Abstract
Objectives:
Methods for pharmacoepidemiologic studies of largescale data repositories are established. While clinical cohorts of older adults often contain critical information to advance our understanding of medication risk and benefit, the methods best-suited to manage medication data in these samples are sometimes unclear and their degree of validation unknown. We sought to provide researchers, in the context of a clinical cohort study of delirium in older adults, with guidance on the methodological tools to use data from clinical cohorts to better understand medication risk factors and outcomes.
Design:
Prospective cohort study.
Setting:
The Successful Aging after Elective Surgery (SAGES) prospective cohort.
Participants:
560 older adults (≥70 years) without dementia undergoing elective major surgery.
Measurements:
Using the SAGES clinical cohort, methods used to characterize medications were identified, reviewed, analyzed and distinguished by appropriateness and degree of validation for characterizing pharmacoepidemiologic data in smaller clinical datasets.
Results:
Medication coding is essential; the American Hospital Formulary System (AHFS), most often used in the U.S., is not preferred over others. Use of equivalent dosing scales (e.g., morphine equivalents) for a single medication class (e.g. opioids) is preferred over multi-class analgesic equivalency scales. Medication aggregation from the same class (e.g. benzodiazepines) is well-established; the optimal prevalence breakout for aggregation remains unclear. Validated scale(s) to combine structurally dissimilar medications (e.g., anticholinergics) should be used with caution; a lack consensus exists regarding the optimal scale. Directed acyclic graph(s) are an accepted method to conceptualize etiologic frameworks when identifying potential confounders. Modelling-based strategies should be used with evidence-based, a priori variable-selection strategies.
Conclusion:
As highlighted in the SAGES cohort, the methods used to classify and analyze medication data in clinically-rich cohort studies vary in the rigor which they have been developed and validated.
Keywords: Pharmacoepidemiology, medication, delirium, older adults, research methods
Background/Introduction
Older adults take an average of ten chronic medications; polypharmacy occurs in up to 40%.1, 2 More than 30% of older adult hospitalizations are directly related to an adverse drug event (ADE); medications commonly contribute to geriatric syndromes (e.g., falls, delirium).3 This high ADE prevalence and the modifiability of medication use makes clinically-driven, rigorous pharmacoepidemiologic research in older adults of great importance.4
Pharmacoepidemiologic studies have traditionally studied medication effects at the population-level with claims data; methodological approaches for these largescale investigations are established.5 However, the detailed information from individual cohort studies may be better suited to address certain clinical questions. For example, investigating medication-related risk factors for delirium requires patient-level medication data, delirium ratings, and clinical outcomes generally not readily available in claims data.6 While clinical cohorts of older adults often contain critical information to advance our understanding of medication risk and benefit, the methods best-suited to manage medication data in these samples are sometimes unclear and their degree of validation unknown.
The aim of this paper is to provide researchers with guidance on the methodological approaches to optimize medication-related analyses in clinical cohort studies. Methods used to characterize medications and related clinical outcomes in the 560-patient, Successful Aging after Elective Surgery (SAGES) cohort study SAGES were identified, reviewed, analyzed and distinguished by appropriateness and degree of validation. A deeper appreciation of these approaches will help optimize medication data analyses when using clinical cohorts to conduct pharmacoepidemiologic research in older adults.
Description of Source Data from SAGES
The SAGES study was developed to better understand the etiology and long-term outcomes of delirium in older adults after elective surgery.7 Within the study, psychoactive medication data (including name and days of administration) were collected pre-hospitalization, for the first 7 days of hospitalization, and upon discharge. Ten classes of psychoactive medications were defined a priori (i.e., anticholinergics, anticonvulsants, antidepressants, antihistamines, antipsychotics, benzodiazepines, opioids, psychostimulants, and sedatives). All medications falling into these classifications were abstracted, including those with intended effects outside of the central nervous system. Pre-hospital medications were obtained by direct review of medication bottles during the baseline in-home interview. All medications administered during hospitalization were abstracted from the hospital medication administration record by a trained physician.8 Medication adherence for pre-hospital and post-discharge medications was not evaluated. The method by which delirium and cognition were assessed and coded are previously reported.7
Methodological Issues to Overcome in Clinical Datasets
Coding of medications
Efficient standardization of medication data requires accurate assignment of each raw medication entry to a recognized drug coding system; errors introduced by alphanumeric data collection should be corrected. Medications infrequently administered can be aggregated if they have similar chemical structures or pharmacologic effects. While medication coding is essential, the best coding system to use remains unclear.9 The American Hospital Formulary System (AHFS),10 the Anatomical Therapeutic Chemical Classification System (ATC),11 and the National Drug Codes (NDC),12 are the most commonly used coding systems; AHFS predominates in the U.S. and ATC in Europe. A new researcher should learn one system well and apply it consistently before considering additional systems. The selection of medication coding systems and scales before data collection commencement will reduce collection errors; standards for brand/generic name use, dose, route, and administration directions should be considered as the systems are implemented.8, 13 Although not a proven strategy, the research team should consider including a medication-use expert (e,g., a clinical pharmacist or pharmacoepidemiologist) or a prescriber (e.g., a physician or advanced practice provider) with geriatric expertise for studies of older adults who can proactively ensure the clinical validity of coding efforts.
Use of individual vs. categorized medication data
The sample size of medication data in cohort studies is often insufficient to examine individual medications. To maximize analytic power while retaining clinically meaningful medication data analyses, different strategies exist. Some are better validated than others. Choice of strategy depends on the question being asked and the nature of the outcome. Analyses of inpatient medication use should always consider the time-varying nature of the medication administration to avoid the introduction of immortal-time bias.14
Strategy 1: Analyze medications with a high prevalence on an individualized basis
Within SAGES, it was decided a priori to use 5% as the prevalence cutoff. While prevalence cutoffs should be individualized based on sample size and power, they remain subjective. This approach is useful for studies where safety and efficacy are being compared among medications prescribed for the same indication (e.g. pain) but have distinct pharmacologic profiles (e.g., duration of action, hydrophilicity, or receptor specificity). In clinical cohorts where data on daily dose is available, well-validated methods exist to convert opioid exposure to daily morphine equivalents15 and antipsychotic exposure to daily haloperidol equivalents.16, 17
Application in SAGES: A lack of availability of daily opioid dosing precluded the calculation of a daily morphine equivalent dose. Given the high prevalence of individual opioid administration after surgery [oxycodone (75%), hydromorphone (73%), tramadol (6%)], we analyzed each opioid individually. While this is the most conservative approach, it precludes the ability to make conclusions about a particular medication class when the rate of delirium occurrence varies by the specific opioid administered18 For example, the risk for post-operative delirium occurrence was nearly two-times higher with tramadol (unadjusted RR 1.83; 95% CI 1.07-3.14) than oxycodone (unadjusted RR 0.64; 95% CI 0.45-0.91).
Strategy 2: Aggregate medications with lower prevalence into classes of similar agents
When AHFS19 coding in SAGES left an individual medication with a prevalence < 5%, aggregation by both AHFS group and therapeutic drug class occurred. While the validity of this approach is not well researched, it is commonly used, even in large pharmacoepidemiologic investigations.20 In this situation, researchers should consult with a biostatistician to determine the most appropriate prevalence cutoff to use given the sample size and power considerations of the cohort.21 For example, using the AHFS system, antihistamines can be grouped by both pharmacologic class (e.g., antihistamines) and clinical use (e.g., second-generation antihistamines). While second-level aggregation improves modelling efforts, it limits evaluation of individual drugs.22
Application in SAGES: Benzodiazepine inpatient use necessitated aggregation by AHFS group (Table 1). Lorazepam (8.2%) and diazepam (7.8%) each met the 5% threshold; the remaining benzodiazepines had a total prevalence of 4.3% - a value deemed too high to ignore. With benzodiazepines having a similar chemical structure and nearly always administered to reach the same therapeutic effect23; all benzodiazepines were grouped together resulting in a prevalence of 20.2%. Unlike benzodiazepines, antipsychotics are structurally unique leading to different neurotransmitter effects. For example, while haloperidol (2.7% prevalence) is primarily a dopamine-2 receptor antagonist,24 quetiapine (1.3% prevalence) antagonizes serotonin-2A, alpha-1, and histamine-1 receptors.25 However, despite concerns about heterogeneity, we still combined all antipsychotics together in an exploratory fashion given the importance of evaluating the association between antipsychotic exposure and delirium occurrence. Sensitivity analyses are planned to further explore the effects of individual antipsychotics.
Table 1.
Examples of medication aggregation within SAGESA
| Medication Group | Medication Class | Individual MedicationB | Inpatient PrevalenceB n (%) | Aggregated Medication Groups | Inpatient Prevalence n (%)A |
|---|---|---|---|---|---|
| Central Nervous System (28.00) | Opiate Agonists (28.08.08) | HYDROmorphone oxyCODONe morphine traMADol HYDROcodone fentaNYL codeine meperidine methadone |
409 (73.0) 421 (75.2) 125 (22.3) 35 (6.3) 20 (3.6) 19 (3.4) 1 (0.2) 1 (0.2) 1 (0.2) |
HYDROmorphone oxyCODONe morphine traMADol Other opioids |
409 (73.0) 421 (75.2) 125 (22.3) 35 (6.3) 42 (7.5) |
| Benzodiazepines (28.24.04) | LORazepam diazePAM ALPRAZolam clonazePAM temazepam triazolam oxazepam midazolam |
46 (8.2) 43 (7.7) 8 (1.4) 7 (1.3) 3 (0.5) 3 (0.5) 2 (0.4) 1 (0.2) |
Benzodiazepines | 113 (20.2) | |
| Antipsychotics (28.16.08) | haloperidol prochlorperazine QUEtiapine OLANZapine chlorproMAZINE |
15 (2.7) 9 (1.6) 7 (1.3) 3 (0.5) 1 (0.2) |
Antipsychotics | 35 (6.3) |
Only medications administered to at least 1 patient from a class are reported.
Numbers in parentheses represent the AHFS code corresponding to the presented categories.19
Strategy 3: Use of validated scales to combine structurally dissimilar medications with similar effects
Evaluating the association between anticholinergic medications and delirium risk has long been recognized as important but is associated with many challenges. The list of medications with anticholinergic activity is long, a lack of consensus regarding which anticholinergic activity scale is the “gold standard” exists, and how each scale should be best summarized and modelled remains unclear.26–28 Two anticholinergic scoring systems [i.e., the Anticholinergic Cognitive Burden scale (ACB) and the Anticholinergic Drug Scale (ADS)], have gained the most widespread use despite each having only moderate concordance with the other.28–32 While the ACB categorizes medications using clinical measures of anticholinergic function (e.g., changes in cognition and mortality within 2 years)30, the ADS categorizes medications using serum anticholinergic activity (a laboratory measure of anticholinergic function).31 These scales can be adapted to account for dose and frequency of medication administration. Although key differences exist between ACB and ADS (Table 2), using both anticholinergic scales in an analysis, and comparing results, ensures findings are robust across both measures.
Table 2.
Notable differences between the Anticholinergic Drug Scale and the Anticholinergic Cognitive Burden Scale
| Anticholinergic Scoring System | Medication Prevalence | ||||
|---|---|---|---|---|---|
| Medication | Anticholinergic Drug ScoreA | Anticholinergic Cognitive Burden | Pre-hospitalization n=560 | Inpatient n=560 | Discharge n=560 |
| Amantadine | 1 | 2 | 3 (0.5%) | 0 (0%) | 3 (0.5%) |
| Doxylamine | 0 | 3 | 1 (0.2%) | 0 (0%) | 0 (0%) |
| Fesoterodine | 0 | 3 | 1 (0.2%) | 0 (0%) | 0 (0%) |
| Methocarbamol | 0 | 3 | 3 (0.5%) | 1 (0.2%) | 3 (0.5%) |
| Olanzapine | 1 | 3 | 0 (0%) | 3 (0.5%) | 1 (0.2%) |
| Paroxetine | 1 | 3 | 4 (0.7%) | 5 (0.9%) | 5 (0.9%) |
| Quetiapine | 0 | 3 | 1 (0.2%) | 7 (1.2%) | 3 (0.5%) |
| Ranitidine | 2 | 1 | 32 (5.7%) | 12 (2.1%) | 24 (4.3%) |
| Solifenacin | 0 | 3 | 6 (1.1%) | 0 (0%) | 4 (0.7%) |
| Trospium | 0 | 3 | 2 (0.4%) | 0 (0%) | 2 (0.4%) |
A score of 0 means that either the medication was judged to have no anticholinergic effect or was not evaluated by the scoring system.
Application in SAGES: Given the lack of consensus on how the anticholinergic properties of medications should be characterized, several approaches were used to summarize and model the scores from each anticholinergic scale for both pre-hospital and hospital medications:
The anticholinergic activity scores for each medication were summed to determine a total score. This resulted in a median [IQR] ADS score of 1 [0-2] and median ACB score of 1 [0-2]. However, this approach could be affected by the total number of medications administered. For example, a patient prescribed 10 medications each with low anticholinergic activity may have a higher summative score than another patient prescribed 5 medications where each medication had a high anticholinergic score.
To address this concern in a similar fashion to other studies,33 we also calculated an average anticholinergic activity score [i.e., total score (from approach 1) divided by the total number of medications administered (not solely medications with anticholinergic activity)]. This method produced a median average ADS of 0.17 [0-0.29] and ACB of 0.17 [0-0.30].
We conducted an additional analysis restricted to only those medications with medium- or high-level anticholinergic activity resulting in a mean per patient score of 0.48 ± 1.16 including medications meeting this threshold for ADS and 0.48 ± 1.26 for ACB.
Controlling for confounding by indication and other factors
Adequate control for confounding is essential to evaluate medication effects independent of underlying patient factors. For any cohort study, the independent variable (i.e., medication exposure) and related confounders (i.e., factors related to both the medication exposure and the clinical outcome but not lying on the causal pathway between the two) should be included in the analysis to provide an unbiased estimate of the medication-related relationship of interest.34 The number of outcome events can limit the the number of variables permitted; in general, no more than one covariable should be explored for every 10-20 binary events. Variables should be limited to true confounders and effect modifiers; those falling directly on the causal pathway should be avoided.35
Strategy 1. A priori approach
For this approach, prior literature and expert consensus are used to develop a directed acyclic graph (DAG)36 that details the relationship between the exposure and outcome, and the relationship of intermediate variables, confounders, and effect modifiers. The DAG development process is a well-established method to inform variable selection for analytic model(s).37
Application in SAGES: This approach was used to determine whether age or urinary retention were important to include in a model evaluating the association between anticholinergic medications and delirium (Figure Part A). While age was included in the model given the risk for delirium increases by 4% per for each year of age,38 urinary retention was not included given it is commonly caused by anticholinergic medication use and urinary retention itself also directly increases delirium risk.39 All other variables considered were evaluated through this same a priori approach.
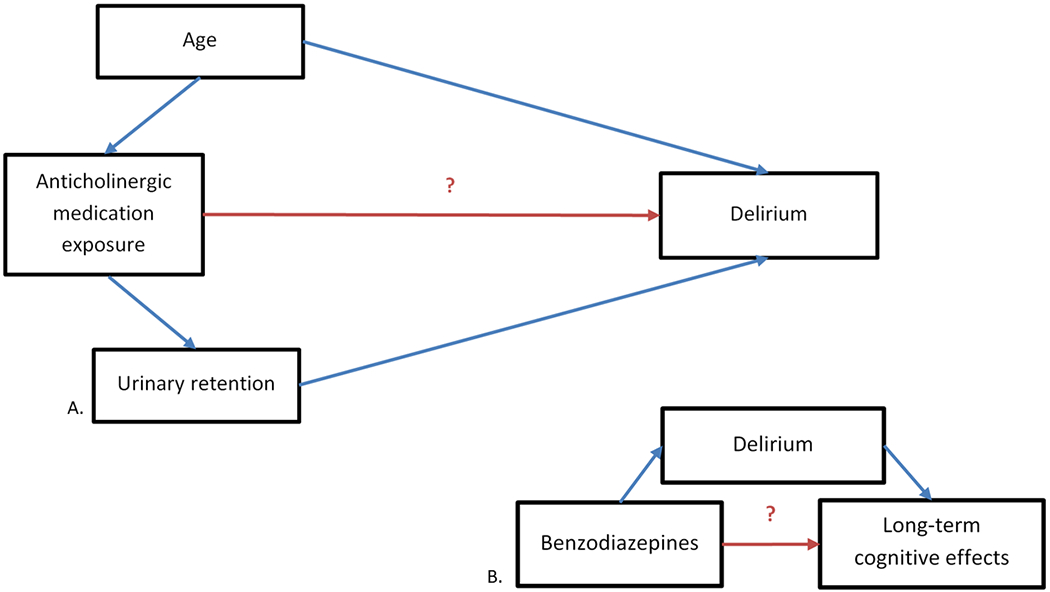
Part A. Diagram of the relationship between medication exposure and post-operative delirium. Part B. Simplified diagram of the potential association between benzodiazepines, delirium, and long-term cognitive effects. The head of the arrow indicates the direction of presumed causality. In this example, age is potentially causally-associated with anticholinergic medication exposure and with delirium, making it a confounder. Urinary retention potentially lies on the causal pathway between anticholinergic medication exposure and delirium, and is therefore excluded from the model, as controlling for it would mask the true association between anticholinergic activity and delirium.
Strategy 2. Modelling-based approaches for variable selection:
While several modelling-based approaches for variable selection exist, limitations exist with all methods and none are preferred.40 These analytic models allow for the exploration of diverse medication-interaction effects including drug-drug interactions and drug-disease interactions. However, these complex interactions can be difficult to evaluate when samples are constrained. Approaches using stepwise procedures should be avoided as these do not correct for issues seen with multiple testing.41 Instead, modelling-based approaches should be combined with clinical knowledge to generate simple explanatory models containing the most important variables.42 By removing nuisance variables, models become more parsimonious, easier to interpret, and more generalizable to clinical practice. Some potential methods of variable selection are detailed in the Supplemental Table. Mediation analysis allows indirect model effects to be measured and is most useful when another outcome is a potential intermediary in the primary medication exposure-clinical outcome pathway.43
Application in SAGES: Since data exists showing an association between benzodiazepines, delirium, and long-term cognitive dysfunction (LTCD)44, 45 mediation analysis will be used to help evaluate whether a delirium-independent association exists between benzodiazepines and LTCD (Figure Part B).
Handling missing data
Given the complexity and multi-morbidity of older adults, data may be unavailable at certain time points due to patient unavailability, nursing issues, inaccurate documentation, or data corruption – a particularly pernicious challenge in studies of aging.46 While different, validated, proactive strategies to prevent missingness are available, there use is unlikely to be fully successful.47 To account for missing records, multiple imputation methods are preferred, as they are less susceptible to inflated variance and biased estimates.48 With any approach used, sensitivity analyses are helpful to evaluate the robustness of the results to varying assumptions used (e.g., best-case and worst-case scenarios).49 Use of open-form medication lists render the usual methods for investigating missingness nearly impossible without independent validation of data fields. When the dataset is created with specific exposures in mind (i.e., where missingness within a field for a specified medication would be apparent), it often remains unclear whether the primary exposure variable should then be imputed.48
Application in SAGES: Missingness within the cohort was uncommon.50 Among baseline variables collected, 2 (0.4%) patients did not have information on their visual impairment status, 6 (1.1%) on alcohol consumption history, and 4 (0.7%) on baseline pain scores. Our method of handling missing data, called multivariate imputation by chained equations, was able to impute variables of multiple types (e.g., binary or continuous) which allows for easier interpretation of outputs.51
Conclusion
We have highlighted methodologic approaches for conducting medication-related analyses in clinical cohort studies, emphasizing the rigor by which each has been developed and validated. We urge researchers to continue to look for ways to improve upon the methods we describe in future research, such as validation studies and comparison to other novel methods.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the contributions of the patients, family members, physicians, nurses, research staff, and study investigators who participate in the SAGES study.
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under award numbers P01AG031720 (SKI), R24AG054259 (SKI), and F31AG066460 (MSD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Sponsor’s Role: The funding source had no role in design, methods, participant recruitment, data collection, statistical analysis and preparation of the paper.
Footnotes
Conflict of Interest: The authors have no conflicts
References:
- 1.Brownlee S, Garber J. Medication overload: America’s other drug problem. Lown Institute, 2019. https://lowninstitute.org/reports/medication-overload-americas-other-drug-problem/ Accessed March 6 2020. [Google Scholar]
- 2.Garpestad E, Devlin JW. Polypharmacy and delirium in critically ill older adults: recognition and prevention. Clin Geriatr Med. 2017;33: 189–203. [DOI] [PubMed] [Google Scholar]
- 3.Jolivot PA, Hindlet P, Pichereau C, et al. A systematic review of adult admissions to ICUs related to adverse drug events. Crit Care. 2014;18: 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.By the American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67: 674–694. [DOI] [PubMed] [Google Scholar]
- 5.Noe MH, Gelfand JM. Research techniques made simple: Pharmacoepidemiology research methods in dermatology. J Invest Dermatol. 2018;138: e13–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113: 941–948. [DOI] [PubMed] [Google Scholar]
- 7.Schmitt EM, Marcantonio ER, Alsop DC, et al. Novel risk markers and long-term outcomes of delirium: The successful aging after elective surgery (sages) study design and methods. J Am Med Dir Assoc. 2012;13: 818 e811–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lockery JE, Rigby J, Collyer TA, et al. Optimising medication data collection in a large-scale clinical trial. PLoS One. 2019;14: e0226868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rønning M, McTaggart S. Classification systems for drugs and diseases. In: Elseviers M, Wettermark B, Almarsdóttir A, et al. , eds. Drug Utilization Research, 2016, pp. 49–57. [Google Scholar]
- 10.American Hospial Formulary Service Pharmacologic-Therapeutic Classification. American Society of Health System Pharmacists, 2020. https://www.ahfsdruginformation.com/ahfs-pharmacologic-therapeutic-classification/. Acccessed August 30 2020. [Google Scholar]
- 11.Anatomical Therapeutic Chemical (ATC) Classification. World Health Organization, 2020. https://www.who.int/medicines/regulation/medicines-safety/toolkit_atc/en/. Accessed August 30 2020.
- 12.National Drug Code Directory. The U.S. Food and Drug Administration, 2020. https://www.fda.gov/drugs/drug-approvals-and-databases/national-drug-code-directory. Acccessed August 30 2020.
- 13.Saczynski JS, McManus DD, Goldberg RJ. Commonly used data-collection approaches in clinical research. Am J Med. 2013;126: 946–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shintani AK, Girard TD, Eden SK, Arbogast PG, Moons KG, Ely EW. Immortal time bias in critical care research: Application of time-varying cox regression for observational cohort studies. Crit Care Med. 2009;37: 2939–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison RS, Dickman E, Hwang U, et al. Regional nerve blocks improve pain and functional outcomes in hip fracture: A randomized controlled trial. J Am Geriatr Soc. 2016;64: 2433–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: A standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67: 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanlon JT, Boudreau RM, Roumani YF et al. Number and dosage of central nervous system medications on recurrent falls in community elders: The Health, Aging and Body Composition study. J Gerontol and Biol Sci Med Sci. 2009;64A: 492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inturrisi CE. Clinical pharmacology of opioids for pain. Clin J Pain. 2002;18: S3–13. [DOI] [PubMed] [Google Scholar]
- 19.AHFS Drug Information®. 60th ed Bethesda, MD: American Society of Health-System Pharmacists, 2018. [Google Scholar]
- 20.Briesacher BA, Mui B, Devlin JW. Koethe B. Nursing homes underreport antipsychotic prescribing. Aging Ment Health. 2020;24: 668–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcantonio ER, Juarez G, Goldman L, et al. The relationship of postoperative delirium with psychoactive medications. JAMA. 1994;272: 1518–1522. [PubMed] [Google Scholar]
- 22.Le HV, Poole C, Brookhart MA, et al. Effects of aggregation of drug and diagnostic codes on the performance of the high-dimensional propensity score algorithm: An empirical example. BMC Med Res Methodol. 2013;13: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin CE 3rd, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13: 214–223. [PMC free article] [PubMed] [Google Scholar]
- 24.Kroeze WK, Hufeisen SJ, Popadak BA, et al. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28: 519–526. [DOI] [PubMed] [Google Scholar]
- 25.Jensen NH, Rodriguiz RM, Caron MG, Wetsel WC, Rothman RB, Roth BL. N-desalkylquetiapine, a potent norepinephrine reuptake inhibitor and partial 5-HTLA agonist, as a putative mediator of quetiapine’s antidepressant activity. Neuropsychopharmacology. 2008;33: 2303–2312. [DOI] [PubMed] [Google Scholar]
- 26.Wolters AE, Zaal IJ, Veldhuijzen DS, et al. Anticholinergic medication use and transition to delirium in critically ill patients: A prospective cohort study. Crit Care Med. 2015;43: 1846–1852. [DOI] [PubMed] [Google Scholar]
- 27.Tune LE, Damlouji NF, Holland A, Gardner TJ, Folstein MF, Coyle JT. Association of postoperative delirium with raised serum levels of anticholinergic drugs. Lancet. 1981;2: 651–653. [DOI] [PubMed] [Google Scholar]
- 28.Khan BA, Zawahiri M, Campbell NL, et al. Delirium in hospitalized patients: Implications of current evidence on clinical practice and future avenues for research--a systematic evidence review. J Hosp Med. 2012;7: 580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boustani M, Campbell N, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: A review and practical application. Aging Health. 2008;4: 311–320. [Google Scholar]
- 30.Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: The medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59: 1477–1483. [DOI] [PubMed] [Google Scholar]
- 31.Carnahan RM, Lund BC, Perry PJ, Pollock BG, Culp KR. The Anticholinergic Drug Ccale as a measure of drug-related anticholinergic burden: Associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46: 1481–1486. [DOI] [PubMed] [Google Scholar]
- 32.Naples JG, Marcum ZA, Perera S, et al. Concordance between anticholinergic burden scales. J Am Geriatr Soc. 2015;63: 2120–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green AR, Reifler LM, Bayliss EA, Weffald LA, Boyd CM. Drugs contributing to anticholinergic burden and risk of fall or fall-related injury among older adults with mild cognitive impairment, dementia and multiple chronic conditions. Drugs Aging. 2019;36: 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenland S Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79: 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenland S Quantifying biases in causal models: Classical confounding vs collider-stratification bias. Epidemiology. 2003;14: 300–306. [PubMed] [Google Scholar]
- 36.Glymour MM. Using causal diagrams to understand common problems in social epidemiology In: Oakes JM, Kaufman JS, editors. Methods in Social Epidemiology. Hoboken, NJ, US: Jossey-Bass/Wiley; 2006. p. 393–428. [Google Scholar]
- 37.Webster-Clark M, Breskin A. Directed acyclic graphs, effect measure modification, and generalizability. Am J Epidemiol. 2020. (ahead of press Aug 25) [DOI] [PubMed] [Google Scholar]
- 38.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354: 1157–1165. [DOI] [PubMed] [Google Scholar]
- 39.Drake MJ, Nixon PM, Crew JP. Drug-induced bladder and urinary disorders. Incidence, prevention and management. Drug Saf. 1998;19: 45–55. [DOI] [PubMed] [Google Scholar]
- 40.Heinze G, Wallisch C, Dunkler D. Variable selection - a review and recommendations for the practicing statistician. Biom J. 2018;60: 431–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steyerberg EW, Eijkemans MJ, Habbema JD. Stepwise selection in small data sets: A simulation study of bias in logistic regression analysis. J Clin Epidemiol. 1999;52: 935–942. [DOI] [PubMed] [Google Scholar]
- 42.Ginzburg LR, Jensen CX. Rules of thumb for judging ecological theories. Trends Ecol Evol. 2004;19: 121–126. [DOI] [PubMed] [Google Scholar]
- 43.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51: 1173–1182. [DOI] [PubMed] [Google Scholar]
- 44.Devore EE, Fong TG, Marcantonio ER, et al. Prediction of long-term cognitive decline following postoperative delirium in older adults. J Gerontol A Biol Sci Med Sci. 2017;72: 1697–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Zhou XH, Meranus DH, Wang L, Kukull WA. Benzodiazepine use and cognitive decline in elderly with normal cognition. Alzheimer Dis Assoc Disord. 2016;30: 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Ness PH, Charpentier PA, Ip EH, et al. Gerontologic biostatistics: The statistical challenges of clinical research with older study participants. J Am Geriatr Soc. 2010;58: 1386–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin JY, Lu Y, Tu X. How to avoid missing data and the problems they pose: Design considerations. Shanghai Arch Psychiatry. 2012;24: 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karahalios A, Baglietto L, Carlin JB, English DR, Simpson JA. A review of the reporting and handling of missing data in cohort studies with repeated assessment of exposure measures. BMC Med Res Methodol. 2012;12: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thabane L, Mbuagbaw L, Zhang S, et al. A tutorial on sensitivity analyses in clinical trials: The what, why, when and how. BMC Med Res Methodol. 2013;13: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmitt EM, Saczynski JS, Kosar CM, et al. The Successful Aging after Elective Surgery (SAGES) study: Cohort description and data quality procedures. J Am Geriatr Soc. 2015;63: 2463–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: What is it and how does it work? Int J Methods Psychiatr Res. 2011;20: 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


