still of concern and requires further investigation for human applications due to previous reports demonstrating inflicted immune responses as a result of decellularized scaffolds [48]. Overall, further investigation of the artCP is needed to conduct adequately powered large animal studies over a longer study period in conjunction with more robust biocompatibility testing, but the promising study results and reported long-term stability emphasize the motivation to engineer solutions with minimal translational hurdles and enhanced potential for clinical translation.
Figure 3: Large animal in vivo assessment of artificial cardiac patch (artCP).
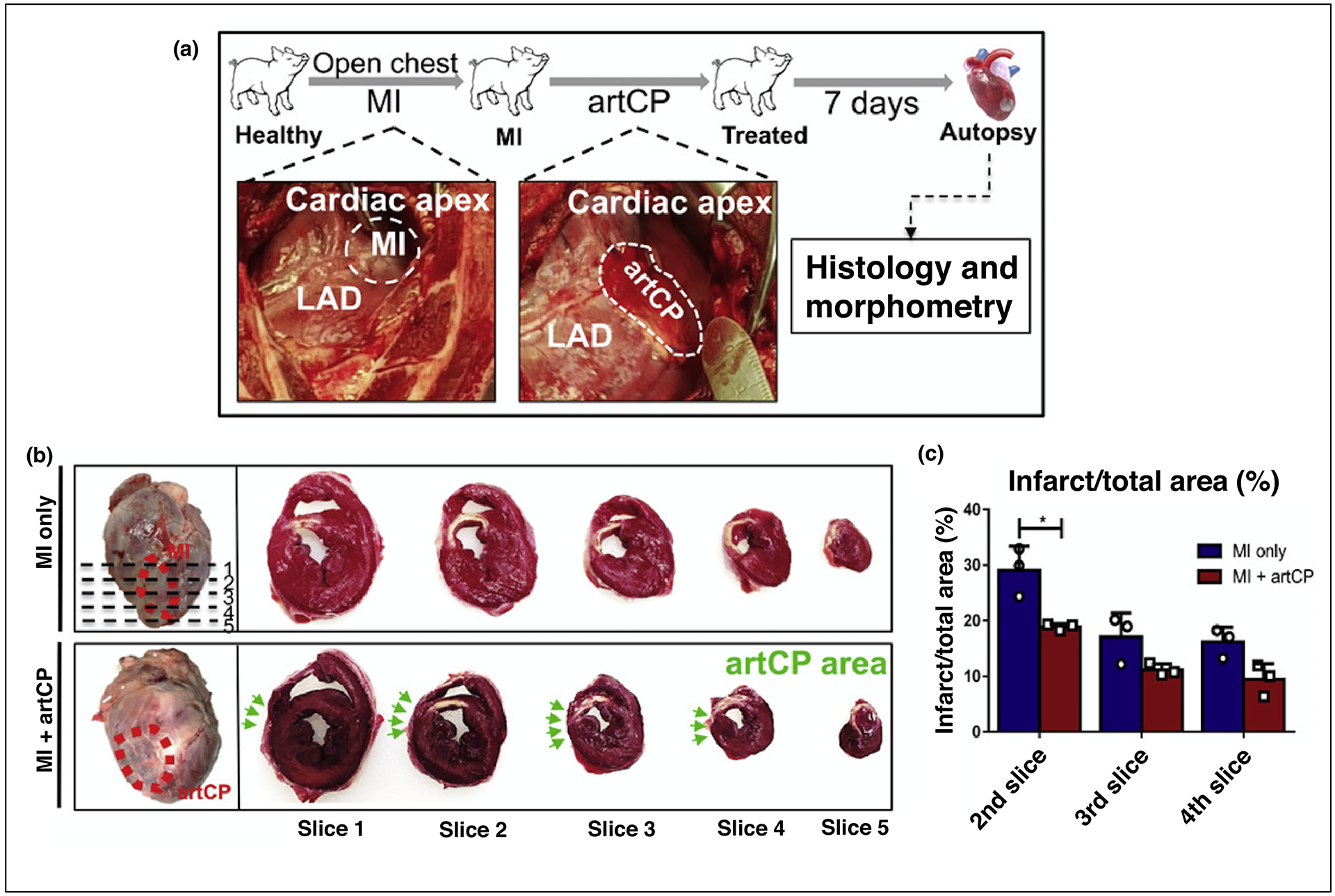
A. Schematic showing the study design. The representative pictures show the porcine MI model creation via LAD ligation (left) and artCP transplantation (right). D. Heart sectioning for gross assessment of infarct size. The top left image (MI control) shows the area of infarction due to successful MI creation (red dashed circle) and five sections (1 cm in thickness; dashed line) cut from apex to level of ligation. The bottom left image shows the artCP transplanted area (red dashed circle). The images on the right show the TTC staining of five heart sections from one heart in the MI-only group (top) and the artCP-treated group (bottom). The white area in the TTC-stained heart sections indicates infarction. The position of artCP was indicated with green arrows. E. Infarction area percentage measured in heart slices 2, 3, and 4 using ImageJ software (n=3). All data area means ± SD. Comparisons among groups were performed using one-way ANOVA followed by post hoc Bonferroni test. The comparisons between samples are indicated by lines, and the statistical significance is indicated by asterisks above the lines. *P<0.05 and ***P<0.001. LAD = left anterior descending artery; TTC = 2,3,5-Triphenyltetrazolium chloride. Reproduced with edits and permissions from The American Association for the Advancement of Science.
Injectable Hydrogels
Injectable hydrogels are water-swollen networks of crosslinked polymers composed of natural and/or synthetic polymers and are mostly classified based on chemical or physical crosslinking mechanisms [49,50]. While these injectable biomaterials do not allow for specific organizational control afforded by the engineered cardiac patches previously discussed, a significant benefit is the potential for minimally-invasive, catheter-based delivery without the need of an invasive, surgical procedure in order to administer the treatment [27]. Similar to the cardiac scaffold approach discussed previously, researchers have demonstrated therapeutic efficacy following myocardial injection of hydrogel alone, without cellular or molecular cargo [51]. Composite, or hybrid, hydrogels have demonstrated promise by combining optimal natural and synthetic material components for improved biochemical and biomechanical material properties [52]. For example, ECM-fibrin, alginate-chitosan, and ECM-polyethylene glycol hydrogels have previously demonstrated improved cardiac repair following MI [51,53,54]. Additionally, composite hydrogels composed of fibrin and alginate resulted in attenuated LV wall thickness and decreased infarct expansion in a porcine chronic MI model [55]. To date, there have been few clinical trials resulting from the vast preclinical work investigating hydrogel-based interventions for myocardial ischemia [5]. In 2015, an alginate-based hydrogel delivered via direct myocardial injection was evaluated in patients with advanced heart failure and exhibited improved exercise capacity, though further clinical studies with larger patient cohorts followed over longer time periods are needed to further validate these results [56]. Recently, Traverse et al. published the results of a Phase 1 clinical trial (AUGMENT-HF) investigating VentriGel, an extracellular matrix hydrogel derived from decellularized porcine myocardium. The study evaluated the safety and feasibility of transendocardial injections of VentriGel to patients with early and late post-MI patients with LV dysfunction. The results of this study support the safety and feasibility of this therapeutic and suggest improvements in exercise capacity and reductions in New York Heart Association functional class across the cohort of patients, warranting further investigation in a larger, randomized, controlled clinical trial [57].
Expanding upon the methodology of hydrogel delivery alone, other studies have investigated the advantageous, tunable cargo delivery characteristics of injectable hydrogels. Several groups have evaluated the therapeutic efficacy of loading therapeutic cargo such as proteins, stem cells, DNA, RNA, small molecules, cytokines, and/or growth factors into the hydrogel, which can then act as a depot for sustained local release [52,58,59]. Steele et al. investigated the sustained delivery of two protein-engineered cytokines via a catheter deliverable hydrogel in small and large animal MI models. In this study, the dual-stage release of dimeric fragment of hepatocyte growth factor (HGFdf) and engineered stromal cell-derived factor 1a (ESA) activate separate, but synergistic reparative pathways yielding improved cardiac function in the small animal MI model and observed reduction in scar size in the large animal, preclinical MI model (Figure 4A–E) [60]. The erosion profile of the hydrogel was investigated in vitro and resulted in 47% of the hydrogel eroding after 14 days. However, while the small animal MI model yielded enhanced functional outcomes, the large animal model resulted in minimal changes in functional parameters that lead to insignificant findings. The improvement in myocardial scar size observed in the preclinical model still suggests the potential for therapeutic benefit, but further investigation into the appropriate dosing required for large animal myocardium is needed. It is also possible that a chronic MI model may be required to better investigate the most optimal dosing for clinical efficacy instead of the acute MI model utilized in this study. A chronic MI model would more accurately reflect the clinical scenario and increase the potential of successful translation with the most optimal therapeutic dose.
Figure 4: Injectable hydrogel approaches and in vivo efficacy.
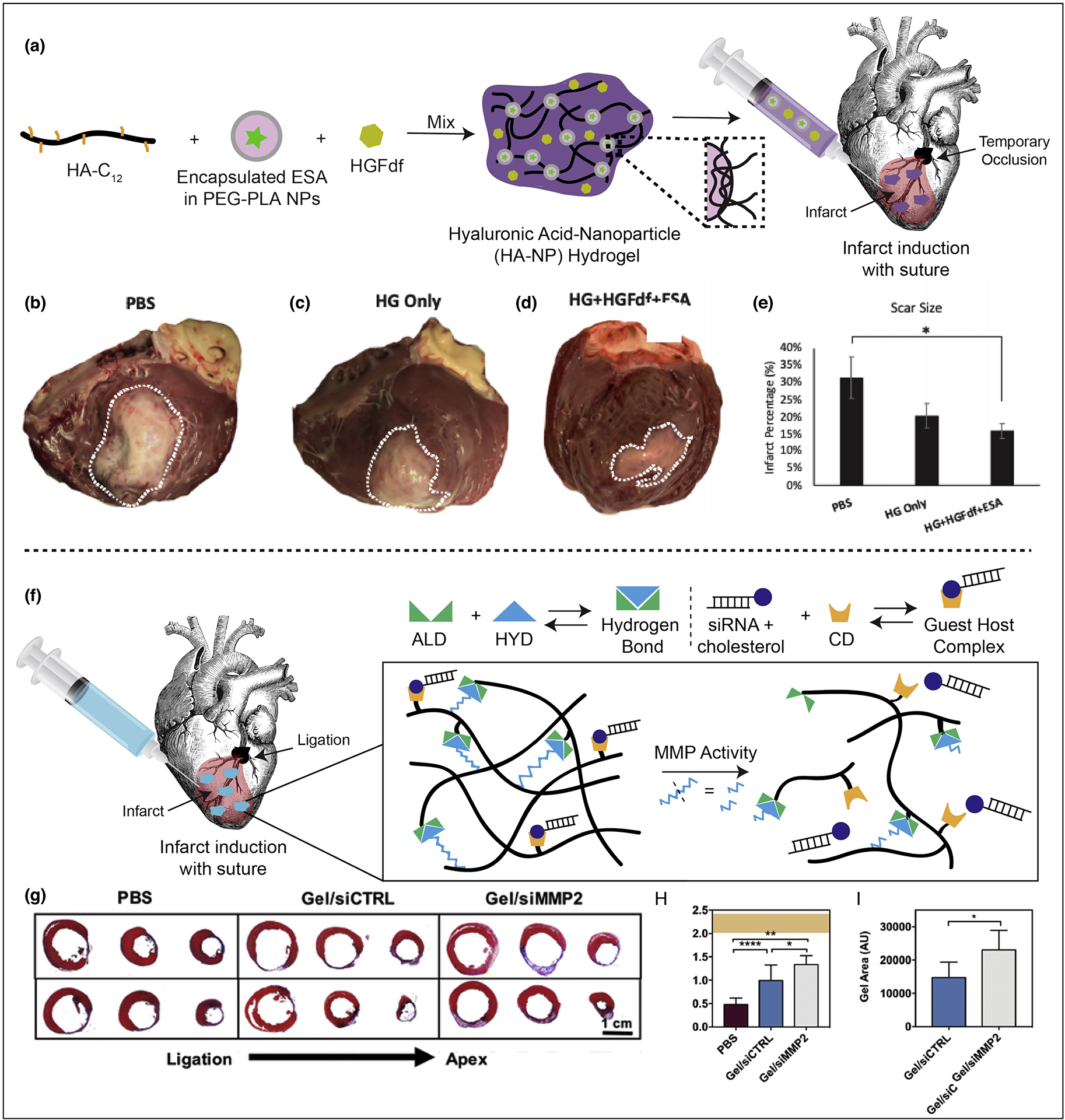
A. Demonstration of hydrogel formation with encapsulated cytokines. HA-NP hydrogel is composed of a hydrophobically-modified hyaluronic acid (HA) which is crosslinked by hydrophobic polyethyleneglycol block polylactic acid (PEGPLA) nanoparticles. The ESA is encapsulated into the nanoparticle phase and HGFdf is encapsulated into the aqueous phase of the hydrogel. B–E. Left ventricle infarct area. Hearts were explanted and opened longitudinally. The infarct was photographed for quantification and representative images of hearts from each group are presented. (B) PBS treated (C) HG only treated and (D) HG + HGFdf + ESA treated animals were evaluated. (E) HG + HGFdf + ESA demonstrated a significantly reduced infarct size compared to PBS animals and smaller average infarcts compared to HG only animals. ANOVA with a Bonferroni correction for multiple comparisons, *p < 0.05. F. Schematic demonstrating siRNA-cholesterol association with hydrogel via cholesterol/CD interactions and illustrating hydrogel erosion in response to MMPs. G. Representative Masson’s trichrome sections (3 representative sections from ligation to apex from left to right in 2 representative animals per group, 1 animal per row). H. Quantification of infarct thickness from Masson’s trichrome sections across three representative axial/transverse sections per animal (mean ± SD, *p<0.05, **p<0.01, ****p<0.001, PBS: n=6, gel/siCTRL: n=7, gel/siMMP2: n=6) and presented in context of healthy controls (orange, mean ± SD, n=7). I. Quantification of hydrogel area from Masson’s trichrome sections across three representative axial/transverse sections per animal (mean ± SD, *p<0.05, PBS: n=6, gel/siCTRL: n=7, gel/siMMP2: n=6). Reproduced with edits and permissions from Elsevier.
Lastly, research groups are further innovating and investigating advanced hydrogel systems or “smart” hydrogels in which internal or external events trigger the release of therapeutic cargo allowing for additional spatiotemporal control over release kinetics [61]. Recently, Burdick et al. engineered a protease-responsive hydrogel delivery platform capable of an “on-demand” release of siRNA in response to the myocardial proteolytic activity contributing LV dilation and mechanical compromise following MI. In this study, a hyaluronic acid (HA) hydrogel with encapsulated siRNA against matrix metalloprotease 2 (siMMP2) is injected directly into the infarct region and erodes in response to the local protease activity releasing siMMP2 (Figure 4F). Delivery of the protease-responsive hydrogel in a rodent model of MI improved myocardial thickness and enhanced cardiac function including increased ejection fraction, stroke volume, and cardiac output. Hydrogel volumes were retained in the infarct wall due to decreased hydrogel erosion as a result of the siMMP2, responsible for attenuating hydrogel erosion by 46% when compared to control siRNA hydrogels. These results suggest promising, synergistic effects of preserved hydrogel volumes for wall bulking in conjunction with a positive feedback loop that responds to the native, infarcted myocardial environment prompting the release of therapeutic cargo (Figure 4G–I) [62]. Additional studies in large animal, preclinical models as well as additional therapeutic combinations should be performed to further address the potential for clinical efficacy and other upregulated factors contributing to complex, adverse LV remodeling events.
Conclusion
This current opinion/review began with an overview of the clinical challenges associated with ischemic heart disease. Based on current literature, we classified and analyzed current biomaterial approaches that contribute to the next generation of translational myocardial ischemia interventions. These were divided into three categories: vascular grafts, cardiac patches, and injectable depot therapeutics. The goals of these approaches are to (1) improve upon existing cardiovascular interventions following myocardial infarction, (2) optimize therapeutic efficacy by utilizing synergistic approaches, (3) provide minimally-invasive, targeted delivery platforms, and/or (4) minimize translational hurdles associated with many traditional approaches. While these recent studies have yielded exciting results and warrant further experimental investigation, many clinical challenges remain to be addressed including long-term efficacy, systemic toxicity, pharmacokinetics, pharmacodynamics, long-term side effects, and concurrent treatment options. It is also important to note the importance of animal models in minimizing the translational hurdle to human application. Prior to clinical translation, these engineered biomaterials should be investigated in relevant large animal preclinical models that most closely resemble the human anatomy and clinical scenario. Ovine or porcine chronic MI models and cardiac bypass models provide an optimal relevancy to the human disease and clinical application that should be considered following small animal acute and chronic MI models. The future of engineering biomaterials for heart disease is continually shifting towards developing the ideal combination of therapeutic innovation and translational potential for facile clinical adoption and true bench-to-beside research. Furthermore, the collaboration between bioengineering, material science, cardiovascular medicine, and cardiothoracic surgery is crucial in solving the complex sequelae related to heart disease. As the research community continues to innovative and address both therapeutic and translational limitations, the future of biomaterials as it relates to heart disease will remain promising with the potential to improve the quality of life and life expectancy of patients with ischemic heart disease.
Acknowledgements
This work was supported, in part, by the National Institute of Health (NIH) grant R01 HL089315-01 (YJW), American Heart Association Predoctoral Fellowship (LMS), and Stanford Interdisciplinary Graduate Fellowships (LMS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Bibliography
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. : Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al. : Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 3.Araszkiewicz A, Grajek S, Lesiak M, Prech M, Pyda M, Janus M, Cieslinski A: Effect of impaired myocardial reperfusion on left ventricular remodeling in patients with anterior wall acute myocardial infarction treated with primary coronary intervention. Am. J. Cardiol 2006, 98:725–728. [DOI] [PubMed] [Google Scholar]
- 4.Boodhwani M, Sodha NR, Laham RJ, Sellke FW: The future of therapeutic myocardial angiogenesis. Shock 2006, 26:332–341. [DOI] [PubMed] [Google Scholar]
- 5.Di Franco S, Amarelli C, Montalto A, Loforte A, Musumeci F: Biomaterials and heart recovery: cardiac repair, regeneration and healing in the MCS era: a state of the “heart”. J Thorac Dis 2018, 10:S2346–S2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pashneh-Tala S, MacNeil S, Claeyssens F: The Tissue-Engineered Vascular Graft-Past, Present, and Future. Tissue Eng. Part B Rev 2015, doi: 10.1089/ten.teb.2015.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skovrind I, Harvald EB, Juul Belling H, Jørgensen CD, Lindholt JS, Andersen DC: Concise Review: Patency of Small-Diameter Tissue-Engineered Vascular Grafts: A Meta-Analysis of Preclinical Trials. Stem Cells Transl. Med 2019, 8:671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinberg CB, Bell E: A blood vessel model constructed from collagen and cultured vascular cells. Science 1986, 231:397–400. [DOI] [PubMed] [Google Scholar]
- 9.Shin’oka T, Imai Y, Ikada Y: Transplantation of a tissue-engineered pulmonary artery. N. Engl. J. Med 2001, 344:532–533. [DOI] [PubMed] [Google Scholar]
- 10.Shin’oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, Sakamoto T, Nagatsu M, Kurosawa H: Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J. Thorac. Cardiovasc. Surg 2005, 129:1330–1338. [DOI] [PubMed] [Google Scholar]
- 11.Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C, Shinoka T: Late-term results of tissue-engineered vascular grafts in humans. J. Thorac. Cardiovasc. Surg 2010, 139:431–6, 436.e1. [DOI] [PubMed] [Google Scholar]
- 12.Poh M, Boyer M, Solan A, Dahl SLM, Pedrotty D, Banik SSR, McKee JA, Klinger RY, Counter CM, Niklason LE: Blood vessels engineered from human cells. Lancet 2005, 365:2122–2124. [DOI] [PubMed] [Google Scholar]
- 13.McKee JA, Banik SSR, Boyer MJ, Hamad NM, Lawson JH, Niklason LE, Counter CM: Human arteries engineered in vitro. EMBO Rep. 2003, 4:633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.L’Heureux N, Germain L, Labbé R, Auger FA: In vitro construction of a human blood vessel from cultured vascular cells: a morphologic study. J. Vasc. Surg 1993, 17:499–509. [DOI] [PubMed] [Google Scholar]
- 15.L’Heureux N, Pâquet S, Labbé R, Germain L, Auger FA: A completely biological tissue-engineered human blood vessel. FASEB J. 1998, 12:47–56. [DOI] [PubMed] [Google Scholar]
- 16.McAllister TN, Maruszewski M, Garrido SA, Wystrychowski W, Dusserre N, Marini A, Zagalski K, Fiorillo A, Avila H, Manglano X, et al. : Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet 2009, 373:1440–1446. [DOI] [PubMed] [Google Scholar]
- 17.L’Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, Chronos NAF, Kyles AE, Gregory CR, Hoyt G, et al. : Human tissue-engineered blood vessels for adult arterial revascularization. Nat. Med 2006, 12:361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahl SLM, Koh J, Prabhakar V, Niklason LE: Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant. 2003, 12:659–666. [DOI] [PubMed] [Google Scholar]
- 19.Dahl SLM, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, Manson RJ, Tente WE, DiBernardo L, Hensley MT, et al. : Readily available tissue-engineered vascular grafts. Sci. Transl. Med 2011, 3:68ra9. [DOI] [PubMed] [Google Scholar]
- ••20.von Bornstädt D, Wang H, Paulsen MJ, Goldstone AB, Eskandari A, Thakore A, Stapleton L, Steele AN, Truong VN, Jaatinen K, et al. : Rapid Self-Assembly of Bioengineered Cardiovascular Bypass Grafts From Scaffold-Stabilized, Tubular Bilevel Cell Sheets. Circulation 2018, 138:2130–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]; A three-layered arterial vascular graft was generated using bilevel cell sheets in as short as 2 weeks with excellent short-term outcome in rodents. This is one of the first studies that demonstrated the feasibility in creating engineered vascular conduit that can withstand arterial circulatory pressure within a clinically relevant window.
- 21.Hasan A, Memic A, Annabi N, Hossain M, Paul A, Dokmeci MR, Dehghani F, Khademhosseini A: Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014, 10:11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ju YM, Choi JS, Atala A, Yoo JJ, Lee SJ: Bilayered scaffold for engineering cellularized blood vessels. Biomaterials 2010, 31:4313–4321. [DOI] [PubMed] [Google Scholar]
- •23.van Uden S, Vanerio N, Catto V, Bonandrini B, Tironi M, Figliuzzi M, Remuzzi A, Kock L, Redaelli ACL, Greco FG, et al. : A novel hybrid silk-fibroin/polyurethane three-layered vascular graft: towards in situ tissue-engineered vascular accesses for haemodialysis. Biomed Mater 2019, 14:025007. [DOI] [PubMed] [Google Scholar]; A three-layered silk fibroin/polyurethane vascular graft was developed by electrospinning for use as long-term haemodialysis vascular access. This study represents an important advance towards an in situ engineered hybrid vascular conduit for vein-graft anastomosis stability, early cannulation, and biointegration.
- 24.Fernández-Colino A, Wolf F, Rütten S, Schmitz-Rode T, Rodríguez-Cabello JC, Jockenhoevel S, Mela P: Small Caliber Compliant Vascular Grafts Based on Elastin-Like Recombinamers for in situ Tissue Engineering. Front. Bioeng. Biotechnol 2019, 7:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ju YM, Ahn H, Arenas-Herrera J, Kim C, Abolbashari M, Atala A, Yoo JJ, Lee SJ: Electrospun vascular scaffold for cellularized small diameter blood vessels: A preclinical large animal study. Acta Biomater. 2017, 59:58–67. [DOI] [PubMed] [Google Scholar]
- 26.Norotte C, Marga FS, Niklason LE, Forgacs G: Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009, 30:5910–5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radisic M, Christman KL: Materials science and tissue engineering: repairing the heart. Mayo Clin. Proc 2013, 88:884–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rane AA, Christman KL: Biomaterials for the treatment of myocardial infarction: a 5-year update. J. Am. Coll. Cardiol 2011, 58:2615–2629. [DOI] [PubMed] [Google Scholar]
- 29.Cui Z, Yang B, Li R-K: Application of biomaterials in cardiac repair and regeneration. ENG. 2016, 2:141–148. [Google Scholar]
- 30.Lister Z, Rayner KJ, Suuronen EJ: How Biomaterials Can Influence Various Cell Types in the Repair and Regeneration of the Heart after Myocardial Infarction. Front. Bioeng. Biotechnol 2016, 4:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chi N-H, Yang M-C, Chung T-W, Chou N-K, Wang S-S: Cardiac repair using chitosan-hyaluronan/silk fibroin patches in a rat heart model with myocardial infarction. Carbohydr. Polym 2013, 92:591–597. [DOI] [PubMed] [Google Scholar]
- 32.Callegari A, Bollini S, Iop L, Chiavegato A, Torregrossa G, Pozzobon M, Gerosa G, De Coppi P, Elvassore N, Sartore S: Neovascularization induced by porous collagen scaffold implanted on intact and cryoinjured rat hearts. Biomaterials 2007, 28:5449–5461. [DOI] [PubMed] [Google Scholar]
- 33.Naveed M, Han L, Khan GJ, Yasmeen S, Mikrani R, Abbas M, Cunyu L, Xiaohui Z: Cardio-supportive devices (VRD & DCC device) and patches for advanced heart failure: A review, summary of state of the art and future directions. Biomed. Pharmacother 2018, 102:41–54. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J: Engineered tissue patch for cardiac cell therapy. Curr Treat Options Cardiovasc Med 2015, 17:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Streeter BW, Davis ME: Therapeutic cardiac patches for repairing the myocardium. Adv. Exp. Med. Biol 2019, 1144:1–24. [DOI] [PubMed] [Google Scholar]
- 36.Chachques JC, Trainini JC, Lago N, Cortes-Morichetti M, Schussler O, Carpentier A: Myocardial Assistance by Grafting a New Bioartificial Upgraded Myocardium (MAGNUM trial): clinical feasibility study. Ann. Thorac. Surg 2008, 85:901–908. [DOI] [PubMed] [Google Scholar]
- 37.Serpooshan V, Wu SM(Eds): Cardiovascular regenerative medicine: tissue engineering and clinical applications. Springer International Publishing; 2019. [Google Scholar]
- •38.Park S-J, Kim RY, Park B-W, Lee S, Choi SW, Park J-H, Choi JJ, Kim S-W, Jang J, Cho D-W, et al. : Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction. Nat. Commun 2019, 10:3123. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article reports the use of combinatorial cardiac patch where both human induced pluripotent stem cells and human mesenchymal stem are delivered to the heart and provide a complimentary treatment strategy for enhanced vascularization and cardiac function.
- 39.Steele AN, MacArthur JW, Woo YJ: Stem cell therapy: healing or hype? why stem cell delivery doesn’t work. Circ. Res 2017, 120:1868–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen PK, Neofytou E, Rhee J-W, Wu JC: Potential strategies to address the major clinical barriers facing stem cell regenerative therapy for cardiovascular disease: A review. JAMA Cardiol. 2016, 1:953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neofytou E, O’Brien CG, Couture LA, Wu JC: Hurdles to clinical translation of human induced pluripotent stem cells. J. Clin. Invest 2015, 125:2551–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Golpanian S, Schulman IH, Ebert RF, Heldman AW, DiFede DL, Yang PC, Wu JC, Bolli R, Perin EC, Moyé L, et al. : Concise review: review and perspective of cell dosage and routes of administration from preclinical and clinical studies of stem cell therapy for heart disease. Stem Cells Transl. Med 2016, 5:186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fisher SA, Brunskill SJ, Doree C, Mathur A, Taggart DP, Martin-Rendon E: Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst. Rev 2014, doi: 10.1002/14651858.CD007888.pub2. [DOI] [PubMed] [Google Scholar]
- 44.Fisher SA, Zhang H, Doree C, Mathur A, Martin-Rendon E: Stem cell treatment for acute myocardial infarction. Cochrane Database Syst. Rev 2015, doi: 10.1002/14651858.CD006536.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang J-N, Cores J, Huang K, Cui X-L, Luo L, Zhang J-Y, Li T-S, Qian L, Cheng K: Concise review: is cardiac cell therapy dead? embarrassing trial outcomes and new directions for the future. Stem Cells Transl. Med 2018, 7:354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Svystonyuk DA, Mewhort HEM, Fedak PWM: Using acellular bioactive extracellular matrix scaffolds to enhance endogenous cardiac repair. Front. Cardiovasc. Med 2018, 5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••47.Huang K, Ozpinar EW, Su T, Tang J, Shen D, Qiao L, Hu S, Li Z, Liang H, Mathews K, et al. : An off-the-shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs. Sci. Transl. Med 2020, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]; Authors improve upon current limitations in cardiac patch therapeutics and demonstrate an off the shelf, completely artificial cardiac patch that maintains therapeutic efficacy following long-term cryopreservation.
- 48.Cravedi P, Farouk S, Angeletti A, Edgar L, Tamburrini R, Duisit J, Perin L, Orlando G: Regenerative immunology: the immunological reaction to biomaterials. Transpl Int 2017, 30:1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uman S, Dhand A, Burdick JA: Recent advances in shear-thinning and self-healing hydrogels for biomedical applications. J. Appl. Polym. Sci 2020, 137:48668. [Google Scholar]
- •50.Mealy JE, Chung JJ, Jeong H-H, Issadore D, Lee D, Atluri P, Burdick JA: Injectable Granular Hydrogels with Multifunctional Properties for Biomedical Applications. Adv. Mater. Weinheim 2018, 30:e1705912. [DOI] [PubMed] [Google Scholar]; This article discusses another class of injectable hydrogels called granular hydrogels that expand the complexity of currently available hydrogel systems.
- 51.Deng B, Shen L, Wu Y, Shen Y, Ding X, Lu S, Jia J, Qian J, Ge J: Delivery of alginate-chitosan hydrogel promotes endogenous repair and preserves cardiac function in rats with myocardial infarction. J. Biomed. Mater. Res. A 2015, 103:907–918. [DOI] [PubMed] [Google Scholar]
- 52.Peña B, Laughter M, Jett S, Rowland TJ, Taylor MRG, Mestroni L, Park D: Injectable hydrogels for cardiac tissue engineering. Macromol Biosci 2018, 18:e1800079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams C, Budina E, Stoppel WL, Sullivan KE, Emani S, Emani SM, Black LD: Cardiac extracellular matrix-fibrin hybrid scaffolds with tunable properties for cardiovascular tissue engineering. Acta Biomater. 2015, 14:84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grover GN, Rao N, Christman KL: Myocardial matrix-polyethylene glycol hybrid hydrogels for tissue engineering. Nanotechnology 2014, 25:014011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mukherjee R, Zavadzkas JA, Saunders SM, McLean JE, Jeffords LB, Beck C, Stroud RE, Leone AM, Koval CN, Rivers WT, et al. : Targeted myocardial microinjections of a biocomposite material reduces infarct expansion in pigs. Ann. Thorac. Surg 2008, 86:1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anker SD, Coats AJS, Cristian G, Dragomir D, Pusineri E, Piredda M, Bettari L, Dowling R, Volterrani M, Kirwan B-A, et al. : A prospective comparison of alginate-hydrogel with standard medical therapy to determine impact on functional capacity and clinical outcomes in patients with advanced heart failure (AUGMENT-HF trial). Eur. Heart J 2015, 36:2297–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Traverse JH, Henry TD, Dib N, Patel AN, Pepine C, Schaer GL, DeQuach JA, Kinsey AM, Chamberlin P, Christman KL: First-in-Man Study of a Cardiac Extracellular Matrix Hydrogel in Early and Late Myocardial Infarction Patients. JACC Basic Transl. Sci 2019, 4:659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sepantafar M, Maheronnaghsh R, Mohammadi H, Rajabi-Zeleti S, Annabi N, Aghdami N, Baharvand H: Stem cells and injectable hydrogels: Synergistic therapeutics in myocardial repair. Biotechnol. Adv 2016, 34:362–379. [DOI] [PubMed] [Google Scholar]
- 59.Wang LL, Liu Y, Chung JJ, Wang T, Gaffey AC, Lu M, Cavanaugh CA, Zhou S, Kanade R, Atluri P, et al. : Local and sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischemic injury. Nat. Biomed. Eng 2017, 1:983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steele AN, Paulsen MJ, Wang H, Stapleton LM, Lucian HJ, Eskandari A, Hironaka CE, Farry JM, Baker SW, Thakore AD, et al. : Multi-phase catheter-injectable hydrogel enables dual-stage protein-engineered cytokine release to mitigate adverse left ventricular remodeling following myocardial infarction in a small animal model and a large animal model. Cytokine 2020, 127:154974. [DOI] [PubMed] [Google Scholar]
- 61.Purcell BP, Lobb D, Charati MB, Dorsey SM, Wade RJ, Zellars KN, Doviak H, Pettaway S, Logdon CB, Shuman JA, et al. : Injectable and bioresponsive hydrogels for on-demand matrix metalloproteinase inhibition. Nat. Mater 2014, 13:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••62.Wang LL, Chung JJ, Li EC, Uman S, Atluri P, Burdick JA: Injectable and protease-degradable hydrogel for siRNA sequestration and triggered delivery to the heart. J. Control. Release 2018, 285:152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study investigates an injectable hydrogel capable of responding to upregulated proteolytic activity following a myocardial infarction in a small and large animal model. The protease-responsive hydrogel provides a platform for the “on demand” release of therapeutics.


