Abstract
Nematode Caenorhabditis elegans (C. elegans) exhibited a vigorous swimming behavior in liquid medium. Addition of dopamine inhibited the swimming behavior, causing paralysis in 65% of wild-type nematodes. Interestingly, phytocannabinoids cannabidiol (CBD) or cannabidivarin (CBDV), caused paralysis in 40% of the animals. Knockout of DOP-3, the dopamine D2-like receptor critical for locomotor behavior, eliminated the paralysis induced by dopamine, CBD, and CBDV. In contrast, both CBD and CBDV caused paralysis in animals lacking CAT-2, an enzyme necessary for dopamine synthesis. Co-administration of dopamine with either CBD or CBDV caused paralysis similar to that of either phytocannabinoid treatment alone. These data support the notion that CBD and CBDV act as functional partial agonists on dopamine D2-like receptors in vivo. The discovery that dopamine receptor is involved in the actions of phytocannabinoids moves a significant step toward our understanding of the mechanisms for medical uses of cannabis in the treatment of neurological and psychiatric disorders.
Keywords: Phytocannabinoid, Cannabidiol, Cannabidivarin, C. elegans
1. Introduction
The Cannabis sativa-derived non-psychoactive compound cannabidiol (CBD) and its propyl analog cannabidivarin (CBDV) are purported to have a broad range of therapeutic benefits stemming from anti-inflammatory, anti-nausea, anti-tumor, anti-convulsant, anxiolytic, and neuroprotective properties [1, 2] While these phytocannabinoids are known to act on a variety of molecular targets throughout the body and especially in the nervous system, our understanding of their mechanisms of action remains limited [1, 2].
The neurotransmitter dopamine plays a central role in the regulation of learning, memory, emotion, cognition, and locomotion [3]. Numerous neurodegenerative and neuropsychiatric disorders have been associated with dopaminergic dysfunction [3]. Phytocannabinoids have been suggested as potential therapeutic agents for pathologies involving the dopamine system [4, 5]. However, there is limited information on the interactions between phytocannabinoids and dopamine receptors[6]. In this study, we investigated the ability of two non-psychotropic compounds, CBD and CBDV, to modulate dopaminergic signaling in vivo.
The nematode Caenorhabditis elegans shares a similar dopamine system with mammals. This provides a useful in vivo model for identifying pharmacological compounds able to target this key neuromodulatory system [7]. C. elegans possess a number of characteristics advantageous for pharmacological research, including a rapid life cycle, small size, ease of genetic manipulation and transparency, which facilitates visualization of cellular and developmental processes [8]. An estimated 60% of genes associated with human pathologies are present in C. elegans, whose genome was sequenced in 1998 [9–11]. A complete neuronal wiring diagram with extensively characterized synaptic connectivity has been constructed [12]. The molecular mechanisms underlying G protein-coupled receptor (GPCR) signaling in the nervous system are largely conserved between nematodes and humans. Key GPCRs of biogenic amines, including dopamine, present in nematodes are homologs of mammalian neurotransmitter receptors [13].
When immersed in a liquid environment, C. elegans exhibit vigorous swimming or ‘thrashing’ behavior that is regulated by endogenous dopamine and the DOP-3 (D2-like) receptor signaling pathway [14]. Swimming-based paralysis assays measuring locomotor behavior have been used extensively to study the functional roles of dopamine system, including receptors and transporters [14–19]. In this study, we used the C. elegans swimming assay to test the hypothesis that phytocannabinoids CBD and CBDV exert their actions in vivo by acting on the dopamine system.
2. Materials and Methods
2.1. Nematode strains and maintenance
The following C. elegans strains were obtained from the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN) and handled using standard conditions [20]: N2 Bristol wild-type, LX703 dop-3(vs106)Xand CB1112 cat-2(e1112)II. nematodes were grown on E. coli OP50-seeded NGM agar plates at 15 °C for stock maintenance and 25 °C for behavioral assays.
2.2. Drugs
CBD and CBDV were purchased from Cayman Chemicals (Ann Arbor, MI) and dissolved in ethanol to generate 0.03, 0.3 and 3 mM stock solutions. Dopamine was purchased from Cayman Chemicals (Ann Arbor, MI) and dissolved in dH2O to generate a 1 M stock solution.
2.3. Swimming assay
Behavioral assays were performed as previously described with some modifications [21]. Briefly, ten to fifteen late L4 hermaphrodite larvae were placed in a well containing 100μL K medium (2.36 g KCl, 3g NaCl in 1 L dH2O) [22] and 1μL of either CBD, CBDV, or vehicle. The number of nematodes paralyzed versus swimming were recorded after 30 minutes. For each treatment group per genotype, six wells were scored per experiment and experiments were repeated on five separate days (n=30).
2.4. Statistics
All data was processed using GraphPad Prism (GraphPad Software, San Diego, CA). The ratios of paralyzed to non-paralyzed nematodes were compared between strains and drug treatments using one-way ANOVA, followed by Dunnett’s post post-tests. Data are expressed as mean ± standard deviation and p-values of <0.05 were considered significant.
3. Results
3.1. Phytocannabinoid-induced paralysis of wild type nematodes
In the first set of experiments, a dose-response relationship analysis for two phytocannabinoids, CBD and CBDV, in N2 wild type nematodes swimming assay was performed (Figures 1A and 1B). No significant difference in swimming behavior was observed between K medium- and vehicle-treated nematodes. Dopamine, a known promoter of paralysis, was used as a positive control [23, 24]. As expected, wild type C. elegans showed an increase in paralysis when exposed to 50 mM dopamine, with 64.9% ± 6.5% of the nematodes paralyzed. Furthermore, both CBD and CBDV elicited a significant increase in paralysis at 30 μM concentration, compared to vehicle control, with 40.4% ± 9.4% and 39.8% ± 5.0% of animals paralyzed respectively (Figures 1A and 1B). Notably, their effect was about 60% in magnitude as compared to those produced by 50 mM dopamine treatment. Lower concentrations (0.3 μM and 3 μM) of the two phytocannabinoids failed to significantly induce paralysis.
Figure 1. Phytocannabinoid-induced paralysis in N2 wild type nematodes.
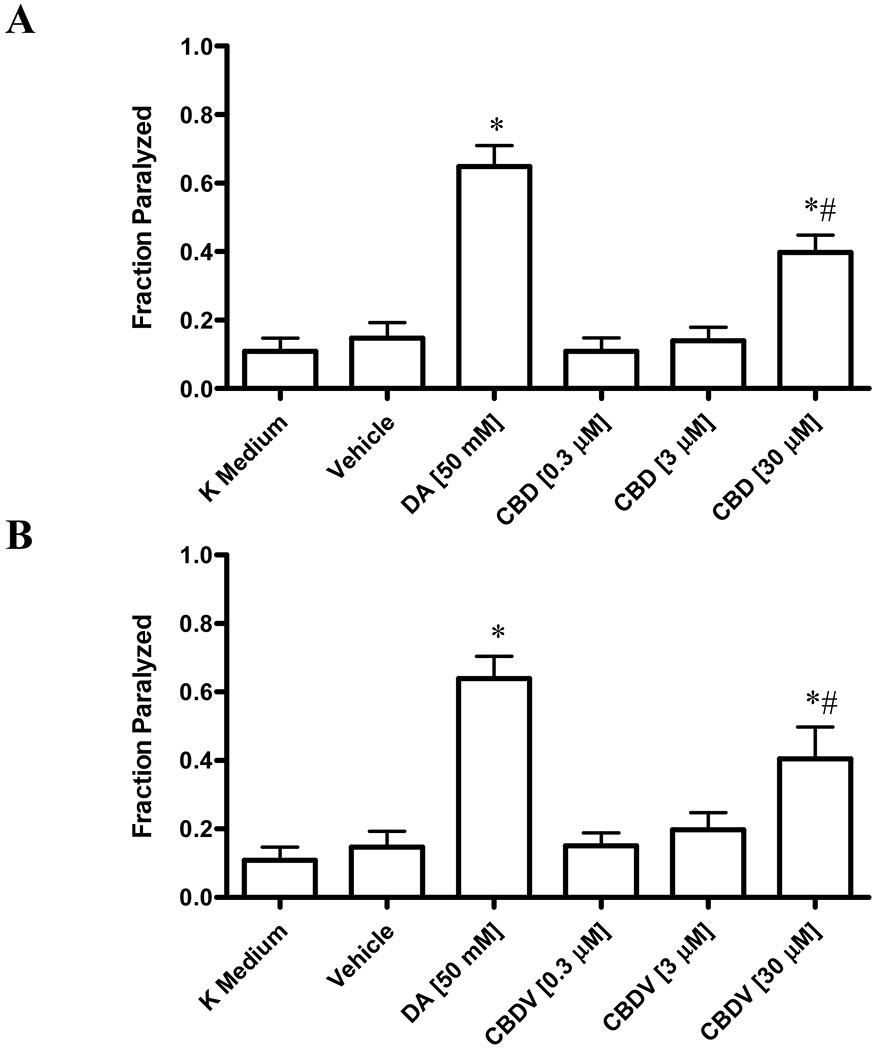
Extent of paralysis induced by CBD (Fig. 1A) and CBDV (Fig. 1B) in N2 wild type nematodes. Data are expressed as mean ± standard deviation. Data were analyzed using one-way ANOVA followed by Dunnett’s post hoc test. p-values of <0.05 were considered significant. * indicates statistically significant difference compared to vehicle. # indicates statistically significant difference compared to dopamine.
3.2. Phytocannabinoid-induced paralysis in dop-3 mutants
Based on these results, the mechanism by which these cannabinoids promote paralysis was next examined. C. elegans express the DOP-3 receptor, a homologue of the mammalian D2-like receptor. Researchers have established that the DOP-3 signaling pathway is necessary for the induction of paralysis, and that the dop-3 knockout mutant vs106 fails to undergo paralysis when treated with exogenous dopamine [23]. We confirmed this finding, as 50 mM dopamine failed to induce paralysis in dop-3 mutants (Figures 2A and 2B). Similarly, when treated with either CBD or CBDV at concentrations ranging from 0.3 μM to 30 μM, dop-3 mutants exhibited normal swimming behavior (Figures 2A and 2B).
Figure 2. Lack of paralysis in phytocannabinoid-treated dop-3 mutants.
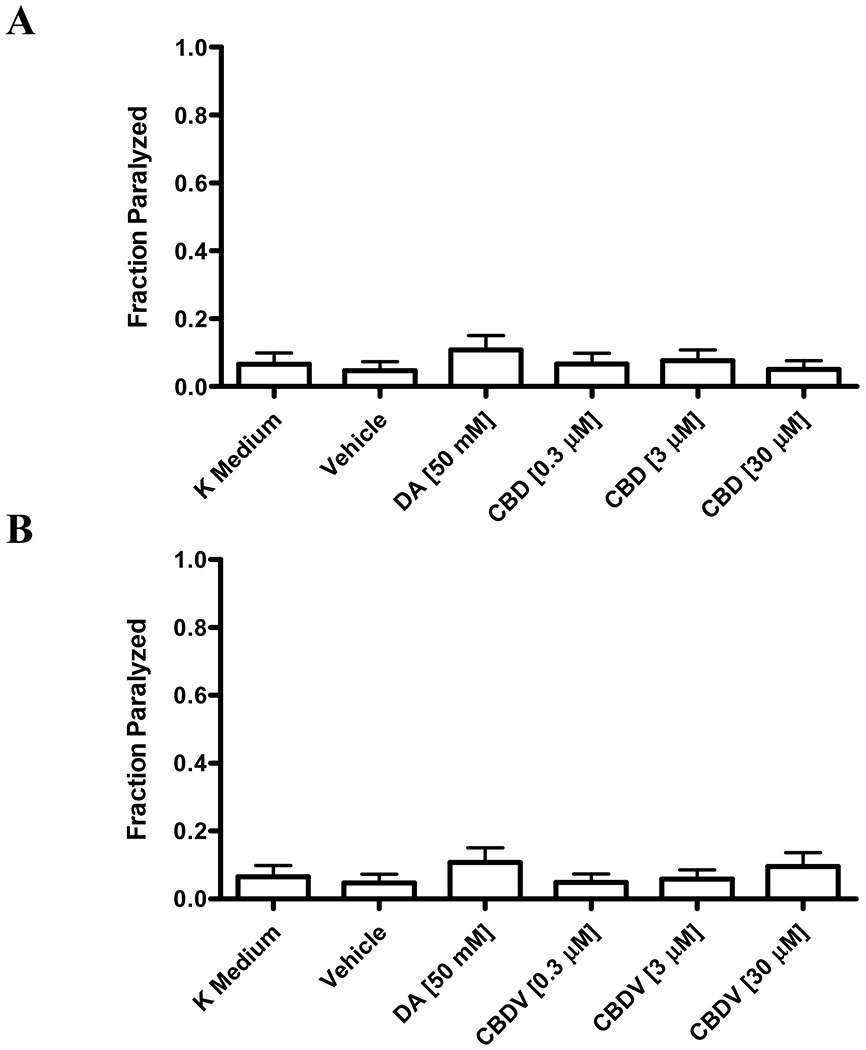
CBD (Fig. 2A) and CBDV (Fig. 2B) fail to significantly induce paralysis in dop-3 mutants lacking the D2-like DOP-3 receptor (p > 0.05). Data were analyzed using one-way ANOVA followed by Dunnett’s post hoc test.
3.3. Phytocannabinoid-induced paralysis in cat-2 mutants
The cat-2 gene encodes tyrosine hydroxylase, which catalyzes the rate-limiting step in the dopamine biosynthetic pathway. Cat-2 mutants cannot produce endogenous DA [14]. Paralysis was not observed in cat-2 mutants treated with K medium alone or vehicle. Treatment with 50 mM dopamine resulted in the paralysis of 60.9% ± 6.7% of the cat-2 mutants. Similarly, cat-2 mutants exposed to either CBD or CBDV showed a dose-dependent increase in paralysis, compared to vehicle. Specifically, CBD induced paralysis in 24.0% ± 3.7% of cat-2 mutants, while CBDV elicited paralysis in 32,7% ± 4.6% of animals (Figures 3A and 3B).
Figure 3. Phytocannabinoids-induced paralysis in cat-2 mutants.
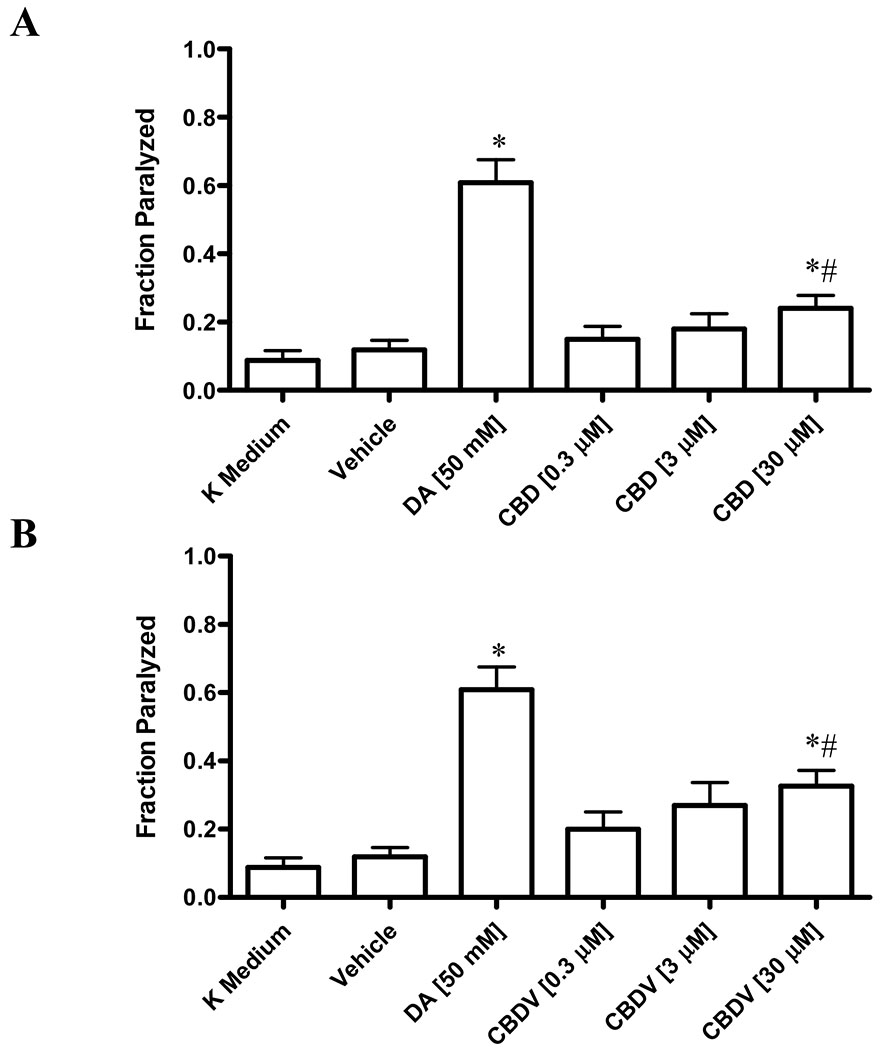
Extent of paralysis induced by CBD (Fig. 3A) and CBDV (Fig. 3B) in cat-2 mutants. Data are expressed as mean ± standard deviation. Data were analyzed using one-way ANOVA followed by Dunnett’s post hoc test. p-values of <0.05 were considered significant. * indicates statistically significant difference compared to vehicle. # indicates statistically significant difference compared to dopamine.
3.4. Paralysis of wild type nematodes induced by phytocannabinoid and dopamine co-treatment
The effects of phytocannabinoid and dopamine co-treatment on wild type animals were analyzed. Compared to dopamine treatment alone, the fraction of paralyzed animals was significantly less in wild-type nematodes co-treated with dopamine and CBD (30.3% ± 5.8%) or dopamine and CBDV (36.3% ± 8.2%) (Figures 4A and 4B). Interestingly, there was no significant difference between the fraction of paralyzed animals following co-treatment compared to phytocannabinoid treatment alone, suggesting that phytocannabinoids are occupying the same receptors as dopamine, so that the latter could not exert its maximum effects.
Figure 4. Co-treatment of phytocannabinoids and dopamine induced paralysis in N2 wild type nematodes.
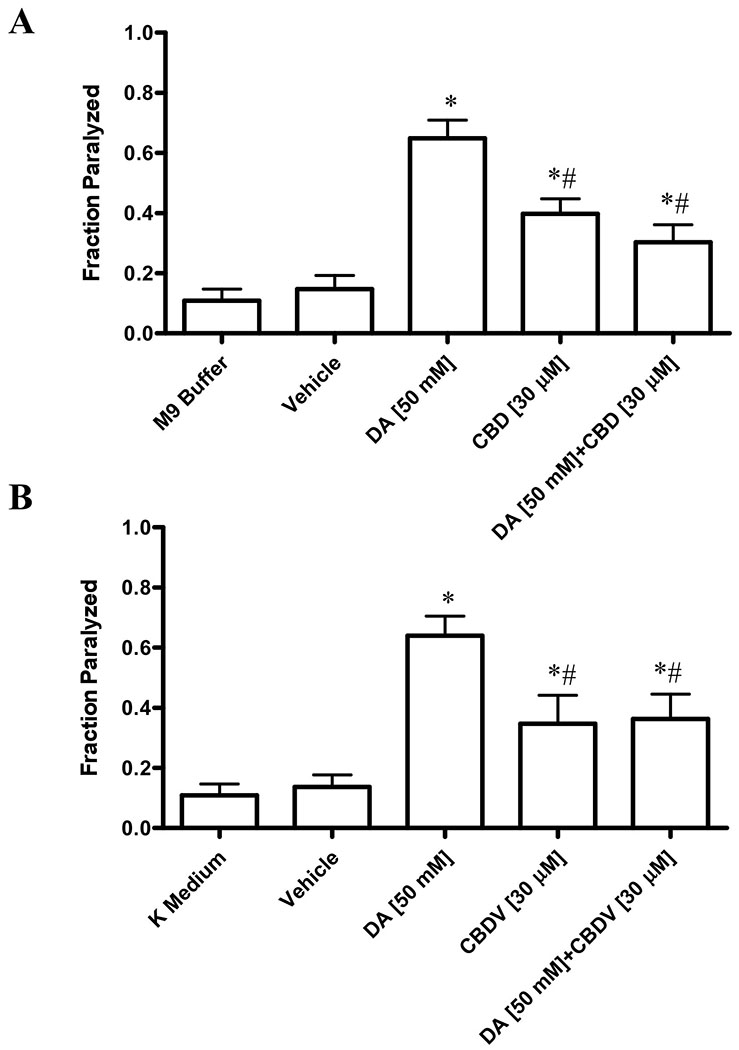
Co-treatment of dopamine with 30 μM of CBD (Fig. 4A) or CBDV (Fig. 4B) induces lower levels paralysis compared to dopamine treatment alone. Data are expressed as mean ± standard deviation. Data were analyzed using one-way ANOVA followed by Dunnett’s post hoc test. p-values of <0.05 were considered significant. * indicates statistically significant difference compared to vehicle. # indicates statistically significant difference compared to dopamine.
4. Discussion
4.1. Phytocannabinoids as paralysis inducers via DOP-3
The dopaminergic system, consisting of endogenous dopamine, D1-like and D2-like dopamine receptors, and pre-synaptic dopamine transporters, is implicated in a broad range of neuropathologies and has consequently become an attractive drug target [3]. This neurotransmitter system is highly conserved in C. elegans, with homologs and orthologs for many of the mammalian genes responsible for dopamine synthesis, vesicular loading, dopamine reuptake and dopamine signaling[13].
In this study, we first used wild type nematodes to investigate the effects of two non-psychoactive phytocannabinoids on swimming behavior. Our results showed that both CBD and CBDV induce paralysis in wild type animals at concentrations in the micromolar range, suggesting that these phytocannabinoids may be acting on the dopamine pathway. We must note that we found that 50 mM dopamine induced paralysis in ~65% of wild type nematodes, while other groups have observed ~100% [23, 24]. This difference may be due to experimental conditions as dopamine was given on the agar plate in the previous studies [23, 24].
A lower concentration of either phytocannabinoids (30 μM), compared to 50 mM of dopamine, was needed to induce paralysis. This could suggest that these phytocannabinoids are more potent than dopamine. Alternatively, the requirement of a higher dopamine concentration may be associated with the difference in lipid solubility: hydrophobic phytocannabinoids versus polar, hydrophilic dopamine. The phytocannabinoids may more easily pass through the nematode cuticle to achieve their effects. In the current study, 30 μM was the highest concentration that could be achieved due to limited solubility of these phytocannabinoids. At this concentration, CBD- and CBDV-induced paralysis was at lower levels compared with those induced by dopamine. This could suggest that these phytocannabinoids are less efficacious than dopamine and act as partial agonists on dopamine receptors.
To investigate the potential mechanism by which CBD and CBDV exerting their effects, two knock-out strains of C. elegans with loss-of-function mutations in the genes involved in the dopamine system, dop-3 and cat-2, were examined.
The nematode DOP-3 receptor shares structural similarity to the mammalian D2-like dopamine receptors [7]. DOP-3 is expressed in the ventral cord motor neurons, which innervate the body wall muscles to directly control locomotion [7]. Specifically, high expression of DOP-3 is found in the GABAergic motor neurons that inhibit contractions; with only moderate expression in the cholinergic motor neurons that stimulate muscle contraction (overlapping co-expression of DOP-1 in cholinergic neurons). The dop-3 (vs106) mutant contains deletions in sequences that encode the conserved transmembrane domains. As a results dop-3 mutant has a loss of Swimming-induced paralysis phenotype [14]. In the current study, CBD and CBDV failed to induce paralysis in the dop-3 mutant, which supports our hypothesis that CBD and CBDV act through the DOP-3 signaling pathway. The absence of paralysis in dop-3 mutants and presence in N2 wild type animals suggest that CBD and CBDV act via the DOP-3 receptor to promote paralysis.
The C. elegans tyrosine hydroxylase ortholog CAT-2 mediates the rate-limiting step in dopamine biosynthesis, catalyzing the conversion of tyrosine to L-dopa [7]. The cat-2 mutant (e1112) is deficient in dopamine, and as a result, does not demonstrate paralysis when immersed in solution under normal conditions. Instead, these animals can only undergo paralysis in the presence of exogenous dopamine or another DOP-3 agonist [7]. As expected, the cat-2 mutants exhibited the paralysis phenotype when treated with dopamine. More importantly, treatment with CBD or CBDV induced paralysis in cat-2 mutants, further confirming the agonist activity of CBD and CBDV on the dopamine pathway, presumably through dopamine receptor DOP-3. However, similar to the situation in N2 wild type, the levels of paralysis induced by these two phytocannabinoids in the cat-2 mutant are only about 40-50% of that dopamine, suggesting again that these phytocannabinoids are partial agonists on DOP-3.
Our results suggest that CBD and CBDV may be acting as a partial agonist at DOP-3. We were then interested in whether these phytocannabinoids can block the effects of dopamine, because a partial agonist should compete and block the effects of a full agonist. To test this, we treated the wild type nematodes with the concentrations of dopamine and either CBD or CBDV that elicited the greatest paralysis effects. Co-administration of dopamine with either CBD or CBDV had reduced effects for dopamine to induce paralysis, and co-administration had similar effects on parylysis to either CBD or CBDV alone. These data further support the conclusion that phytocannabinoids CBD and CBDV act as partial agonists on dopamine receptor DOP-3.
4.2. Physiological significance and implications
Growing evidence supports the use of phytocannabinoids for the treatment of various neurological and psychiatric disorders, including Alzheimer’s disease [25, 26], Parkinson’s disease [25, 26], neuropathic pain [27], epilepsy [28], anxiety [29], and schizophrenia [5, 30–32]. Interestingly, many of these pathologies are characterized by aberrant dopaminergic signaling.
It has been suggested that cannabinoids modulate mesocorticolimbic dopaminergic signaling [5]. Previously, ligand binding experiments in vitro has shown that CBD maybe a partial agonist at dopamine D2High receptors in rat striatal tissues[6]. Notably, this phytocannabinoid behaved similarly to the antipsychotic drug aripiprazole, an established dopamine partial agonist, suggesting a potential therapeutic use of CBD in the treatment of schizophrenia, where aberrant dopamine signaling is a hallmark [6]. The current study provides in vivo data, for the first time, to support the concept that the phytocannabinoids CBD and CBDV act as partial agonists on dopamine D2-like receptors. The dopamine hypothesis for schizophrenia posits that striatal hyper-dopaminergia causes the associated psychopathological symptoms. Urs et al. (2017) have further shown that it may be beneficial to downregulate overactive dopaminergic pathways in striatal tissues, while upregulating underactive dopaminergic pathways in cortical tissues [36]. Our finding has important implications for the mechanisms of actions of these phytocannabinoids for treating neurodegenerative and neuropsychiatric disorders, e.g. schizophrenia. One can imagine that a partial dopamine agonist such as CBD, CBDV as well as aripiprazole might be able to achieve this goal by blocking the D2 receptors where they are overreactive and activating the D2 receptor where they are underactive.
To our knowledge, this is the first report of CBD and CBDV as functional partial agonists for dopamine D2-like receptors in vivo. C elegans are simple but valuable models for studying molecular targets of pharmaceuticals. Now that a role of dopamine signaling has been established in nematodes for phytocannabinoids, further studies are warranted to extend the in vivo studies to the mammals, such as using mice with dopamine receptors knocked out, to explore the potential therapeutic effects and elucidate the detailed mechanisms of actions of phytocannabinoids on neurodegenerative and neuropsychiatric disorders.
Supplementary Material
Highlights.
C. elegans were used to analyze the actions of cannabidiol and cannabidivarin
These phytocannabinoids exhibited partial efficacy of dopamine in causing paralysis
Knockout D2-like receptor, but not dopamine making enzyme, abrogated the effects
Dopamine D2-like receptor is involved in the in vivo actions of phytocannabinoids
This is a significant step toward understanding the mechanisms of phytocannabinoids
Abbreviations
- CBD
cannabidiol
- CBDV
cannabidivarin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ibeas Bih C, et al. , Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics, 2015. 12(4): p. 699–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morales P, Hurst DP, and Reggio PH, Molecular Targets of the Phytocannabinoids: A Complex Picture. Prog Chem Org Nat Prod, 2017. 103: p. 103–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein MO, et al. , Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell Mol Neurobiol, 2019. 39(1): p. 31–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.More SV and Choi DK, Promising cannabinoid-based therapies for Parkinson’s disease: motor symptoms to neuroprotection. Mol Neurodegener, 2015. 10: p. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hudson R, Rushlow W, and Laviolette SR, Phytocannabinoids modulate emotional memory processing through interactions with the ventral hippocampus and mesolimbic dopamine system: implications for neuropsychiatric pathology. Psychopharmacology (Berl), 2018. 235(2): p. 447–458. [DOI] [PubMed] [Google Scholar]
- 6.Seeman P, Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Transl Psychiatry, 2016. 6(10): p. e920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chase DL and Koelle MR, Biogenic amine neurotransmitters in C. elegans. Worm Book, 2007: p. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaletta T and Hengartner MO, Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov, 2006. 5(5): p. 387–98. [DOI] [PubMed] [Google Scholar]
- 9.Consortium C.e.S., Genome sequence of the nematode C. elegans: a platform for investigating biology. Science, 1998. 282(5396): p. 2012–8. [DOI] [PubMed] [Google Scholar]
- 10.Culetto E and Sattelle DB, A role for Caenorhabditis elegans in understanding the function and interactions of human disease genes. Hum Mol Genet, 2000. 9(6): p. 869–77. [DOI] [PubMed] [Google Scholar]
- 11.Kuwabara PE and O’Neil N, The use of functional genomics in C. elegans for studying human development and disease. J Inherit Metab Dis, 2001. 24(2): p. 127–38. [DOI] [PubMed] [Google Scholar]
- 12.White JG, et al. , The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci, 1986. 314(1165): p. 1–340. [DOI] [PubMed] [Google Scholar]
- 13.Komuniecki RW, et al. , Biogenic amine receptors in parasitic nematodes: what can be learned from Caenorhabditis elegans? Mol Biochem Parasitol, 2004. 137(1): p. 1–11. [DOI] [PubMed] [Google Scholar]
- 14.McDonald PW, et al. , Vigorous motor activity in Caenorhabditis elegans requires efficient clearance of dopamine mediated by synaptic localization of the dopamine transporter DAT-1. J Neurosci, 2007. 27(51): p. 14216–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardaway JA, et al. , Forward genetic analysis to identify determinants of dopamine signaling in Caenorhabditis elegans using swimming-induced paralysis. G3 (Bethesda), 2012. 2(8): p. 961–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carvelli L, Blakely RD, and DeFelice LJ, Dopamine transporter/syntaxin 1A interactions regulate transporter channel activity and dopaminergic synaptic transmission. Proc Natl Acad Sci U S A, 2008. 105(37): p. 14192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bermingham DP, et al. , Acute blockade of the Caenorhabditis elegans dopamine transporter DAT-1 by the mammalian norepinephrine transporter inhibitor nisoxetine reveals the influence of genetic modifications of dopamine signaling in vivo. Neurochem Int, 2016. 98: p. 122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson SB, et al. , Dopamine-dependent, swimming-induced paralysis arises as a consequence of loss of function mutations in the RUNX transcription factor RNT-1. PLoS One, 2019. 14(5): p. e0216417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Refai O and Blakely RD, Blockade and reversal of swimming-induced paralysis in C. elegans by the antipsychotic and D2-type dopamine receptor antagonist azaperone. Neurochem Int, 2019. 123: p. 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stiernagle T, Maintenance of C. elegans. WormBook, 2006: p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kudumala S, Sossi S, and Carvelli L, Swimming Induced Paralysis to Assess Dopamine Signaling in Caenorhabditis elegans. J Vis Exp, 2019(146). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyd WA, Smith MV, and Freedman JH, Caenorhabditis elegans as a model in developmental toxicology. Methods Mol Biol, 2012. 889: p. 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chase DL, Pepper JS, and Koelle MR, Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nat Neurosci, 2004. 7(10): p. 1096–103. [DOI] [PubMed] [Google Scholar]
- 24.Schafer WR and Kenyon CJ, A calcium-channel homologue required for adaptation to dopamine and serotonin in Caenorhabditis elegans. Nature, 1995. 375(6526): p. 73–8. [DOI] [PubMed] [Google Scholar]
- 25.Pisanti S, et al. , Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol Ther, 2017. 175: p. 133–150. [DOI] [PubMed] [Google Scholar]
- 26.Aymerich MS, et al. , Cannabinoid pharmacology/therapeutics in chronic degenerative disorders affecting the central nervous system. Biochem Pharmacol, 2018. 157: p. 67–84. [DOI] [PubMed] [Google Scholar]
- 27.Donvito G, et al. , The Endogenous Cannabinoid System: A Budding Source of Targets for Treating Inflammatory and Neuropathic Pain. Neuropsychopharmacology, 2018. 43(1): p. 52–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg EC, Patra PH, and Whalley BJ, Therapeutic effects of cannabinoids in animal models of seizures, epilepsy, epileptogenesis, and epilepsy-related neuroprotection. Epilepsy Behav, 2017. 70(Pt B): p. 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blessing EM, et al. , Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics, 2015. 12(4): p. 825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katzman MA, Furtado M, and Anand L, Targeting the Endocannabinoid System in Psychiatric Illness. J Clin Psychopharmacol, 2016. 36(6): p. 691–703. [DOI] [PubMed] [Google Scholar]
- 31.Leweke FM, et al. , Therapeutic Potential of Cannabinoids in Psychosis. Biol Psychiatry, 2016. 79(7): p. 604–12. [DOI] [PubMed] [Google Scholar]
- 32.McGuire P, et al. , Cannabidiol (CBD) as an Adjunctive Therapy in Schizophrenia: A Multicenter Randomized Controlled Trial. Am J Psychiatry, 2018. 175(3): p. 225–231. [DOI] [PubMed] [Google Scholar]
- 33.Kahn RS, et al. , Schizophrenia. Nat Rev Dis Primers, 2015. 1: p. 15067. [DOI] [PubMed] [Google Scholar]
- 34.Fletcher PC and Frith CD, Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci, 2009. 10(1): p. 48–58. [DOI] [PubMed] [Google Scholar]
- 35.Masri B, et al. , Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc Natl Acad Sci U S A, 2008. 105(36): p. 13656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urs NM, Peterson SM, and Caron MG, New Concepts in Dopamine D2 Receptor Biased Signaling and Implications for Schizophrenia Therapy. Biol Psychiatry, 2017. 81(1): p. 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


