Anti‐Ro60 is one of the most important and common autoantibodies found in systemic autoimmune diseases. Using a range of traditional diagnostic laboratory assays and innovative mass spectrometry, authors dissect low from high expression of anti‐Ro60 and the clinical implications of this. Low expression of anti‐Ro60 was found to be a serologically and molecularly distinct entity that has important clinical relevance.

Keywords: antibodies, anti‐Ro60, autoantibodies, diagnostics, laboratory medicine
Summary
Anti‐Ro60 is one of the most common and clinically important serum autoantibodies that has a number of diagnostic and predictive capabilities. Most diagnostic laboratories report this simply as a qualitative positive/negative result. The objective of this study was to examine the clinical and serological relevance of a novel subset of anti‐Ro60 in patients who display low levels of anti‐Ro60 (anti‐Ro60low). We retrospectively identified anti‐Ro60 sera during a 12‐month period at a major immunopathology diagnostic laboratory in Australia. These all were anti‐Ro60‐precipitin‐positive on the diagnostic gold standard counter‐immuno‐electrophoresis (CIEP). Lineblot immunoassay was used to stratify patients into either anti‐Ro60low or anti‐Ro60high subsets. We compared the medical and laboratory parameters associated with each group. Enzyme‐linked immunosorbent assay (ELISA) and mass spectrometry techniques were used to analyse the serological and molecular basis behind the two subsets. Anti‐Ro60low patients displayed less serological activity than anti‐Ro60high patients with less intermolecular spreading, hypergammaglobulinaemia and less tendency to undergo anti‐Ro60 isotype‐switching than anti‐Ro60high patients. Mass spectrometric typing of the anti‐Ro60low subset showed restricted variable heavy chain subfamily usage and amino acid point mutations. This subset also displayed clinical relevance, being present in a number of patients with systemic autoimmune rheumatic diseases (SARD). We identify a novel anti‐Ro60low patient subset that is distinct from anti‐Ro60high patients serologically and molecularly. It is not clear whether they arise from common or separate origins; however, they probably have different developmental pathways to account for the stark difference in immunological maturity. We hence demonstrate significance to anti‐Ro60low and justify accurate detection in the diagnostic laboratory.
Introduction
One of the most common anti‐extractable nuclear antigens (ENA) in clinical practice and the diagnostic laboratory, anti‐Ro60/SSA is an autoantibody that has considerable clinical significance [1]. Historically, it has been classed with its closely related anti‐Ro52/tripartite motif containing 21 (TRIM21) as part of the anti‐SSA entity; however, this autoantibody has its own unique clinicopathological characteristics [2, 3].
Ro60 is a 60‐kDa intracellular antigen that forms a ribonucleoprotein complex with non‐coding RNA proteins known as Y RNAs. This complex is involved in RNA processing and regulation [4]. Consistent with its role in molecular quality control, Ro60 knock‐out mice develop autoantibodies, glomerulonephritis and photosensitivity, analogous to a systemic lupus erythematosus (SLE)‐like syndrome [5]. Ro60 and anti‐Ro60 is, hence, purported to be vital in the immunopathogenesis of systemic autoimmunity [6].
It is important that anti‐Ro60 is detected accurately, as it has several significant clinical uses. It is used diagnostically for a number of systemic autoimmune rheumatic diseases (SARD) such SLE and Sjögren’s syndrome (SS) [7]. Clinically, anti‐Ro60 associates with features such as skin lesions, photosensitivity, interstitial lung disease and haematological cytopaenias [1, 8]. In mothers who are anti‐Ro60‐positive, there is a small risk of giving birth to a baby with neonatal lupus erythematosus (NLE), which may entail devastating cardiac manifestations or cutaneous lesions [9]. Thus, anti‐Ro60 is a clinically important antibody that has a number of diagnostic and predictive uses.
There is no ‘gold‐standard’ method for detection of anti‐Ro60 in the diagnostic laboratory; however, gel‐based methods such as counter‐immuno‐electrophoresis (CIEP) and Ouchterlony double immunodiffusion assays offer better specificities than other methods [10]. Line immunoblots (LIB) are thought to be sensitive assays to detect various autoantibodies. In fact, one study found that they can be too sensitive and create ‘false positives’ [11]. Hence, often more than one method is required in the laboratory to confidently detect anti‐ENA antibodies [12, 13].
Our laboratory uses a screening in‐house CIEP assay using the human myeloid leukaemia cell line K562 (in‐house) and rabbit thymus extract (RTE) (Bacto Laboratories Pty Ltd, Sydney, Australia) as antigen sources. We then use a combination of CIEP and a commercial LIB (EuroImmun AG, Lübeck, Germany) to confirm the identity of the CIEP antibody precipitin. We have identified a number of patient serum samples which have a negative/low anti‐Ro60 on LIB but positive on CIEP. The clinical significance and molecular profiles of these anti‐Ro60 antibodies is unknown. Therefore, the aim of this study was to determine if CIEP‐positive LIB‐low anti‐Ro60 (anti‐Ro60low) differs from CIEP‐positive LIB‐high (anti‐Ro60high) patients from clinical, serological and molecular points of view. We establish that these groups appear mutually exclusive and exhibit distinct pathological and clinical characteristics. Therefore, we establish significance to low or equivocal anti‐Ro60 results as quantified by LIB assays.
Methods
Patients
The study was conducted at a major public pathology service in South Australia, Australia that has an approximate population of 1·7 million people. All patients who had an anti‐Ro60 detected on CIEP in our diagnostic laboratory was retrospectively identified in a 12‐month period between 2018 and 2019. All patients had a reflex LIB performed to confirm the presence of anti‐Ro60 and other specificities. Banked patient serum was stored at −20°C. Patient medical and pathology records were accessed to ascertain demographic and medical data. Healthy control (HC) serum was obtained with informed consent and confirmed to be CIEP‐negative and, hence, lacking anti‐Ro60. Ethical approval for the study was provided by the Southern Adelaide Clinical Research Ethics Committee (no. 39.034).
Diagnostic assays
CIEP was performed as our laboratory’s anti‐ENA screen using K562 and RTE antigen sources. Briefly, 20 μl of patient serum or characterized control was subjected to CIEP in wells of 1% agarose gel in Electra B1 buffer (Helena Laboratories, Victoria, Australia). Samples were run at 4°C at 100 V. After washing, the gels were stained with Coomassie blue, destained (distilled water, methanol and acetic acid) and dried. Anti‐Ro60 precipitates out on K562 cell extract only and anti‐Ro60 was confirmed by observing a precipitated line of identity in control serum monospecific for anti‐Ro60 (Supporting information, Fig. S1).
Figure 1.
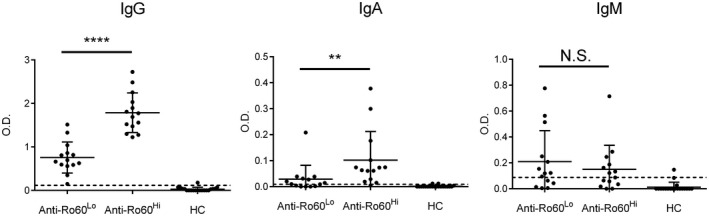
Anti‐Ro60 isotype levels in anti‐Ro60low, anti‐Ro60high and healthy control (HC) patients. **P < 0·01; ****P < 0·0001; n.s. = not significant.
LIBs were performed using Euroline ANA profile 3 test kit (EuroImmun) as per the manufacturer’s instructions. Purified Ro60 antigen was purified from affinity chromatography using bovine spleen and thymus and used in the LIB [14]. Signal intensities of each anti‐Ro60 immunoglobulin (Ig)G band was semi‐quantitatively analysed using EUROLineScan (EuroImmun) software. The ENA LIB recommends interpretation of the result based on different classes of signal intensity: 0–5 (negative), 6–10 (borderline), 11–25 (weak positive), 26–50 (positive) and > 50 (strongly positive) [14]. For the purpose of our study, low levels of anti‐Ro60 (anti‐Ro60low) were defined as signal intensity ≤ 25 and high levels of anti‐Ro60 (anti‐Ro60high) as signal intensity ≥ 26.
Enzyme‐linked immunosorbent assay (ELISA)
Indirect ELISAs were performed on flat‐bottomed 96‐well plates (Nunc MaxiSorp; ThermoFisher Scientific, Loughborough, UK) by coating native bovine Ro60 antigen (Arotec Diagnostics Ltd, Lower Hutt, New Zealand) at a concentration of 1 μg/ml. Briefly, plates were blocked using Block Ace buffer (Bio‐Rad, Hercules, CA, USA) for 1 h at 37°C. Following washing with phosphate‐buffered saline (PBS) and 0·05% Tween (Sigma‐Aldrich, St Louis, MO, USA), wells were incubated with serum sample 1 : 100 in PBS/0·1% bovine serum albumin for 1 h at 37°C, washed, incubated with goat anti‐human IgG, IgA (Sigma‐Aldrich) or IgM (Invitrogen, Carlsbad, CA, USA) conjugated with alkaline phosphatase 1 : 1000 in PBS/1% skimmed milk for 1 h at 37°C, washed, then developed using phosphatase substrate 5 mg/ml (Sigma‐Aldrich). Plates were read at 30 min at 405 nm using a microplate reader (680XR; Bio‐Rad). No‐antigen controls were performed on all samples. Samples were performed in technical duplicates.
Mass spectrometry
Mass spectrometry (MS) was performed on anti‐Ro60 isolated from sera. To minimize background noise, only sera confirmed to be monospecific for anti‐Ro60 (single line of identity with confirmed monospecific anti‐Ro60 sample on K562, no RTE precipitin and monospecific anti‐Ro60 on LIB) were used. Briefly, sera were concentrated ×5 using 10 K spin columns (Merck). At least 30 μl of concentrated sera was run on CIEP (see Diagnostic assays) using K562 extract. Precipitins were washed thoroughly, excised, boiled at 95°C for 5 min with standard sample buffer [0·5 M Tris‐HCl pH 6.8, 40% glycerol, 8% sodium dodecyl sulphate (SDS), 400 mM dithiothreitol (DTT), 0·04% bromophenol blue] and ran non‐reduced on Mini‐Protean TGX stain‐free sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS‐PAGE) gels (Bio‐Rad) for 25 min at 300 V. IgG bands (150 kDa) were then excised and digested with Pierce trypsin protease (ThermoFisher Scientific). Digested peptides were analysed using a Thermo Scientific Orbitrap Exploris 480 mass spectrometer coupled to an Ultimate 3000 UHPLC (Dionex, Sunnyvale, CA, USA). Anti‐Ro60 peptides sequencing was analysed by Peaks studio version X plus software (Bioinformatics Solution Inc., Waterloo, ON, Canada) using International ImMunoGeneTics (IMGT) databases. Parameters for database searches, data refinement and IgV gene family assignments have been described previously [15].
Statistics
Simple descriptive statistics were performed. Student’s t‐test or the Mann‐Whitney U test were used for continuous variables, depending on the distribution of data, and χ2/Fisher’s exact test for binary variables. spss version 19 statistical software package (SPSS, Chicago, IL, USA) was used to perform analyses. Unless stated otherwise, the alpha value was set at 0·05. Graphs are displayed with data points and mean. Error bars represent standard deviations (SD).
Results
Patients and demographics
A total of 534 CIEPs with anti‐Ro60 present were performed during a 12‐month period in 2018–19; each had a corresponding LIB performed. We removed 55 episodes which were duplicate tests on the same patient, leaving 479 patients for analysis. This consisted of 71 anti‐Ro60low (14·8%) and 408 anti‐Ro60high patients (85·2%) (Supporting information, Fig. S2). Anti‐Ro60low comprised 10 LIB‐negative (intensity 0–5), 5 LIB borderline (intensity 6–10) and 56 LIB weakly positive (intensity 11–25) results. Of the anti‐Ro60high group, 92 patients were LIB‐positive (intensity 26–50) and 316 were LIB strongly positive (intensity > 50).
Figure 2.
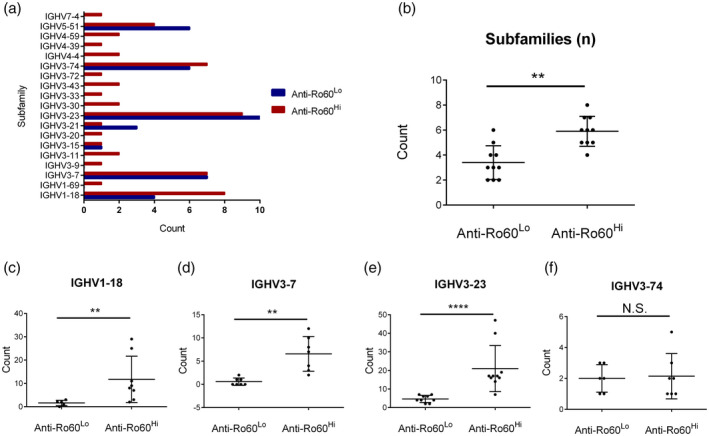
Mass spectrometric profiling of anti‐Ro60 in anti‐Ro60low and anti‐Ro60high subsets. **P < 0·01, ****P < 0·0001; n.s. = not significant.
The mean ages ± SD (53·0 ± 17·9 versus 55·4 ± 18·4 years) and proportion of females (88·7 versus 84·3%) of the anti‐Ro60low and anti‐Ro60high groups, respectively, did not differ significantly (P = 0·295 and P = 0·471, respectively).
Anti‐Ro60low is less immunologically active than anti‐Ro60high sera
Interestingly, we found that anti‐Ro60low sera were less likely to have other anti‐ENA detected on the LIB than anti‐Ro60high (mean = 0·49 ± 0·65 other anti‐ENA per patient versus 1·25 ± 0·87; P < 0·001). Hence, anti‐Ro60low tended to be more monospecific for anti‐Ro60 and co‐exist less with anti‐Ro52/TRIM21 and anti‐La antibodies than anti‐Ro60high (Table 1). ANA is screened on the HEp‐2000 substrate (Immunoconcepts, Sacramento, CA, USA) at a dilution of 1 : 80. As expected, the anti‐Ro60high group had more instances of positive ANA and the SSA pattern on the HEp‐2000 substrate (Table 1), which forms a distinct nucleolar pattern in transfected HEp‐2 cells when anti‐Ro60 is present [16, 17].
Table 1.
Associated pathology results of anti‐Ro60 patients stratified according to low and high expression
| Anti‐Ro60low (%) | Anti‐Ro60high (%) | P‐value | |
|---|---|---|---|
| Monospecific anti‐Ro60 | 42/71 (59·2) | 80/408 (19·6) | < 0·001 |
| Co‐existing anti‐Ro52 | 24/71 (33·8) | 309/408 (75·7) | < 0·001 |
| Co‐existing anti‐La | 1/71 (1·4) | 122/408 (29·9) | < 0·001 |
| Positive ANA | 64/71 (90·1) | 402/408 (98·5) | 0·001 |
| SSA pattern (HEp‐2000) | 34/64 (45·7) | 299/402 (74·4) | 0·001 |
| High CRP | 9/46 (19·6) | 46/257 (17·9) | 0·787 |
| High ESR | 16/50 (32·0) | 89/239 (37·2) | 0·522 |
| Low C3 | 2/30 (6·7) | 23/180 (12·8) | 0·542 |
| Low C4 | 1/30 (3·3) | 16/180 (8·9) | 0·477 |
| Positive RF | 5/18 (2·8) | 107/170 (62·9) | 0·004 |
| Positive anti‐dsDNA | 3/55 (5·4) | 44/264 (16·7) | 0·033 |
| Hypergammaglobulinaemia | 0/13 (0·0) | 28/60 (46·7) | 0·001 |
| Lymphopaenia | 16/57 (28·1) | 181/315 (57·5) | < 0·001 |
Bolded values represent significant P values < 0·0042 (Bonferroni correction).
ANA = anti‐nuclear antibody; CRP = C‐reactive protein; ESR = erythrocyte sedimentation rate; RF = rheumatoid factor; dsDNA = double‐stranded DNA.
Next, we extracted other immunological and biochemical parameters from the same episode of anti‐Ro60 testing and compared across the two groups (Table 1). Using Bonferroni’s correction for multiple comparisons, we adjusted the α value to 0·0042. In line with the above results, anti‐Ro60low patients were less immunologically active, as determined by lower proportions of patients with positive rheumatoid factor (RF), hypergammaglobulinaemia and lymphopaenia (Table 1). Other parameters, such as elevated C‐reactive protein (CRP) and erythrocyte sedimentation rate (ESR), did not show any significant differences across the groups (Table 1).
ELISA was used to quantify the amount of anti‐Ro60 IgG, IgA and IgM in sera from anti‐Ro60low and anti‐Ro60high patients. CIEP‐negative healthy controls (HCs) were used to determine the cut‐off optical density (OD) and background, defined as 2 SDs above the geometric mean of HCs [18]. Fourteen anti‐Ro60low (13 females), 14 anti‐Ro60high (11 females) and 20 HC sera (12 females) were selected for ELISA. As expected, anti‐Ro60high patients had significantly higher levels of anti‐Ro60 IgG than the anti‐Ro60low group and, in line with a more mature response, higher anti‐Ro60 IgA was seen in the former group (Fig. 1). No differences in anti‐Ro60 IgM were appreciated (Fig. 1). Including an additional 14 anti‐Ro60 samples (three anti‐Ro60low and 11 anti‐Ro60high) for IgG analysis, there was a very good correlation between LIB band intensity and ELISA OD for pooled samples (n = 42, densitometry range = 2–146) (Pearson’s r = 0·79, P < 0·001). These data indicate that the anti‐Ro60low patients demonstrate evidence of a restricted immunological response compared to their anti‐Ro60high counterparts.
Clinical relevance of anti‐Ro60 subsets
Accompanying request form clinical notes as well as medical records were perused for the reasons for ordering the test, as well as eventual diagnosis of the 71 anti‐Ro60low patients. Clinical notes and/or medical records were available for 60 patients (85·4%). Thirty‐five patients (58·3%) were seeing rheumatology or immunology departments and had a SARD such as SLE or SS (Table 2). Thirteen patients (21·7%) had another localized autoimmune disease (e.g. uveitis), had manifestations of a possible autoimmune disease (e.g. Raynaud’s phenomenon) with no definite diagnosis or had abnormal previous blood tests which indicated possible autoimmunity (e.g. positive ANA). Five patients (8·3%) had polyarthralgias/polyarthritis and three patients (5·0%) had haematological manifestations as their reasons for ordering an anti‐ENA. Four patients (6·7%) had other miscellaneous diagnoses or reasons.
Table 2.
Clinical diagnoses and reasons for requesting the anti‐Ro60 test
| Anti‐Ro60low (n, %) | Anti‐Ro60high (n, %) | P‐value | |
|---|---|---|---|
| Systemic autoimmune rheumatic disease | 35 (58·3) | 53 (62·4) | 0·730 |
| Sjögren’s syndrome | 7 (11·7) | 19 (22·4) | 0·125 |
| SLE | 15 (25·0) | 20 (23·5) | 0·846 |
| Rheumatoid arthritis | 4 (6·7) | 3 (3·5) | 0·448 |
| Mixed CTD | 4 (6·7) | 1 (1·2) | 0·160 |
| Undifferentiated CTD | 4 (6·7) | 7 (8·2) | 0·763 |
| Systemic sclerosis | 1 (1·7) | 2 (2·4) | 1·000 |
| Neonatal lupus erythematosus | 0 (0) | 1 (1·2) | 1·000 |
| Autoimmune phenomena, e.g. Raynaud’s phenomenon | 11 (18·3) | 9 (10·6) | 0·224 |
| Previous abnormal investigations, e.g. raised ANA | 2 (3·3) | 3 (3·5) | 1·000 |
| Haematological manifestations e.g. neutropaenia | 3 (5·0) | 4 (4·7) | 0·692 |
| Polyarthralgias/arthritis | 5 (8·3) | 3 (3·5) | 0·276 |
| Miscellaneous conditions | 4 (6·7) | 13 (15·3) | 0·125 |
| Total | 60 | 85 |
SLE = systemic lupus erythematosus; CTD = connective tissue disease; ANA = anti‐nuclear antibody.
We then chose, at random, 100 anti‐Ro60high patients to compare the prevalence of these clinical parameters; clinical notes were available for 85 of these patients (85·0%). Surprisingly, we observed no difference throughout all clinical parameters including diagnoses (Table 2), indicating that the anti‐Ro60low group has the same clinical relevance as anti‐Ro60high patients.
Natural evolution of anti‐Ro60 subsets
To examine the natural evolution of the two anti‐Ro60 subsets, we examined historical data (available for the preceding 7 years) for 25 randomly selected patients from each group for (a) the acquisition or loss of other LIB antibodies and (b) change in densitometry intensity group. Because historical samples were not kept in the laboratory, we therefore could not examine for changes at the densitometry unit level by running old and new samples concurrently.
Both subsets had a comparable mean duration of follow‐up (Table 3). Consistent with our earlier data (see Table 1), the anti‐Ro60high subset had more LIB specificities than anti‐Ro60low which emerged at the end of the follow‐up period. The vast majority of patients had no change in their anti‐Ro60 intensity, regardless of the anti‐Ro60 subset (Table 3). These data reinforce that anti‐Ro60low patients follow a similar immunological evolution as anti‐Ro60high patients, are a distinct subset and do not ‘evolve’ into the anti‐Ro60high group.
Table 3.
Longitudinal follow‐up of anti‐Ro60low and anti‐Ro60high groups
| Anti‐Ro60low (n = 25) | Anti‐Ro60high (n = 25) | P‐value | |
|---|---|---|---|
| Mean follow‐up period (years ± SD) | 2·15 ± 1·20 | 2·68 ± 1·69 | 0·207 |
| Additional LIB specificities at baseline (mean ± SD) | 0·64 ± 1·04 | 1·08 ± 1·19 | 0·170 |
| Loss/no change/gain of additional LIB specificities by end of follow‐up (n) | 3/21/1 | 4/14/6 | 0·307 |
| Additional LIB specificities by end of follow‐up (mean ± SD) | 0·52 ± 0·92* | 1·24 ± 1·01* | 0·011 |
| Decreased/same/increased anti‐Ro60 intensity group by end of follow‐up (n) | 4/18/3 | 8/16/1 | 0·294 |
P > 0·05 compared to baseline within the same anti‐Ro60 subset.
Bold value represents significant P < 0·05.
SD = standard deviation; LIB = line immunoblot assay.
Molecular basis of each anti‐Ro60 subset
We wondered about the molecular determinant of the anti‐Ro60low subset and how it compared to its counterpart anti‐Ro60high subset. To shed light on this, we subjected patients from each subset to MS proteomic typing to identify immunoglobulin heavy chain variable (IGHV) region usage and amino acid (AA) mutational analyses in their anti‐Ro60.
Ten randomly selected patients from each subset were analysed. Consistent with established data [19], most patients displayed the canonical anti‐Ro60 subfamilies: IGHV1‐18 (12 of 20), IGHV3‐7 (14 of 20), IGHV3‐23 (19 of 20) and IGHV3‐74 (13 of 20), among other subfamilies (Fig. 2a). In general, anti‐Ro60low patients had fewer anti‐Ro60 subfamily usage compared to anti‐Ro60high (Fig. 2b). AA mutational analyses also revealed that in relation to germline transcripts there were fewer AA substitutions, and hence less somatic hypermutation (SHM) in the anti‐Ro60low subset in IGHV1‐18 (Fig. 2c), IGHV3‐7 (Fig. 2d) and IGHV3‐23 (Fig. 2e), but not IGHV3‐74 (Fig. 2f).
Of note, we observed a conserved serine to arginine (S→R) AA substitution in the complementarity‐determining region (CDR) 1 of IGHV3‐23 in 10 of 10 anti‐Ro60high and nine of nine anti‐Ro60low patients. Similarly, there was a conserved serine to phenylalanine (S→F) AA substitution immediately adjacent and upstream to the CDR2 region of IGHV3‐74 in six of seven anti‐Ro60high patients and five of five anti‐Ro60low patients. As CDR regions of variable chains are known to be hypervariable, it is not surprising that these regions demonstrate a high degree of mutations.
Together, these data provide molecular evidence that the anti‐Ro60 response in anti‐Ro60low patients represents both a less mature and immunologically restricted response and a distinct subset from the classical anti‐Ro60 response in anti‐Ro60high patients. Despite a reduced frequency of mutations in the anti‐Ro60 IGHV subfamilies in anti‐Ro60low patients, there is a remarkable degree of conservation between mutations around CDRs in anti‐Ro60 IGHVs from anti‐Ro60high patients. This suggests common mechanisms of SHM between the two subsets.
Discussion
In this study, we describe a novel subset of anti‐Ro60 IgG (anti‐Ro60low) that was quantitatively low on LIB and ELISA platforms. Compared to anti‐Ro60high patients, these patients have a more restricted serological and molecular profile, undergo less isotype‐switching of their anti‐Ro60 responses, but are as clinically relevant as patients with a high anti‐Ro60 level (anti‐Ro60high).
Interestingly, the anti‐Ro60low subset were more likely to be restricted in terms of displaying other antibody specificities and were no more likely to evolve into other specificities than the anti‐Ro60high group (Tables 1 and 3). This is in contrast to our earlier observations, that found a temporal emergence of anti‐Ro52/TRIM21 antibodies after mice were immunized with recombinant Ro60 protein and vice versa [20].
It is entirely possible that the origins of anti‐Ro60 in the anti‐Ro60low group is a result of immunological epiphenomena and has alternate origins from the anti‐Ro60high group. This begs the question concerning the drivers for the two distinct subsets: is it a result of immunological convergence or divergence? Although it is tempting to think that perhaps the anti‐Ro60low subset evolves (diverges) into anti‐Ro60high, we do not find any evidence for this in our longitudinal analysis (Table 3). As we did not observe any significant change in autoantibody profile in anti‐Ro60 patients longitudinally, this study also supports the minimization of unnecessary serial autoantibody testing in patients unless there has been a change in the clinical picture [21, 22]. This stability in the overall anti‐Ro60 response is consistent with our previous observations where, despite a relentless anti‐Ro60 turnover in patients, there is little change in anti‐Ro60 affinity over time [23]. Of course, this area is limited by the short follow‐up duration and the retrospective review of results, as we could not test historical and present samples in parallel. As such, interassay variation may have accounted for some of the apparent changes in anti‐Ro60 densitometry readings.
One explanation for the differing subsets of anti‐Ro60 may lie with the origins of these autoantibodies. With limited evidence of SHM in the anti‐Ro60low subset (Fig. 2), it is conceivable that this subset originated from the T cell‐independent extrafollicular pathway where it is now recognized that SHM may occur, but not to the extent of the follicular pathway [24, 25]. Following ‘selection’ in the extrafollicular pathway, a plasmablast may be promoted to a long‐lived plasma cell[25], which may explain the persistent anti‐Ro60low response in our group. This theory is also supported by the demonstration of lower isotype‐switching in the anti‐Ro60low subset (Fig. 1), as naive B cells in the extrafollicular pathway undergo considerably less isotype‐switching than the germinal centre pathway [26]. Curiously, there was no difference serologically of the Ro60‐IgM response, which may reflect similar rates of developing plasmablast/plasma cell turnover in the two groups. Indeed, the process of selecting which developing B cell will enter the follicular or extrafollicular response depends upon a fine balance of checkpoints, transcriptional regulation, B cell receptor affinity and chemokine signalling [25, 27].
Furthermore, the higher frequency of AA mutations in the anti‐Ro60high subset over anti‐Ro60low (Fig. 2) may also be consistent with the opposing origins, as the follicular pathway of antibody production has a more extensive SHM process. However, we cannot discount the diverging molecular profiles based on the acquisition of mutations in the B cell receptor clones, adding diversity to the B cell repertoire [28]. This may also reflect the constant turnover of anti‐Ro60 clones, which adds to the molecular diversity seen in our mass spectrometric analyses [23].
We also established that the anti‐Ro60low subset was found in as many patients with a SARD as those in the anti‐Ro60high group. This implies that the finding of the anti‐Ro60low in these patients are clinically relevant. However, we did not assess if those patients found in the latter represented more severe forms of disease, or if specific clinical features (such as rashes) differed in frequency across the two subsets, as our study was not designed and is underpowered to detect such differences. Furthermore, documentation for each patient was unreliable, and clinical manifestations could not be confidently established. Intuitively, it makes sense that the anti‐Ro60high subset may display more severe disease, given that the anti‐Ro60high subset displays evidence of greater serological activity (Table 1). For example, patients with primary SS and greater RF activity has been associated with greater risk of hypocomplementaemia, hypergammaglobulinaemia and greater disease severity [29, 30]. However, it is not uncommon for patients to have serological manifestations of SS and be completely asymptomatic, which would complicate association of disease activity with each subset [31].
Another clinical use for stratifying anti‐Ro60 responses is in identifying female patients at higher risk of giving birth to babies affected by NLE. Higher maternal anti‐Ro60 titres (equivalent to our anti‐Ro60high subset) confer a higher risk of the development of neonatal congenital heart block [32, 33], and together with other autoantibodies such as anti‐La [34] can offer important predictive information. Fundamentally, risk may also be related to the epitopes recognized by these antibodies [35]. It is possible that differential epitope targets have specific disease correlations, but this was not explored in this study and remains a possibility for future research. This phenomenon has been seen in the related anti‐Ro52/TRIM21 antibody, where specific systemic autoimmune diseases demonstrate preferential anti‐Ro52/TRIM21 against certain peptide targets [36].
Our study also highlights important implications for the diagnostic immunopathology laboratory. We give clinical significance to the low‐level of anti‐Ro60 detection, which may be missed on LIB due to loss of conformational epitopes [10], and lend support to reporting weak positive anti‐Ro60 results. In the Australasia region, nearly half (47·2%) of the diagnostic immunology laboratories enrolled into the Royal College of Pathologists of Australasia (RCPA) Quality Assurance Program use the LIB assay as part of anti‐ENA characterization [37], highlighting an important limitation to this assay to a large proportion of laboratories. Furthermore, it emphasizes the need to have at least a second method for confirming indeterminate or borderline anti‐ENA results on one method, consistent with international recommendations [38]. Our laboratory uses a third method (HEp‐2000 substrate), which increases our sensitivity of picking up anti‐Ro60; nearly half of the anti‐Ro60low patients also display the SSA nuclear pattern on HEp‐2000 substrate (Table 1).
Conversely, it is unlikely that the detection of anti‐Ro60low, by itself, will be diagnostic for a single SARD, as a diagnosis relies upon the integration of clinical presentation, examination findings and other auxiliary pathology testing. Indeed, our finding of the same amount of SARD clinical diagnoses in the anti‐Ro60low subset as the anti‐Ro60high subset could be coincidental, as other clinical and serological parameters (including other autoantibodies) may have secured the diagnosis. In order to investigate this, it would be useful to repeat this analysis at another laboratory that does not report low/weak anti‐Ro60 results and/or retest the sera using our laboratory’s algorithm (Supporting information, Fig. S2).
Our study has some important limitations. First, as a retrospective study, we are limited by the clinical information available to us in medical records. Diagnoses may not be accurate, and in some cases medical records were not accessible at all. Furthermore, our longitudinal study of anti‐Ro60 subsets was not performed prospectively; hence, samples could not be run side‐by‐side to minimize interassay variation and therefore accurate characterization of changes in the anti‐Ro60 densitometry. Our average follow‐up period (2–2·5 years) may also be insensitive to detect changes in anti‐Ro60 profiles. Prospective studies with accurate clinical correlations are required to further investigate and qualify our findings. However, our outcome of largely no change in anti‐Ro60 antibody intensity is congruent with a study that found virtually no change in anti‐Ro60 status in a group of SLE patients [22].
Future studies to dissect the immunological basis of the anti‐Ro60 subsets will facilitate our understanding of the immunopathogenesis of anti‐Ro60‐associated autoimmune diseases. It is very likely that similar subsets of other significant autoantibodies exist and can be broken down by the clinical and molecular phenotypes. This has similarly been seen with anti‐La, where we previously identified precipitating and non‐precipitating anti‐La subsets, each with distinct serological phenotypes [39]. Thus, recognizing distinct subsets of antibodies will also help to prognosticate patients and may identify those who are at higher risk of more severe immunological complications.
Disclosure
T. P. G. has received grants from Immuno Concepts Inc.
Author contributions
A. Y. S. L. contributed to concept design, experiments, data analysis and drafted the manuscript. D. B. contributed to concept design, data collection and experiments. L. B., C. L., A. K., L. G., N. H., Y. H. N. and O. S. contributed to data collection and experiments. T. P. G. contributed to concept design and data analysis. J. J. W. contributed to concept design, experiments and data analysis. All authors reviewed and revised the manuscript for intellectual content.
Supporting information
Fig S1. Example counterimmuno‐electrophoresis (CIEP) gel. Precipitin lines of identity (arrow) in 3 patients form with the monospecific anti‐Ro60 control. K562 antigen extract is used as a source of Ro60 antigen. A second band (*) is seen in Patient 3 of different specificity (anti‐La).
Fig S2. Workflow and division of samples in the 12‐month study period. CIEP, counterimmuno‐electrophoresis, LIB, line immunoblot assay.
Acknowledgements
The authors wish to thank the technical expertise of Drs Tim Chataway and Alex Colella at the Flinders University Proteomics Facility and Dr Louise Wienholt from the Royal College of Pathologists of Australasia. The authors also acknowledge and extend gratitude to the patients involved in this study. This work was supported by an Australian National Health and Medical Research Council Early Career Fellowship grant (J. J. W.). The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Contributor Information
T. P. Gordon, Email: t.gordon@flinders.edu.au, Email: jing.wang@flinders.edu.au.
J. J. Wang, Email: jing.wang@flinders.edu.au.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Yoshimi R, Ueda A, Ozato K, Ishigatsubo Y. Clinical and pathological roles of Ro/SSA autoantibody system. Clin Dev Immunol 2012; 2012:606195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee AYS. A review of the role and clinical utility of anti‐Ro52/TRIM21 in systemic autoimmunity. Rheumatol Int 2017; 37:1323–33. [DOI] [PubMed] [Google Scholar]
- 3. Robbins A, Hentzien M, Toquet S et al Diagnostic utility of separate anti‐Ro60 and anti‐Ro52/TRIM21 antibody detection in autoimmune diseases. Front Immunol 2019; 10:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boccitto M, Wolin SL. Ro60 and Y RNAs: structure, functions, and roles in autoimmunity. Crit Rev Biochem Mol Biol 2019; 54:133–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xue D, Shi H, Smith JD et al A lupus‐like syndrome develops in mice lacking the Ro 60‐kDa protein, a major lupus autoantigen. Proc Natl Acad Sci USA 2003; 100:7503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scofield RH. Genetic knock out of 60 kD Ro (or SSA), a common lupus autoantigen, induces lupus. Trends Immunol 2004; 25:1–3. [DOI] [PubMed] [Google Scholar]
- 7. Menendez A, Gomez J, Escanlar E, Caminal‐Montero L, Mozo L. Clinical associations of anti‐SSA/Ro60 and anti‐Ro52/TRIM21 antibodies: Diagnostic utility of their separate detection. Autoimmunity 2013; 46:32–9. [DOI] [PubMed] [Google Scholar]
- 8. Menéndez A, Gómez J, Caminal‐Montero L, Díaz‐López JB, Cabezas‐Rodríguez I, Mozo L. Common and specific associations of anti‐SSA/Ro60 and anti‐Ro52/TRIM21 antibodies in systemic lupus erythematosus. Sci World J 2013; 2013:832789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee LA. Cutaneous lupus in infancy and childhood. Lupus 2010; 19:1112–7. [DOI] [PubMed] [Google Scholar]
- 10. Phan TG, Wong RCW, Adelstein S. Autoantibodies to extractable nuclear antigens: making detection and interpretation more meaningful. Clin Diagn Lab Immunol 2002; 9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bundell C, Rojana‐Udomsart A, Mastaglia F, Hollingsworth P, McLean‐Tooke A. Diagnostic performance of a commercial immunoblot assay for myositis antibody testing. Pathology 2016; 48:363–6. [DOI] [PubMed] [Google Scholar]
- 12. Bizzaro N, Tozzoli R, Tonutti E et al Variability between methods to determine ANA, anti‐dsDNA and anti‐ENA autoantibodies: a collaborative study with the biomedical industry for the Italian Society of Laboratory Medicine (SIMeL) Study Group on the Diagnosis of Autoimmune Diseases. J Immunol Methods 1998; 219:99–107. [DOI] [PubMed] [Google Scholar]
- 13. Wienholt L, Adelstein S. Modification of thresholds for detection of extractable nuclear antigens (ENA) using a commercial ELISA. Pathology 2012; 44:S99–S100. [Google Scholar]
- 14. EuroImmun Medizinische Labordiagnostika AG . EUROLINE ANA Profile 3 plus DFS70 (IgG) test instruction. Lübeck, Germany: EuroImmun AG, 2019. [Google Scholar]
- 15. Wang JJ, Colella AD, Beroukas D, Chataway TK, Gordon TP. Precipitating anti‐dsDNA peptide repertoires in lupus. Clin Exp Immunol 2018; 194:273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keech CL, Howarth S, Coates T, Rischmueller M, McCluskey J, Gordon TP. Rapid and sensitive detection of anti‐Ro (SS‐A) antibodies by indirect immunofluorescence of 60kDa Ro HEp‐2 transfectants. Pathology 1996; 28:54–7. [DOI] [PubMed] [Google Scholar]
- 17. Lee AYS, Beroukas D, Roberts‐Thomson PJ. Utility of the HEp‐2000 antinuclear antibody substrate. Ann Rheum Dis 2019; 79:e67. [DOI] [PubMed] [Google Scholar]
- 18. Sharma B, Jain R. Right choice of a method for determination of cut‐off values: a statistical tool for a diagnostic test. Asian J Med Sci 2014; 5:30–4. [Google Scholar]
- 19. Wang JJ, Al Kindi MA, Colella AD et al IgV peptide mapping of native Ro60 autoantibody proteomes in primary Sjögren’s syndrome reveals molecular markers of Ro/La diversification. Clin Immunol 2016; 173:57–63. [DOI] [PubMed] [Google Scholar]
- 20. Keech CL, Gordon TP, McCluskey J. The immune response to 52‐kDa Ro and 60‐kDa Ro is linked in experimental autoimmunity. J Immunol 1996; 157:3694–9. [PubMed] [Google Scholar]
- 21. Lee AYS, Hudspeth AR, Adelstein S. The concordance of serial ANA tests in an Australian tertiary hospital pathology laboratory. Pathology 2016; 48:597–601. [DOI] [PubMed] [Google Scholar]
- 22. Raissi TC, Hewson C, Pope JE. Repeat testing of antibodies and complements in systemic lupus erythematosus: when is it enough? J Rheumatol 2018; 45:827–34. [DOI] [PubMed] [Google Scholar]
- 23. Lindop R, Arentz G, Bastian I et al Long‐term Ro60 humoral autoimmunity in primary Sjogren’s syndrome is maintained by rapid clonal turnover. Clin Immunol 2013; 148:27–34. [DOI] [PubMed] [Google Scholar]
- 24. Herlands RA, Christensen SR, Sweet RA, Hershberg U, Shlomchik MJ. T cell‐independent and Toll‐like receptor‐dependent antigen‐driven activation of autoreactive B cells. Immunity 2008; 29:249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. MacLennan ICM, Toellner K‐M, Cunningham AF et al Extrafollicular antibody responses. Immunol Rev 2003; 194:8–18. [DOI] [PubMed] [Google Scholar]
- 26. Liu YJ, Malisan F, de Bouteiller O et al Within germinal centers, isotype switching of immunoglobulin genes occurs after the onset of somatic mutation. Immunity 1996; 4:241–50. [DOI] [PubMed] [Google Scholar]
- 27. Allen CDC, Okada T, Cyster JG. Germinal‐center organization and cellular dynamics. Immunity 2007; 27:190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hershberg U, Prak ETL. The analysis of clonal expansions in normal and autoimmune B cell repertoires. Phil Trans R Soc Lond B Biol Sci 2015; 370:20140239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. García‐Carrasco M, Mendoza‐Pinto C, Jiménez‐Hernández C, Jiménez‐Hernández M, Nava‐Zavala A, Riebeling C. Serologic features of primary Sjögren’s syndrome: clinical and prognostic correlation. Int J Clin Rheumtol 2012; 7:651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maślińska M, Mańczak M, Kwiatkowska B. Usefulness of rheumatoid factor as an immunological and prognostic marker in PSS patients. Clin Rheumatol 2019; 38:1301–7. [DOI] [PubMed] [Google Scholar]
- 31. Jonsson R, Theander E, Sjöström B, Brokstad K, Henriksson G. Autoantibodies present before symptom onset in primary Sjögren syndrome. JAMA 2013; 310:1854–5. [DOI] [PubMed] [Google Scholar]
- 32. Anami A, Fukushima K, Takasaki Y et al The predictive value of anti‐SS‐A antibodies titration in pregnant women with fetal congenital heart block. Mod Rheumatol 2013; 23:653–8. [DOI] [PubMed] [Google Scholar]
- 33. Friedman DM, Kim MY, Copel JA et al Utility of cardiac monitoring in fetuses at risk for congenital heart block: the PR Interval and Dexamethasone Evaluation (PRIDE) prospective study. Circulation 2008; 117:485–93. [DOI] [PubMed] [Google Scholar]
- 34. Jaeggi E, Laskin C, Hamilton R, Kingdom J, Silverman E. The importance of the level of maternal anti‐Ro/SSA antibodies as a prognostic marker of the development of cardiac neonatal lupus erythematosus a prospective study of 186 antibody‐exposed fetuses and infants. J Am Coll Cardiol 2010; 55:2778–84. [DOI] [PubMed] [Google Scholar]
- 35. Silverman ED, Buyon J, Laxer RM et al Autoantibody response to the Ro/La particle may predict outcome in neonatal lupus erythematosus. Clin Exp Immunol 1995; 100:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gkoutzourelas A, Liaskos C, Mytilinaiou MG et al Anti‐Ro60 seropositivity determines anti‐Ro52 epitope mapping in patients with systemic sclerosis. Front Immunol 2018; 9:2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Royal College of Pathologists of Australasia . Rheumatic Disease Serology, Cycle 21, Survey 7. Surry Hills, Australia: Royal College of Pathologists of Australasia Quality Assurance Programs (RCPAQAP), 2019. [Google Scholar]
- 38. Agmon‐Levin N, Damoiseaux J, Kallenberg C et al International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti‐nuclear antibodies. Ann Rheum Dis 2014;73:17‐23. [DOI] [PubMed] [Google Scholar]
- 39. Gordon T, Mavrangelos C, McCluskey J. Restricted epitope recognition by precipitin‐negative anti‐La/SS‐B‐positive sera. Arthritis Rheum 1992; 35:663–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Example counterimmuno‐electrophoresis (CIEP) gel. Precipitin lines of identity (arrow) in 3 patients form with the monospecific anti‐Ro60 control. K562 antigen extract is used as a source of Ro60 antigen. A second band (*) is seen in Patient 3 of different specificity (anti‐La).
Fig S2. Workflow and division of samples in the 12‐month study period. CIEP, counterimmuno‐electrophoresis, LIB, line immunoblot assay.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


