Abstract
Background:
The phenomenology and neurobiology of depressive symptoms in anxious youth is poorly understood.
Methods:
Association networks of anxiety and depressive symptoms were developed in adolescents with generalized anxiety disorder (GAD; N=52, mean age: 15.4±1.6 years) who had not yet developed major depressive disorder. Community analyses were used to create consensus clusters of depressive and anxiety symptoms and to identify “bridge” symptoms between the clusters. In a subset of this sample (n=39), correlations between cortical thickness and depressive symptom severity was examined.
Results:
Ten symptoms clustered into an anxious community, 5 clustered into a depressive community and 5 bridged the two communities: impaired schoolwork, excessive weeping, low self-esteem, disturbed appetite, and physical symptoms of depression. Patients with more depressive cluster burden had altered cortical thickness in prefrontal, inferior and medial parietal (e.g., precuneus, supramarginal) regions and had decreases in cortical thickness-age relationships in prefrontal, temporal and parietal cortices.
Limitations:
Data are cross-sectional and observational. Limited sample size precluded secondary analysis of comorbidities and demographics.
Conclusions:
In youth with GAD, a sub-set of symptoms not directly related to anxiety bridge anxiety and depression. Youth with greater depressive cluster burden had altered cortical thickness in cortical structures within the default mode and central executive networks. These alternations in cortical thickness may represent a distinct neurostructural fingerprint in anxious youth with early depressive symptoms. Finally, youth with GAD and high depressive symptoms had reduced age-cortical thickness correlations. The emergence of depressive symptoms in early GAD and cortical development may have bidirectional, neurobiological relationships.
Keywords: Depression, Adolescent, Generalized anxiety disorder (GAD), Network
Introduction
Anxiety disorders—which frequently emerge in childhood or adolescence—typically precede the onset of depressive symptoms (Bittner et al., 2004) and increase the risk of developing major depressive disorder (MDD) (Beesdo et al., 2010). Moreover, anxiety disorders, when they co-occur in depressed youth, increase morbidity and treatment resistance beyond what might be expected with either MDD or an anxiety disorder alone. Indeed, the combination of these disorders increase the likelihood of suicidal ideation and suicide attempts (Husky et al., 2012). Yet, little is known regarding: (1) risk factors for developing depressive symptoms in adolescents with anxiety disorders and (2) how features of an anxiety disorders are linked to early depressive symptoms.
Prior work demonstrated that anxiety disorders increase the risk of developing depressive disorders. Three reports from the Early Developmental Study of Psychopathology (EDSP) (Wittchen et al., 1998) reveal that certain anxiety disorders (i.e., social anxiety disorder), when present in adolescence, increase the risk of developing MDD in early adulthood (Stein et al., 2001). Additional analyses of this sample (Bittner et al., 2004) reveal that social anxiety disorder, panic disorder, agoraphobia and generalized anxiety disorder (GAD) increase the risk of developing depressive disorders while having multiple anxiety disorders, more severe impairment, comorbid panic attacks specifically contribute to this risk of developing MDD. Interestingly, the severity of anxiety-related impairment represents the strongest independent predictor of developing MDD (OR: 2.2 [95% CI: 1.0 to 4.4]) (Bittner et al., 2004). Also in this cohort, more than half of individuals who had social anxiety disorder during adolescence developed MDD during the follow-up period (OR: 3.2 [95% CI: 2.5 to 4.1]) (Beesdo et al., 2007) and social anxiety disorder represented the first disorder to emerge in most of these patients. Providing further evidence of the temporal association of depressive and anxiety disorders, the age-of-onset distribution for GAD parallels the age-of-onset distribution for depressive disorders although the age-of-onset distribution for GAD emerges 2 years earlier than that of the depressive disorders (Beesdo et al., 2010).
Studies that examine GAD and MDD as distinct disorders are complicated by diagnostic, phenomenologic, epidemiologic and clinical issues. First, diagnostic criteria for GAD and MDD overlap (e.g., dyssomnia, fatigue or low energy, and poor concentration). Second, these overlapping symptoms and features such as interference with schoolwork, somatic symptoms and appetite disturbances are assessed in rating scales for both depressive and anxiety disorders (Birmaher et al., 1997; Isa et al., 2014; Kovacs, 1985; March et al., 1997; Mossman et al., 2017). Third, multiple formulations have been offered to explain this comorbidity. Tyrer, et al, for example, have proposed that MDD and GAD when present concurrently in the same patient should constitute a distinct diagnosis of “cothymia” (Tyrer et al., 2001). Indeed, given shared symptoms, genetic risk factors, and psychopharmocologic interventions some have argued MDD and GAD are differing phenotypes of a similar underlying genotype perhaps due to separate environmental triggers (Kendler, 1996; Kendler et al., 2007; Roy et al., 1995). However, more recent studies (Kessler et al., 2008) have argued against this conceptualization given each disorder’s distinct risk factors. GAD and MDD share certain risk factors (e.g., childhood adversity and harm avoidance appear) (Beesdo et al., 2010; Blanco et al., 2014). However, family history of GAD, behavioral inhibition, childhood separation events, and parental overprotection specifically increase the risk of developing anxiety disorders. Likewise, parental depression, low parental emotional warmth, and parental rejection are specifically associated with the development of MDD (Beesdo et al., 2010). Finally, a 15 year, longitudinal study of young adults (aged 19-20 years) suggests that anxiety disorders increase the likelihood of developing anxious-depression or depressive disorders (Merikangas et al., 2003). Disorder-specific risk was not evaluated (e.g., unique risk associated with panic disorder vs. generalized anxiety disorder vs. social anxiety disorder).
Multiple biological, environmental and social factors influence the complex relationship between depressive and anxiety symptoms during childhood and adolescence. Anxiety symptoms in children (N=2220) who were prospectively followed from late childhood through late adolescence, initially decreased from age 10 until age 12-14 and then increased during mid-to-late adolescence (Van Oort et al., 2009). In these adolescents, sex affects anxiety symptom severity and this effect appears to contribute to the severity of depressive symptoms, while time-dependent variability in anxiety and depressive symptoms differ significantly between sexes. Thus, anxiety symptoms appear to predict later depressive symptoms in youth but the mechanism of this phenomenon remains poorly understood (Van Oort et al., 2009).
Few studies have evaluated the neurobiology of depressive symptoms in youth with anxiety disorders. Most prior work in youth has focused on the functional neurocircuitry of either MDD or anxiety disorders. These studies have observed non-disorder specific differences in amygdala activation (i.e., amygdala hyperactivation in both MDD and anxiety disorders), but differences in task-specific amygdala activation in youth with MDD and those with anxiety disorders (Beesdo et al., 2009). Additionally, in a sample of youth who were at risk for developing MDD (e.g., parental history of MDD), (Monk et al., 2008) functional differences were observed in the amygdala and nucleus accumbens. However, in this small sample, 59% of the at risk youth had a history of an anxiety disorder; this high prevalence precluded specific examination of the neurofunctional risk contribution of anxiety disorders and the “contribution of anxiety disorders to neural perturbations in at-risk individuals” could not be specifically determined (Monk et al., 2008). Neurostructural evaluations of anxious depression are rare in children, despite numerous studies of the neurostructural basis of anxiety disorders and depressive disorders (as separate conditions) (Adleman et al., 2012; Gold et al., 2016; Mueller et al., 2013; Strawn et al., 2015, 2014). In one voxel-based morphometry study of adolescents with anxiety disorders, depressive disorders or anxiety disorders + MDD (anxious depression), adolescents with anxious depression exhibited decreased gray matter volumes in the dorsolateral prefrontal cortex compared to patients with MDD alone, while compared to healthy subjects, adolescents with anxious depression had increased gray matter volumes in the pre- and post-central gyri (Wehry et al., 2015). To date, no studies have examined the neurobiology of depressive symptoms in youth with anxiety disorders.
To explore the relationship between depressive and anxiety symptom clusters, we focused on patients with GAD. The decision to focus on GAD was based on (1) symptomatic distinctiveness of GAD from other anxiety disorders with overlapping symptoms (Cho et al., 2019) (2) reliability and validity of the diagnosis in adolescents (Lyneham et al., 2007; Rappaport et al., 2017); (3) evidence that it is distinct from depressive disorders (Cummings et al., 2014); longitudinal stability during childhood, adolescence and early adulthood (Beesdo et al., 2010). Beyond this, most DSM-5 anxiety disorders in youth have specific foci (e.g., separation from a primary attachment figure in separation anxiety disorder, fear of humiliation or embarrassment in social anxiety disorder) whereas the anxiety that typifies GAD is diffuse and prototypic of central cognitive aspects of anxiety—“repetitive chains of thoughts about potentially adverse consequences of issues that present with some degree of uncertainty” (Cho et al., 2019).
Although the comorbidity of anxiety disorders and major depression in youth has been extensively studied (Cummings et al., 2014), there are substantial knowledge gaps regarding subthreshold depressive symptoms in anxious youth. Identifying specific symptoms that bridge anxiety and depressive disorders in anxious youth, will inform early intervention development and precision medicine approaches. Targeted screening could prevent the development of depressive disorders and decrease the morbidity of anxiety and depressive disorders. With these considerations in mind, we sought to (1) identify predictors of depressive symptoms in adolescents with a primary anxiety disorder; (2) explore potential associations among features of anxiety and depressive symptoms; and (3) examine neurostructural correlates of depressive symptoms in anxious youth.
Methods
The protocol was approved by the Institutional Review Board for the University of Cincinnati and conducted in accordance with Good Clinical Practice guidelines. Written, informed consent and assent were provided by parent/legal guardian and patients respectively and the study was conducted at a single academic, outpatient site.
Study population
Study participants (N=52) were outpatient youth aged 12 through 17 years who met DSM-IV-TR criteria for GAD, assessed by unstructured and semi-structured assessments by a board-certified child and adolescent psychiatrist (JRS). The diagnosis of GAD was confirmed with the Anxiety Disorders Interview Schedule for DSM-IV (ADIS-IV) (Silverman et al., 2001). We included only patients who met criteria for GAD and excluded patients with MDD or PTSD. Patients treated with psychotropic medications were not included. Patients with active substance abuse or dependence within the past 6 months were excluded and patients had a negative urine drug screen. Data used for this study was extracted from the screening phase of a randomized controlled trial of escitalopram in the treatment of GAD including functional neuroimaging before and during escitalopram treatment. Importantly, not all patient who were screened were randomized. Results and further detail regarding this randomized controlled trial were recently published (Strawn et al., 2020).
Measures
Demographic data including age, sex, and race were collected. Additionally, pubertal status was determined using the Duke Self-Rated Tanner Scale (Self Report) (Duke et al., 1980). The CGI-S, a clinician-administered instrument of global severity—which is performed by a clinician—is based both on observed and reported symptoms, functional impairment and behavior over a 7 day period (Guy, 1976).
The Children’s Depression Rating Scale-Revised (CDRS-R) is a 18-item semi-structured, interview-based assessment is commonly employed in clinical research to monitor depressive symptom severity in youth (Rintelmann et al., 1996). Each item represents a feature or symptom of depression: (1) impaired schoolwork (difficulty with concentration); (2) difficulty having fun (anhedonia); (3) social withdrawal; (4) sleep disturbance; (5) appetite disturbance; (6) excessive fatigue; (7) physical complaints; (8) irritability; (9) excessive guilt; (10) low self-esteem, (11) depressed mood; (12) morbid ideation; (13) suicidal ideation; (14) excessive weeping; (15) depressed facial affect; (16) listless speech and (17) hypoactivity. These items are rated from 1 to 5 or 1 to 7 (total possible raw score range: 17-113). For the purposes of imaging analysis, we used the CDRS-R to categorize patients into “high” and “low” burdens of depressive symptoms which we defined as CDRS-R score greater than or less than to our sample-specific median, 35.
The Pediatric Anxiety Rating Scale (PARS), is a clinician-rated instrument that is commonly used in clinical trials of anxious youth, queries anxiety symptoms, severity, and impairment (RUPP, 2002). The PARS consists of 7 sub-scales, each rated 1-5, including: overall number of anxiety symptoms (based on a symptom checklist), overall frequency of anxiety symptoms, overall severity of anxiety feelings, overall severity of physical symptoms of anxiety, overall avoidance of anxiety-provoking situations, interference with family relationships and/or performance at home, and interference with peer and adult relationships and/or performance outside of home.
Association network
Descriptive statistics were calculated using Microsoft Excel. All statistical analyses were performed in R (version 3.4.2). First, Pearson’s correlation coefficients calculated for each pairwise combination of the following: (1) total PARS score, (2) each PARS item, and (3) each CDRS item. Based on this correlation matrix, association networks were created using qgraph (R package). Each partial correlation coefficient then constituted an “edge” for the association network while each symptom subscale represented a “node.” qgraph uses the Fruchterman-Reingold algorithm (Fruchterman and Reingold, 1991), ported from the SNA package (Butts, 2010) to create a force-directed layout which clusters correlated nodes and prevents overlapping edges. This algorithm causes all nodes to repulse each other while correlated or connected nodes also attract each other. The result is a representation of the network of symptoms in which highly correlated nodes visually ‘cluster’ together in proportion to their correlational coefficient. We included only edges with partial correlations greater than 0.25, a cutoff used in previous network analyses (Epskamp et al., 2018; Jones et al., 2018) to limit spurious correlations. Networks were developed to contain (1) PARS and CDRS sub-scales, as well as (2) total PARS score and CDRS sub-scales. A sensitivity analysis examining the associations between age and each node in the association network was performed to assess the impact of age on depressive and anxious symptoms was also performed.
Community Analysis
We performed a community analysis to assess which anxiety and depressive symptoms formed highly correlated clusters. This approach has been previously used to identify syndromic networks of psychopathology (Robinaugh et al., 2014). To detect communities, we used five algorithms from the R package igraph (Csardi and Nepusz, 2006): (1) spin-glass (spinglass.community), (2) walk-trap (walktrap.community), (3) leading eigenvector (cluster_leading_eigen), (4) edge-betweenness (cluster_edge_betweenness), and (5) fast-greedy (cluster_fast_greedy).
Neuroimaging
In a subset of adolescents (imaging cohort, n=39), high resolution T1-weighted images were obtained using a 3.0 Tesla Phillips Achieva MRI scanner. During each scan, subjects were recumbent in the bed of the scanner and a magnetization-prepared radio-frequency pulses and rapid gradient-echo (MPRAGE) was acquired in the sagittal orientation with 1 mm slice thickness, over contiguous slices. Images were acquired with a repetition time (TR) of 6.8 ms, an echo time (TE) of 2.9 ms, a flip angle of 9°; a matrix size of 256 x 256, a field of view of 256×256×160 mm.
Image Processing
Initial image processing was completed automatically using FreeSurfer package 6.0.0 (http://surfer.nmr.mgh.harvard.edu). Processing included motion correction, averaging of multiple volumetric T1 weighted images, skull striping using a deformable template model, automated registration to Talairach space, segmentation of subcortical white matter and deep gray matter volumetric structures, normalization of intensity, tessellation of the gray matter, and white matter boundaries, automated topology correction and surface deformation (based on intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid borders) (Fischl et al., 2002). The segmentation procedure was visually inspected for inaccuracies (HKS, MJL), and manual corrections were performed as needed by incorporating control points. This was followed by surface inflation and registration to a spherical atlas (based on subject cortical folding patterns) to harmonize the cortical geometry across the patients. The cerebral cortex was subsequently auto-parcellated into 34 different gyral regions per hemisphere using gyral and sulcal anatomy as previously described (Desikan et al., 2006). Individual cortical reconstructions were smoothed using a Gaussian kernel of 10mm FWHM and fit to an average subject through recognition of cortical curvature for visualization of results. Differences in cortical thickness, at each vertex of the cortical surface, between groups (see next section) were determined using a general linear model (GLM). This was performed in Freesurfer’s QDEC (Query, Design, Estimate, Contrast) graphic user interface using a cross-subject GLM with a different offset different slope (DODS) design matrix that co-varied for age with gender as a nuisance factor. A vertex-wise threshold of p<0.001 was used and, to correct for multiple comparisons, a Monte Carlo simulation with 10,000 iterations was used to identify findings at a cluster-level significance threshold of 0.05, as previously described (Strawn et al., 2014; Sylvester et al., 2016).
Relationship between depressive symptoms and cortical thickness
To investigate distinct anatomic correlates of depressive symptoms (i.e., the core depressive symptoms identified in the community analysis), anxious adolescents were divided into 2 groups (“high” and “low” depressive symptoms) based on a core CDRS score greater than or less than 35—the median core CDRS-R score in our sample. These groups were then evaluated with regard to differences in cortical thickness as described in Image Processing. Additionally, to understand the neurodevelopmental effects of depressive symptoms in this age range and given that this period of time—adolescence—represents a critical neurodevelopmental period, we performed a post-hoc analysis of age-cortical thickness relationships in anxious adolescents with high core depressive symptoms and a separate analysis of age-cortical thickness relationship in those with low levels of core depressive symptoms (i.e., “high” and “low” depressive symptom groups). A dichotomous approach to our analysis was chosen due to concern that a continuous analysis may be more sensitive to multicollinearity given the multidimensionality of our measures, however it should be noted that dichotomization of continuous variables can lead to several disadvantages including decreased power and loss of patient-level information (MacCallum et al., 2002).
RESULTS
Demographics and Suicidality
Of the 52 patients included in this analysis, 40 were female (76.9%) and 12 were male (23.1%). Racially, our sample included 46 White patients (88.5%), 3 patients of mixed parentage (5.8%), 2 African American patients (3.8%), and 1 Asian patient (1.9%). The patients were aged 15.4±1.6 years and all patients were post-pubertal (12.2% Tanner 3, 42.9% Tanner 4, 42.9% Tanner 5). Family history was available for 51 of the 52 patients in this study. Among these, many patients had a family history of a first degree relative with an anxiety disorder (66.6%) or with major depressive disorder (37.3%). While all patients had GAD, co-morbid anxiety was common, including panic disorder (55.8%), social anxiety disorder (50.0%), separation anxiety disorder (25.0%), specific phobia (23.1%), ADHD (21.2%) and dysthymia (7.7%). Anxiety symptoms were moderate to severe as reflected by a total PARS score of 25.2±3.7 (clinical trial scoring: 19.1±2.7). The average CGI-S score was 4.2 ± 1.0. Lifetime suicidality was assessed with the Columbia-Suicide Severity Rating Scale (CSSRS) (Posner et al., 2011). Of the 52 patients for whom suicidality data were collected, 32 (61.5%) endorsed CSSRS 1 ideation (“wishing to be dead”), 13 (25%) had previous non-specific suicidal thoughts (CSSRS 2 ideation), 8 (15.4%) had previous suicidal thoughts with a specific method, but without intent to act, 4 (7.7%) had previous suicidal thoughts with intent to act but without a specific plan, and 3 (5.8%) had previous suicidal thoughts with both a specific plan and some intent to act. 2 (3.8%) patients had actually attempted suicide in the past.
Association network
Our association networks are included as Figure 1–2. With correlations of R>0.25 included, all anxious and depressive symptoms connected to all other symptoms, either directly or indirectly. All correlations were positive, except between anhedonia and overall number of anxiety Symptoms. Anxious symptoms were highly correlated with each other and correlated with six depressive symptoms: physical complaints (to overall number of anxiety symptoms, overall severity of anxiety feelings, overall severity of physical symptoms of anxiety, and interference with family relationships and/or performance at home), morbid ideation (to overall frequency of anxiety symptoms), anhedonia (to overall number of anxiety symptoms and overall severity of physical symptoms of anxiety), impaired schoolwork (to overall severity of physical symptoms of anxiety and overall severity of physical symptoms of anxiety), social withdrawal (to overall severity of physical symptoms of anxiety, overall avoidance of anxiety-provoking situations, and interference with peer and adult relationships and/or performance outside of home), and sleep disturbance (to overall severity of physical symptoms of anxiety, overall avoidance of anxiety-provoking situations, interference with family relationships and/or performance at home, and interference with peer and adult relationships and/or performance outside of home). Total PARS score correlated with the same depressive symptoms, with the exception of morbid ideation. Age, which was not included in our association network, was weakly associated with excessive weeping (R=0.29, p=0.038), but not with any other depressive/anxiety symptoms.
Figure 1:
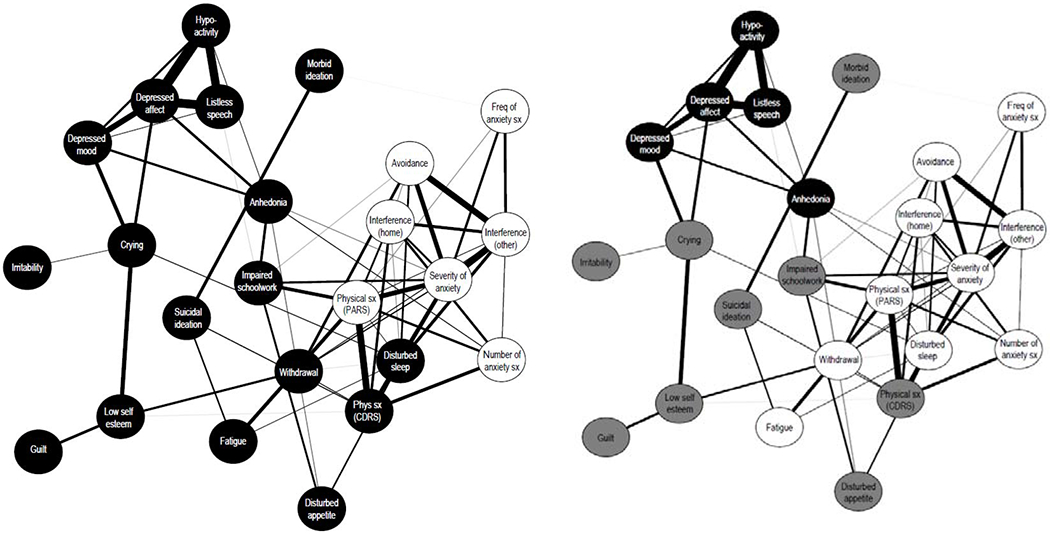
Network association plot of anxiety and depression symptoms. In Figure 1a, symptoms are coded by their rating scale of origin (CDRS-R nodes in black, PARS nodes in white). In Figure 1b, symptoms are grouped by consistent clustering in 4 out of 5 clustering algorithms (see Table 1) with the depressive cluster in black, the anxious cluster in white, and symptoms clustering to neither group in grey.
Avoidance = overall avoidance of anxiety-provoking situations; CDRS-R = Children’s Depression Rating Scale-Revised; Freq = frequency; Interference (home) = interference with family relationships and/or performance at home; Interference (other) = interference with peer and adult relationships and/or performance outside of home; PARS = Pediatric Anxiety Rating Scale; Phys = physical; Withdrawal = social withdrawal; Sx = symptoms
Figure 2: Cortical thickness in anxious adolescents with core depressive symptom burdens that are greater than the median core depression symptom score compared to those with fewer than the median core depression symptom score.
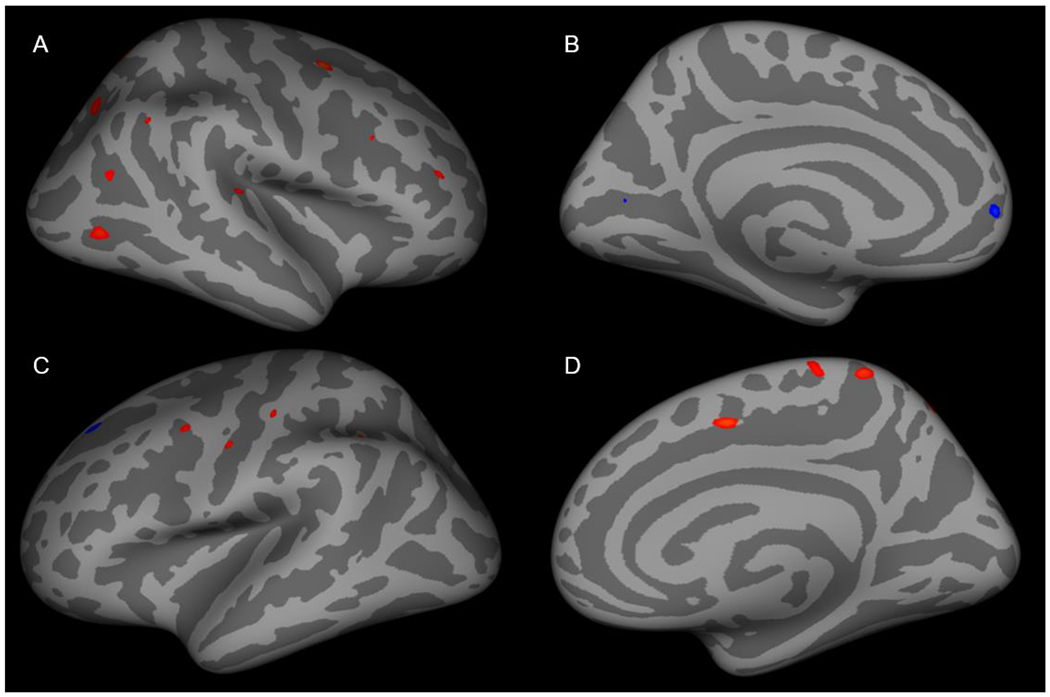
(A) Cortical thickness was increased in the caudal middle frontal, lateral occipital, rostral middle frontal, superior parietal, superior temporal and inferior parietal regions in the right lateral cortex. (B) Cortical thickness was decreased in the pericalcarine and superior frontal regions. In the left lateral cortex (C) cortical thickness was increased in the precentral, postcentral, supramarginal and superior parietal regions and decreased was observed in the superior frontal region. (D) Cortical thickness was increased in superior frontal, precuneus and paracentral regions.
Community analysis
In the community analysis (Table 1), all 7 PARS sub-scores were grouped in a single community (anxiety) in each of the five algorithms except for the “edge-between” algorithm which overall number of anxiety symptoms formed a separate community with physical complaints (CDRS-R). In each of the five algorithms, sleep disturbances was included in the anxiety community. In four out of five algorithms, social withdrawal was included in the anxiety community. In three out of five, excessive fatigue and physical complaints (CDRS-R) were also included in the anxiety community. Similarly, depressive symptoms were found in distinct communities. All five algorithms clustered anhedonia, depressed mood, depressed affect, listless speech, and hypoactivity in a single community (depression). With some algorithms, the Depression community included impaired schoolwork (3 of 5), irritability (3 of 5), morbid ideation (3 of 5), and appetite disturbances (2 of 5). A third cluster containing the core symptoms excessive guilt and low self-esteem, which we will refer to as Internalizing, emerged in three of the five community analyses. Other symptoms clustered in the Internalizing community include (in two analyses) suicidal ideation and (in one analysis each) impaired schoolwork, social withdrawal, appetite disturbance, excessive fatigue, physical complaints (CDRS-R), morbid ideation, and excessive weeping.
Table 1:
Study sample demographic and diagnostic features characterized, with imaging group compared to group without imaging data available.
| Imaging (n=39)* | No Imaging (n=13) | Total (N=52)* | P value | |
|---|---|---|---|---|
| Age ± SD (in years) | 15.5 ± 0.91 | 15.0 ± 1.8 | 15.4 ± 1.6 | 0.3674 |
| Females (%) | 31 (79.4) | 9 (69.2) | 40 (76.9) | 0.4661 |
| Race | ||||
| White (%) | 35 (89.7) | 11 (84.6) | 46 (88.5) | 0.6323 |
| Non-white (%) | 4 (10.3) | 2 (15.4) | 6 (11.5) | |
| SAD (%) | 9 (23.1) | 4 (30.8) | 13 (25.0) | 0.7137 |
| Social anxiety disorder (%) | 21 (53.8) | 5 (38.5) | 26 (50.0) | 0.5218 |
| Specific phobia (%) | 10 (25.6) | 2 (15.4) | 12 (23.1) | 0.7063 |
| Panic disorder (%) | 20 (51.3) | 9 (69.2) | 29 (55.8) | 0.4202 |
| ADHD (%) | 7 (17.9) | 4 (30.8) | 11 (21.2) | 0.4350 |
| Dysthymia (%) | 3 (7.7) | 1 (7.7) | 4 (7.7) | 1 |
| Family History* of Anxiety Disorder (%) | 26 (68.4) | 8 (61.5) | 38 (66.6) | 0.7379 |
| Family History* of MDD (%) | 17 (44.7) | 2 (15.4) | 19 (37.3) | 0.0960 |
| CGI-S ± SD | 4.1 ± 1.0 | 4.3 ± 0.9 | 4.2 ± 1.0 | 0.5819 |
| Total | 39 | 13 | 52 |
ADHD = attention deficit hyperactivity disorder; CGI-S = Clinical Global Impression-Severity; MDD = major depressive disorder; SAD = separation anxiety disorder; SD = standard deviation
Family History refers to first degree relatives, no information available for one patient (adopted)
An alternative association network with signs and symptoms group by community analysis clustering (rather than whether they arose from PARS or CDRS-R) is presented in Figure 1B. We included signs and symptoms that clustered with depressed mood and anhedonia in at least 4 of our 5 clustering methods in the depression group, while symptoms clustering with the majority of the PARS items in 4 out of 5 clustering methods are grouped in an anxiety group.
Identification of Bridge Symptoms
We define a “bridge symptom” as a symptom found on the shortest direct route from the anxiety group to the depression group, without being a member of either group, in our clustered association network. Five symptoms were identified as bridge symptoms: low self-esteem, impaired schoolwork, appetite disturbances, physical complaints relating to depression and excessive weeping (Table 2). In a post hoc analysis, the average value of bridge symptom was compared in subjects with and without each comorbid social anxiety disorder, separation anxiety disorder, panic disorder, ADHD and dysthymia using a two-tailed t-test. No statistically significant differences in these co-morbidities were detected.
Table 2:
Identified symptoms bridging the cluster of anxious symptoms and the cluster of depressive symptoms.
| Anxiety Cluster | Bridge Symptoms | Depression Cluster | ||
|---|---|---|---|---|
| Sleep disturbance | Excessive weeping | Depressed mood; Depressed affect | ||
| Social withdrawal | Low self-esteem | Excessive weeping | Depressed mood; Depressed affect | |
| Anxiety-related distress, physical symptoms (PARS) | Impaired schoolwork | Anhedonia | ||
| Sleep Disturbance | Appetite disturbance | Anhedonia | ||
| Number of anxiety sx, Anxiety-related distress, Physical symptoms (PARS), Anxiety interference at home | Physical symptoms (CDRS-R) | Low self-esteem | Excessive weeping | Depressed mood; Depressed affect |
Cortical Thickness and Depressive Symptoms
Cortical thickness differed between adolescents with “high” and “low” depressive symptoms (i.e., greater or less than CDRS-R score of 35) in an ensemble of regions: bilateral precuneus, bilateral cuneus, bilateral supramarginal, bilateral lateral occipital, bilateral superior parietal, bilateral superior frontal, bilateral postcentral, bilateral inferior temporal, bilateral inferior parietal, right posterior cingulate, right medial orbitofrontal, right middle temporal, left precentral, left fusiform, left insula, left superior temporal sulcus, left entorhinal and left superior temporal regions (Supplemental Table 3, Figure 3). Additionally, in our post-hoc examination of age-cortical thickness relationships in patients with “high” core depressive symptom burden, we observed decreased age-thickness correlations when compared with patients with “low” core depressive symptom burden in the following: bilateral caudal middle frontal, bilateral superior frontal, bilateral superior parietal, left superior temporal, left precuneus, left lateral occipital, left rostral middle frontal, left paracentral, left inferior parietal, right supramarginal, right precentral, right postcentral, and right pericalcarine regions (Supplemental Table 3, Figure 4). Family history of a first degree relative with an anxiety disorder or with major depressive disorder did not statistically differ between adolescents with “high” or “low” depressive symptoms in this sample (p=0.9204 and p=0.4458, respectively).
Figure 3: Age-cortical thickness correlations are decreased in anxious adolescents with greater core depressive symptoms.
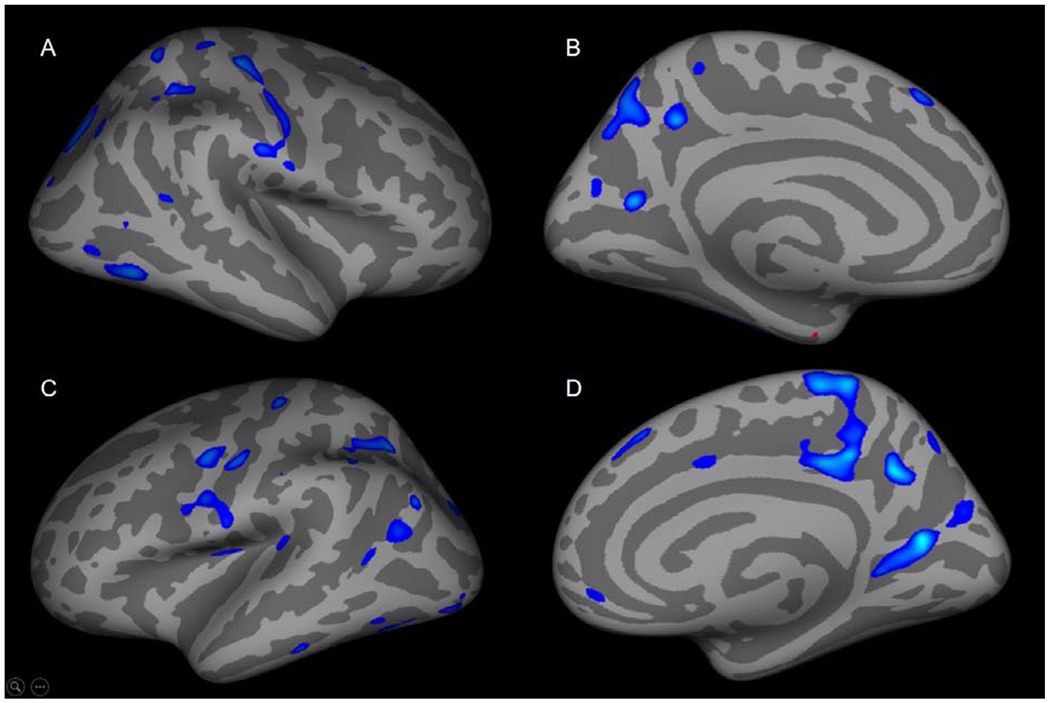
In anxious adolescents with greater depressive symptoms, cortical thickness-age correlations were decreased in (A) inferior parietal, postcentral, lateral occipital, mid-temporal, superior frontal, inferior temporal, superior parietal and supramarginal regions. (B) Cortical thickness-age correlations were decreased in the precuneus, cuneus, superior frontal regions and was increased in the entorhinal region. (C) Cortical thickness was decreased in the superior parietal, pre- and postcentral, inferior parietal, supramarginal, inferior/superior temporal, lateral occipital, insula, superior temporal regions. (D) Cortical thickness-age relationships were decreased in the medial orbital frontal, posterior cingulate, precuneus, cuneus and superior frontal.
Discussion
This study is the first to identify a constellation of symptoms that correlatively “bridge” clusters of anxiety and depressive symptoms in adolescents with primary anxiety disorders. Understanding the putative links between clusters of anxiety and depressive symptoms in younger patients provides insights into this relationship before it is modified by the onset of full, syndromic MDD. From a developmental psychopathology standpoint, bridging symptoms (e.g., excessive weeping, low self-esteem, impaired schoolwork, appetite disturbances, and physical symptoms), early in the course of illness may play critical roles in the pathogenesis of depressive disorders in youth with anxiety disorders and may represent specific treatment targets. Additionally, our findings of two distinct symptom clusters representing anxious and depressive symptoms extend the findings of a longitudinal symptom network study, which focused on a broader and younger population (age 5-14 years) of developing children rather than those with anxiety disorders. In this prior analysis, maternally-reported depressive and anxious symptoms did not form distinct networks and symptoms highly correlated regardless of which diagnostic family to which a symptom belonged (McElroy et al., 2018). Taken together with the current findings, clustering of depressive and anxiety symptoms may emerge following the onset of an anxiety disorder.
For nearly two centuries, psychiatric diagnoses have been based on the identification of symptom clusters that represent reported and observed symptoms. Within the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), (American Psychiatric Association, 2013) diagnoses represent the consensus of symptom clusters which represent distinct disorders. This approach represents the backbone of structured diagnostic assessments and the DSM, however, it has been challenged in recent years. The last decade saw dimensional approaches (e.g., Research Domain Criteria [RDoC]) to psychiatric illness based on domains and valence which promised to elucidate the pathoetiology of these disorders at genetic, molecular, circuit and behavioral levels (Insel, et al., 2010; Insel et al., 2015). Although both RDoC and cluster-based (i.e., conventional) constructs classify psychiatric illnesses based on empirical data, cluster-based approaches focus on reported symptoms (e.g. excessive guilt or anhedonia) or observed signs (e.g., psychomotor retardation or agitation). By contrast, RDoC focuses on “dimensions of observable behavior and neurobiological measures” with little attention given to subjective experience of patients (Cuthbert, 2015). Implementation of such construct-based based on specific domains (i.e., RDoC approaches) raises concerns with regard to (1) underlying developmental processes, (2) classification of specific disorders within a dimensional framework, (3) shared symptoms as well as circuits (Franklin et al., 2015). Additionally, some argued that RDoC approaches are problematic an create biological reductionism (Franklin et al., 2015). Regardless of whether neurobiologically based constructs such as RDoC or a symptom-focused cluster-based construct are preferred, it is imperative that both the unique symptomatology of disorders in youth and symptoms linking comorbid diagnoses are considered in research settings and in the clinic.
The analyses presented herein suggest the validity of cluster-based constructs. Specifically, we observed that patients with predominantly anxiety-related symptoms included all seven PARS items as well as dysomnia and fatigue whereas a second group featured predominantly of depression-related symptoms and included listless speech, depressed affect, hypoactivity, depressed mood, and anhedonia. The symptoms in the depressive cluster are specific for depression and include anhedonia and depressed mood—core depressive symptoms. Within the depressive cluster, anhedonia is the only directly linked symptom to the anxiety cluster (through anxiety-related distress). Given the link between anxiety-related distress and anhedonia (but not depressed mood), the emergence of anhedonia in anxious youth may be a more sensitive risk marker for developing MDD than depressed mood. This finding is consistent with lower animal studies in which chronic stress reduces dopamine release in response to pleasurable stimuli (Di Chiara et al., 1999) and decreases engagement in pleasurable activities (Papp et al., 1991; Treadway and Zald, 2011). Therefore, a treatment focusing on discriminating between rewarding and non-rewarding behaviors, such as behavioral activation, may be useful in preventing the development of depression in at-risk anxious youth (Treadway and Zald, 2011).
We identified five symptoms that bridge depression and anxiety clusters (low self-esteem, impaired schoolwork, appetite disturbances, physical symptoms of depression (CDRS-R) and excessive weeping). Some of these symptoms (e.g., self-esteem and excessive weeping) represent sequelae of anxiety (e.g., crying in times of severe anxiety or when experiencing anxiety-related distress when being overwhelmed by anxiety, etc.). Additionally, some bridging symptoms reflect functional impairment (e.g., impaired schoolwork) and could be attributed to either the anxiety disorder or to an emerging depressive disorder. To this end, both severe anxious symptoms and emerging depressive symptoms could result in poor schoolwork but would not necessarily be due to the same underlying symptoms or disease process. Specific bridge symptoms (e.g., impaired schoolwork) may lend themselves to specific intervention (i.e., increased educational support and accommodations). Many of these symptoms can be recognized and reported by family members (e.g., appetite disturbances, excessive weeping, impaired schoolwork) and thus, clinicians might increase vigilance in monitoring these specific symptoms with the hopes that early identification could forestall the development of MDD. Finally, the bridging role of physical symptoms which represent somatic manifestations of internalizing disorders in pediatric populations (Bernstein et al., 1997; Crawley et al., 2014; Ginsburg et al., 2006), may reflect a shared neurobiologic/psychological etiology. Specifically, in patients with anxiety disorders and in those with depressive disorders, neurostructural and neurofunctional abnormalities in the anterior insula and salience network have been described (Craig, 2009; Peters et al., 2016). This network processes interoceptive information and somatic afferent signals and the processing of this information is disrupted in both anxiety and depressive disorders. Whether altered interoceptive processing represents a specific endophenotype of youth with anxiety or depressive disorders remains to be determined. At this time, the relationship between abnormal interoceptive functioning and high somatic symptom burden in individuals with anxiety disorders and those who develop depressive symptoms represents a significant void in our understanding of the relationship between anxiety and depression. Importantly, although comorbidities and family history were not significantly related to bridge symptoms, these results should be interpreted with caution given our limited power to detect these differences. Further research with larger samples is needed to elucidate the relationship between genetic loading and various comorbidities and symptoms bridging anxious and depressive symptom clusters.
Social withdrawal, a symptom found in our anxiety cluster, is the starting point for a bridge to our depressive cluster (specifically, depressed mood and depressed affect). That social withdrawal begins a cascade leading to depressive symptoms is consistent with previous observations that more than half of patients with social anxiety disorder in childhood or adolescence later develop MDD (Beesdo et al., 2007). Importantly, the correlation between social withdrawal and depressed mood and affect is not direct, but rather linked through low self-esteem and excessive weeping. We postulate that future longitudinal studies will find these linking symptoms to precede more frank and specific signs of MDD (i.e., anhedonia and depressed mood). Further, by addressing low self-esteem, specifically in adolescents with anxiety-related social withdrawal we may thwart the development of depressive disorders in youth with primary anxiety disorders. Similarly, disturbed sleep clustered with other anxiety-related symptoms but form the start point to a bridge to anhedonia, a depressive-cluster symptom. Previous studies have found that sleep deprivation can confer greater risk for psychopathology and medical illness in adolescents and may portend poorer outcomes irrespective of diagnoses (Owens, 2014). For this reason, researchers have begun targeting sleep from a transdiagnostic perspective (Dong et al., 2020) which could be particularly useful in youth with mixed depressive and anxious symptoms.
In anxious adolescents with more depressive symptoms, abnormalities were observed in key cognitive networks including the salience, default mode and ventral attention networks. These three networks have been associated with the pathophysiology of anxiety disorders but also the pathophysiology of depressive disorders in adults and in pediatric populations (Williams, 2016). Thus, cortical abnormalities in these regions likely relate to shared cognitive processes and functions. First, we observed cortical thickness differed in the salience network (i.e., cingulo-opercular network) and specifically within the bilateral superior frontal, left insula, bilateral supramarginal cortex. This network putatively processes negative affect, pain, and cognitive control, (Seminowicz et al., 2007; Shackman et al., 2011) functions that are shared by both depressive and anxiety disorders. Second, we observed differences in cortical thickness in the bilateral precuneus and cuneus, as well as the bilateral inferior temporal, right posterior cingulate, right medial frontal, left superior temporal cortices. These regions collectively belong to the default mode network which has been implicated in the pathophysiology of both anxiety and depressive disorders (Ho et al., 2015; McVoy et al., 2019) in children and adolescents. It should be noted that within our sample anxious adolescents with high depressive symptom burden showed relative increased and decreases in cortical thickness contingent on specific foci involved. It has been hypothesized that depressive and anxiety disorder are the result of abnormal trajectories of childhood brain development (Bale et al., 2010; Insel et al., 2010). Early adolescence is a major period of brain development that is characterized by dynamic structural and functional brain maturation as well as reorganization. While the specific cellular and molecular changes underlying these developmental changes are unknown, likely mechanisms include increasing myelination and axon caliber, pruning and synaptogenesis which have variable importance relative to age and neuroanatomical region (Benes et al., 1994; Tamnes et al., 2017). These factors ultimately result in region-specific cortical thickness and surface area changes that are likely result from a complex interplay of age, sex, genetic and environmental factors (Mills et al., 2014; Whitaker et al., 2016).
Within the default mode network, previous work has examined functional connectivity in patients who were at high and low risk for developing depressive and anxiety disorders and observed that children who are at high risk (based on the presence of behavioral inhibition) have decreased default mode network connectivity, although salience network connectivity did not differ between the two groups (Bellgowan et al., 2015). Taken together with the current findings, these results raise the possibility that structural and functional abnormalities within these networks may be related. Additionally, the two regions within the ventral and dorsal attention networks (bilateral interior and superior parietal cortex) differed between those patients with and without high levels of depressive symptoms. Within this network, resting state functional connectivity differs between those youth 8-12 years of age with a history of anxiety or depressive disorders and healthy, age-matched youth (Sylvester et al., 2013). Further, these networks may already exhibit distinct connectivity patterns in infants who are at risk for developing depressive disorders (Sylvester et al., 2018)—which typically precede the development of anxiety disorders (Asselmann et al., 2014; Pine et al., 1998) suggesting the neurobiological underpinnings of anxiety and depressive disorders may far precede clinical manifestations of illness. Although functional connectivity analyses are beyond the scope of the present study, processing of fMRI data in this sample before and during treatment as part of a larger randomized control trial is ongoing (clinicaltrials.gov, NCT02818751).
Finally, in our post-hoc examination of cortical thickness-age relationships, anxious adolescents with more depressive symptoms had thicker cortices than expected for their age in many of the same structures within with salience, default mode, and ventral attention networks. During adolescence, these regions typically undergo pruning (Blakemore, 2008; Blakemore et al., 2006) although environmental factors can alter network connectivity in these regions during childhood and early adolescence (Rebello et al., 2018). Importantly, neurostructural changes (e.g., changes in cortical thickness, synaptic pruning, and myelination) impact network dynamics (He et al., 2007; Sur et al., 2005) and likewise network dynamics can modify the physical structure of a network (Katz et al., 1996; Majewska et al., 2006). The aberrancies in cortical thickness-age relationship that we observed could suggest delayed cortical maturation (ie, slowed cortical migration or pruning) in anxious adolescents who develop more severe depressive symptoms. This delay in cortical maturation could then disrupt network connectivity and pathogenically contribute to depressive symptoms in anxious adolescents.
The present article has important limitations. First, our data are observational and cross-sectional. We cannot comment on the time course of the development of these depressive symptoms or predict in which patients these depressive symptoms will indeed later present as full MDD. Second, our sample size is relatively small, which increases the risk of Type II error. Third, whether these individuals go on to develop MDD or continue to experience persistent levels of depressive symptoms remains to be determined. Fourth, the primary rating scales used in this study (ie, CDRS-R and PARS) were developed to characterize symptoms as they relate to depressive and anxiety disorders, respectively. Network clustering of one scale’s items with other items on the same scale could be due to biased terminology used in the rating scale rather than unbiased appraisal of a given symptom. For example, the CDRS-R defines severely “impaired schoolwork” as “no motivation to perform” which implies impaired schoolwork is related to an amotivational state rather than avoidance or poor concentration due to anxiety (Mayes et al., 2010).
While these cross-sectional analyses cannot causally link anxiety and depressive symptoms nor can they establish that anxiety-cluster symptoms predate depression-cluster symptoms, our findings are consistent with several lines of evidence concerning the relationship between anxiety and depression. First, anxiety disorders typically precede depressive disorders in youth (Beesdo et al., 2007; Bittner et al., 2004). Second, our patients’ primary diagnosis of GAD, rather than MDD, makes these bridge symptoms areas of interest for further, longitudinal studies in the pathogenesis of MDD in anxious adolescents. Third, the clustering of classically depression-related symptoms (e.g., social withdrawal, poor sleep, and fatigue) within our anxious cluster underscores their poor specificity for depression; they may not constitute true “bridge” symptoms. Further, these three symptoms do not directly correlate with either core depression symptom (i.e., anhedonia and depressed mood) in our association network. Therefore, in the case of youth with anxiety disorders, these three symptoms can arise solely due to the anxiety disorder and should not be seen as evidence of an emerging depressive disorder.
Conclusions
Anxiety and depressive symptoms form distinct clusters in adolescents with primary anxiety disorders who do not meet criteria for MDD. These clusters mirror groupings in common assessment instruments (i.e., CDRS-R and PARS) for youth with anxiety or depression; however, some key symptoms (e.g., excessive weeping, low self-esteem, impaired schoolwork, appetite disturbances, physical symptoms) fail to cluster within either the anxiety or depressive cluster and form correlative links between anxious and depressive clusters. These “bridge” symptoms may be important in the pathogenesis of depressive disorders in anxious youth. Further, adolescents with GAD and high depressive symptom burden show specific changes in default mode and central executive networks which may serve as a neurostructural fingerprint in anxious youth with early depressive symptoms. Moreover, these same youth have reduced age-cortical thickness correlations relative to anxious youth with low depressive symptom burden suggesting the emergence of depressive symptoms in early GAD and cortical development may have bidirectional, neurobiological relationships. Finally, longitudinal studies are needed to explore the development of depression in anxious youth.
Supplementary Material
Highlights.
-Anxious and depressive symptoms form separate correlative clusters in adolescents with generalized anxiety disorder
-Key symptoms bridge depressive and anxious symptoms in youth with generalized anxiety disorder
-Anxious adolescents with depressive symptoms have altered cortical thickness in regions that comprise the default mode and central executive networks
Acknowledgements:
The authors thank the patients and their families for participating in this study and the Data Safety Monitoring Board for their oversight of the study. Additionally, we thank the MR technologists from the Imaging Research Center at Cincinnati Children’s Hospital Medical Center and Blaise V. Jones, MD, Chief of Neuroradiology, Cincinnati Children’s Hospital Medical Center for his over-read of the patients’ neuroimaging.
Funding Source:
This work was supported by the National Institute of Mental Health (K23 MH106037, JRS), the National Institute of Child Health and Development (R01 HD098757, JRS), and the National Institute of Environmental Health Sciences (R01 ES027224, KMC).
Disclosures:
Dr. Dobson has received support from the American Academy of Child & Adolescent Psychiatry (Campaign for America’s Kids). Dr. Strawn has received research support from Allergan, Neuronetics, Lundbeck, Otsuka and the National Institutes of Health. He receives royalties from Springer Publishing for two texts and has received material support from Myriad. He has also provided consultation to Myriad and to Intra-cellular Therapeutics. Dr. Croarkin has received research grant support from the National Institute of Mental Health, Brain and Behavior Research Foundation, and Pfizer, Inc.; equipment support from Neuronetics, Inc.; and has received supplies and genotyping services from Assurex Health, Inc. for investigator-initiated studies. He is the primary investigator for a multicenter study funded by Neuronetics, Inc. and a site primary investigator for a study funded by NeoSync, Inc., and he has served as a consultant for Procter & Gamble Company and Myriad Neuroscience. The views expressed within this article represent those of the authors and are not intended to represent the position of NIMH, NICHD, the National Institutes of Health (NIH), or the Department of Health and Human Services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adleman NE, Fromm SJ, Razdan V, Kayser R, Dickstein DP, Brotman MA, Pine DS, Leibenluft E, 2012. Cross-sectional and longitudinal abnormalities in brain structure in children with severe mood dysregulation or bipolar disorder. J. Child Psychol. Psychiatry Allied Discip 53, 1149–1156. 10.1111/j.1469-7610.2012.02568.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. DSM-5, Diagnostic and statistical manual of mental disorders. [Google Scholar]
- Asselmann E, Wittchen H-U, Lieb R, Hofler M, Beesdo-Baum K, 2014. Associations of fearful spells and panic attacks with incident anxiety, depressive, and substance use disorders: A 10-year prospective-longitudinal community study of adolescents and young adults. J. Psychiatr. Res 55, 8–14. 10.1016/j.jpsychires.2014.04.001 [DOI] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nemeroff CB, Reyes TM, Simerly RB, Susser ES, Nestler EJ, 2010. Early life programming and neurodevelopmental disorders. Biol. Psychiatry 68, 314–319. 10.1016/j.biopsych.2010.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, Bittner A, Pine DS, Stein MB, Höfler M, Lieb R, Wittchen H-U, 2007. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Arch. Gen. Psychiatry 64, 903–12. 10.1001/archpsyc.64.8.903 [DOI] [PubMed] [Google Scholar]
- Beesdo K, Lau JY, Guyer AE, McClure-Tone EB, Monk CS, Nelson EE, Fromm SJ, Goldwin MA, Wittchen HU, Leibenluft E, Ernst M, Pine DS, 2009. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry 66, 275–285. 10.1001/archgenpsychiatry.2008.545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, Pine DS, Lieb R, Wittchen H-U, 2010. Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Arch. Gen. Psychiatry 67, 47–57. 10.1001/archgenpsychiatry.2009.177 [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P, 1994. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch. Gen. Psychiatry 51, 477–484. 10.1001/archpsyc.1994.03950060041004 [DOI] [PubMed] [Google Scholar]
- Bernstein GA, Massie ED, Thuras PD, Perwien AR, Borchardt CM, Crosby RD, 1997. Somatic symptoms in anxious-depressed school refusers. J Am Acad Child Adolesc Psychiatry 36, 661–668. 10.1097/00004583-199705000-00017 [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM, 1997. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J. Am. Acad. Child Adolesc. Psychiatry 36, 545–553. 10.1097/00004583-199704000-00018 [DOI] [PubMed] [Google Scholar]
- Bittner A, Goodwin RD, Wittchen H-U, Beesdo K, Höfler M, Lieb R, 2004. What characteristics of primary anxiety disorders predict subsequent major depressive disorder? J. Clin. Psychiatry 65, 618–26, quiz 730. [DOI] [PubMed] [Google Scholar]
- Blakemore S-J, 2008. Development of the social brain during adolescence. Q. J. Exp. Psychol. (Hove). 61, 40–49. 10.1080/17470210701508715 [DOI] [PubMed] [Google Scholar]
- Blakemore S-J, Choudhury S, 2006. Development of the adolescent brain: implications for executive function and social cognition. J. Child Psychol. Psychiatry. 47, 296–312. 10.1111/j.1469-7610.2006.01611.x [DOI] [PubMed] [Google Scholar]
- Blanco C, Rubio J, Wall M, Wang S, Jiu CJ, Kendler KS, 2014. Risk factors for anxiety disorders: common and specific effects in a national sample. Depress. Anxiety 31, 756–764. 10.1002/da.22247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts CT, 2010. Tools for social network analysis. R Packag. version 2. [Google Scholar]
- Cho S, Przeworski A, Newman M, 2019. Pediatric Generalized Anxiety Disorder, pp. 251–275. 10.1016/B978-0-12-813004-9.00012-8 [DOI] [Google Scholar]
- Craig AD, 2009. How do you feel — now? The anterior insula and human awareness. Nat. Rev. Neurosci 10, 59–70. 10.1038/nrn2555 [DOI] [PubMed] [Google Scholar]
- Crawley SA, Caporino NE, Birmaher B, Ginsburg G, Piacentini J, Albano AM, Sherrill J, Sakolsky D, Compton SN, Rynn M, McCracken J, Gosch E, Keeton C, March J, Walkup JT, Kendall PC, 2014. Somatic complaints in anxious youth. Child Psychiatry Hum. Dev 45, 398–407. 10.1007/s10578-013-0410-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csardi G, Nepusz T, 2006. The igraph software package for complex network research. Inter Journal Complex Sy, 1695. [Google Scholar]
- Cummings CM, Caporino NE, Kendall PC, 2014. Comorbidity of anxiety and depression in children and adolescents: 20 years after. Psychol. Bull 140, 816–845. 10.1037/a0034733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, 2015. Research Domain Criteria: toward future psychiatric nosologies. Dialogues Clin. Neurosci 17, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ, 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Loddo P, Tanda G, 1999. Reciprocal changes in prefrontal and limbic dopamine responsiveness to aversive and rewarding stimuli after chronic mild stress: implications for the psychobiology of depression. Biol. Psychiatry 46, 1624–1633. [DOI] [PubMed] [Google Scholar]
- Dong L, Dolsen MR, Martinez AJ, Notsu H, Harvey AG, 2020. A transdiagnostic sleep and circadian intervention for adolescents: six-month follow-up of a randomized controlled trial. J. Child Psychol. Psychiatry. 61, 653–661. 10.1111/jcpp.13154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke PM, Litt IF, Gross RT, 1980. Adolescents’ self-assessment of sexual maturation. Pediatrics 66, 918–920. [PubMed] [Google Scholar]
- Epskamp S, Borsboom D, Fried EI, 2018. Estimating psychological networks and their accuracy: A tutorial paper. Behav. Res. Methods 50, 195–212. 10.3758/s13428-017-0862-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM, 2002. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. 10.1016/s0896-6273(02)00569-x [DOI] [PubMed] [Google Scholar]
- Franklin JC, Jamieson JP, Glenn CR, Nock MK, 2015. How Developmental Psychopathology Theory and Research Can Inform the Research Domain Criteria (RDoC) Project. J. Clin. Child Adolesc. Psychol 44, 280–290. 10.1080/15374416.2013.873981 [DOI] [PubMed] [Google Scholar]
- Frost Bellgowan J, Molfese P, Marx M, Thomason M, Glen D, Santiago J, Gotlib IH, Drevets WC, Hamilton JP, 2015. A neural substrate for behavioral inhibition in the risk for major depressive disorder. J. Am. Acad. Child Adolesc. Psychiatry. 10.1016/j.jaac.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruchterman TMJ, Reingold EM, 1991. Graph drawing by force-directed placement. Softw. Pract. Exp 21, 1129–1164. [Google Scholar]
- Ginsburg GS, Riddle MA, Davies M, 2006. Somatic symptoms in children and adolescents with anxiety disorders. J. Am. Acad. Child Adolesc. Psychiatry 45, 1179–1187. [DOI] [PubMed] [Google Scholar]
- Gold AL, Brotman MA, Adleman NE, Lever SN, Steuber ER, Fromm SJ, Mueller SC, Pine DS, Leibenluft E, 2016. Comparing Brain Morphometry Across Multiple Childhood Psychiatric Disorders, in: Journal of the American Academy of Child and Adolescent Psychiatry, pp. 1027–1037. e3 10.1016/j.jaac.2016.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group, T.R.U. on P.P.A.S., 2002. The Pediatric Anxiety Rating Scale (PARS): development and psychometric properties. J. Am. Acad. Child Adolesc. Psychiatry 41, 1061–9. [DOI] [PubMed] [Google Scholar]
- Guy W, 1976. CGI Clinical Global Impressions, in: ECDEU Assessment Manual, pp. 217–222. [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M, 2007. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron 53, 905–918. 10.1016/j.neuron.2007.02.013 [DOI] [PubMed] [Google Scholar]
- Ho TC, Connolly CG, Henje Blom E, LeWinn KZ, Strigo IA, Paulus MP, Frank G, Max JE, Wu J, Chan M, Tapert SF, Simmons AN, Yang TT, 2015. Emotion-dependent functional connectivity of the default mode network in adolescent depression. Biol. Psychiatry. 10.1016/j.biopsych.2014.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husky MM, Olfson M, He J, Nock MK, Swanson SA, Merikangas KR, 2012. Twelve-month suicidal symptoms and use of services among adolescents: results from the National Comorbidity Survey. Psychiatr. Serv 63, 989–96. 10.1176/appi.ps.201200058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel Thomas R, Cuthbert Bruce, Garvey Marjorie, Heinssen R, Pine Daniel S., Quinn Kevin, Sanislow Charles, & Wang P, 2010. Research Domain Criteria ( RDoC): Toward a new classification framework for research on mental disorders. Am. J. Psychiatry 167, 748–751. [DOI] [PubMed] [Google Scholar]
- Insel TR, Cuthbert BN, 2015. Brain disorders? Precisely. Science (80-,). 348, 499–500. 10.1126/science.aab2358 [DOI] [PubMed] [Google Scholar]
- Isa A, Bernstein I, Trivedi M, Mayes T, Kennard B, Emslie G, 2014. Childhood depression subscales using repeated sessions on Children’s Depression Rating Scale - revised (CDRS-R) scores. J. Child Adolesc. Psychopharmacol 24, 318–324. 10.1089/cap.2013.0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PJ, Mair P, Riemann BC, Mugno BL, McNally RJ, 2018. A network perspective on comorbid depression in adolescents with obsessive-compulsive disorder. J. Anxiety Disord 53, 1–8. 10.1016/j.janxdis.2017.09.008 [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ, 1996. Synaptic activity and the construction of cortical circuits. Science 274, 1133–1138. 10.1126/science.274.5290.1133 [DOI] [PubMed] [Google Scholar]
- Kendler KS, 1996. Major depression and generalised anxiety disorder. Same genes, (partly)different environments-revisited. Br. J. Psychiatry. Suppl 68–75. [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Gatz M, Pedersen NL, 2007. The sources of co-morbidity between major depression and generalized anxiety disorder in a Swedish national twin sample. Psychol. Med 37, 453–462. 10.1017/S0033291706009135 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Gruber M, Hettema JM, Hwang I, Sampson N, Yonkers KA, 2008. Co-morbid major depression and generalized anxiety disorders in the National Comorbidity Survey follow-up. Psychol. Med 38, 365–374. 10.1017/S0033291707002012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, 1985. The Children’s Depression, Inventory (CDI). Psychopharmacol. Bull 21, 995–998. [PubMed] [Google Scholar]
- Lyneham HJ, Abbott MJ, Rapee RM, 2007. Interrater reliability of the Anxiety Disorders Interview Schedule for DSM-IV: child and parent version. J. Am. Acad. Child Adolesc. Psychiatry 46, 731–736. 10.1097/chi.0b013e3180465a09 [DOI] [PubMed] [Google Scholar]
- MacCallum RC, Zhang S, Preacher KJ, Rucker DD, 2002. On the practice of dichotomization of quantitative variables. Psychol. Methods 7, 19–40. 10.1037/1082-989x.7.1.19 [DOI] [PubMed] [Google Scholar]
- Majewska AK, Sur M, 2006. Plasticity and specificity of cortical processing networks. Trends Neurosci. 29, 323–329. 10.1016/j.tins.2006.04.002 [DOI] [PubMed] [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, Conners CK, 1997. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. J. Am. Acad. Child Adolesc. Psychiatry 36, 554–65. 10.1097/00004583-199704000-00019 [DOI] [PubMed] [Google Scholar]
- Mayes TL, Bernstein IH, Haley CL, Kennard BD, Emslie GJ, 2010. Psychometric properties of the Children’s Depression Rating Scale-Revised in adolescents. J. Child Adolesc. Psychopharmacol 20, 513–516. 10.1089/cap.2010.0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy E, Fearon P, Belsky J, Fonagy P, Patalay P, 2018. Networks of Depression and Anxiety Symptoms Across Development. J. Am. Acad. Child Adolesc. Psychiatry 57, 964–973. 10.1016/jjaac.2018.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVoy M, Aebi ME, Loparo K, Lytle S, Morris A, Woods N, Deyling E, Tatsuoka C, Kaffashi F, Lhatoo S, Sajatovic M, 2019. Resting-state quantitative electroencephalography demonstrates differential connectivity in adolescents with major depressive disorder. J. Child Adolesc. Psychopharmacol 10.1089/cap.2018.0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Zhang H, Avenevoli S, Acharyya S, Neuenschwander M, Angst J, 2003. Longitudinal trajectories of depression and anxiety in a prospective community study: the Zurich Cohort Study. Arch. Gen. Psychiatry 60, 993–1000. 10.1001/archpsyc.60.9.993 [DOI] [PubMed] [Google Scholar]
- Mills KL, Tamnes CK, 2014. Methods and considerations for longitudinal structural brain imaging analysis across development. Dev. Cogn. Neurosci 9, 172–190. 10.1016/j.dcn.2014.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HMC, Chen G, McClure-Tone EB, Ernst M, Pine DS, 2008. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch. Gen. Psychiatry 65, 568–576. 10.1016/S0084-3970(08)79329-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman SA, Luft MJ, Schroeder HK, Varney ST, Fleck DE, Barzman DH, Gilman R, DelBello MP, Strawn JR, 2017. The Generalized Anxiety Disorder 7-item scale in adolescents with generalized anxiety disorder: Signal detection and validation. Ann. Clin. Psychiatry 29, 227–234A. [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Aouidad A, Gorodetsky E, Goldman D, Pine DS, Ernst M, 2013. Gray matter volume in adolescent anxiety: an impact of the brain-derived neurotrophic factor Val(66)Met polymorphism? J. Am. Acad. Child Adolesc. Psychiatry 52, 184–95. 10.1016/j.jaac.2012.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J, 2014. Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics 134, e921–32. 10.1542/peds.2014-1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp M, Willner P, Muscat R, 1991. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl) 104, 255–259. [DOI] [PubMed] [Google Scholar]
- Peters SK, Dunlop K, Downar J, 2016. Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: A Central Pathway in Psychiatric Disease and Treatment. Front. Syst. Neurosci 10 10.3389/fnsys.2016.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y, 1998. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch. Gen. Psychiatry 55, 56–64. 10.1001/archpsyc.55.1.56 [DOI] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, Mann JJ, 2011. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am. J. Psychiatry 168, 1266–1277. 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport BI, Pagliaccio D, Pine DS, Klein DN, Jarcho JM, 2017. Discriminant validity, diagnostic utility, and parent-child agreement on the Screen for Child Anxiety Related Emotional Disorders (SCARED) in treatment- and non-treatment-seeking youth. J. Anxiety Disord 51, 22–31. 10.1016/jjanxdis.2017.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebello K, Moura LM, Pinaya WHL, Rohde LA, Sato JR, 2018. Default Mode Network Maturation and Environmental Adversities During Childhood. Chronic Stress (Thousand Oaks, Calif.) 2, 2470547018808295 10.1177/2470547018808295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintelmann JW, Emslie GJ, Rush AJ, Varghese T, Gullion CM, Kowatch RA, Hughes CW, 1996. The effects of extended evaluation on depressive symptoms in children and adolescents. J. Affect. Disord 41, 149–156. [DOI] [PubMed] [Google Scholar]
- Robinaugh DJ, LeBlanc NJ, Vuletich HA, McNally RJ, 2014. Network analysis of persistent complex bereavement disorder in conjugally bereaved adults. J. Abnorm. Psychol 123, 510–522. 10.1037/abn0000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy MA, Neale MC, Pedersen NL, Mathé AA, Kendler KS, 1995. A twin study of generalized anxiety disorder and major depression. Psychol. Med 25, 1037–1049. 10.1017/S0033291700037533 [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Davis KD, 2007. Pain enhances functional connectivity of a brain network evoked by performance of a cognitive task. J. Neurophysiol, 10.1152/jn.01210.2006 [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ, 2011. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci 10.1038/nrn2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman WK, Saavedra LM, Pina AA, 2001. Test-retest reliability of anxiety symptoms and diagnoses with the Anxiety Disorders Interview Schedule for DSM-IV: child and parent versions. J. Am. Acad. Child Adolesc. Psychiatry 40, 937–44. 10.1097/00004583-200108000-00016 [DOI] [PubMed] [Google Scholar]
- Stein MB, Fuetsch M, Müller N, Höfler M, Lieb R, Wittchen H-U, 2001. Social anxiety disorder and the risk of depression: a prospective community study of adolescents and young adults. Arch. Gen. Psychiatry 58, 251–256. 10.1001/archpsyc.58.3.251 [DOI] [PubMed] [Google Scholar]
- Strawn JR, Hamm L, Fitzgerald DA, Fitzgerald KD, Monk CS, Phan KL, 2015. Neurostructural abnormalities in pediatric anxiety disorders. J Anxiety Disord 32, 81–88. 10.1016/j.janxdis.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, John Wegman C, Dominick KC, Swartz MS, Wehry AM, Patino LR, Strakowski SM, Adler CM, Eliassen JC, DelBello MP, 2014. Cortical surface anatomy in pediatric patients with generalized anxiety disorder. J Anxiety Disord 28, 717–723. 10.1016/jjanxdis.2014.07.012 [DOI] [PubMed] [Google Scholar]
- Strawn JR, Mills JA, Schroeder H, Mossman SA, Varney ST, Ramsey LB, Poweleit EA, Desta Z, Cecil K, DelBello MP, 2020. Escitalopram in Adolescents With Generalized Anxiety Disorder: A Double-Blind, Randomized, Placebo-Controlled Study. J. Clin. Psychiatry 81 10.4088/JCP.20m13396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur M, Rubenstein JLR, 2005. Patterning and plasticity of the cerebral cortex. Science 310, 805–810. 10.1126/science.1112070 [DOI] [PubMed] [Google Scholar]
- Sylvester CM, Barch DM, Corbetta M, Power JD, Schlaggar BL, Luby JL, 2013. Resting state functional connectivity of the ventral attention network in children with a history of depression or anxiety. J. Am. Acad. Child Adolesc. Psychiatry, 10.1016/jjaac.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester CM, Barch DM, Harms MP, Belden AC, Oakberg TJ, Gold AL, White LK, Benson BE, Troller-Renfree S, Degnan KA, Henderson HA, Luby JL, Fox NA, Pine DS, 2016. Early Childhood Behavioral Inhibition Predicts Cortical Thickness in Adulthood. J. Am. Acad. Child Adolesc. Psychiatry 55, 122–129 e1 10.1016/jjaac.2015.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester CM, Smyser CD, Smyser T, Kenley J, Ackerman JJ, Shimony JS, Petersen SE, Rogers CE, 2018. Cortical functional connectivity evident after birth and behavioral inhibition at age 2. Am. J. Psychiatry 175, 180–187. 10.1176/appi.ajp.2017.17010018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Herting MM, Goddings A-L, Meuwese R, Blakemore S-J, Dahl RE, Güroğlu B, Raznahan A, Sowell ER, Crone EA, Mills KL, 2017. Development of the Cerebral Cortex across Adolescence: A Multisample Study of Inter-Related Longitudinal Changes in Cortical Volume, Surface Area, and Thickness. J. Neurosci 37, 3402–3412. 10.1523/JNEUROSCI.3302-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH, 2011. Reconsidering Anhedonia in Depression: Lessons from Translational Neuroscience. Neurosci. Biobehav. Rev 35, 537–555. 10.1016/j.neubiorev.2010.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrer P, Seivewright H, Simmonds S, Johnson T, 2001. Prospective studies of cothymia (mixed anxiety-depression): how do they inform clinical practice? Eur. Arch. Psychiatry Clin. Neurosci 251 Suppl 2, II53–6. 10.1007/BF03035128 [DOI] [PubMed] [Google Scholar]
- Van Oort FV, Greaves-Lord K, Verhulst FC, Ormel J, Huizink AC, 2009. The developmental course of anxiety symptoms during adolescence: the TRAILS study. J Child Psychol Psychiatry 50, 1209–1217. 10.1111/j.1469-7610.2009.02092.x [DOI] [PubMed] [Google Scholar]
- Wehry AM, McNamara RK, Adler CM, Eliassen JC, Croarkin P, Cerullo M. a, DelBello MP, Strawn JR, 2015. Neurostructural impact of co-occurring anxiety in pediatric patients with major depressive disorder: A voxel-based morphometry study. J. Affect. Disord 171, 54–9. 10.1016/j.jad.2014.09.004 [DOI] [PubMed] [Google Scholar]
- Whitaker KJ, Vértes PE, Romero-Garcia R, Váša F, Moutoussis M, Prabhu G, Weiskopf N, Callaghan MF, Wagstyl K, Rittman T, Tait R, Ooi C, Suckling J, Inkster B, Fonagy P, Dolan RJ, Jones PB, Goodyer IM, Bullmore ET, 2016. Adolescence is associated with genomically patterned consolidation of the hubs of the human brain connectome. Proc. Natl. Acad. Sci. U. S. A 113, 9105–9110. 10.1073/pnas.1601745113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, 2016. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. The lancet. Psychiatry 3, 472–480. 10.1016/S2215-0366(15)00579-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen HU, Perkonigg A, Lachner G, Nelson CB, 1998. Early developmental stages of psychopathology study (EDSP): Objectives and design. Eur. Addict. Res 10.1159/000018921 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


