Abstract
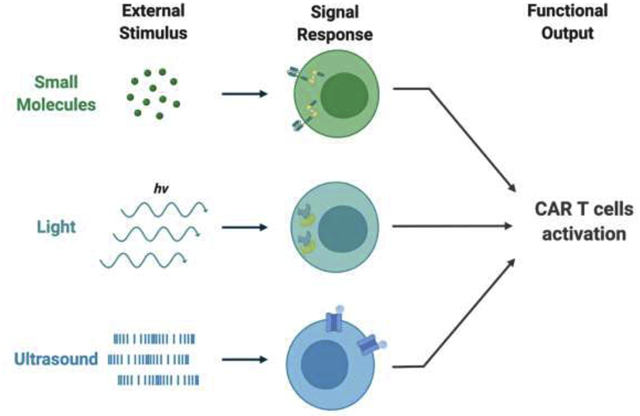
Recent synthetic biology advancements have shown that cells can be engineered to respond to external stimuli such as chemical compounds and light, which significantly improves the specificity and controllability of CAR T therapy. However, the lack of both spatiotemporal and depth control is still the main issue in the clinic of CAR T treatment. At the same time, mechanogenetics, capable of penetrating deep tissues with high spatiotemporal precision, is rapidly evolving and advancing to reveal its potential for cancer immunotherapy. In the past few years, researchers have demonstrated the precise and remote control of engineered cells with mechanical perturbation originated from ultrasound, which may become a new solution to circumvent the limitations of CAR T therapy in the future. This review will discuss mechanobiology and the state-of art designs of controllable CAR T cells. A specific focus of this review will be on the mechanical control of CAR T therapy.
Keywords: CAR T therapy, ultrasound, Mechanobiology, Mechanogenetics, Synthetic biology
Graphical Abstract
Introduction
Unlike traditional chemotherapy and radiotherapy which do not distinguish normal cells from tumor, cancer immunotherapy utilizes the patient’s own immune system to kill the tumor cells via tumor specific antigens and/or checkpoint inhibitors [1]. This leaves healthy cells relatively unharmed, which significantly improves the prognosis of cancer patients. Indeed, both checkpoint inhibitors and immune cell engineering become popular methods for cancer immunotherapy [1]. Among these, Chimeric Antigen Receptor (CAR) T cell therapy has been demonstrated to be very successful for blood cancers [2–6]. However, the tumor immune-inhibitory microenvironment, on-target off-tumor effect, and cytokine release syndrome have largely limited broad applications of CAR T therapy, particularly against solid tumors [7–9]. In order to mitigate these issues, particularly non-specificity related to on-target off-tumor effect, CAR T cells have been genetically re-engineered to be controllable by chemical and optical stimulations for higher precision in therapeutics [10]. However, chemical stimuli typically lack spatial control and light cannot penetrate deep into the tissues.
It has been known that cells can not only sense biochemical and optical cues but also process mechanical information from the environment. In fact, cells have evolved a whole complicated system for mechanosensation, mechanotransduction, and mechanoresponse [11]. Numerous mechano-sensors have been reported, including mechanosensitive ion channels, receptors, and cytoskeletons [11]. Fortunately, mechanical stimulation can be achieved remotely and deep in the tissue with high spatial and temporal resolution through magnetic field [12] and ultrasound [13], paving the way for its application in cancer immunotherapy.
In this review, we will first give an introduction to CAR T therapy and the state-of-art designs for better controllability, followed by discussing the current studies of mechanosensitive proteins in the context of mechanogenetics and particularly, mechanogenetic designs in CAR T immunotherapy.
Cancer immunotherapy
Cancer immunotherapy is the treatment of cancer by utilizing and boosting a patient’s own immune system. It includes the use of antibodies, cytokines, and cell infusions. Among these, CAR T cell based cellular immunotherapy has emerged as a powerful treatment. In CAR T therapy, T cells from the patient are equipped with CARs through genetic engineering, rendering them capable of targeting tumor cells with the tumor associated antigen (TAA) (Figure 1a). These CAR T cells are then infused back into the patient following ex vivo expansion to treat cancer. With its first successful case report of a partial response in a B cell lymphoma patient in 2010 and a complete remission in a chronic lymphocytic leukemia (CLL) patient in 2011 [14–16], with both treated with anti-CD19 CAR T cells, CAR T therapy has received unprecedented attention. In 2017, FDA approved CAR T therapies for acute lymphocytic leukemia (ALL) and diffuse large B cell lymphoma (DLBCL) [14].
Figure 1. Controllable CAR T cell designs.
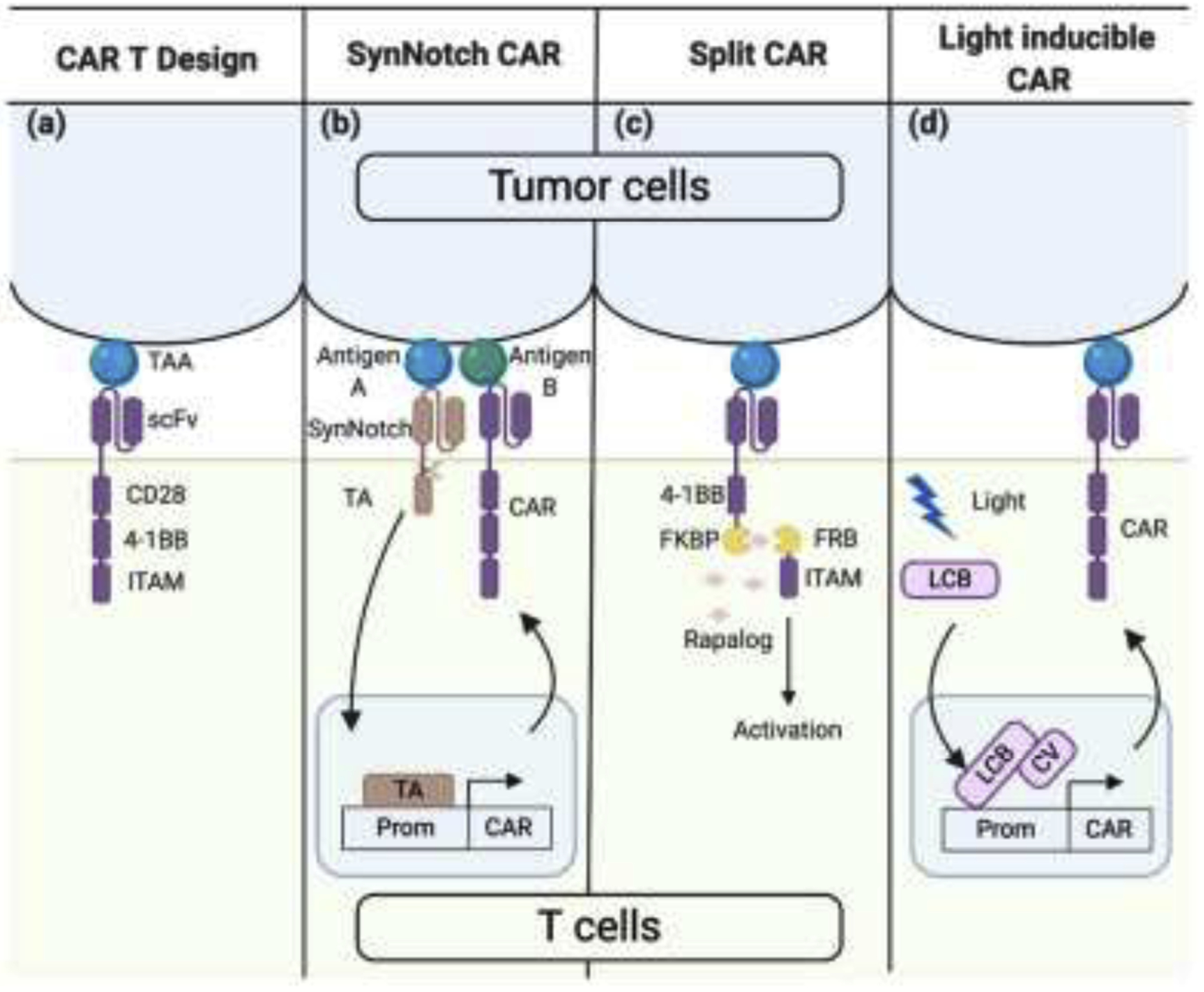
(a) Design of third generation CAR. A CAR is composed of an extracellular single-chain variable fragment (scFv) that specifically binds to the tumor associated antigen (TAA), co-stimulatory domain CD28 and 4–1BB, and the signal transduction immunoreceptor tyrosine-based activation motif (ITAM). (b) Design of SynNotch CAR. Upon cognate antigen A binding, SynNotch undergoes self-cleavage and release the transcription activator (TA) from the membrane. The TA then translocates into the nucleus and starts the CAR expression, which targets antigen B. (c) Design of Split CAR. The co-stimulatory domain 4–1BB and the signaling ITAM are separated into two molecules initially. When the small molecule Rapalog is added, FKBP and FRB dimerize, and the intact and functional CAR is formed. (d) Design of light inducible CAR. Upon light, LexA-CIB1-biLINus (LCB) translocate into the nucleus and bind to the promoter of CAR. At the same time, light can also induce the binding between LCB and CRY2-VPR (CV) to recruit a TA at the promoter region, which initiates CAR expression. Prom, promoter.
Despite the promising results in treating blood cancers, there has been limited success in applying CAR T therapy in solid tumors [17]. Meanwhile, CAR T therapy possesses a toxicity profile distinct from other cancer therapies, which requires specific management [18]. One common adverse effect is cytokine release syndrome (CRS), where the infused constitutive CAR T cells induce strong cytokine releases upon engagement with target cancer cells. CRS can be self-limited at lower grades while becoming life-threatening in severe cases, but can be mitigated by IL-6 receptor antagonists in most cases [19]. Another adverse effect is the on-target off-tumor toxicity, where CAR T cells attack normal tissues expressing the same antigen as tumors albeit at low levels. While this is manageable in the case of B cell aplasia caused by anti-CD19 CAR T therapy, it can be lethal when nonmalignant vital organs are attacked [7,20].
To tackle the on-target off-tumor toxicity, CAR T cells that rely on two antigens have been developed. Such approaches include inhibitory CARs (iCARs), combinatorial CARs, and SynNotch CARs. T cells engineered with iCARs or combinatorial CARs demonstrated enhanced specificity against target tumors, although delicate balancing and characterization among different components of the design is required to achieve optimal performance [21,22]. In SynNotch CAR T cells, the engagement of tumor-associated antigen A leads to the expression of a CAR for tumor-associated antigen B via the mechanical activation mechanism of the Notch receptor (Figure 1b) [23]. The activation of SynNotch CAR T cells requires dual-antigen tumors, thus sparing normal tissues expressing only one target antigen.
Another strategy to mitigate the on-target off-tumor side effect and to achieve finer controllability is to develop inducible CAR T cells. This approach allows the “user” to control the activation of the CAR T cells through external stimuli (e.g., chemical and optical cues) after infusion. One category of inducible CARs is controlled by small molecules including the rapamycin-inducible split CAR and the doxycycline-inducible CAR (Figure 1c) [24,25]. However, drug-inducible control is limited by its lack of spatial and tissue specificity. We have recently developed light-controllable CAR T cells based on a light-inducible nuclear translocation and dimerization (LINTAD) gene activation system, and demonstrated their activation and cytotoxicity against target tumor cells upon blue light stimulation in vitro and in vivo with spatiotemporal controllability (Figure 1d) [26]. But, the penetration depth of light limits the application of this technology to a wide range of solid tumors, particularly those embedded in deep tissues.
Mechanobiology
Cells are subject to various types of mechanical cues in their local microenvironments, such as mechanical loading on bone and cartilage, flow-induced shear stress on vascular endothelial cells, and cyclic stretch on cardiac cells [27]. Many of these mechanical cues can be transmitted deep into different tissues. In fact, cellular responses to these physical inputs are crucial for a large array of physiological processes. At the protein level, mechanosensitive ion channels, receptors, and membrane-bound proteins associated with the extracellular matrix (ECM) or cytoskeleton, all play a role in converting external mechanical stimuli into internal biochemical signals, also known as a mechanotransduction. Since the first identification of mechanosensitive ion channels in embryonic chick skeletal muscle [28], numerous prokaryotic and eukaryotic mechanosensitive proteins have been discovered and characterized. For instance, the bacterial large conductance mechanosensitive channel (MscL) has been extensively studied in E. coli, and its crystal structure, gating mechanisms, and physiological functions are well documented [29]. Several members of the transient receptor potential (TRP) family of channels have also been identified to be involved in a variety of mechanotransduction processes in eukaryotic cells [30]. In recent years, extensive investigation has been focused on the evolutionarily conserved Piezo ion channel family, which can also be activated by mechanical stimuli [31].
Similar to the discovery of the light-gated ion channel channelrhodopsin having paved the way for the development of optogenetics, identifying mechano-sensors responding to a specific user-induced mechanical perturbation could be a critical step for the establishment of mechanogenetics (i.e. employing mechanical stimulation to control genetic regulations and cellular functions). In evaluating different mechano-sensors candidates for mechanogenetics, several factors should be considered. First, is this candidate protein itself sufficient for mechanosensing? Or does it require other sensing components to be activated? For example, the opening of Cx43, a mechanosensitive hemichannel, requires the conformational activation of integrin ɑ5β1 [32]. In contrast, the non-pore-containing region of Piezo1 has been found to be sufficient for mechanosensing and opening of its central pore region [33], thus it is expected to retain its mechanosensitive characteristics exogenously expressed in different organisms. Secondly, what biochemical signals does the candidate sensor induce upon activation? In the context of mechanogenetics, it is essential that the transduced signals can be readily converted into gene expression. Calcium signaling, for example, can be triggered by numerous mechanically gated ion channels and couples with NFAT-dependent transcriptional activity in regulating cellular functions [34]. Lastly, in what microenvironment will the engineered cells reside? Many mechanosensitive proteins are also susceptible to a variety of other stimuli, such as pH and temperature, which can either up- or downregulate the mechanosensitivity of the protein [30,35]. The comprehensive characterization and careful assessment of candidate proteins could expedite the successful development of mechanogenetics.
Mechanogenetics and cancer immunotherapy
Different from light and small molecules, mechanical perturbations allow precise spatial and temporal control with deep penetration, and hence can be applied to control cell functions and cancer immunotherapy in deep tissues. For example, magnetic or radiofrequency fields can exert force on cells coated with magnetic nanoparticles. Stanley et al. developed a system which utilizes the endogenous iron contained ferritins as magnetic nanoparticles to control the expression of insulin in vivo [36]. In this design, transient receptor potential vanilloid 1(TRPV1) is fused to GFP antibody (α-GFP) on its N-terminal, and ferritin is tagged with GFP. Inside the cells, GFP tagged ferritin binds to iron and assembles into a 24-mer superparamagnetic iron oxide nanoparticle. These nanoparticles then bind to the α-GFP fused TRPV1 channel, which allows the transduction of magnetic or radiofrequency field into the activation of TRPV1 and hence calcium influx. Meanwhile, a genetic cassette containing a calcium response promoter and bioengineered insulin was developed and introduced into the same cells. The influx of calcium will trigger the nuclear factor of activated T-cells (NFAT) dephosphorylation and translocation into the nucleus. The translocated NFAT then binds to the calcium response promoter and drives the expression of bioengineered insulin. In this study, it was demonstrated that blood glucose levels in mice can be downregulated remotely through magnetic or radiofrequency field. In a recent study, Seo et al. have developed a magnetic toolkit to study the mechanisms of Notch and VE-cadherin signaling with high spatiotemporal resolution [37]. In the design, the authors covalently coupled SNAP-tag fused receptors (Notch and VE-cadherin) of a cell with benzyl guanine (BG) modified magnetoplasmonic nanoparticles (MNP). A piezo-controlled micromagnetic tweezer was positioned on top of the cell. By tuning the focused magnetic field gradient generated by the tweezer across the cell membrane, the authors were able to precisely control the force exerted on these mechano-sensors. However, the application of magnetic field induced mechanogenetics in cancer immunotherapy has yet to be developed.
Ultrasound is used primarily as an imaging tool for diagnosis in the clinic, but recently, its usage has been greatly expanded to other therapeutics as the acoustic wave can be focused on a confined region in deep tissues with millimeter precision [38]. In a recent study, we engineered CAR T cells with a genetic transducing module capable of converting ultrasound stimulation into gene expression [39]. In this study, T cell surfaces were coated with streptavidin-microbubbles via the biotinylated RGD peptide engaging the surface receptor integrins. These microbubbles can convert and amplify the mechanical energy transmitted through ultrasound waves and activate the Piezo1 channel endogenously expressed on the plasma membrane of T cells. Upon activation, Piezo1 channel allows an influx of calcium ions into the cell. The elevation of calcium level in the cell can trigger the CAR expression through a mechanism mediated by the activation of calcineurin to dephosphorylate NFAT, which subsequently translocates into the nucleus to bind to its promoter for the induction of CAR and hence rendering the T cells cytotoxic against tumor cells (Figure 2a). However, microbubbles used in this study may potentially limits its application in clinic due to the short lifetime of microbubbles in vivo.
Figure 2. Ultrasound guided mechanogenetics for CAR T engineering.
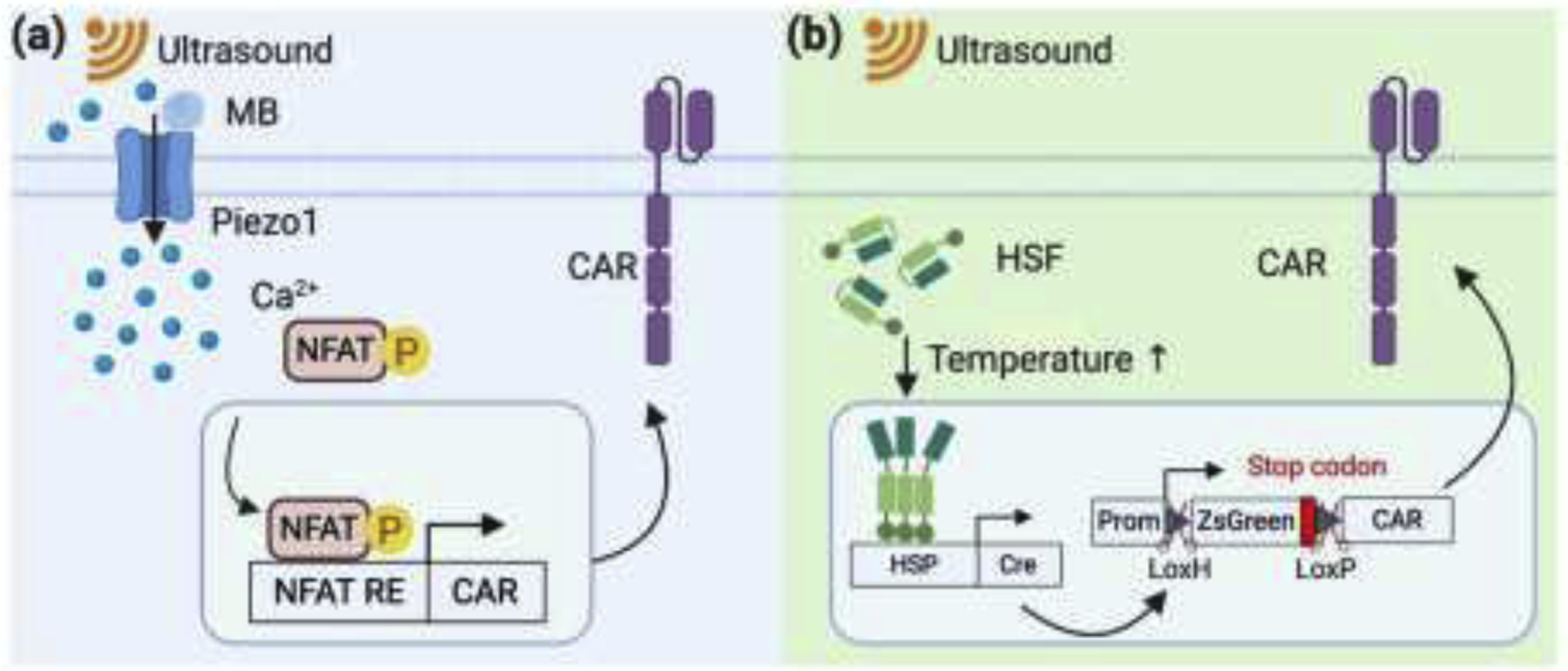
(a) Design of mechanical inducible CAR. Piezo1 channel is coupled with microbubbles (MBs). Upon low-intensity pulsed ultrasound stimulation, Piezo1 channel opens and induces increase of cytosol calcium (Ca2+) level. NFAT, a Ca2+-dependent transcription factor, is then dephosphorylated and translocates into the nucleus, which drives CAR expression through a synthetic genetic circuit. (b) Design of heat inducible CAR by ultrasound. Focused ultrasound stimulation with relatively high-intensity generates heat in the targeted tissue. The increase of temperature is sensed by heat shock factors (HSFs), which form active trimer and drive DNA recombinase Cre expression. The subsequent excision of lox-flanked stop codon by Cre enables the expression of CAR.
The mechanical energy from focused ultrasound (FUS) can also be converted into thermal energy through internal friction, causing local temperature increase in biological tissues. For example, Piraner et al. demonstrated the control of bacteria gene expression through the hyperthermia effect of FUS [40]. Recently, we have developed a new class of FUS-inducible CAR T cells utilizing a heat-sensitive genetic module [41]. In this design, we utilized the endogenous mammalian cell temperature sensor heat shock factor (HSF) and its cognate response element heat shock element (HSE). When FUS is applied to T cells, local temperature will increase due to internal friction. HSF then senses the heat and activate the expression of Cre recombinase, which is under the control of HSE. The Cre recombinase then will cleave the stop codon flanked by two lox sites, which allows the CAR molecules to be transcribed (Figure 2b). We investigated this technology in a bilateral subcutaneous tumor mouse model by applying short-pulsed FUS precisely toward the tumor on one side. Inhibited tumor growth was observed on the side with FUS stimulation compared to the control side. These results suggest the ultrasound-guided mechanical stimulation can be applied to guide the genetically engineered CAR T cells for cancer immunotherapy.
Conclusions and future perspectives
It is an era witnessing an amazing convergence of multiple cutting-edge frontiers in molecular/cellular immunoengineering, genetic and genomic engineering, mechanobiology, synthetic biology, engineering technology, as well as therapeutic medicine. Genetic tools and synthetic biology have been applied to reengineer immune cells, including but not limited to T cells, nature killer cells [42], and monocytes/macrophages [43], such that these engineered cells can gain new functions targeting, suppressing, and even eradiating tumor cells in various forms. Specifically, synthetically engineered CAR T cells have already had tremendous success in treating blood cancers. It becomes clear that the next frontier on cellular engineering for cancer immunotherapy will be on solid tumors. As we discussed in this paper, several issues remain to be addressed for cell-based cancer immunotherapy against solid tumors, including on-target off-tumor effect, cytokine release syndrome. Boolean logic circuits including SynNotch and split CARs have been applied to improve the specificity. However, given the complicated physiological system in the body, controllable CAR T cell activation specifically at local tumor sites will be in great needs to avoid systemic side effects.
While there are numerous studies on mechanical sensitive molecules and their related signaling transductions in relaying external mechanical cues into the regulation of gene expression and cellular functions, there is a lack of research utilizing mechanical sensors and their interrelated signaling network to control cellular functions, particularly for cancer immunotherapy. Since ultrasound signals as mechanical pressure waves can be delivered in relatively long distance within soft tissues, it is attractive if ultrasound-guided mechanical signals can be applied to directly and remotely control mechano-sensors and molecular events at depth in live animals without any co-factor. Presumably, T cells from the patients can be engineered with genetic cassettes encoding molecular elements sensitive to mechanical cues and infused back to the patients. These engineered cells can localize and enrich around tumor sites. Ultrasound transducers can then be guided to aim at tumor sites to deliver focused mechanical waves for the remote activation of these localized T cells. At the same time, various modes and parameters of ultrasound have been shown to directly activate Piezo1 channel without the help of microbubbles, which may help circumvent the limitation of microbubbles in clinic. [44–46]. Recently, stretchable electronic circuits have been developed to produce wearable patches and ultrasound transducer arrays [47,48]. It is expected that wearable patches carrying ultrasound transducers capable of controlling genetics, molecular and cellular functions will be available in the future, which can be further integrated with smart phones and internet for the remote control of genetics and physiology in the body (Figure 3). In fact, we envision that ultrasound controllers and transducers, genetic mechano-sensors and mechanotransducing modules, will continue to evolve for greater precision and sensitivity. As such, ultrasound can be integrated with genetic mechano-sensors and mechanobiology to have transformative impact on translational medicine and pave the way for revolutionary telemedicine approaches in the future.
Figure 3. Wearable ultrasound patches for cancer immunotherapy.
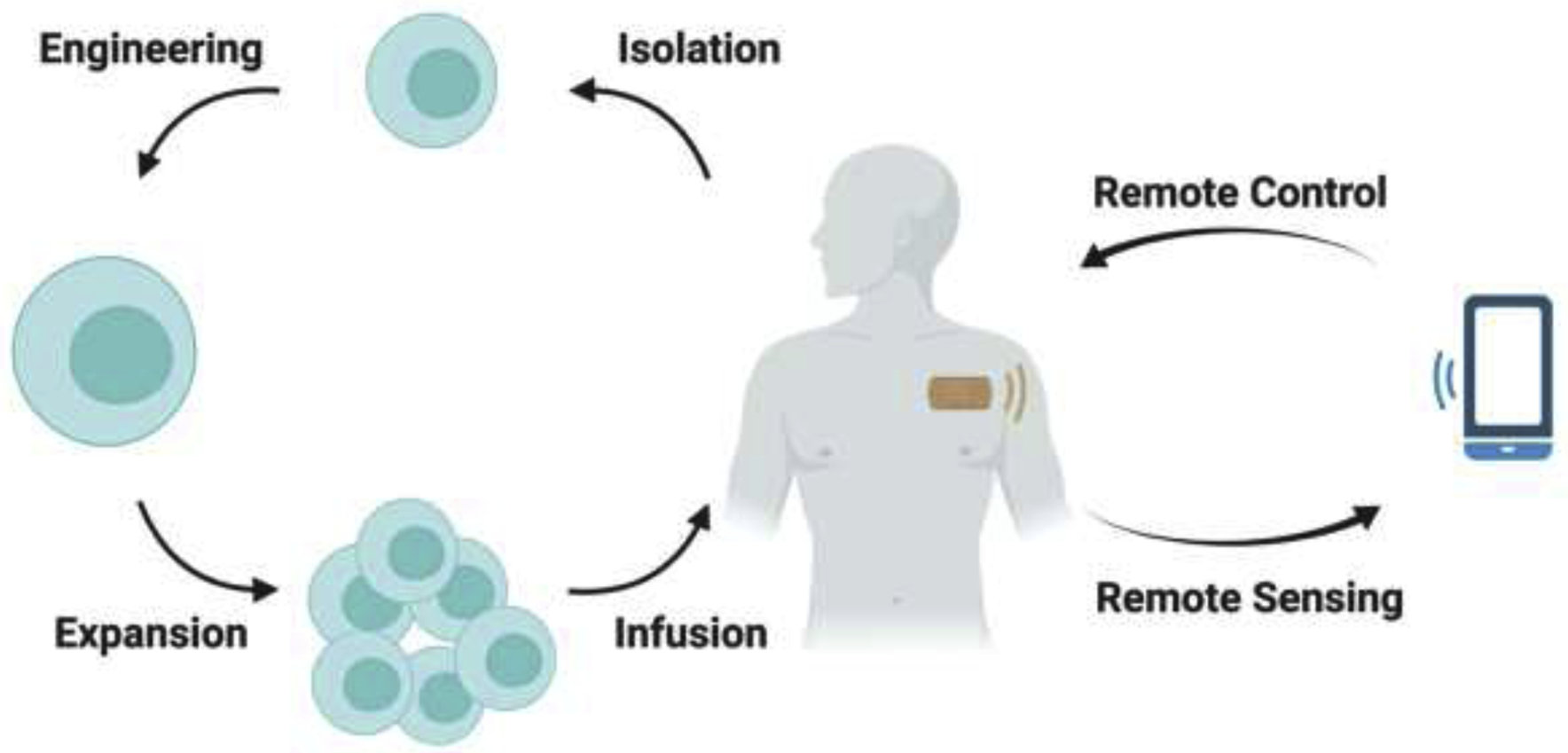
Primary T cells are isolated from the patients and then engineered with controllable genetic cassettes. After in vitro expansion, these engineered T cells are transferred back to the patients. At the target site (i.e. tumor site), a wearable ultrasound transducer patch is put right above the tumor. Doctors can hence remotely control the function of the wearable ultrasound transducer through mobile phones, which allows precise temporal and spatial control over engineered T cells at the tumor site.
Acknowledgements
This work was supported in part by grants from NIH HL121365, GM125379, GM126016, CA204704 and CA209629 (Y. Wang).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Y.Wang is a scientific co-founder of Cell E&G Inc and Acoustic Cell Therapy Inc. These financial interests do not affect the design, conduct or reporting of this research.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References.
- 1.Kruger S, Ilmer M, Kobold S, Cadilha BL, Endres S, Ormanns S, Schuebbe G, Renz BW, D’Haese JG, Schloesser H, et al. : Advances in cancer immunotherapy 2019 - latest trends. J Exp Clin Cancer Res 2019, 38:268. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A systematic review of the current clinical and pre-clinical studies of CAR T therapy and checkpoint inhibitors.
- 2.Liu X, Jiang S, Fang C, Yang S, Olalere D, Pequignot EC, Cogdill AP, Li N, Ramones M, Granda B, et al. : Affinity-Tuned ErbB2 or EGFR Chimeric Antigen Receptor T Cells Exhibit an Increased Therapeutic Index against Tumors in Mice. Cancer Res 2015, 75:3596–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eyquem J, Mansilla-Soto J, Giavridis T, van der Stegen SJ, Hamieh M, Cunanan KM, Odak A, Gonen M, Sadelain M: Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 2017, 543:113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu B, Ren J, Luo Y, Keith B, Young RM, Scholler J, Zhao Y, June CH: Augmentation of Antitumor Immunity by Human and Mouse CAR T Cells Secreting IL-18. Cell Rep 2017, 20:3025–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feucht J, Sun J, Eyquem J, Ho YJ, Zhao Z, Leibold J, Dobrin A, Cabriolu A, Hamieh M, Sadelain M: Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat Med 2019, 25:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamieh M, Dobrin A, Cabriolu A, van der Stegen SJC, Giavridis T, Mansilla-Soto J, Eyquem J, Zhao Z, Whitlock BM, Miele MM, et al. : CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 2019, 568:112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA: Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 2010, 18:843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, et al. : Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014, 6:224ra225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maus MV, Grupp SA, Porter DL, June CH: Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood 2014, 123:2625–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zamat A, Zhu L, Wang Y: Engineering Molecular Machines for the Control of Cellular Functions for Diagnostics and Therapeutics. Advanced Functional Materials n/a:1904345. [Google Scholar]; • A comprehensive summary of current small molecules and light gated genetics circuits for the control of cellular functions.
- 11.Argentati C, Morena F, Tortorella I, Bazzucchi M, Porcellati S, Emiliani C, Martino S: Insight into Mechanobiology: How Stem Cells Feel Mechanical Forces and Orchestrate Biological Functions. Int J Mol Sci 2019, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Summary of the mechanisms of cell mechanosensing, mechanotransduction and mechanoresponse.
- 12.Barbic M: Possible magneto-mechanical and magneto-thermal mechanisms of ion channel activation in magnetogenetics. Elife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan Y, Yoon S, Zhu L, Wang Y: Acoustic mechanogenetics. Curr Opin Biomed Eng 2018, 7:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyiadzis MM, Dhodapkar MV, Brentjens RJ, Kochenderfer JN, Neelapu SS, Maus MV, Porter DL, Maloney DG, Grupp SA, Mackall CL, et al. : Chimeric antigen receptor (CAR) T therapies for the treatment of hematologic malignancies: clinical perspective and significance. J Immunother Cancer 2018, 6:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porter DL, Levine BL, Kalos M, Bagg A, June CH: Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011, 365:725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, Maric I, Raffeld M, Nathan DA, Lanier BJ, et al. : Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010, 116:4099–4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez M, Moon EK: CAR T Cells for Solid Tumors: New Strategies for Finding, Infiltrating, and Surviving in the Tumor Microenvironment. Front Immunol 2019, 10:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy LB, Salama AKS: A review of cancer immunotherapy toxicity. CA Cancer J Clin 2020. [DOI] [PubMed] [Google Scholar]
- 19.Yanez L, Sanchez-Escamilla M, Perales MA: CAR T Cell Toxicity: Current Management and Future Directions. Hemasphere 2019, 3:e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ: Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics 2016, 3:16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M: Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol 2013, 31:71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fedorov VD, Themeli M, Sadelain M: PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med 2013, 5:215ra172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roybal KT, Rupp LJ, Morsut L, Walker WJ, McNally KA, Park JS, Lim WA: Precision Tumor Recognition by T Cells With Combinatorial Antigen-Sensing Circuits. Cell 2016, 164:770–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakemura R, Terakura S, Watanabe K, Julamanee J, Takagi E, Miyao K, Koyama D, Goto T, Hanajiri R, Nishida T, et al. : A Tet-On Inducible System for Controlling CD19-Chimeric Antigen Receptor Expression upon Drug Administration. Cancer Immunol Res 2016, 4:658–668. [DOI] [PubMed] [Google Scholar]
- 25.Wu CY, Roybal KT, Puchner EM, Onuffer J, Lim WA: Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science 2015, 350:aab4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Z, Wu Y, Allen ME, Pan Y, Kyriakakis P, Lu S, Chang YJ, Wang X, Chien S, Wang Y: Engineering light-controllable CAR T cells for cancer immunotherapy. Sci Adv 2020, 6:eaay9209. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Development of a light-inducible nuclear translocation and dimerization (LINTAD) system for the control of CAR T cells.
- 27.Uhler C, Shivashankar GV: Regulation of genome organization and gene expression by nuclear mechanotransduction. Nat Rev Mol Cell Biol 2017, 18:717–727. [DOI] [PubMed] [Google Scholar]
- 28.Guharay F, Sachs F: Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol 1984, 352:685–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haswell ES, Phillips R, Rees DC: Mechanosensitive channels: what can they do and how do they do it? Structure 2011, 19:1356–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinac B: Mechanosensitive ion channels: molecules of mechanotransduction. J Cell Sci 2004, 117:2449–2460. [DOI] [PubMed] [Google Scholar]
- 31.Murthy SE, Dubin AE, Patapoutian A: Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat Rev Mol Cell Biol 2017, 18:771–783. [DOI] [PubMed] [Google Scholar]; • A state-of-art discussion of the cell mechanosensors Piezo1 and Piezo2.
- 32.Batra N, Riquelme MA, Burra S, Jiang JX: 14-3-3theta facilitates plasma membrane delivery and function of mechanosensitive connexin 43 hemichannels. J Cell Sci 2014, 127:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Q, Wu K, Geng J, Chi S, Wang Y, Zhi P, Zhang M, Xiao B: Ion Permeation and Mechanotransduction Mechanisms of Mechanosensitive Piezo Channels. Neuron 2016, 89:1248–1263. [DOI] [PubMed] [Google Scholar]
- 34.Dobner S, Amadi OC, Lee RT: Chapter 14 - Cardiovascular Mechanotransduction In Muscle. Edited by Hill JA, Olson EN. Academic Press; 2012:173–186. [Google Scholar]
- 35.Scimia MC, Hurtado C, Ray S, Metzler S, Wei K, Wang J, Woods CE, Purcell NH, Catalucci D, Akasaka T, et al. : APJ acts as a dual receptor in cardiac hypertrophy. Nature 2012, 488:394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanley SA, Sauer J, Kane RS, Dordick JS, Friedman JM: Remote regulation of glucose homeostasis in mice using genetically encoded nanoparticles. Nat Med 2015, 21:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seo D, Southard KM, Kim JW, Lee HJ, Farlow J, Lee JU, Litt DB, Haas T, Alivisatos AP, Cheon J, et al. : A Mechanogenetic Toolkit for Interrogating Cell Signaling in Space and Time. Cell 2016, 165:1507–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang C, Li Y, Du M, Chen Z: Recent advances in ultrasound-triggered therapy. J Drug Target 2019, 27:33–50. [DOI] [PubMed] [Google Scholar]
- 39.Pan Y, Yoon S, Sun J, Huang Z, Lee C, Allen M, Wu Y, Chang YJ, Sadelain M, Shung KK, et al. : Mechanogenetics for the remote and noninvasive control of cancer immunotherapy. Proc Natl Acad Sci U S A 2018, 115:992–997. [DOI] [PMC free article] [PubMed] [Google Scholar]; ••This work established a mechanogenetic toolkit for remote control of cellular functions through ultrasound. Particularly, this toolkit is shown to be able to remotely regulate CAR T cell function against target tumor cells.
- 40.Piraner DI, Abedi MH, Moser BA, Lee-Gosselin A, Shapiro MG: Tunable thermal bioswitches for in vivo control of microbial therapeutics. Nat Chem Biol 2017, 13:75–80. [DOI] [PubMed] [Google Scholar]
- 41.Wu Y, Liu Y, Huang Z, Wang X, Jin Z, Li J, Limsakul P, Zhu L, Allen M, Pan Y, et al. : Acoustogenetic Control of CAR T Cells via Focused Ultrasound. bioRxiv 2020:2020.2002.2018.955005. [Google Scholar]; ••This work ultized the endogeneous heat sensing system of cells and engineered a heat-inducible CAR T cell, which can be remotely and directly controlled by ultrasound.
- 42.Li Y, Hermanson DL, Moriarity BS, Kaufman DS: Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell 2018, 23:181–192 e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, Schmierer M, Gabrusiewicz K, Anderson NR, Petty NE, et al. : Human chimeric antigen receptor macrophages for cancer immunotherapy. Nature Biotechnology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prieto ML, Firouzi K, Khuri-Yakub BT, Maduke M: Activation of Piezo1 but Not Na(V)1.2 Channels by Ultrasound at 43 MHz. Ultrasound Med Biol 2018, 44:1217–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liao D, Li F, Lu D, Zhong P: Activation of Piezo1 mechanosensitive ion channel in HEK293T cells by 30MHz vertically deployed surface acoustic waves. Biochem Biophys Res Commun 2019, 518:541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiu Z, Guo J, Kala S, Zhu J, Xian Q, Qiu W, Li G, Zhu T, Meng L, Zhang R, et al. : The Mechanosensitive Ion Channel Piezo1 Significantly Mediates In Vitro Ultrasonic Stimulation of Neurons. iScience 2019, 21:448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rogers JA, Someya T, Huang Y: Materials and mechanics for stretchable electronics. Science 2010, 327:1603–1607. [DOI] [PubMed] [Google Scholar]
- 48.Mierzwa AP, Huang SP, Nguyen KT, Culjat MO, Singh RS: Wearable Ultrasound Array for Point-of-Care Imaging and Patient Monitoring. Stud Health Technol Inform 2016, 220:241–244. [PubMed] [Google Scholar]


