Abstract
Background/Objectives:
Due to high rates of multimorbidity, polypharmacy, hazardous alcohol and opioid use, middle-aged Veterans are at risk for serious falls (those prompting a visit with a healthcare provider), posing significant risk to their forthcoming geriatric health and quality of life. We developed and validated a predictive model of the six-month risk of serious falls among middle-aged Veterans.
Design:
Cohort study
Setting:
Veterans Health Administration
Participants:
Veterans aged 45–65 years who presented for care within the VA between 2012 and 2015 (N=275,940).
Exposures:
The exposures of primary interest were substance use (alcohol, prescription opioid use), multimorbidity, and polypharmacy. Hazardous alcohol use was defined as an AUDIT-C score ≥3 for women and ≥4 for men. We used ICD9 codes to identify alcohol and illicit substance use disorders and identified prescription opioid use from pharmacy fill-refill data. We included counts of chronic medications and of physical and mental health comorbidities.
Measurements:
We identified serious falls using Ecodes and a machine learning algorithm that identified serious falls in radiology reports. We used multivariable logistic regression with general estimating equations to calculate risk. We used an integrated predictiveness curve to identify intervention thresholds.
Results:
Most of our sample (54%) was ≤60 years of age. Duration of follow-up was up to 4 years. Veterans who fell were more likely to be female (11% vs 7%) and White (72% vs 68%). They experienced 43,641 serious falls during follow-up. We identified 16 key predictors of serious falls and five interaction terms. Model performance was enhanced by addition of opioid use as evidenced by overall category-free net reclassification improvement of 0.32 (p<0.001). Discrimination (C-statistic 0.76) and calibration were excellent for both development and validation datasets.
Conclusion:
We developed and internally validated a model to predict six-month risk of serious falls among middle-aged Veterans with excellent discrimination and calibration.
Keywords: serious falls, middle-age, Veterans
INTRODUCTION
Although the rate of falls among middle-aged adults ranges from 8.7% to 31.1%,1–4 falls are understudied in this population.1 In this age group, falls are the third leading cause of death from unintentional injury, and a leading cause of non-fatal injury.5 They also result in the annual loss of 422,000 disability-adjusted life-years3,6 and presage higher rates of injury and disability as these individuals age, suggesting that fall prevention during middle-age may improve quality of life among older adults.
To prevent falls in this population, we must identify those at risk and understand what drives this risk. Our previous work focused on serious falls -- those occurring in the community that were serious enough to require a visit with a healthcare provider – among persons living with HIV (PLWH), a population that is predominantly middle-aged. Many of the risk factors that we identified overlapped with those found in older adults: polypharmacy, mental health medications, other CNS-active medications, and proton pump inhibitors.7 We also identified serious fall risk factors not previously considered for older adults such as hazardous alcohol use and having an opioid prescription.8 While HIV-specific factors were associated with serious falls, the factors with the strongest associations were those that are highly prevalent in middle-aged populations, such as substance use and multimorbidity.
There are no screening algorithms to identify middle-aged persons at risk for serious falls. To address this gap, we explored serious falls in the context of middle-aged Veterans aging with complex chronic disease and developed and internally validated a six-month risk prediction model for serious falls in this population.
METHODS
Our manuscript follows the guidelines of the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) consensus document.9
Sample
We used data from the Veterans Health Administration (VA) Birth Cohort.10 The VA Birth Cohort is a national, electronic health record (EHR)-based cohort that includes data from all Veterans born between 1945 and 1965 (“Baby Boomers”) who accessed care at a VA facility at least twice from January 1st 2000 through December 31st 2013.10 Available data include administrative data (ICD9 codes), demographics (race, sex, year of birth), laboratory results, pharmacy fill/refill data, health factors (pain, alcohol use), vital signs, and clinical text notes (including radiology reports). Our sample was randomly drawn from the full cohort (N= 4,521,077) and restricted to Veterans who presented for VA care at least twice between 2012 and 2015 (N=325,514). We used 09/30/2015 as the upper cut off because this was the last date through which we had access to radiology reports for use with our machine learning algorithm. We then restricted the cohort to individuals aged 45–65 years (N=275,940) (Figure 1). Using random sampling as implemented in the SAS® “surveyselect” procedure,11 participants were randomized such that 2/3 (n=184,047) were assigned to the development sample and 1/3 (n=91,816) to the validation sample. This random allocation protected against selection bias and allowed sufficient power to develop and internally validate the risk prediction model.
Figure 1.

Consort Diagram: Derivation of sample
Observation time
The unit of analysis was a six-month person-interval. Explanatory variables were fixed at the beginning and updated at the start of each subsequent interval. We used a six-month person-interval because the mean time between patient visits was approximately 7 months. A maximum of four years of follow-up (up to eight six-month intervals) was possible for each participant. The outcome was an indicator of any serious fall within the six-month interval.12
Serious falls
Serious falls are those falls that occur in the community and that are serious enough to require a visit with a healthcare provider. These are not self-reported falls. They are, however, similar to the “injurious falls” identified by Tinetti and colleagues.12 We operationalized serious falls using International Classification of Disease-9 (ICD9) external cause of injury codes (Ecodes): E880.X, E881.X, E884.X, E885.9, E886.9, E888.X13 and using a machine learning algorithm to identify serious falls from radiology reports.14 This algorithm identifies a serious fall in radiology report text. For example, when a provider orders a radiologic study to assess an injury from a fall, they may write an order such as: “Patient fell and landed on right wrist. Assess for fracture.” The order is part of the radiology report. A machine learning algorithm can be trained to “identify” terms related to “fall” in text notes (e.g. fall, fell, trip, slip), thereby allowing us to identify a serious fall that may not have been identified otherwise.
This algorithm was developed in the Veterans Aging Cohort Study (VACS).14 VACS is a prospective, observational cohort of all Veterans diagnosed with HIV who receive care within the VA system and matched, uninfected Veterans.15 VACS is a national, EHR-based cohort that includes administrative, demographic, pharmacologic, laboratory, and clinical data including text notes. To evaluate how well the machine learning algorithm identified serious falls or their absence, two clinicians (JW and CB) reviewed 100 randomly selected radiology reports that had been classified by the algorithm. Of these reports, there was one false positive and no false negatives.14 The false positive came from a report that identified a place name that included “fall” (e.g. Fall River). This incorrect identification of place names was corrected during algorithm validation. Overall performance metrics were excellent: positive predictive value: 93%; sensitivity: 95%; F measure: 94%.
We then validated the algorithm in the VA Birth Cohort using the approach described above and obtained similar metrics: positive predictive value: 93%; sensitivity: 99%; F measure: 96%. For the purposes of our study, a person was identified as experiencing a serious fall if they had either an Ecode or a radiology report that indicated a serious fall.
Primary predictors
We selected risk factors for consideration in a multivariable risk prediction model of serious falls among middle-aged Veterans.4,7,16 These included sex, race/ethnicity, and age. We also included body mass index (BMI) as a categorical variable (< or ≥ 25 kg/m2), and any history of a fall within the past 12 months. We included a count of physical comorbidities identified by ICD9 codes (one inpatient ICD9 code or two outpatient ICD9 codes) (anemia, asthma, COPD, coronary artery disease, heart failure, cirrhosis/end stage liver disease, cerebrovascular disease/stroke, hepatitis B virus, hepatitis C virus (HCV), HIV, hypertension, dyslipidemia, peripheral vascular disease, osteoarthritis, diabetes, renal insufficiency, dementia/cognitive impairment, blindness, cataracts, convulsions/seizures, abnormal gait, peripheral artery disease, incontinence, dizziness, nocturia, urinary frequency), and a count of mental comorbidities identified by ICD9 codes (one inpatient or two outpatient) (major depression, mild depression, bipolar disorder, psychosis, PTSD, schizophrenia, schizoaffective disorder, Alzheimer’s disease, cognitive impairment, and dementia). To adjust for comorbid disease severity, we included the VACS Index 2.0 score17 as a continuous variable in increments of five (score range: 9–153). The Index uses demographic information and routinely assessed laboratory measures associated with all-cause mortality: age, hemoglobin, FIB-4 ((age[years]xAST[IU/L]/platelet count [expressed as platelets x 109/L] x (ALT1/2[IU/L])), eGFR ((186.3 x serum creatinine-1.154) x (age-0.203) x (1.21 if black)), HCV status, BMI, albumin, and white blood cell (WBC) count. The VACS Index has been validated in PLWH (where it includes CD4 count and HIV-1RNA) and in uninfected populations.17,18 Hazardous alcohol use was defined as an AUDIT-C score ≥3 for women and ≥4 for men.19 We used ICD9 codes to identify alcohol use disorder (291.X. 303.XX, 305.XX, 357.5, 425.5, 535.3, 571.X, V11.3) and illicit substance use (292.0, 292.11, 292.12, 304.XX). We included a count of active, chronic medications defined as a prescription for at least 90 consecutive days allowing for a 30 day refill window.20 We also included specific CNS-active medications (benzodiazepines, muscle relaxants, opioids, anticonvulsants, SSRIs, SNRIs) and proton pump inhibitors as well as continuous pain score from the numeric rating scale (NRS). Demographic variables were identified at baseline. Medications and pain scores were identified in the six months preceding the interval of interest. All other predictors were assessed at the start of the index interval.
Ethics
The VA Birth Cohort was approved by the Institutional Review Boards of VA Connecticut Healthcare System and Yale University. It has been granted a waiver of informed consent and is HIPAA compliant.
Statistical Analysis
We generated descriptive statistics, using means and standard deviations or medians and interquartile ranges for continuous variables, and percentages for categorical variables. Under an assumption of missing-at-random, we multiply imputed the data five times. All variables were imputed using fully conditional specification, drawing from all 23 candidate variables for model selection. Due to its tendency to select the most parsimonious model relative to other criteria, we used backwards selection based on the Bayesian information criterion to select the final variables from a pool of the five imputations of development data.21 Additional details on imputation, model selection, and evaluation of linearity and model fit are included in the Statistical Appendix.
The predictors selected were subsequently used in a multivariable logistic regression model fit separately to each imputation using generalized estimating equations to adjust for the clustering of repeated intervals within patients. The coefficients estimated from the separate imputations were combined using Rubin’s rules to yield final model coefficients.22 For validation, these final model coefficients were directly applied to the predictors in the validation sample. In both development and validation samples, discrimination of the final model was assessed using the area-under-the-curve (AUC) where values > 70% represent good discrimination. Calibration was evaluated by plotting the observed probabilities within deciles against the average predicted probabilities as stipulated by the TRIPOD statement.9
To gain further insight regarding the model’s predictive utility, we evaluated the incremental value of adding prescription for opioids using category-free (continuous) net reclassification improvement (NRI).23.
To identify intervention thresholds that would help providers advise patients about meaningful levels of risk, we created an integrated predictive-ness curve24 based on the predicted probabilities across the development cohort. To provide references that inform the choice of thresholds for decision-making, we also identified the marginal rate of serious falls for the entire sample and the marginal risk of a serious fall for those who experienced a serious fall in the previous year.
All analyses were performed in SAS Version 9.4. with SAS/STAT 14.3.11
Role of the funding source
This research was supported by the Veterans Health Administration Health Services Research and Development Nursing Research Initiative [16–344 to JAW], by the Yale Claude D. Pepper Older Americans Independence Center [P30AG021342 to TEM and TMG], and by the National Institute of Alcohol Abuse and Alcoholism [U01 AA026224 to ACJ]. Funding sources had no role in study design, conduct, or reporting.
RESULTS
Sample Characteristics
The full cohort included 275,940 individuals (Table 1). Of these, 39,220 (14.2%) had at least one serious fall over the four years of follow-up, resulting in a total of 43,641 serious falls. Mean age at baseline was 59±5 years; 54% of the sample was less than 60 years of age. The annual rate of serious falls was 5.1% (95% confidence interval (CI) 4.9%, 5.1%) and was appreciably higher among specific subgroups: women (7.2%), those with an opioid prescription (7.4%), those taking more than five medications (6.8%); those with two or more physical comorbidities (6.6%), those with at least one mental health diagnosis (7.8%), and those with a previous fall (20%). Fallers and non-fallers differed on all characteristics except for age. Fallers were more likely to be female (11% vs 7%, p<0.001), White (72% vs 68%, p<0.001), to die during following up (16% vs. 10%, p<0.001), and to experience a fragility fracture (19% vs 3%, p<0.001) or hip fracture (1.6% vs 0.1%, p<0.001). They had a higher mean count of physical (3 vs 2, p<0.001) and mental (1 vs 0, p<0.001) comorbidities, were more likely to engage in hazardous alcohol use (29% vs 17%, p<0.001) and illicit substance use (20% vs 11%, p<0.001), and were more likely to have a diagnosis of alcohol use/abuse (27% vs 15%, p<0.001). They also were more likely to be prescribed the individual medications of interest, to have a higher mean count of chronic medications (11 vs 8, p<0.001), and to have a higher VACS Index score than non-fallers (37±11 vs 35±10, p<0.001).
Table 1.
Sample description by fall status
| Variables | Non-Fallers N= 236,720 | Missing (%) | Fallers N= 39,220 | Missing (%) | P value |
|---|---|---|---|---|---|
| Age | 59±5 | 0 | 59±5 | 0 | 0.28 |
| White | 68% | 72% | |||
| Other | 4% | 3% | |||
| Female sex | 7% | 0 | 11% | 0 | <0.001 |
| Died during follow-up | 10% | 0 | 16% | 0 | <0.001 |
| VACS Index Score 2.0 (mean±SD) | 35±10 | 31% | 37±11 | 22% | <0.001 |
| Wrist, hip, upper arm fracture | 3% | 0 | 19% | 0 | <0.001 |
| Hip fracture | 0.1% | 0 | 1.6% | 0 | <0.001 |
| <18.5 | 0.7% | 0.9% | |||
| >30 | 40% | 44% | |||
| Never | 27% | 25% | |||
| Past | 25% | 24% | |||
| Pain score in the prior six-month period (mean±SD) | 3±3 | 34% | 4±3 | 26% | <0.001 |
| Physical comorbidity count (mean±SD) | 2±2 | 0 | 3±3 | 0 | <0.001 |
| Mental health comorbidity count (mean±SD) | 0.4±0.7 | 0 | 0.7±0.9 | 0 | <0.001 |
| Drug use/abuse | 11% | 0 | 20% | 0 | <0.001 |
| Hazardous alcohol use | 29% | 50% | 17% | 48% | <0.001 |
| Diagnosis of alcohol use/abuse | 27% | 0 | 15% | 0 | <0.001 |
| Antithrombotics | 16% | 25% | <0.001 | ||
| Nitrates | 11% | 16% | <0.001 | ||
| SSRI | 32% | 46% | <0.001 | ||
| Atypical antidepressants | 33% | 48% | <0.001 | ||
| Antipsychotics | 14% | 24% | <0.001 | ||
| Benzodiazepines | 23% | 37% | <0.001 | ||
| Opioids | 60% | 83% | <0.001 | ||
| Antihistamines | 38% | 54% | <0.001 | ||
| Hypoglycemics | 23% | 0 | 29% | 0 | <0.001 |
| Proton pump inhibitors | 44% | 0 | 61% | 0 | <0.001 |
| Medication count | 8±6 | 0 | 11±8 | 0 | <0.001 |
Multivariable results
Our development cohort included 184,047 individuals who experienced 29,155 serious falls (Supplemental Table 1). Our final multivariable model included 16 predictors and 5 interaction terms (Figure 2). All coefficients demonstrated face validity. With the exception of BMI ≥ 25 kg/m2 (odds ratio [OR] 0.87; 95% confidence interval [CI] 0.84, 0.90) and non-white race/Hispanic ethnicity (OR 0.73; 95% CI 0.71, 0.76), all predictors were associated with increased risk of serious falls: female sex (OR 1.69; 95% CI 1.58, 1.80), fall within the past 12 months (OR 4.14; 95% CI 3.79, 4.51), count of physical comorbidities (OR 1.10; 95% CI 1.10, 1.11), chronic medication count (OR 1.06; 95% CI 1.05, 1.06), count of mental health comorbidities (OR 1.09; 95% CI 1.07, 1.10), hazardous alcohol use (OR 1.03; 95% CI 1.03, 1.04), diagnosis of alcohol use/abuse (OR 1.30; 95% CI 1.25, 1.35), pain (OR 1.03; 95% CI 1.02, 1.03), VACS Index Score 2.0 (in increments of 5) (OR 1.02; 95% CI 1.02, 1.03), having prescriptions for opioids (OR 1.14; 95% CI 1.07, 1.14), benzodiazepines (OR 1.16; 95% CI 1.12, 1.19), anticonvulsants (OR 1.10; 95% CI 1.08, 1.14), muscle relaxants (OR 1.06; 95% CI 1.03, 1.10), and SSRIs (OR 1.08; 95% CI 1.05, 1.11). Interaction terms were sub-additive and of modest impact (Supplemental Table 3). Model covariates in descending order by standardized estimates are noted in Supplemental Table 4. The AUC of 0.76 reflects very good discrimination. Calibration was excellent (Figure 3).
Figure 2.
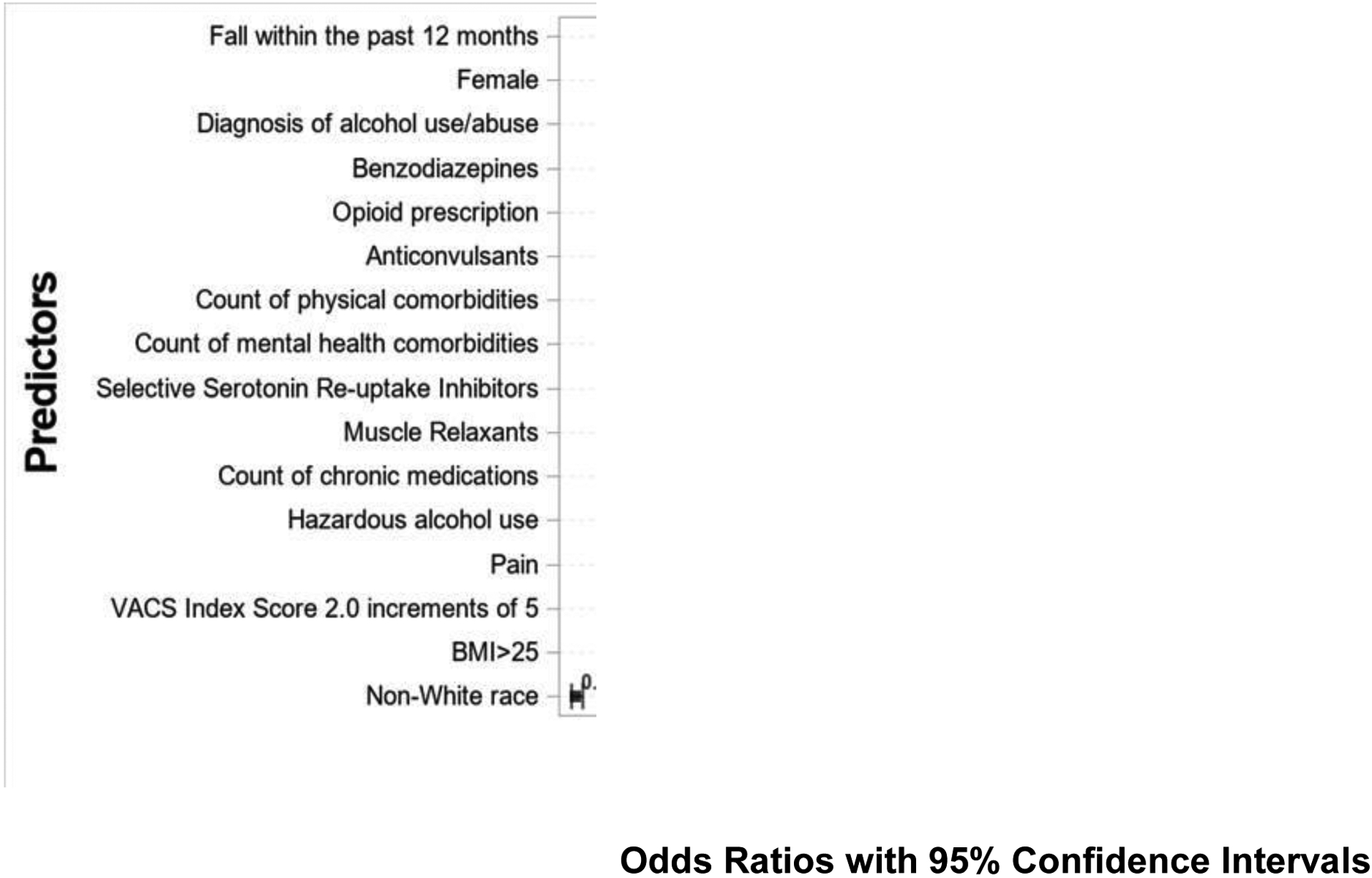
Associations between predictors and serious falls*
* We included only the main effects of the predictive model. There were also five interaction terms: three of these were with falls within the past 12 months and two were with female sex. All interaction terms were sub-additive. Please see Supplemental Table 3 for further information on these interactions.
Figure 3.
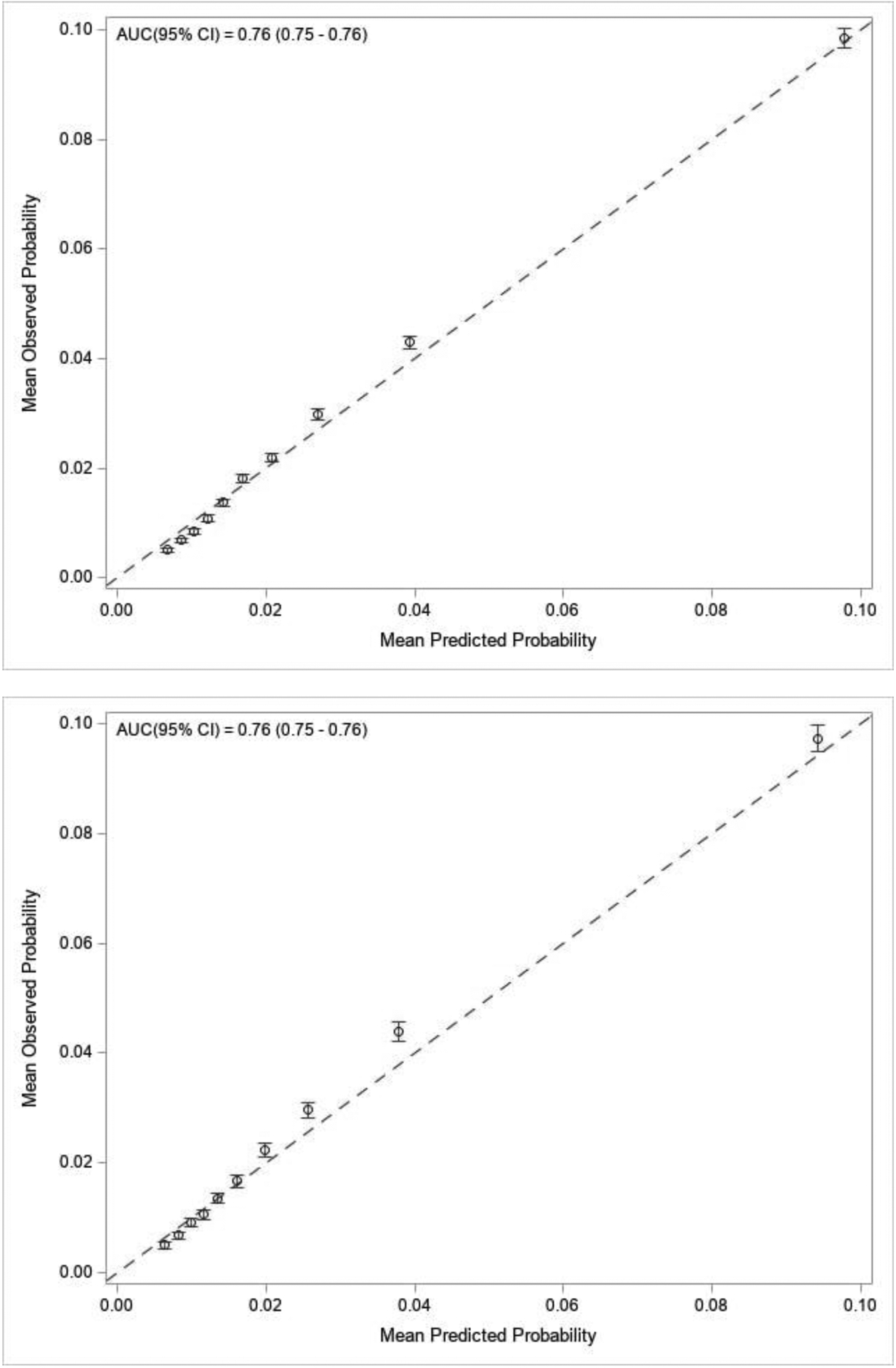
Calibration plot for the predictive model in the development sample (a) and Calibration plot for the predictive model in the validation sample (b)
The validation cohort (Supplemental Table 2) included 91,816 individuals who experienced 14,486 serious falls. In this dataset, the model demonstrated very good discrimination (AUC of 0.76) and excellent calibration (Figure 3), suggesting robust internal validation.
As measured by category-free net reclassification improvement (NRI),25 augmentation of the model with the opioid prescription variable – typically not part of fall risk prediction models – enhanced the predictive ability of the model. Addition of this variable yielded an event-NRI of 1.6% (95% CI 1.48%, 1.76%) and a nonevent-NRI of 30.4% (30.3%, 30.5%) for a combined NRI of 0.32 (p<0.001).
To decide when to advise patients of meaningful levels of risk, we created an integrated predictiveness curve24 based on the predicted probabilities across the development cohort (Figure 4). The six-month marginal rate of serious falls for the entire sample was 2.6%. The six-month marginal risk of serious falls among those with a serious fall in the past year was 10.1%. We then identified important risk cut-offs between these two values. The eighth decile of predictive probability intersected with the integrated predictiveness curve at a probability of a serious fall of 3.1%. The ninth decile intersected with the curve at a probability of 5.1%. When a patient’s probability of a fall lies between the eighth and ninth deciles, they should be warned about their risk for a fall and key risks identified. Anyone with a probability of a serious fall greater than 5.1% (i.e., those in the top 10% of predicted risk) require more substantial intervention, as discussed in the next section.
Figure 4.

Identifying intervention cut-points using an integrated predictiveness curve
Blue line: Integrated predictiveness curve
Purple dotted line: The marginal rate of falls in a six-month period
Red dotted line: The marginal risk of a serious fall with a history of a serious fall in the past year
Orange dashed line: The probability of a fall above which providers should identify and discuss risk factors for serious falls
Red dashed line: The probability of a serious fall at which providers need more substantive interventions to reduce risk of a serious fall
DISCUSSION
Serious falls are an important concern among high-risk subgroups of middle-aged Veterans. These include women, those with an opioid prescription, those taking more than five medications, those with two or more physical comorbidities, those with at least one mental health diagnosis, and those with a previous serious fall. The annual rate of occurrence of serious falls in these subgroups was comparable to that identified by Tinetti and colleagues12 in a population aged 70+ years.
We developed a predictive model that calculates the probability of a serious fall within a six-month period for middle-aged Veterans. We included demographic risk factors, comorbidity and medication count, individual medications, and laboratory values that capture disease severity. Our model had very good discrimination and was well-calibrated in both the development and validation samples. Augmentation with opioid prescriptions enhanced the predictive ability of the model as demonstrated by category-free NRI index. To our knowledge, no other predictive model for serious falls has been validated in middle-aged adults.
Appropriate and timely intervention to prevent serious falls may prevent disability and death in middle-aged Veterans during the years when they are most economically and socially productive, thereby supporting a high quality of life as they age. To this end, we used an integrated predictiveness curve to identify probability cutoffs when interventions should be implemented to prevent serious falls. An intervention cut-off used by the CDC’s STEADI program is anyone who has had a fall within the past year. In our sample, the marginal six-month risk of a serious fall among individuals with a fall in the past year was 10% -- almost double the risk of an individual in the ninth decile of predicted probability. This seemed to be too high a threshold to reduce the risk of serious falls in this population. We posit that those between the eighth and ninth deciles (equivalent to a probability of a serious fall of 3.1% to 5.1%) merit a warning about their risk. Their modifiable risk factors should be identified from the modifiable variables in the predictive model, and efforts should be made to address them. Among individuals with expected risk greater than the ninth decile (5.1%), a more in-depth assessment is recommended. In addition to emphasizing those variables emphasized in fall prevention for older adults, programs targeted to middle-aged Veterans will need to confront additional risk factors such as hazardous alcohol use, pain management that avoids opioids, and deprescribing that balances the medical needs of this middle-aged population against their elevated risk of serious falls. The exact nature of this intervention is the subject of ongoing research.
In addition to benefitting Veterans, interventions to prevent serious falls may reduce costs for the VA. In 2017, Veterans aged 45–64 constituted 31% of the VA population (2.6 million individuals).26 Estimates suggest that serious falls among middle-aged Veterans cost the VA a minimum of $675 million per year in outpatient or emergency department visits.27 If we implemented an intervention that could reduce serious falls in this population by 10%,12,13 we would reduce annual costs to the VA by at least $68 million. Next steps in this research include developing a clinical decision support tool based on this algorithm to help providers identify patients at risk for serious falls, and to provide information to help them mitigate this risk and to develop effective interventions.
This study has important strengths and limitations. The VA Birth Cohort included a large sample of middle-aged Veterans. Because of the computational intensity of running the machine learning algorithm, we randomly selected a subset of this population. Even so, we were well powered to develop a predictive model of serious falls for this population. Because the VA Birth Cohort is an electronic health record-based cohort, we had access to a wide range of clinical variables. We also had access to detailed information on medication exposure and substance use including opioid prescriptions and alcohol use. Our analytic approach ensured that we recorded exposures of interest prior to occurrence of the outcome, thereby reducing the risk of indication bias.
A limitation is our operationalization of serious falls. Our definition was restricted to those falls that caused a patient to present for health care, reflecting that used by Tinetti and colleagues.12 Although this approach did not identify all falls, it likely identified those that were most concerning to both patient and provider. We were also unable to adjust for all potentially significant risk factors, e.g. we could not identify those with peripheral neuropathy. Peripheral neuropathy is notoriously underassessed, implying that neither administrative codes nor use of machine-learning algorithms reliably identify this condition. Other conditions that we did not include (e.g. Parkinson’s disease) are extremely rare in middle-aged populations. We were also unable to assess for gait or balance impairments. An obvious limitation was that only 7% of the sample were women. However, we explored the fit of our predictive model in sample populations stratified by sex and found equally good fit among males and females (data not shown). We also had a significant amount of missing data for four variables: VACS Index 2.0 (30%), hazardous alcohol use (50%), body mass index (20%), and pain (33%). The VACS Index is comprised, primarily, of laboratory results. Although follow-up time in our study was divided into six-month intervals, patients do not necessarily present for care every six months. In our sample, the mean time between appointments was 228 days. The high level of “missingness” in these variables reflects the longitudinal structure of the dataset. Because we had one or more observations of these variables for each participant and over 20 other correlated measures across all time points, our assumption of missingness at random is plausible. Finally, our model has not yet been externally validated.
Future research will involve using this algorithm as the foundation for a clinical decision support tool that can be used by providers to identify middle-aged Veterans at risk of a serious fall. It will also identify key risk factors so that prevention efforts can be undertaken in a timely manner and focused on the most important risk factors of serious falls for that patient. Data will be entered by the patient, the provider, or as part of an algorithm that is routinely run on data from all middle-aged patients who present to clinic and can present the data to patient and clinician as part of a routine visit. Interventions to reduce serious falls in this population must also be developed.
In conclusion, we developed and validated a model for risk of serious falls within a six-month period for middle-aged Veterans that considered traditional and novel risk factors. This model demonstrated strong internal validation and will form the foundation of future interventions.
Supplementary Material
Impact statement:
We certify that this work is novel. Falls are typically considered the purview of adults 65+ years of age. Our research suggests that some middle-aged individuals (ages 45–64 years) are also at high risk. We developed a predictive model that calculates middle-aged Veterans’ probability of experiencing a serious fall within a six-month period. We also identify the probabilities at which treatment should be considered.
ACKNOWLEDGEMENTS
Sponsor’s role: This research was supported by VA HSR&D NRI 16-344, by the Yale Claude D. Pepper Older Americans Independence Center (P30AG021342), and by NIAAA U01 AA026224. Funders played no role in the design, methods, data collection, analysis and preparation of the manuscript.
Footnotes
Conflict of interest: None of the authors report a conflict of interest.
REFERENCES
- 1.Peeters G, van Schoor NM, Cooper R, Tooth L, Kenny RA. Should prevention of falls start earlier? Co-ordinated analyses of harmonised data on falls in middle-aged adults across four population-based cohort studies. PLoS One. 2018;13(8):e0201989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verma SK, Willetts JL, Corns HL, Marucci-Wellman HR, Lombardi DA, Courtney TK. Falls and Fall-Related Injuries among Community-Dwelling Adults in the United States. PLoS One. 2016;11(3):e0150939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talbot LA, Musiol RJ, Witham EK, Metter EJ. Falls in young, middle-aged and older community dwelling adults: perceived cause, environmental factors and injury. BMC Public Health. 2005;5:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White AM, Tooth LR, Peeters G. Fall Risk Factors in Mid-Age Women: The Australian Longitudinal Study on Women’s Health. Am J Prev Med. 2018;54(1):51–63. [DOI] [PubMed] [Google Scholar]
- 5.Kool B, Chelimo C, Robinson E, Ameratunga S. Deaths and hospital admissions as a result of home injuries among young and middle-aged New Zealand adults. N Z Med J. 2011;124(1347):16–26. [PubMed] [Google Scholar]
- 6.Li W, Keegan TH, Sternfeld B, Sidney S, Quesenberry CP Jr., Kelsey JL. Outdoor falls among middle-aged and older adults: a neglected public health problem. Am J Public Health. 2006;96(7):1192–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Womack JA, Murphy TE, Rentsch CT, et al. Polypharmacy, Hazardous Alcohol and Illicit Substance Use, and Serious Falls Among PLWH and Uninfected Comparators. J Acquir Immune Defic Syndr. 2019;82(3):305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC. STEADI -- Older Adult Fall Prevention. 2017. Accessed 25 October, 2017.
- 9.Collins GS, Reitsma JB, Altman DG, Moons KGM, members of the Tg. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. Eur Urol. 2015;67(6):1142–1151. [DOI] [PubMed] [Google Scholar]
- 10.Sarkar S, Esserman DA, Skanderson M, Levin FL, Justice AC, Lim JK. Disparities in Hepatitis C Testing in U.S. Veterans Born 1945–1965. J Hepatol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inc SI. SAS 9.4 Procedures Guide SAS Institute; 2013. [Google Scholar]
- 12.Tinetti ME, Baker DI, King M, et al. Effect of dissemination of evidence in reducing injuries from falls. N Engl J Med. 2008;359(3):252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tinetti ME, Baker DI, McAvay G, et al. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med. 1994;331(13):821–827. [DOI] [PubMed] [Google Scholar]
- 14.Bates J, Fodeh SJ, Brandt CA, Womack JA. Classification of radiology reports for falls in an HIV study cohort. J Am Med Inform Assoc. 2016;23(e1):e113–e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44(8 Suppl 2):S25–30. [DOI] [PubMed] [Google Scholar]
- 16.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319(26):1701–1707. [DOI] [PubMed] [Google Scholar]
- 17.Tate JP, Sterne JAC, Justice AC, Veterans Aging Cohort S, the Antiretroviral Therapy Cohort C. Albumin, white blood cell count, and body mass index improve discrimination of mortality in HIV-positive individuals. AIDS. 2019;33(5):903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tate JP, Brown ST, Rimland D, Rodriguez-Barradas M, Justice AC. Comparison of VACS Index Performance in HIV-Infected and Uninfected Veterans from 2000 to 2010 18th International Workshop on HIV Observational Databases; March, 2014; Sitges, Spain. [Google Scholar]
- 19.Bush K, Kivlahan DR, McDonell MB The AUDIT Alcohol Consumption Questions (AUDIT-C): An effective brief screening test for problem drinking. Archives of Internal Medicine. 1998;158(16):1789–1795. [DOI] [PubMed] [Google Scholar]
- 20.Edelman EJ, Gordon KS, Glover J, McNicholl IR, Fiellin DA, Justice AC. The next therapeutic challenge in HIV: polypharmacy. Drugs Aging. 2013;30(8):613–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy TE, Tsang SW, Leo-Summers LS, et al. Bayesian Model Averaging for Selection of a Risk Prediction Model for Death within Thirty Days of Discharge: The SILVER-AMI Study. Int J Stat Med Res. 2019;8:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin D. Inference and missing data. Biometrika. 1976;63(3):581–592. [Google Scholar]
- 23.Pencina MJ, D’Agostino RB Sr., D’Agostino RB Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172; discussion 207–112. [DOI] [PubMed] [Google Scholar]
- 24.Pepe MS, Feng Z, Huang Y, et al. Integrating the predictiveness of a marker with its performance as a classifier. Am J Epidemiol. 2008;167(3):362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leening MJ, Vedder MM, Witteman JC, Pencina MJ, Steyerberg EW. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician’s guide. Ann Intern Med. 2014;160(2):122–131. [DOI] [PubMed] [Google Scholar]
- 26.Huang G, Muz B, Kim S, Gasper J. 2017 Survey of Veterans Enrollees’ Health and Use of Health Care: Data Findings Report. Rockville, Maryland: Veterans Health Administration, Department of Veterans Affairs; April 2018 2018. [Google Scholar]
- 27.Hoffman GJ, Hays RD, Shapiro MF, Wallace SP, Ettner SL. Claims-based Identification Methods and the Cost of Fall-related Injuries Among US Older Adults. Med Care. 2016;54(7):664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


