Our goal was to explore immunoserological relations between IgM and IgG isotypes of natural autoantibodies (nAAbs) and pathogen (or vaccine)‐induced or disease‐related antibodies in systemic autoimmune diseases (SAD); systemic sclerosis (SSc), systemic lupus erythematosus (SLE), rheumatoid arthritis (RA). The found significant associations between IgG isotype nAbs and specific humoral antibodies may underscore the immune response‐inducible nature of the diseases investigated, and may corroborate the notion that nAbs can act as a mediator between the innate‐like and the adaptive arms of the immune system .The relationship between protective anti‐dsDNA IgM and the IgM isotype of anti‐F4 and anti‐CS may provide immuno‐serological evidence for the beneficial roles of nAAbs in SLE patients.
![]()
Keywords: autoimmunity, autoantibodies, antibodies, vaccination, systemic lupus erythematosus
Summary
Infection or vaccine‐induced T cell‐dependent immune response and the subsequent high‐affinity neutralizing antibody production have been extensively studied, while the connection between natural autoantibodies (nAAbs) and disease‐specific antibodies has not been thoroughly investigated. Our goal was to find the relationship between immunoglobulin (Ig)M and IgG isotype nAAbs and infection or vaccine‐induced and disease‐related autoantibody levels in systemic autoimmune diseases (SAD). A previously described indirect enzyme‐linked immunosorbent assay (ELISA) test was used for detection of IgM/IgG nAAbs against citrate synthase (anti‐CS) and F4 fragment (anti‐F4) of DNA topoisomerase I in 374 SAD samples, with a special focus on systemic lupus erythematosus (SLE) (n = 92), rheumatoid arthritis (n = 73) and systemic sclerosis (n = 157) disease groups. Anti‐measles IgG and anti‐dsDNA IgG/IgM autoantibodies were measured using commercial and in‐house indirect ELISA tests. In all SAD groups the anti‐measles IgG‐seropositive cases showed significantly higher anti‐CS IgG titers (P = 0·011). In anti‐dsDNA IgG‐positive SLE patients, we detected significantly higher levels of anti‐CS and anti‐F4 IgG nAAbs (P = 0·001 and < 0·001, respectively). Additionally, we found increased levels of IgM isotypes of anti‐CS and anti‐F4 nAAbs in anti‐dsDNA IgM‐positive SLE patients (P = 0·002 and 0·016, respectively). The association between IgG isotypes of pathogen‐ or autoimmune disease‐related antibodies and the IgG nAAbs may underscore the immune response‐inducible nature of the diseases investigated. The relationship between protective anti‐dsDNA IgM and the IgM isotype of anti‐F4 and anti‐CS may provide immunoserological evidence for the beneficial roles of nAAbs in SLE patients.
Introduction
Since the discovery of natural autoantibodies, a great effort has been devoted to describing their generation, regulation and function [1]. It has been determined that natural immunoglobulin (Ig)M antibodies are present prior to antigen stimulation, and that their reactivity profiles are highly conserved between individuals [2]. The presence of natural antibodies recognizing citrate synthase (CS) both in healthy individuals and in patients with systemic autoimmune disease has already been demonstrated in our previous study. The majority of these antibodies proved to be of IgM isotype. Their presence in infants and their unaltered serum level during ≥ 5 years in adults indicates that these antibodies belong to the natural autoantibody (nAAb) repertoire established early in postnatal life [3]. Natural IgM autoantibodies have been also proposed to convey protection from pathological autoimmune reactions [4, 5, 6, 7, 8]. The impaired anti‐apoptotic function of natural IgM autoantibodies, may, leading to their pathological elimination of dying cells and subsequent maintenance of autoinflammation [5]. Natural IgM antibodies are produced by the relatively class‐restricted B1 cells, while IgG antibodies are known to be produced via the T cell‐dependent interactions of follicular B2 cells [2, 9]. Although natural and pathological antibodies of IgG isotype may show mutual characteristics, it is important to differentiate between them. Since its discovery, the role of natural IgG, which pre‐exists in neonates and uninfected individuals, has remained unclear due to the common view that natural antibodies lack affinity for pathogens [10]. Although it is already known that one of the most prevalent functions of nAAbs is the ability to provide protection against bacterial, viral and fungal infections, the connection between immunization and nAAbs has not yet been thoroughly investigated.
The potential role of natural IgGs in controlling inflammation has been also demonstrated [11, 12]. Many autoimmune diseases are initiated by the appearance of IgG autoantibodies to specific cellular and tissue components [13, 14, 15]; for instance, by the presence of pathological anti‐dsDNA IgG antibodies in systemic lupus erythematosus (SLE). Like those of pathological origin, levels of IgG isotype nAAbs have been proved to fluctuate over time [3, 16], and are reported to be abundant and ubiquitous in human sera. Their levels are influenced by age, gender and disease [2, 3, 17]. Moreover, they were found to play a role in transplantation‐related complications, such as graft injury [18], which further confirms their adaptive immune system‐derived nature and role in pathological conditions.
Herein we describe our investigations in SLE, systemic sclerosis (SSc) and rheumatoid arthritis (RA) sample groups, focusing on associations among levels of vaccine‐ or infection‐induced antibodies [pathological; anti‐measles IgG, SLE‐associated autoantibodies (naturally and pathological; anti‐dsDNA IgG and IgM) and nAAbs (anti‐CS), anti‐dsDNA topoisomerase I F4 fragment (anti‐F4)] aiming to find an immunoserological proof for the co‐existence of IgG isotype pathogen‐ or disease‐related antibodies and nAAbs. Secondly, we wanted to evidence the simultaneous presence of the known protective anti‐dsDNA IgM autoantibodies and IgM isotype of anti‐CS and anti‐F4 in SLE patients, confirming their potential regulatory and beneficial role.
Materials And Methods
Samples
Serum samples of patients suffering from different systemic autoimmune diseases (SAD) were obtained from the serum bank of the Department of Rheumatology and Immunology, University of Pécs (Hungary). The samples were stored and analyzed anonymously in the laboratories of the Department of Immunology and Biotechnology (University of Pécs Medical School) according to quality assurance criteria (ISO 17025) (Ethical License: 2015/5726 by the Regional Research Ethics Committee at the University of Pécs). The number of sera derived from different systemic autoimmune patients comprised the following: SSc n = 157, SLE n = 92, RA n = 73, other = 52 (total n = 374). Mean age (rounded values; years) within sample groups was the following: SSc, 56; SLE, 44; RA, 59, 53 (overall, 52).
Methods
In order to investigate associations among vaccine‐induced antibodies (anti‐measles IgG), SLE‐related autoantibodies (anti‐dsDNA IgG/M) and natural (auto)antibodies [anti‐DNA topoisomerase‐I (or anti‐Scl‐70) fragment F4 (anti‐F4) IgG/M, anti‐CS IgG/M] indirect enzyme‐linked immunosorbent assay (ELISA) tests were performed using manual sample dilution followed by programmed assay execution on automated Siemens BEP 2000 Advance® platform (Siemens AG, Frankfurt, Germany).
Anti‐F4 IgG/M ELISA
We used the recombinant fragment‐4 (F4) of topoisomerase I [amino acid (AA) 450–600] as antigen for detection of IgG and IgM nAAbs. Similarly, as described earlier [3, 19], 96‐well polystyrene plates (Nunc, Roskilde, Denmark) were coated with recombinant topo I F4 fragment or with maltose‐binding protein (MBP) on the other half of the plate at 2·5 µg/ml in ELISA coating buffer (Bio‐Rad BUF030) (50 µl/well, 4–6°C, overnight; Biorad, Hercules, CA, USA). Plates were washed with washing buffer (WB) [100 mM phosphate‐buffered saline (PBS), pH 7.4 + 1 ml/l Tween 20, (350 µl/well] and blocked with 0·5 m/m% polyvinyl alcohol (PVA) (~72 000 Mw, 300 µl/well, room temperature, ≥ 2 h). Serum samples were incubated in 100‐fold dilution in WB (IgG: 50 µl/well, 37°C, 35 min/IgM: 50 µl/well, 37°C, 35 min). Standards, blanks (wells containing only WB), high and low controls (positive and negative samples identified in a previous run) were processed as patient sera, and were also automatically assigned to both plate halves. Subsequently, plates were incubated with horseradish peroxidase (HRP)‐conjugated anti‐human‐IgG or anti‐human IgM secondary antibody (Dako, Glostrup, Denmark) for 30 min at 37°C. Color reaction was developed using 3,3´,5,5´‐tetramethylbenzidine (TMB) (Bio‐Rad BUF056A); finally, 0·18 M H2SO4 stop solution was applied (50 µl/well). Reading was performed at λ = 450/620 nm.
To achieve a better comparability between vast numbers of results, the formerly used operational protocol was optimized to the automated setting. The former extinction [optical density (OD)]‐based result evaluation was replaced by the conversion of optical densities into quantitative results, using an in‐house anti‐F4 standard made of pooled known positive sera. Doubling, five‐point dilution series of standard points were applied in triplicate (starting dilution point: 50‐fold). For the calculation of the standard curve, a four‐point sigmoid fit was applied.
As described by Simon et al. [19], the F4 fragment of DNA topoisomerase I (topo I) was expressed as recombinant maltose‐binding protein (MBP) fusion protein. Consequently, results obtained for the MBP antigen coating were used to measure the potential background. The final result calculation in terms of absorbance (OD) was performed as follows:
OD sample (anti‐topo I F4 fragment) = OD sample F4 well–OD sample MBP well.
Anti‐CS IgG/M ELISA
Anti‐CS (IgG/M) indirect ELISA measurements were also performed as described earlier [3, 16, 17, 19] with the already detailed slight modifications, due to high sample numbers and the need of signal‐to‐noise ratio optimization required by the automated setting. Nunc MaxiSorp™ ELISA plates were coated with CS from porcine heart (Sigma‐Merck C3260) at a concentration of 2·25 µg/ml in coating buffer (Bio‐Rad BUF030) (50 µl/well, 4–6°C, overnight). Plates were washed and blocked as described above. Serum samples were incubated in 100‐fold dilution in WB for 35 min at room temperature (RT). Standards, blanks, high and low controls were processed as patient sera. After three washing steps, anti‐human IgM or IgG secondary antibody (Dako) was incubated for 30 min followed by TMB substrate for 15 min and H2SO4 stop solution (50 µl/well). Automation and reading was performed as described earlier. A five‐point dilution series of our in‐house anti‐CS standard was used for result quantitation, with subsequent four‐point sigmoid curve fitting.
Anti‐measles IgG ELISA
Anti‐measles antibody (IgG)‐level data were considered an adequate model for pathogen‐derived (or infection‐induced) antibodies that are present in the great majority of the Hungarian population. Based on our previous findings [20, 21] and in accordance with data of other Hungarian colleagues [22, 23], the overall ratio of the Hungarian population in possession of sufficient anti‐measles IgG antibody levels is ≈90%, with a well‐characterized, more susceptible cluster being responsible for the lagging ≈10%. Age group categorization based on historical changes in the measles, mumps and rubella (MMR) immunization schedule, recurring epidemics, subsequent revaccination protocols and shifts between vaccine valency (mono‐, bi‐ or trivalent) and manufacturers have been thoroughly detailed previously [20, 21, 24]. Considering that we already had a population‐level result [more‐fold verified by multiple measurement techniques (ELISA, IIF)] and independent research groups] [20, 21, 23, 25] focusing on anti‐measles IgG antibody titers, it was obvious to use anti‐measles data as one of the means of our comparisons. Anti‐measles antibody (IgG) measurements were performed using our self‐developed ELISA assays validated by well‐established commercial kits (Novalisa, Immunolab, Euroimmun, Sekisui‐Virotech, Serion, Siemens Enzygnost), as previously reported [20, 21]. For the anti‐measles (IgG) in‐house ELISA, Nunc MaxiSorp™ plates were coated with measles virus Edmonston strain (Bio‐Rad PIP013) as antigen at a concentration of 2·8 µg/ml in coating buffer (Bio‐Rad BUF030) at 4–6°C overnight. After three washing steps, plates were blocked with PVA at RT overnight. After pretreatment of sera with IgM reducing assay diluent (Bio‐Rad BUF038) and subsequent centrifugation, 25 µl of supernatant was transferred to a microplate prefilled with 75 µl of WB, resulting in a further fourfold dilution. The Third WHO International Standard for Anti‐Measles (NIBSC code: 97/648) was used in five‐point doubling dilutions (starting concentration: ~5000 mIU/ml). Standards, blanks, high and low controls were processed as patient sera, and were automatically assigned to plates. Primary and secondary antibodies (analytes and the Dako, rabbit anti‐human HRP‐conjugated polyclonal IgG) were incubated for 20–20 min (100 µl/well, 37°C). Color reaction development and stopping were carried out as detailed above. Automation and reading were performed using the Siemens BEP 2000 Advance System, λ = 450/620 nm. For the qualitative evaluation, cut‐off values were based on area under the receiver operating characteristic (AUROC) analysis, as described earlier [21].
Anti‐dsDNA IgG/M ELISA
Anti‐dsDNA IgG/M measurements were performed using commercial anti‐dsDNA ELISA kits (ORG604 and ORG604M; Orgentec Diagnostika GmbH, Mainz, Germany). In the test, human recombinant double‐stranded DNA (dsDNA) is bound to microwells. Quantitative anti‐dsDNA antibody titer evaluation was performed as per the manufacturer’s instructions; optical density values were read at 450 nm (reference 600 690 nm). Qualitative (positive/negative) evaluation of anti‐dsDNA IgM results was carried out as per the kit manual. For the anti‐dsDNA IgG qualitative result evaluation a corrected cut‐off was used (30 IU/ml) that had been optimized based on the past 5 years’ clinical–practical feedbacks.
Statistical analysis
Statistical analysis was carried out in the undivided serum bank of SAD, and also in the individual SAD subgroups who had sufficient sample numbers to yield representative data (SSc, SLE and RA). Statistical evaluation was performed using spss version 25.0 statistics package (IBM, Armonk, NY, USA). Spearman’s correlation analysis, Mann–Whitney U and Kruskal–Wallis tests were used as appropriate; P‐values < 0·05 were considered significant.
The data that support the findings of this study are available from the corresponding author (S.D.) upon reasonable request
Results
Infection‐ or vaccine‐induced anti‐measles antibody levels in systemic autoimmune diseases
In our previous studies [20, 21] we investigated the immunological protection against measles infection at population‐level in healthy individuals categorized into age or vaccination groups, based on changes introduced in measles immunization schedules and in vaccine components since the introduction of the first measles vaccine in Hungary. In the current study, in order to explore relationships between infection‐ or vaccine‐induced antibodies and natural antibodies of IgG isotype, anti‐measles IgG levels in samples obtained from SAD patients (total n = 374) were measured. Anti‐measles IgG seronegativity ratios in different SAD groups are listed in Table 1. As already described in the epidemiological literature, and in accordance with our previously published findings [20, 21, 22, 24, 26], measles seronegativity ratios showed significant correlation with age (P < 0·001 correlation coefficient; 0·323). Accordingly, enhanced seronegativity ratios were detected in patient groups with mean ages connected to the early vaccination era, which was characterized by an incomplete measles/MMR immunization routine and vaccine inefficiency. The highest seronegativity ratio was found in SLE patients, with a mean age of 44 years.
Table 1.
Age division and ratio of anti‐measles (IgG)‐seronegative samples in systemic autoimmune disease groups
| SAD | No. of sera | Mean age (rounded years) | No. of anti‐measles IgG seronegative sera | Ratio (%) |
|---|---|---|---|---|
| SSc | 157 | 56 | 13 | 8·28 |
| SLE | 92 | 44 | 13 | 14·13 |
| RA | 73 | 59 | 3 | 4·10 |
| Other* | 52 | 53 | 4 | 7·69 |
| Total | 374 | 52 | 33 | 8·82 |
Other = myositis, non‐differentiated collagen disease, arthritis psoriatica, mixed connective tissue disease, morphea, primary Raynaud syndrome, secondry Raynaud syndrome, Sjögren’s syndrome.
Ig = immunoglobulin; SAD = systemic autoimmune diseases; SLE = systemic lupus erythematosus; SSc = systemic sclerosis; RA = rheumatoid arthritis.
Comparison of natural autoantibody and infection‐induced antibody levels
Based on previous findings nAAbs (IgG/M) against the mitochondrial inner membrane, enzyme CS and topo I F4 could be detected in sera of healthy individuals and patients with SSc, SLE and in other autoimmune rheumatic diseases [19]. Natural autoantibodies form a network in healthy individuals, but at the same time can also recognize other antigens (nucleosome) in systemic autoimmune patients [3]. When analyzing the undivided totality of SAD samples, the same trend was observed as in the case of the three accentuated disease groups (SSc, SLE, RA). Considering all SAD samples together (total n = 374), significantly higher anti‐CS IgG titers were detected in the anti‐measles IgG‐seropositive patient group (P = 0·011) compared to the anti‐measles IgG seronegative individuals (Fig. 1). Analyzing the association between virus‐ or vaccine‐induced (anti‐measles IgG) and natural (anti‐CS IgG) antibody titers in the singularly investigated autoimmune diseases (RA, SLE and SSc), the same trend described above was observable; in all three groups the anti‐measles IgG‐seropositive samples showed significantly higher anti‐CS IgG titers (Fig. 2). A similar, but statistically non‐significant, trend was observed in the case of anti‐F4 IgG nAAbs. No association was found between infection‐ or vaccine‐induced IgG and IgM isotype nAAbs.
Fig. 1.
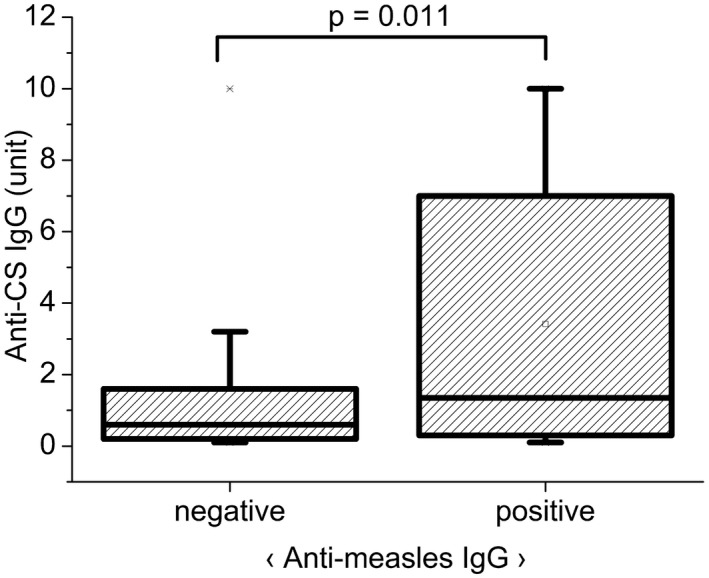
Relationship between anti‐measles immunoglobulin (Ig)G and anti‐citrate synthase (CS) IgG antibody titers in the undivided serum bank of systemic autoimmune diseases (SAD) serum samples (n SAD = 374 = n RA 73 + n SLE 92 + n SSc 157 + n other 52). Qualitative (positive, negative) anti‐measles IgG results were compared to quantitative anti‐CS IgG results (expressed in arbitrary units, based on our in‐house standard), using Mann–Whitney U analysis. Significantly higher anti‐CS IgG titers were detected in the anti‐measles IgG‐seropositive compared to ‐seronegative SAD patients. Boxes show interquartile ranges (IQR); whiskers indicate lowest and highest values; horizontal lines represent medians.
Fig. 2.
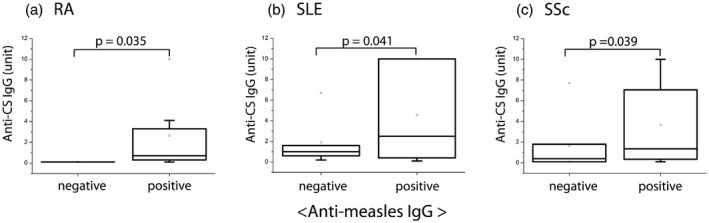
Relationship between anti‐measles immunoglobulin (Ig)G and anti‐citrate synthase (CS) IgG natural autoantibody titers [in systemic sclerosis (SSc), systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) disease groups (n RA = 73; n SLE = 92; n SSc = 157]. Qualitative (positive, negative) anti‐measles IgG results were compared to quantitative anti‐CS IgG results (expressed in arbitrary units, based on our in‐house standard), using Mann–Whitney U analysis. Significantly higher levels of natural anti‐CS IgG were detected in anti‐measles IgG‐seropositive compared to seronegative samples in RA, SLE and SSc. Boxes show interquartile ranges (IQR); whiskers indicate lowest and highest values; horizontal lines represent medians.
Relationship between IgG natural autoantibody levels and anti‐dsDNA IgG in SLE patients
In order to investigate the association between autoimmune disease‐specific pathological autoantibodies and IgG isotype nAAbs, anti‐dsDNA IgG measurement was chosen, as anti‐dsDNA IgG is a highly specific disease marker in SLE. In those SLE patient samples that proved to be positive for the disease‐specific marker anti‐dsDNA IgG, significantly higher levels of anti‐F4 IgG (P = 0·001) and anti‐CS IgG (P < 0·001) nAAbs were measured, as shown in Fig. 3. Anti‐dsDNA IgG and nAAbs levels also showed significant correlation (P/correlation coefficient: 0·006/0·321 and 0·000/0·510 for anti‐F4 IgG and anti‐CS IgG, respectively). No correlation was found between IgM nAAbs and disease‐specific anti‐dsDNA IgG autoantibody levels.
Fig. 3.
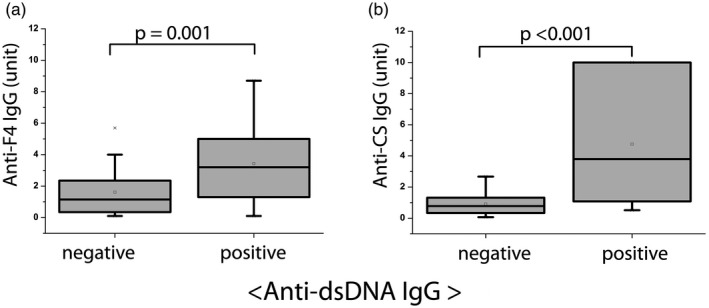
Relationship between disease‐specific anti‐dsDNA immunoglobulin (Ig)G autoantibody levels and natural anti‐F4 and anti‐citrate synthase (CS) IgG natural autoantibody titers in systemic lupus erythematosus (SLE) patients (n SLE = 92). Qualitative (positive, negative) anti‐dsDNA IgG results were compared to quantitative anti‐DNA topoisomerase I F4 fragment (anti‐F4) IgG and anti‐CS IgG results (expressed in arbitrary units, based on our in‐house standard), using Mann–Whitney U analysis. The levels of anti‐F4 and anti‐CS IgG antibodies were significantly increased in anti‐dsDNA IgG‐positive compared to anti‐dsDNA IgG‐negative SLE patients. Boxes show interquartile ranges (IQR); whiskers indicate lowest and highest values; horizontal lines represent medians.
Anti‐dsDNA IgM and natural IgM autoantibody levels showed association in SLE
Previous reports have proposed that anti‐dsDNA IgM antibodies may play a protective role in lupus nephritis [7, 8, 27]. Herein we compared anti‐dsDNA IgM levels with nAAb titers in SLE patients. Significantly higher levels of anti‐F4 IgM and anti‐CS IgM nAAbs were detectable (P = 0·002 and 0·016, respectively) in anti‐dsDNA IgM‐positive SLE patient samples, as shown in Fig. 4. Anti‐dsDNA IgM titers and nAAb levels also showed significant correlation (P/correlation coefficient: 0·002/0·344 and 0·018/0·252 for anti‐F4 IgM and anti‐CS IgM, respectively).
Fig. 4.
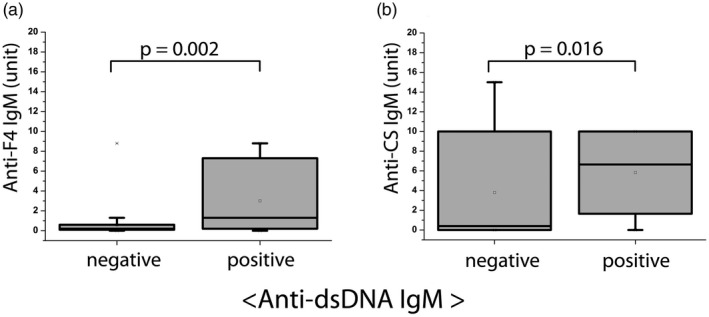
Relationship between anti‐dsDNA immunoglobulin (Ig)M autoantibody levels and anti‐F4, anti‐citrate synthase (CS) IgM natural autoantibody titers in systemic lupus erythematosus (SLE) patients (n SLE = 92). Qualitative (positive, negative) anti‐dsDNA IgM results were compared to quantitative anti‐DNA topoisomerase I F4 fragment (anti‐F4) IgM and anti‐CS IgM results (expressed in arbitrary units, based on our in‐house standard), using Mann–Whitney U analysis. The levels of anti‐F4 and anti‐CS IgM natural antibodies were significantly elevated in anti‐dsDNA IgM‐positive compared to anti‐dsDNA IgM‐negative SLE patients. Boxes show interquartile ranges (IQR); whiskers indicate lowest and highest values; horizontal lines represent medians.
Discussion
Regarding the evaluation of anti‐measles antibody titers, we would like to note that all examined samples belonged to individuals who were aged a minimum of 15 years [the age range of the undivided serum bank (n = 374) was from 15 to 81 years]. Since 1992, the MMR trivalent vaccine has been administered in Hungary at age 15 months, and the reminder vaccine is given at age 11 years. Consequently, the problem of questionable sero‐epidemiological data due to inadequate seroconversion as a consequence of an immature immune response or a scarce time interval between the measles (or MMR) vaccination and the measurement of the antibody titers can be excluded.
According to known epidemiological data, the early measles/MMR vaccination era was characterized by poorly defined age at vaccination. Moreover, disregarded thermolability of the inoculum and the lack of suitable adjuvants may have also contributed to vaccine inefficiency [22, 24]. Based on our previous findings [21], and in accordance with recent publication data [25], immunization gaps have been formed in the age group of individuals vaccinated between 1978 and 1987 (≈20% of seronegativity) [21]. To this potentially susceptible cluster belong today’s 35–42‐year‐old adults, explaining the high ratio of samples with low anti‐measles antibody (IgG) titers. SLE typically affects young or middle‐aged individuals; the mean age of SLE patients in our serum bank was 44 years, justifying the higher anti‐measles seronegativity ratio in this patient group. We found a significant association between infection‐ or vaccine‐induced anti‐measles IgG and anti‐CS IgG natural autoantibodies in the undivided sample multitude of SAD samples and also in the distinct autoimmune disease groups. The concomitance of pathogen‐ or vaccine‐associated and natural antibodies is also verified by the literature. A recent study reported the enhancement of natural antibody repertoire by immunization [28] in laboratory rats. The study suggested that immunization‐induced natural antibodies may also contribute to wound repair and tumor surveillance [28]. Another animal study performed on cod juveniles reports an increased natural antibody response of vaccinated compared to unvaccinated fish (against Vibrio anguillarum) [29]. Scientific data also describe a significant correlation between levels of natural autoantibodies and response to vaccination in elderly, physically active individuals [30]. Based on our serological findings, supported by cumulative scientific data, it can be hypothesized that natural autoantibodies may play a role in efficient vaccination and the subsequent formation of long‐lasting immunological memory.
The connection between disease‐specific anti‐dsDNA IgG and the IgG isotypes of antibodies of natural origin in SLE samples may support the notion regarding the disease‐associated nature of the IgG isotype nAAbs. According to our previous findings, titers of anti‐CS antibodies with IgG isotype fluctuate over time; consequently, the presence of these antibodies can be a result of an adaptive‐like immune response [3, 16]. Natural IgG autoantibodies are reported to be abundant and ubiquitous in human sera, and their number is influenced by age, gender and disease [2].
Anti‐topoisomerase‐I (anti‐Scl‐70) autoantibodies are considered highly specific for SSc, although they have been found occasionally in a small number of patients with SLE [31, 32]. In our previous study [19], fragment F4 of topo I was recognized by all SSc and SLE patients’ sera which were positive for anti‐topo I IgG antibodies by a conventional ELISA kit used for the detection of anti‐Scl‐70 autoantibodies. The serum level of anti‐F4 IgG antibodies was significantly higher in SLE and SSc patients than in healthy individuals. Anti‐F4 IgM was found in both SAD and healthy controls [19].
Recent publications suggest that IgM autoantibodies may also play a protective role in SLE [7, 8, 33, 34, 35]. According to literature data, secretory IgM deficiency in mice showed a connection with increased susceptibility to autoimmunity [36]; in human studies, a reduction in IgM levels was also associated with SLE [27]. The impaired function of natural IgM in clearance of dying cells can result in the accumulation of apoptotic remnants and fragments of necrotic cells, which aids their pathological elimination and thus contributes to the maintenance of autoinflammation [5]. Protective IgM autoantibodies in SLE are of particular interest. It has been reported that increased levels of IgM are negatively associated with the prevalence of atherosclerotic plaques in patients with SLE [37]. Beneficial clinical associations between natural IgM and autoimmunity, as well as opportunities for potential therapeutic implications, are widely studied [4]. IgM antibodies against dsDNA are frequent in SLE [35]. Additionally, highly significant negative correlation between IgM anti‐dsDNA antibodies and glomerulonephritis was observed in mice and in humans [7]. The clearance of pathogenic immune complexes may be improved by IgM, therefore IgM antibodies against dsDNA certainly appear to be protective, and may be a new treatment modality of lupus nephritis in humans [8]. As expected, IgM isotypes of natural anti‐F4 and anti‐CS autoantibody levels showed correlation with anti‐dsDNA IgM titers, supporting the hypothesis that these IgM autoantibodies are part of the natural immune repertoire in SLE.
The current immunoserological data – supported by recent scientific literature [38] – shows associations between IgG isotype natural antibodies (nAbs) and specific humoral antibodies. This finding may corroborate the notion that nAbS not only play an important role in the first line of defense of the immune system, but also contribute to an effective adaptive response through the maintenance of immune homeostasis [38, 39, 40] and priming of the adaptive immune functions. Consequently, nAbs can act as a mediator between the innate‐like and the adaptive arms of the immune system [3, 12, 38]. The dogma that high‐affinity IgG response is the major goal of immunization and low‐affinity antibodies should be avoided has positively contributed to the dearth of information regarding the role of nAbs in vaccination [38]. However, it has been recently proposed that nAbs may serve as potential screening targets to predict the strength of antigen‐induced immune response [41]. The serological investigations presented herein should lead to more focus on nAbs as a first‐line component of the adaptive immune response [38], and may promote further research in the potential use of nAbs as predictive tools for vaccine development. Furthermore, measuring the levels of nAAbs may have clinical relevance in SAD, especially in SLE. Therapeutic applications could harness the potency of immune‐regulatory nAAbs either by boosting in‐vivo natural IgM production or via therapeutic infusions of monoclonal or polyclonal IgM preparations [4, 42].
Disclosures
None declared.
Acknowledgements
This work was supported by research grants of the Hungarian National Scientific Research Fund OTKA K‐112939, PTE ÁOK‐KA 2020/10, GINOP‐232‐15‐2016‐00050, EFOP 3.6.1‐16‐2016‐00004 grants, and also by the HUHR/1901/3.1.1/0032 CABCOS3 grant. The research was also co‐financed by the Higher Education Institutional Excellence Programme of the Ministry for Innovation and Technology in Hungary, within the framework of the Proof of Principle programme of the University of Pécs.
Data availability statement
The data that support the findings of this study are available from the corresponding author [Simon D], upon reasonable request.
References
- 1. Avrameas S, Alexopoulos H, Moutsopoulos HM. Natural autoantibodies: An undersugn hero of the immune system and autoimmune disorders – a point of view. Front Immunol 2018; 9:1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nagele EP, Han M, Acharya NK, DeMarshall C, Kosciuk MC, Nagele RG. Natural IgG autoantibodies are abundant and ubiquitous in human sera, and their number is influenced by age, gender, and disease. PLOS ONE 2013; 8:e60726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Czömpöly T, Olasz K, Simon D et al A possible new bridge between innate and adaptive immunity: are the anti‐mitochondrial citrate synthase autoantibodies components of the natural antibody network? Mol Immunol 2006; 43:1761–8. [DOI] [PubMed] [Google Scholar]
- 4. Grönwall C, Silverman GJ. Natural IgM: beneficial antibodies protect against atherosclerosis in SLE? Nature Rev Rheum 2016; 12:442–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mũoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheum 2010; 6:280–9. [DOI] [PubMed] [Google Scholar]
- 6. McMahon M, Skaggs B. Autoimmunity: do IgM antibodies protect against atherosclerosis in SLE? Nat Rev Rheum 2016; 12:442–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jiang C, Zhao ML, Scearce RM, Diaz M. Activation‐induced deaminase‐deficient MRL/LPR mice secrete high levels of protective antibodies against lupus nephritis. Arthritis Rheum 2011; 63:1086–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Witte T. IgM antibodies against dsDNA in SLE. Clin Rev Allergy Immunol 2008; 34:345–7. [DOI] [PubMed] [Google Scholar]
- 9. Tarlinton DM, McLean M, Nossal GJV. B1 and B2 cells differ in their potential to switch immunoglobulin isotype. Eur J Immunol 1995; 25:3388–93. [DOI] [PubMed] [Google Scholar]
- 10. Panda S, Zhang J, Tan NS, Ho B, Ding JL. Natural IgG antibodies provide innate protection against ficolin‐opsonized bacteria. EMBO J 2013; 32:2905–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palma J, Tokarz‐Deptuła B, Deptuła J, Deptuła W. Natural antibodies – facts known and unknown. Cent Eur J Immunol 2018; 43:466–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Panda S, Ding JL. Natural antibodies bridge innate and adaptive immunity. J Immunol 2015; 194:13–20. [DOI] [PubMed] [Google Scholar]
- 13. Sokolove J, Bromberg R, Deane KD et al Autoantibody epitope spreading in the pre‐clinical phase predicts progression to rheumatoid arthritis. PLOS ONE 2012; 7:e35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pisetsky DS. Antinuclear antibodies in rheumatic disease: a proposal for a function‐based classification. Scand J Immunol 2012; 76:223–8. [DOI] [PubMed] [Google Scholar]
- 15. Bournia VK, Vlachoyiannopoulos PG. Subgroups of Sjögren syndrome patients according to serological profiles. J Autoimmun 2012; 39:15–26. [DOI] [PubMed] [Google Scholar]
- 16. Czömpöly T, Olasz K, Nyárády Z, Simon D, Bovári J, Németh P. Detailed analyses of antibodies recognizing mitochondrial antigens suggest similar or identical mechanism for production of natural antibodies and natural autoantibodies. Autoimmun Rev 2008; 7:463–7. [DOI] [PubMed] [Google Scholar]
- 17. Simon D, Gilicze O, Farkas N, Najbauer J. Correlation of natural autoantibodies and cardiovascular disease‐related anti‐bacterial antibodies in pericardial fluid of cardiac surgery patients. Clin Exp Immunol 2018; 193:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zorn E, See SB. Polyreactive natural antibodies in transplantation. Curr Opin Organ Transplant 2017; 22:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simon D, Czömpöly T, Berki T et al Naturally occurring and disease‐associated auto‐antibodies against topoisomerase I: a fine epitope mapping study in systemic sclerosis and systemic lupus erythematosus. Int Immunol 2009; 21:415–22. [DOI] [PubMed] [Google Scholar]
- 20. Böröcz K, Csizmadia Z, Markovics Á et al Development of a robust and standardized immunoserological assay for detection of anti‐measles IgG antibodies in human sera. J Immunol Methods 2019; 464:1–8. [DOI] [PubMed] [Google Scholar]
- 21. Böröcz K, Csizmadia Z, Markovics Á et al Application of a fast and cost‐effective ‘three‐in‐one’ MMR ELISA as a tool for surveying anti‐MMR humoral immunity: the Hungarian experience. Epidemiol Infect 2020; 148:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agócs MM, Markowitz LE, Straub I, Dömök I. The 1988–1989 measles epidemic in Hungary: assessment of vaccine failure. Int J Epidemiol 1992; 21:1007–13. [DOI] [PubMed] [Google Scholar]
- 23. Lengyel G, Ánosi N, Marossy A, Mátyus M, Bosnyákovits T, Orosz L. Az európai és a magyarországi kanyaróhelyzet összefoglalása és tanulságai [Summary and lessons from the measles situation in Europe and Hungary]. Orv Hetil 2019; 160:767–73. [DOI] [PubMed] [Google Scholar]
- 24. International Notes Measles – Hungary [internet] [cited 2020 May 3]. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/00001472.htm.
- 25. Lengyel G, Marossy A, Ánosi N et al Screening of more than 2000 Hungarian healthcare workers’ anti‐measles antibody level: results and possible population‐level consequences. Epidemiol Infect 2019; 147:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. MMWR Weekly . MMWR Publications. [cited 2020 May 20]. Available at: https://www.cdc.gov/mmwr/publications/index.html.
- 27. Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol 2010; 10:778–86. [DOI] [PubMed] [Google Scholar]
- 28. Beinart D, Ren D, Pi C et al Immunization enhances the natural antibody repertoire. Exp Clin Sci 2017; 16:1018–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gudmundsdó S, Gudmundsdó BK. Specific and natural antibody response of cod juveniles vaccinated against Vibrio anguillarum . Fish Shellfish Immunol 2009; 26:619–24. [DOI] [PubMed] [Google Scholar]
- 30. Bachi ALL, Suguri VM, Ramos LR, Mariano M, Vaisberg M, Lopes JD. Increased production of autoantibodies and specific antibodies in response to influenza virus vaccination in physically active older individuals. Results Immunol 2013; 3:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Whyte J, Earnshaw WC, Champouxt JJ et al Detection of anti‐topoisomerase I antibodies using a full length human topoisomerase I recombinant protein purified from a baculovirus expression system. Clin Exp Immunol 1995; 100:214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stojanov L, Satoh M, Dooley MA, Kuwana M, Jennette JC, Reeves WH. Autoantibodies to topoisomerase I in a patient with systemic lupus erythematosus without features of scleroderma. Lupus. 1995; 4:314–7. [DOI] [PubMed] [Google Scholar]
- 33. Grönwall C, Vas J, Silverman GJ. Protective roles of natural IgM antibodies. Front Immunol 2012; 3:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shoenfeld Y, Toubi E. Protective autoantibodies: Role in homeostasis, clinical importance, and therapeutic potential. Arthritis Rheum 2005; 52:2599–606. [DOI] [PubMed] [Google Scholar]
- 35. Hahn BH. Antibodies to DNA. N Engl J Med 1998; 338:1359–68. [DOI] [PubMed] [Google Scholar]
- 36. Nguyen TTT, Baumgarth N. Natural IgM and the development of B cell‐mediated autoimmune diseases. Crit Rev Immunol 2016; 36:163–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McMahon M, Skaggs B. Autoimmunity: Do IgM antibodies protect against atherosclerosis in SLE? Nat Rev Rheumatol 2016; 12:442–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maddur MS, Lacroix‐Desmazes S, Dimitrov JD, Kazatchkine MD, Bayry J, Kaveri SV. Natural antibodies: from first‐line defense against pathogens to perpetual immune homeostasis. Clin Rev Allergy Immunol 2020; 58:213–28. [DOI] [PubMed] [Google Scholar]
- 39. Notley CA, Brown MA, Wright GP, Ehrenstein MR. Natural IgM is required for suppression of inflammatory arthritis by apoptotic cells. J Immunol 2011; 186:4967–72. [DOI] [PubMed] [Google Scholar]
- 40. Maddur MS, Kaveri SV, Bayry J. Circulating normal IgG as stimulator of regulatory T cells: lessons from intravenous immunoglobulin. Trends Immunol 2017; 38:789–92. [DOI] [PubMed] [Google Scholar]
- 41. Kohler H, Bayry J, Nicoletti A, Kaveri SV. Natural autoantibodies as tools to predict the outcome of immune response? Scand J Immunol 2003; 58:285–9. [DOI] [PubMed] [Google Scholar]
- 42. Kaveri SV, Silverman GJ, Bayry J. Natural IgM in immune equilibrium and harnessing their therapeutic potential. J Immunol 2012; 188:939–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author [Simon D], upon reasonable request.


