Abstract
Background:
Synaptic loss is a feature of MS pathology that can be seen even in normal-appearing grey matter. Opsonization of synapses with complement components may underlie pathologic synapse loss.
Objective:
We sought to determine whether circulating neuronal-enriched and astrocytic-enriched extracellular vesicles (NEVs and AEVs) provide biomarkers reflecting complement-mediated synaptic loss in MS.
Methods:
From plasma of 61 people with MS (46 RRMS, 15 Progressive MS) and 31 healthy controls, we immunocaptured L1CAM+ NEVs and GLAST+ AEVs. We measured pre-and post-synaptic proteins synaptopodin and synaptophysin in NEVs and complement components (C1q, C3, C3b/iC3b, C4, C5, C5a, C9, Factor B, Factor H) in AEVs, total circulating EVs and neat plasma.
Results:
We found lower levels of NEV synaptopodin and synaptophysin in MS compared to controls (p < 0.0001 for both). In AEVs, we found higher levels of multiple complement cascade components in people with MS compared to controls; these differences were not noted in total EVs or neat plasma. Strikingly, there were strong inverse correlations between NEV synaptic proteins and multiple AEV complement components in MS, but not in controls.
Conclusion:
Circulating EVs could identify synaptic loss in MS and suggest a link between astrocytic complement production and synaptic loss.
Keywords: extracellular vesicles, complement, synaptic dysfunction, astrocytes, multiple sclerosis, biomarkers
INTRODUCTION
Multiple sclerosis (MS) is a chronic neurological disorder with autoimmune and degenerative components [1]. While imaging and pathology studies have long focused on white matter demyelinating lesions, recent studies have shown the presence of extensive synaptic pathology in MS [2]–[5]. This is evident even in grey matter that does not demonstrate evidence of demyelination and could, perhaps, account for disease progression in MS independent of demyelinating episodes. The etiology and pathogenesis of synaptic loss in MS remains unknown, but glial cells are considered major effectors of synaptopathy across neurological disorders [6].
There is also a growing interest in the role of complement in MS pathophysiology. Complement refers to interconnected cascades of sequentially activated humoral and cellular proteins that connect innate and adaptive immunity and comprise a classical pathway (activated by IgM or IgG immunocomplexes), an alternative pathway (activated by pathogenic antigens) and a lectin pathway (activated by mannose residues) [7]. In the periphery, complement is mainly produced by the liver, whereas, in the brain, local synthesis occurs, primarily by astrocytes and microglia [8]. Early complement components play a pivotal role in pruning of synapses during development [9], however this mechanism may become active in aging and pathological states causing synaptic loss [10],[11]. Complement activation and deposition is observed in MS cortical grey matter and in association with glia and in relation to synaptic loss [12]–[14]. We recently demonstrated that genetic variants for early complement components are related to retinal neurodegeneration in MS [15]. The role of various brain cell populations in complement production and activation has not been completely elucidated, although astrocytes have been of interest following the description of neurotoxic A1 astrocytes that express high levels of C3 [16]. These reactive astrocytes have been noted in increased numbers in several neurodegenerative diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and also MS [16].
Extracellular vesicles (EVs) are membranous nanoparticles produced by all cells that play important roles in inter-cellular communication [17], whereas their cargo can be an important source of biomarkers. We and others have demonstrated that neuronal-enriched and astrocytic-enriched EVs (NEVs and AEVs) can be isolated from plasma and provide biomarkers for neurological disorders, including AD [18]–[20], PD [21], Restless Legs Syndrome [22], and Traumatic Brain Injury [23]. EV biomarkers are useful for diagnosis and preclinical prediction [24], but may also provide insights into disease mechanisms (e.g. lysosomal dysfunction [25], insulin resistance [26] and synaptic abnormalities [27] in AD). We have shown that circulating AEVs contain multiple complement components and have demonstrated higher levels in AD patients compared to controls [28].
In this study, we simultaneously isolated NEVs and AEVs from plasma of MS patients and healthy controls, quantified their concentration and size distribution, and measured one pre-synaptic (synaptophysin) and one post-synaptic (synaptopodin) protein in NEVs and several complement components in AEVs. We not only identified major differences in the levels of these markers in both sets of EVs between MS and control individuals, but, most strikingly, revealed strong inverse correlations between NEV synaptic proteins and AEV complement components in MS patients, but not in controls. These findings indirectly but strongly suggest the involvement of complement in synaptic damage in MS and suggest that circulating EVs may be used to monitor this pathogenic process.
SUBJECTS/MATERIALS AND METHODS
Study cohort and blood draws
We studied 61 MS patients (46 with Relapsing Remitting (RR), 15 with Progressive (P) MS) and 31 healthy controls (HC, defined as being free of any neurological or autoimmune disease) from the Johns Hopkins MS Center. All participants provided informed consent using a Johns Hopkins Institutional Review Board approved protocol and subsequently underwent collection of demographic and clinical information and a blood draw. Approximately 10 mL of venous blood were collected in plasma separator tubes containing EDTA, incubated for 10 minutes at room temperature (RT) and then centrifuged at 3000 rpm for 15 minutes at RT. Supernatant plasma was divided into 1.0 mL aliquots and stored at −80 ºC as previously described [29]. Samples had not been thawed prior to EV isolation. Pre-analytical factors for blood collection and storage comply with guidelines for EV biomarkers [30],[31].
Neuronal and astrocytic EV isolation
Plasma samples were thawed on ice, divided into two halves and immediately subjected to isolation of AEVs and NEVs using a methodology extensively described[24]. Briefly, fibrinogen and fibrin proteins known to impede efficient EV recovery were removed by incubating 500 μL plasma with 5 U/mL of thrombin for 30 minutes at RT followed by addition of 495 μL of Dulbecco’s PBS-1X (DPBS) supplemented with protease and phosphatase inhibitors and centrifugation at 4,000 g for 20 minutes at 4 °C. The supernatant was transferred to a sterile 1.5 mL microtube and total EVs were sedimented by incubation with 252 μL of ExoQuick® for 1 hour at 4 °C followed by centrifugation at 1,500 g for 20 minutes at 4 °C. Pelleted total EVs were resuspended by overnight gentle rotation mixing at 4 °C in 700 μL of ultrapure distilled water (dH2O) supplemented with protease and phosphatase inhibitors. Resuspended total EVs were incubated with 4 μg of biotinylated antibody against the astrocytic glutamate aspartate transporter 1 (GLAST) or against neural cell adhesion molecule L1CAM (i.e. anti-human CD171) in 100 μL of dH2O supplemented with protease and phosphatase inhibitors and 10% bovine serum albumin (BSA), for 14 hours at RT, to immunocapture AEVs and NEVs respectively. EV-antibody complexes were incubated with 40 μL of washed Pierce™ Streptavidin Plus UltraLink™ Resin in 60 μL of dH2O supplemented with protease and phosphatase inhibitors and 10% BSA, for 30 minutes at RT. EV-antibody-bead complexes were sedimented by centrifugation at 800 g for 10 minutes at 4 °C followed by removal of unbound EVs and soluble proteins in the supernatant. Bound AEVs and NEVs were eluted using 200 μL of 0.1 M glycine followed by vortexing for 10 seconds and centrifugation at 4,500 g for 5 minutes at 4 °C. 10 μL of intact EVs in the supernatant were transferred to a sterile microtube containing 90 μL of DPBS supplemented with protease and phosphatase inhibitors and used for determination of particle concentration and average diameter using nanoparticle tracking analysis (NTA) (Nanosight NS500; Malvern, Amesbury, UK). The remaining intact EVs were transferred to a sterile microtube containing 30 μL of 1 M tris buffer, 50 μL of 10% BSA and 740 μL of MPER lysis buffer (Thermo Fisher Scientific, Cincinnati, OH) supplemented with protease and phosphatase inhibitors and subjected to 2 freeze-thaw cycles for EV lysis. The solution was stored in 100 μL aliquots at −80 °C until used for immunoassays.
All laboratory personnel were blinded to group assignments of samples during the subsequent EV isolation and biomarker measurements.
Synaptic protein measurements
Synaptic protein markers levels were measured in lysed NEVs using ELISAs for Synaptopodin (LS BioScience, Seattle, WA) and Synaptophysin (Cusabio, Houston, TX). A titration ELISA ascertained optimal dilution and subsequently samples were diluted 1:50. We performed ELISAs as per manufacturer protocol in duplicate. As internal control (IC) we included samples from three participants in every plate to assess plate-to-plate variability. The mean coefficient of variation (CV) of ICs across plates were <20% for both synaptophysin and synaptopodin. The average CV of duplicates across plates were <10% for both assays. Limit of Detection (LOD) was defined as mean of the blank plus 2.5 times standard deviation of blank. Values that were below the LOD were imputed to the LOD value of that particular plate. The lower limit of quantification (LLOQ) (defined as the concentration of the standard with i) signal above the LOD, ii) CV among duplicates < 20%, and iii) recovery > 80% and < 120%), was also calculated for each plate, and the mean LLOQ was used as the global LLOQ. 100% and 99% of samples were above the LLOQ and within the linear range of the standard curve for synaptophysin and synaptopodin, respectively.
Complement component measurements
We quantified complement components C5, C5a and C9 in neat plasma and lysed total EVs and AEVs using a customized version of the MILLIPLEX® MAP Human Complement Panel 1 assay (cat no. HCMP1MAG-19K), and C1q, C3, C3b/iC3b, C4, Factor B and Factor H in lysed AEVs using the MILLIPLEX® MAP Human Complement Panel 2 assay (cat no. HCMP2MAG-19K) (EMD Millipore Corporation, Billerica, MA). Plates were read using the Luminex® 200™ System and the xPOTENT® acquisition software (Luminex Corporation, Austin, TX). All samples were measured in duplicate. An internal control (IC) sample prepared from a healthy donor was included in every plate to assess plate-to-plate variability. The mean coefficient of variation (CV) of IC across plates were <5% for all assays. IC measurements for each plate were used to create a correction factor (defined as mean value of IC measurement for that plate divided by the mean IC values across plates), which was used to normalize measurements across plates. Samples with CV among duplicates >20% were excluded from the analysis (Only one sample for C9, no other samples were excluded). The average CV of duplicates across plates were <10% for all analytes. The Limit of Detection (LOD) was defined as the mean of the blank plus 2.5 times the standard deviation of the blank. Fluorescent signals that were below the LOD were imputed to the LOD value of that particular plate. The LLOQ (defined above), and the upper limit of quantification (ULOQ) (defined as the upper asymptote of the four-parameter logistic regression curve) were also calculated for each plate, and the mean LLOQ and ULOQ were used as the global LLOQ and ULOQ. Samples with concentrations bellow the LLOQ and CV among duplicates < 20%, were imputed to the global LLOQ. For the analytes with values above the ULOQ (C1q, C3b/iC3b, C4, Factor B), the raw fluorescent signal intensity was compared instead of the concentration. The LLOQ and ULOQ for various analytes are listed (all in ng/ml except for C5a which was pg/ml)-C1q (LLOQ: 0.92 ± 0.86, ULOQ: 56.7 ± 0.8), C3 (LLOQ: 0.29 ± 0.05, ULOQ: 133.4 ± 79.9), C3b/iC3b (LLOQ: 22.3 ± 9.3, ULOQ: 5186 ± 734), C4 (LLOQ: 0.55 ± 0.00, ULOQ: 356.7 ± 141.3), C5 (LLOQ: 6.3 ± 3.0, ULOQ: 2014.5 ± 14.9), C5a (LLOQ: 4.22 ± 0.05, ULOQ: 935.9 ± 37.8), C9 (LLOQ: 36.7 ± 5.8, ULOQ: 29151 ± 1491), Factor B (LLOQ: 0.08 ± 0.01, ULOQ: 62.97 ± 1.33) and Factor H (LLOQ: 0.46 ± 0.04, ULOQ: 240.0 ± 106.3).
Serum Neurofilament light measurement
We obtained serum neurofilament light measurements on 58 of the 61 people with MS and 27 of the 31 healthy controls on serum samples collected concomitantly with the plasma utilized for the EV analyses. These measurements were performed using single molecule array (SIMOA) technology at Quanterix Corporation, Billerica, MA, USA.
Statistical analyses
We utilized Student’s t-test to compare biomarkers between MS and control groups and one-way ANOVA with Dunnett’s multiple comparisons test to compare HC, RRMS and PMS groups. We also explored linear regression including age, sex and NEV or AEV concentration as additional factors/covariates and found similar results as with univariate comparisons. We utilized Spearman correlation to assess correlation between NEV synaptophysin, synaptopodin, and age and AEV complement components, separately by group (MS or HC). We also utilized Spearman correlations to test correlations between serum NfL levels, age and NEV synaptopodin or synaptophysin. Statistical analyses were performed with either Stata Version 15 or GraphPad Prism 8.0.
RESULTS
Study cohort characteristics are provided in Table 1. The HCs and RRMS groups were significantly younger than PMS (p<0.0001 for both), however there was no significant difference in age between RRMS and HC groups (p=0.19). A schematic overview of the study is provided in Figure 1A. None of the MS patients were in active relapse and none of them had received high-dose steroids within 30 days of blood collection.
Table 1:
Study cohort characteristics
| Characteristic | HC (n=31) | RRMS (n=46) | PMS (n=15) | p value |
|---|---|---|---|---|
| Age (years) | 44.2 ± 10.9 | 48.9 ± 11.4 | 63.1 ± 8.04 | < 0.0001 |
| Mean ± SD | ||||
| Sex | 23:8 | 32:13 | 11:4 | 0.95 |
| F:M | ||||
| Disease duration (years) | - | 11.7 ± 7.1 | 21.1 ± 10.9 | |
| Mean ± SD | ||||
| EDSS | - | 2.5 (1.5 – 3.5) | 6.5 (4.0 – 6.5) | |
| Median (IQR) | ||||
| DMT (%) | - | |||
| None | 3 | 8 | ||
| Injectable | 17 | 2 | ||
| Oral | 11 | 2 | ||
| Natalizumab | 12 | 1 | ||
| Ocrelizumab | 1 | 2 | ||
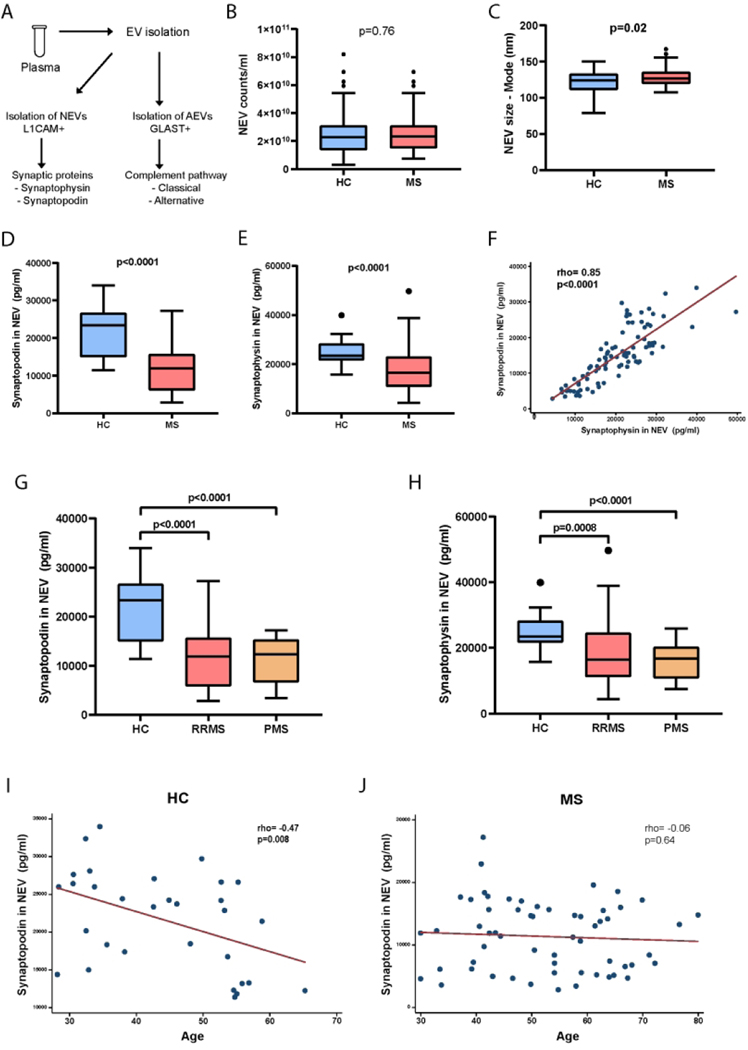
Figure 1. Synaptic proteins in NEVs are reduced in MS.
(A) Schematic representation of the study involving 60 MS patients (45 with RRMS, 15 with PMS) and 31 HC. (B) Concentration of NEVs in MS patient and HC plasma. (C) Average diameter (size) of NEVs in MS and HC groups. (D) NEV Synaptopodin and (E) Synaptophysin concentrations in MS and HC groups. (F) Tight correlation of nEV synaptopodin and synaptophysin concentrations in MS and HC groups. (G) NEV synaptopodin and (H) synaptophysin concentrations in HC, RRMS and PMS groups. (I) Scatter plot of NEV synaptopodin concentration by age in the HC group. (J) Scatter plot of nEV synaptopodin concentration by age in the MS group. p values for B, C, D & E are derived from Student’s t tests. p values for G & H are derived from one-way ANOVA with Dunnett’s multiple comparisons test. rho and p values for F, I & J are derived from Spearman’s correlation.
MS patients have reduced levels of synaptic proteins in NEVs
The concentration of plasma NEVs was not different between MS patients and controls (Figure 1 B), but NEV average diameter was slightly greater in the MS group (Figure 1C). Levels of synaptopodin and synaptophysin in NEVs were markedly lower in MS patients compared to controls (p<0.0001 for both, Figure 1 D-E). These differences were essentially unchanged in linear regression models adjusting for age, sex and NEV counts (p<0.0001 for both). Since the majority of MS patients were on a DMT we performed sensitivity analyses restricting to MS patients not on a DMT and identified similar results with reduced levels of both synaptopodin (p=0.007) and synaptophysin (p=0.005) in MS compared to controls.
We noted a strong correlation between NEV synaptophysin and synaptopodin in both MS and control groups (Figure 1F). We also compared the NEV synaptic markers between HC and MS sub-types (RRMS and PMS) and noted significant differences between RRMS and PMS versus controls for both markers, but no significant differences between RRMS and PMS (Figure 1G & H).
We examined the relationship of synaptophysin and synaptopodin levels with age and noted a strong inverse correlation for synaptopodin with age in the HC group (Figure 1 I); but not in the MS group (Figure 1J). We noted similar trends for synaptophysin (data not shown).
We also examined the relationship between these markers and serum NfL levels. As expected, mean serum NfL levels were higher in the MS group compared to controls (10.78 vs 7.41 pg/ml, p=0.01). Serum NfL levels were positively correlated with age in both control (r=0.71, p=<0.0001) and MS groups (r=0.58, p<0.0001). In the control group there was a significant inverse correlation noted between NEV synaptopodin levels and NfL (r=−0.42, p=0.02) but not for NEV synaptophysin and NfL (r=−0.22, p=0.26). This relationship was not noted when adjusting for age, suggesting that this relationship is driven by ageing in healthy controls. In the MS group there was no correlation noted between serum NfL levels and either NEV synaptopodin or synaptophysin levels either in unadjusted analyses or adjusting for age, sex, DMT and/or disease sub-type.
MS patients have elevated levels of several complement cascade components in AEVs
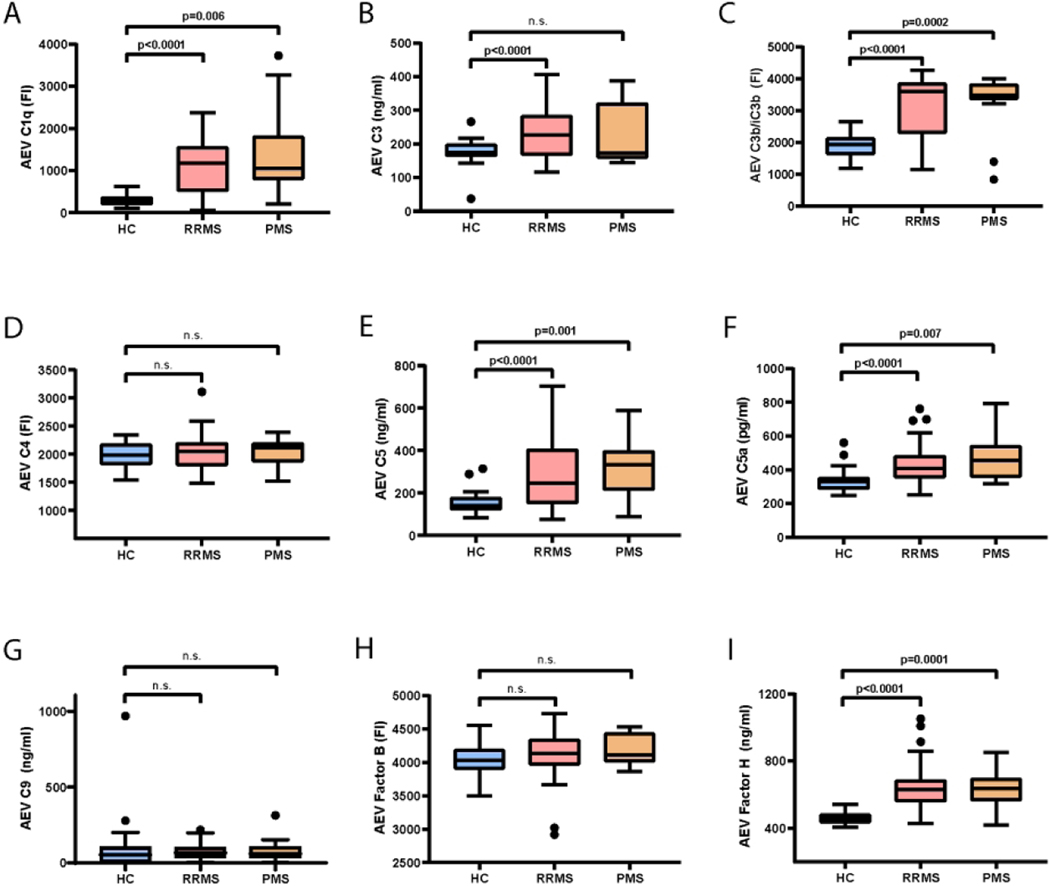
The concentration of AEVs was higher in controls (3.76×1011/ml vs 5.32×1010/ml, p<0.0001) but AEV average diameter did not differ between groups (data not shown). Multiple complement cascade components were markedly elevated in the MS group compared to controls, including early (C1q, C3, C3b/iC3b), as well as late components (C5, C5a) and inhibitory factors (Factor H – alternative pathway) (Figure 2 A-I). These results were essentially unchanged when we adjusted for age, sex and AEV concentration. There were no correlations between age and AEV complement components in the HC or MS groups (all p >0.05).
Figure 2. Complement components in AEVs are increased in MS.
Complement component levels in AEVs from HC, RRMS and PMS groups – (A) C1q, (B) C3, (C) C3b/iC3b, (D) C4, (E) C5, (F) C5a, (G) C9, (H) Factor B and (I) Factor H. p values for A-I are derived from one-way ANOVA with Dunnett’s multiple comparisons test.
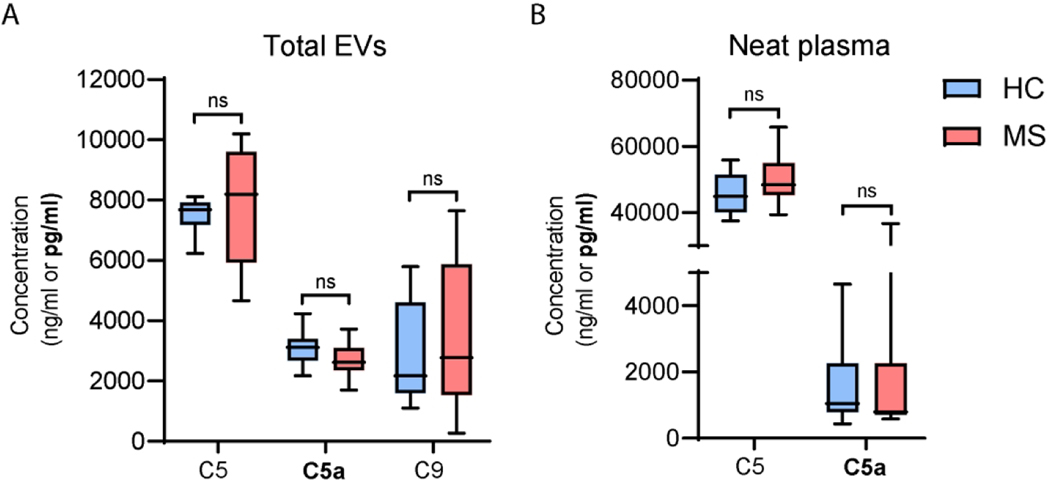
To evaluate whether the higher levels of complement observed in AEVs of MS patients compared to control reflected differences in total circulating EVs or plasma, we selected a sub-cohort of 10 MS patients and 10 controls with AEV complement levels around the mean value for their group. In this sub-cohort, we measured C5, C5a and C9 protein levels in total EVs and neat plasma and found that levels were not significantly different between groups (Figure 3A-B).
Figure 3. Complement levels in total circulating EVs and neat plasma do not differ between MS and HC groups.
C5, C5a and C9 protein levels in total circulating EVs (A) and neat plasma (B) from 10 MS patients and 10 HC. Subjects were selected from the larger cohort so that their complement levels in AEVs were around the group average to serve as group representatives. All C9 signals from neat plasma samples were bellow the LOD and hence not shown. p values for A and B are derived from two-tailed unpaired Student’s t tests.
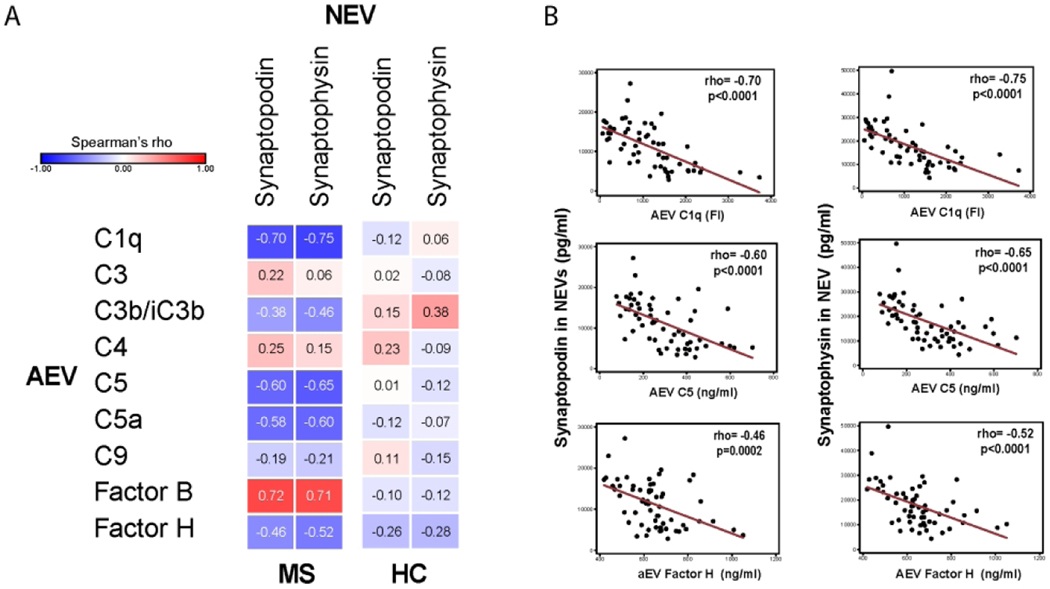
Strong correlations between AEV complement components and NEV synaptic proteins
We noted strong inverse correlations between the levels of multiple complement components in AEVs and the levels of both synaptophysin and synaptopodin in NEVs in MS patients (Figure 4A), but not noted in the HC group (Figure 4A). Some examples of these correlations are depicted in Figure 4B. Interestingly, the alternative complement pathway component-Factor B had a strong positive correlation with synaptophysin and synaptopodin levels in MS patients (Figure 4A).
Figure 4. Complement component levels in AEVs are strongly correlated with NEV synaptic protein levels.
(A) Heat map of correlations between NEV synaptic proteins and AEV complement components in MS and HC groups demonstrates strong correlations in MS but not in HC. (B) Scatter plots depicting correlation between selected AEV complement components (C1q, C5, Factor H) and NEV synaptopodin (left) or synaptophysin (right). Rho and p values for J & K are derived from Spearman’s correlations.
DISCUSSION
In this study, we utilized NEVs to probe biomarkers related to pre-and post-synaptic integrity and AEVs to probe astrocyte complement production in MS patients and controls, revealing striking differences for both types of biomarkers and strong correlations between them, in MS patients but not in controls.
To the best of our knowledge this is the first study to measure NEV synaptic proteins in MS. The reduction in NEV synaptic proteins is similar to that noted in previous studies in neurodegenerative diseases, e.g. frontotemporal disease (FTD) and AD [27],[32]. In FTD and AD, there were lower levels of NEV synaptopodin and synaptophysin compared to controls and their levels were associated with clinical disease severity [27]. These findings were interpreted as reflection of the severity of neurodegeneration and synaptic loss. The strong inverse correlation of age with NEV synaptic proteins in healthy controls in this study is also consistent with this conclusion. The absence of an association between serum NfL, a marker of inflammatory axonal damage, and NEV synaptic proteins in people with MS, also suggests that NEV synaptic protein levels measure a distinct pathological process.
To the best of our knowledge this is also the first study to isolate AEVs and measure complement components in MS. Given the evidence of complement activation in grey matter lesions in MS, it is likely that this cascade plays a role in MS pathophysiology affecting neurons and synapses [12],[13]. Recent studies have demonstrated increased C3 production in neurotoxic astrocytes, and a link of early complement cascade-related genetic polymorphisms with neurodegeneration in MS [15],[16]. Here, we found increased levels of multiple complement components in AEVs in MS compared to controls. We also demonstrated that differences in AEVs cannot be attributed to systemic differences in complement levels in total circulating EVs or neat plasma; this is an important finding since some of these complement components have previously been noted to be increased in either CSF or circulation in MS [33],[34]. A previous study that measured levels of multiple complement components in AEVs in AD also demonstrated elevations in multiple complement components and this was interpreted as a reflection of the increased abundance of reactive neurotoxic astrocytes in the disease [16],[28]. A similar explanation could be invoked for our observations given the description of similar astrocytes in MS [16].
Multiple studies have demonstrated the importance of complement deposition in synapse phagocytosis both during development and pathophysiological states [9],[10]. The striking inverse correlation between NEV synaptic proteins and AEV complement cascade components in MS patients could potentially be explained by a similar mechanism in MS. Indeed, complement activation and deposition has been identified in MS brain tissue including cortical grey matter [13],[35]. A recent study demonstrated evidence of complement deposition specifically at synapses in MS brains and in an animal model inhibition of the complement cascade prevented synaptic loss[11]. Our data also demonstrated relationships between both classical and alternative complement pathway components and synaptic proteins in keeping with pathological data from MS tissue demonstrating the activation of both complement pathways in MS lesions[12]. The presence of significant correlations only in MS and not in healthy controls suggests that our findings may reflect a pathogenic mechanism rather than a normal phenomenon.
Limitations of this study include its cross-sectional nature, with samples drawn from a single center and a significant difference in age between PMS and control groups. We also had participants on various DMTs and for varying durations of treatment, which may have resulted in smaller observed differences between groups, if DMTs are capable fo alleviating the pathological processes causing synaptic loss. On the other hand, it is unlikely that DMTs would worsen these processes and contribute to any of the observed differences. To address this concern, we performed sensitivity analyses restricting NEV synaptic protein comparisons to untreated MS participants and noted similar results when compared to the whole cohort. Future longitudinal studies will be required to assess whether and which DMTs have an impact on NEV or AEV derived measures. While our analyses were adjusted for age, it would be important for future replication studies to utilize age-matched cohorts drawn from multiple sites.
Using subpopulations of plasma EVs to interrogate the state of their cells of origin, we not only identify potential new biomarkers for MS, but also provide novel insights into disease pathophysiology. These findings indirectly but strongly implicate astrocytic complement in synaptopathy in MS and motivate targeting complement therapeutically to alleviate disease progression. EV biomarkers could be utilized as outcomes in proof-of-principle studies targeting glial and complement mediated mechanisms of neurodegeneration in MS, augmenting therapeutic discovery for the disease.
ACKNOWLEDGMENTS
This study was supported in part by the Intramural Research Program of the National Institute on Aging, NIH; in part by a Marilyn Hilton Award for Innovation in MS from the Conrad N. Hilton Foundation to PB and DK; and in part by a Pilot award from the Myelin Repair Foundation to PB.
Footnotes
CONFLICT OF INTEREST
The authors report no conflicts of interest.
REFERENCES
- 1.Reich DS, Lucchinetti CF, Calabresi PA. Multiple Sclerosis Longo DL, ed. N. Engl. J. Med. 2018; 378(2):169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jürgens T, Jafari M, Kreutzfeldt M, et al. Reconstruction of single cortical projection neurons reveals primary spine loss in multiple sclerosis. Brain. 2016; 139(1):39–46. [DOI] [PubMed] [Google Scholar]
- 3.Dutta R, Chang A, Doud MK, et al. Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients. Ann. Neurol. 2011; 69(3):445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wegner C, Esiri MM, Chance SA, Palace J, Matthews PM. Neocortical neuronal, synaptic, and glial loss in multiple sclerosis. Neurology. 2006; 67(6):960–967. [DOI] [PubMed] [Google Scholar]
- 5.Mandolesi G, Gentile A, Musella A, et al. Synaptopathy connects inflammation and neurodegeneration in multiple sclerosis. Nat. Rev. Neurol. 2015; 11(12):711–724. [DOI] [PubMed] [Google Scholar]
- 6.Chung WS, Welsh CA, Barres BA, Stevens B. Do glia drive synaptic and cognitive impairment in disease? Nat. Neurosci. 2015; 18(11):1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noris M, Remuzzi G. Overview of complement activation and regulation. Semin. Nephrol. 2013; 33(6):479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veerhuis R, Nielsen HM, Tenner AJ. Complement in the brain. Mol. Immunol. 2011; 48(14):1592–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens B, Allen NJ, Vazquez LE, et al. The Classical Complement Cascade Mediates CNS Synapse Elimination. [DOI] [PubMed] [Google Scholar]
- 10.Hong S, Beja-Glasser VF, Nfonoyim BM, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science (80-. ). 2016; 352(6286):712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werneburg S, Jung J, Kunjamma RB, et al. Targeted complement inhibition at synapses prevents microglial synaptic engulfment and synapse loss in demyelinating disease. bioRxiv. 2019:841601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watkins LM, Neal JW, Loveless S, et al. Complement is activated in progressive multiple sclerosis cortical grey matter lesions. J. Neuroinflammation 2016; 13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingram G, Loveless S, Howell OW, et al. Complement activation in multiple sclerosis plaques: an immunohistochemical analysis. Acta Neuropathol. Commun. 2014; 2(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michailidou I, Willems JGP, Kooi E-J, et al. Complement C1q-C3-associated synaptic changes in multiple sclerosis hippocampus. Ann. Neurol. 2015; 77(6):1007–1026. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald KC, Kim K, Smith MD, et al. Early complement genes are associated with visual system degeneration in multiple sclerosis. Brain. 2019; 142(9):2722–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017; 541(7638):481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019; 21(1):9–17. [DOI] [PubMed] [Google Scholar]
- 18.Jia L, Qiu Q, Zhang H, et al. Concordance between the assessment of Aβ42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimer’s Dement. 2019; 15(8):1071–1080. [DOI] [PubMed] [Google Scholar]
- 19.Fiandaca MS, Kapogiannis D, Mapstone M, et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers. Dement. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapogiannis D, Mustapic M, Shardell MD, et al. Association of Extracellular Vesicle Biomarkers with Alzheimer Disease in the Baltimore Longitudinal Study of Aging. JAMA Neurol. 2019; 76(11):1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Athauda D, Gulyani S, Karnati H, et al. Utility of Neuronal-Derived Exosomes to Examine Molecular Mechanisms That Affect Motor Function in Patients With Parkinson Disease: A Secondary Analysis of the Exenatide-PD Trial. JAMA Neurol. 2019; 76(4):420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chawla S, Gulyani S, Allen RP, et al. Extracellular vesicles reveal abnormalities in neuronal iron metabolism in restless legs syndrome. Sleep. 2019; 42(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goetzl EJ, Elahi FM, Mustapic M, et al. Altered levels of plasma neuron-derived exosomes and their cargo proteins characterize acute and chronic mild traumatic brain injury. FASEB J. 2019; 33(4):5082–5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mustapic M, Eitan E, Werner JK, et al. Plasma Extracellular Vesicles Enriched for Neuronal Origin: A Potential Window into Brain Pathologic Processes. Front. Neurosci. 2017; 11:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goetzl EJ, Boxer A, Schwartz JB, et al. Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology. 2015; 85(1):40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapogiannis D, Boxer A, Schwartz JB, et al. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J. 2014; 29(2):589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goetzl EJ, Kapogiannis D, Schwartz JB, et al. Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimers disease. FASEB J. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goetzl EJ, Schwartz JB, Abner EL, Jicha GA, Kapogiannis D. High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann. Neurol. 2018; 83(3):544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhargava P, Nogueras-Ortiz C, Chawla S, et al. Altered Levels of Toll-Like Receptors in Circulating Extracellular Vesicles in Multiple Sclerosis. Cells. 2019; 8(9):1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witwer KW, Buzás EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013; 2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Witwer KW, Soekmadji C, Hill AF, et al. Updating the MISEV minimal requirements for extracellular vesicle studies: building bridges to reproducibility. J. Extracell. Vesicles 2017; 6(1):1396823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goetzl EJ, Abner EL, Jicha GA, Kapogiannis D, Schwartz JB. Declining levels of functionally specialized synaptic proteins in plasma neuronal exosomes with progression of Alzheimer’s disease. FASEB J. 2017:fj.201700731R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ingram G, Hakobyan S, Hirst CL, et al. Complement regulator factor H as a serum biomarker of multiple sclerosis disease state. Brain. 2010; 133(Pt 6):1602–11. [DOI] [PubMed] [Google Scholar]
- 34.Ingram G, Hakobyan S, Hirst CL, et al. Systemic complement profiling in multiple sclerosis as a biomarker of disease state. Mult. Scler. J. 2012; 18(10):1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watkins LM, Neal JW, Loveless S, et al. Complement is activated in progressive multiple sclerosis cortical grey matter lesions. J. Neuroinflammation 2016; 13(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]