In this study, IgG‐autoantibodies associated with autoimmune hepatitis and primary biliary cholangitis (PBC) were found in a considerable proportion of well‐characterized patients diagnosed with primary Sjögren’s syndrome and systemic lupus erythematosus (SLE) but without known liver involvement or autoimmune liver disease (AILD). PBC‐associated autoantibodies have previously not been reported in a substantial percentage of cases with SLE. However, the association between autoantibody findings and AILD was poor as no significant difference in liver enzymes was observed between AILD‐associated autoantibody negative and positive patients.

Keywords: autoantibodies, autoimmune hepatitis, primary biliary cholangitis, Sjögren’s syndrome, systemic lupus erythematosus
Summary
Knowledge of concomitant autoimmune liver diseases (AILD) is more detailed in primary Sjögren’s syndrome (pSS) compared to systemic lupus erythematosus (SLE). Herein, the prevalence of autoantibodies associated with autoimmune hepatitis (AIH) and primary biliary cholangitis (PBC) was investigated in stored sera from patients with SLE (n = 280) and pSS (n = 114). Antibodies against mitochondria (AMA), liver–kidney microsomal (LKM) antigen, smooth muscle (SMA) and anti‐nuclear antibodies (ANA) were analysed with immunofluorescence microscopy. In addition, AILD‐associated autoantibodies were tested with immunoblot. Prior to sampling, eight SLE (2·9%) and three pSS (2·6%) cases were diagnosed with AILD. Among SLE‐cases without known AILD (n = 272), 26 (9·6%) had PBC‐associated autoantibodies, 15 (5·5%) AIH‐associated autoantibodies (excluding ANA) and one serological overlap. Most subjects with PBC‐associated autoantibodies had liver enzymes within reference limits (22 of 27, 81%) or mild laboratory cholestasis (two of 27, 7·4%), while one fulfilled the diagnostic PBC‐criteria. AMA‐M2 detected by immunoblot was the most common PBC‐associated autoantibody in SLE (20 of 272, 7·4%). The prevalence of SMA (4·4%) was comparable with a healthy reference population, but associated with elevated liver enzymes in four of 12 (25%), none meeting AIH‐criteria. The patient with combined AIH/PBC‐serology had liver enzymes within reference limits. Among pSS cases without known AILD (n = 111), nine (8·1%) had PBC‐associated, 12 (10·8%) AIH‐associated autoantibodies and two overlapped. PBC‐associated autoantibodies were found as frequently in SLE as in pSS but were, with few exceptions, not associated with laboratory signs of liver disease. Overall, AILD‐associated autoantibodies were predominantly detected by immunoblot and no significant difference in liver enzymes was found between AILD autoantibody‐negative and ‐positive patients.
Introduction
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease which potentially can involve any organ system and predominantly affects women [1]. Serologically, SLE is characterized by an array of autoantibodies typically including anti‐nuclear antibodies (ANA) [2, 3, 4, 5, 6]. Although the liver is not considered as a main target organ in SLE, elevated liver enzymes in serum during the course of SLE have been demonstrated in 19–60% [7, 8]. Signs of liver affection in SLE may have a wide range of causes, including drug‐induced injury, steatosis, autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), lupus hepatitis and viral hepatitis [9, 10, 11]. However, while knowledge of PBC in relation to primary Sjögren’s syndrome (pSS) is well recognized, the co‐existence of autoimmune liver diseases (AILD) in SLE has been less studied [8, 12, 13, 14]. Over time, a subset of approximately 20–25% of SLE cases develop secondary SS at Swedish academic centres [15].
Lupus hepatitis is a liver dysfunction with a reported frequency of 3–6% in SLE patients [7, 16, 17, 18]. It is a diagnosis of exclusion, distinct from AIH, and is characterized by fluctuations in alanine transaminase (ALT) [19]. It has a subclinical course with a low rate of progression to cirrhosis [20]. Histologically, lupus hepatitis has a variable presentation, but usually inflammation of the lobules is seen with a paucity of lymphocytes [7]. Fatty infiltration and mild portal infiltration may also be found [16].
AIH is a chronic progressive hepatitis with a female preponderance [21]. AIH can be classified into two subtypes; type 1 and type 2 [22, 23, 24]. These subtypes differ in their autoantibody profiles and age of onset. Type 1 patients are typically middle‐aged or older with presence of ANA, smooth muscle antibodies (SMA) and/or anti‐soluble liver antigen/liver pancreas antigen antibodies (anti‐SLA/LP) [25]. Type 2 patients are younger, usually children and teenagers, and characterized by the presence of anti‐liver kidney microsome‐1 antibodies (anti‐LKM‐1) and/or anti‐liver cytosol‐1 antibodies (anti‐LC‐1) [22]. AIH can present acutely with jaundice and rapid progression to cirrhosis. However, more frequently it presents as an indolent disease with slightly elevated liver enzymes [22]. The histological picture ranges from normal to patchy or diffuse necrosis [26, 27, 28].
PBC is characterized by inflammatory destruction of intrahepatic bile ducts [29, 30]. The underlying pathogenic mechanisms are incompletely understood, but genetic and environmental factors as well as epigenetic mechanisms are assumed to be of importance [31]. Large as well as extrahepatic bile ducts are usually spared. In line with AIH, there is a strong female preponderance with debut in middle age.
PBC is typically associated with the presence of anti‐mitochondrial antibodies (AMA, type M2) which, in addition to persistently elevated serum alkaline phosphatase (ALP) and liver histology consistent with PBC, constitutes one of the three diagnostic criteria. However, several subtypes of ANA, such as anti‐speckled 100‐kDa (Sp100), anti‐promyelocytic leukaemia protein (PML) and anti‐glycoprotein 210‐kDa (gp210), are also specific markers [32]. PBC can initially be subclinical with elevated cholestatic liver enzymes and over decades progress to cholestatic disease with portal hypertension [29].
The aims of this study were first, to evaluate the prevalence of autoantibodies associated with AILD among two cohorts of well‐characterized Swedish cases of SLE (± secondary SS) and pSS; and secondly, to investigate whether these findings were associated with laboratory signs of liver affection.
Patients and methods
Patients
Serum samples from 280 patients diagnosed with SLE who fulfilled the 1982 ACR criteria (ACR‐82) and/or the Fries’ diagnostic principle [33, 34] were included. 277 of 280 (99%) patients met the Fries’ diagnostic principle and 235 of 280 (84%) fulfilled ACR‐82 (mean number of fulfilled ACR criteria was 4·8, range = 3–9). All patients took part in the prospective follow‐up programme KLURING (a Swedish acronym for Clinical LUpus Register In North‐eastern Gothia) at the Department of Rheumatology, Linköping University Hospital, as previously described [35]. Blood sampling was performed consecutively at inclusion to the register between 2008 and 2018 [36]. In addition, sera from 114 patients diagnosed with pSS meeting the American–European consensus criteria were included [37]. These sera were collected between 2006 and 2014. All serum samples were stored at −70°C until analysis. Description of the study populations are further detailed in Tables 1 and 2.
Table 1.
Characteristics of the included patients with SLE
| Background variables | Total, n = 280 |
|---|---|
| Females, n (%) | 241 (86·1) |
| Age at blood sampling, mean years (range, years) | 48·5 (18–82) |
| SLE duration at sampling, mean years (range, years) | 9·5 (0–45) |
| Caucasian ethnicity, n (%) | 250 (89·3) |
| Ever smoker (former or current), n (%) | 119 (42·5) |
| Disease variables and treatments | |
| Secondary Sjögren’s syndrome (defined by classification † ), n (%) | 61 (21·8) |
| Anti‐phospholipid syndrome (defined by classification ‡ ), n (%) | 55 (19·6) |
| Previously identified autoimmune liver disease, n (%) | 8 (2·8) |
| Autoimmune hepatitis, n (%) | 4 (1·4) |
| Primary biliary cholangitis, n (%) | 4 (1·4) |
| Anti‐hypertensives, n (%) | 137 (48·9) |
| Statins, n (%) | 50 (17·9) |
| Diabetes mellitus, n (%) | 15 (5·4) |
| Patients meeting ≥ 4 ACR‐82 criteria, n (%) | 235 (83·9) |
| Number of fulfilled ACR‐82 criteria, mean (range) | 4·8 (3–9) |
| Clinical phenotypes (ACR‐82 defined), n (%) | |
| (1) Malar rash | 109 (38·9) |
| (2) Discoid rash | 42 (15·0) |
| (3) Photosensitivity | 144 (51·4) |
| (4) Oral ulcers | 34 (12·1) |
| (5) Arthritis | 215 (76·8) |
| (6) Serositis | 106 (37·9) |
| Pleuritis | 104 (37·1) |
| Pericarditis | 98 (35·0) |
| (7) Renal disorder | 78 (27·9) |
| (8) Neurological disorder | 14 (5·0) |
| Seizures | 12 (4·3) |
| Psychosis | 3 (1·1) |
| (9) Haematological disorder | 166 (59·3) |
| Haemolytic anaemia | 14 (5·0) |
| Leukocytopenia | 84 (30·0) |
| Lymphopenia | 105 (37·5) |
| Thrombocytopenia | 30 (10·7) |
| (10) Immunological disorder | 145 (51·8) |
| Anti‐dsDNA antibody (anti‐dsDNA) | 133 (47·5) |
| Anti‐Smith antibody (anti‐Sm) | 18 (6·4) |
| (11) Anti‐nuclear antibody (IF‐ANA)* | 277 (98·9) |
Table 2.
Characteristics of the included patients with pSS
| Background variables | Total, n = 114 |
|---|---|
| Females, n (%) | 109 (95·6) |
| Age at blood sampling, mean years (range, years) | 61·8 (24–82) |
| Caucasian ethnicity, n (%) | 111 (97·4) |
| Disease variables | |
| Patients meeting American–European consensus criteria † , n (%) | 114 (100) |
| Anti‐phospholipid syndrome (defined by classification ‡ ), n (%) | 2 (1·8) |
| Established autoimmune liver disease, n (%) | 3 (2·6) |
| Autoimmune hepatitis, n (%) | 3 (2·6) |
| Primary biliary cholangitis, n (%) | 0 (0) |
| Extra‐glandular manifestations, n (%) | 51 (44·7) |
Autoantibody analyses and definitions
ANA were detected by immunofluorescence (IF) microscopy on HEp2‐cells (IF‐ANA), as previously described [6]. With a screening dilution of 1 : 800, the cut‐off level for a positive ANA corresponds to the 95th percentile among healthy blood donors (n = 752; 50% females, 50% males) [38].
Immunoglobulin (Ig)G‐antibodies against smooth muscle (SMA), mitochondria (AMA) and liver–kidney–microsomal antigen (anti‐LKM) were detected by IF‐microscopy on slides with sections of fixed rat liver, kidney and stomach (NOVA Lite®, Inova, San Diego, CA, USA). For detection of SMA, the slides were incubated for 30 min with serum diluted 1 : 400 in phosphate‐buffered saline (PBS). This serum dilution corresponds to the 95th percentile of positive SMA in healthy blood donor sera (n = 200). After washing and 30 min incubation with fluorescein isothiocyanate (FITC)‐conjugated γ‐chain‐specific anti‐human IgG the slides were mounted in fluorescent mounting medium (Dako, Glostrup, Denmark) and evaluated by indirect IF‐microscopy (Olympus BX43; Olympus, Tokyo, Japan) at ×10/0·30 magnification.
A commercial immunoblot‐test [EUROLINE; ‘Autoimmune Liver Diseases’ (IgG); Euroimmun, Lübeck, Germany] was used according to the manufacturer’s instruction for detection of multiple AILD‐associated autoantibodies; AMA‐M2 (i.e. E2 subunit of pyruvate dehydrogenase), M2‐3E [BPO, i.e. a recombinant fusion protein of the E2‐subunits of the three main M2‐antigens branched‐chain 2‐oxoacid dehydrogenase, pyruvate dehydrogenase complex (PDC) and 2‐oxoglutarate dehydrogenase], sp100, PML, gp210, LKM‐1, LC‐1, SLA/LP and Ro52/SSA. The samples were analysed with EUROBlotmaster (Euroimmun AG, Euroimmun Lübeck, Germany). The manufacturer’s recommended cut‐offs were used for all specificities. Samples that were positive for AMA or anti‐LKM on immunoblot, but negative at screening with IF‐microscopy on rat tissue in serum dilution 1 : 400, were reanalysed on rat tissue in serum dilution 1 : 40 (corresponding to 20 IU AMA; NIBSC 67/183). All autoantibody tests were performed at the accredited laboratory of Clinical Immunology, Linköping, Sweden.
PBC‐associated autoantibodies were defined as AMA type M2 (including BPO), antibodies against sp100, gp210, PML or PBC‐associated IF‐ANA‐patterns (nuclear lamins, AC‐12 and/or nuclear dots, AC‐6). AIH‐associated autoantibodies were defined as SMA, antibodies against SLA/LP, LKM‐1 or LC‐1. Isolated appearance of anti‐Ro52/SSA was not included as an AILD‐associated autoantibody due to its high prevalence in both SLE and pSS.
Review of medical records
Pathological liver enzyme values were defined as persistently elevated values above the upper limit of the reference interval during a 5‐year period before and/or at least 2 years after the time‐point of sampling. Pathological liver enzymes were categorized as cholestatic [i.e. elevated ALP ± elevated γ‐glutamyl transferase (GGT), aspartate aminotransferase (AST) and/or ALT] or hepatocellular (i.e. elevated ALT ± AST).
Routine laboratory measurements
Liver enzymes (ALP, ALT, AST and GGT) were analysed using the spectrophotometric technique according to routine methods at the accredited laboratory of Clinical Chemistry, University Hospital in Linköping (cobas c 701, cobas® 8000; Roche Diagnostics International Ltd, Rotkreuz, Switzerland).
Statistics
Fischer’s exact test was used in comparison of groups. P‐values < 0·05 were defined as significant. The data analysis for this paper was generated using the Real Statistics Resource Pack software release 6.8 [copyright (2013–2020) Charles Zaiontz. www.real-statistics.com].
Ethical approval
Oral and written informed consent was obtained from all participants. The study protocol was approved by the regional ethics review boards in Linköping, regarding SLE (M75‐08/2008) and in Uppsala, regarding pSS (2006/217/2).
Results
Patients with previously identified AILD
Eight cases with SLE (2·9%) had been diagnosed with AILD prior to our review of medical records. Four had AIH type 1 and another four had PBC (1·4%, respectively); all had serology characteristic of AILD (Table 3). Three of the PBC‐diagnoses and two of the AIH‐diagnoses were confirmed by histopathology. At the time‐point of sampling, all eight patients were IF‐ANA positive and all were on immunosuppressive therapy due to different SLE manifestations.
Table 3.
Characteristics of the eight SLE patients with previously identified autoimmune liver disease (AILD).
| Sex/year of birth | F‐1939 | F‐1946 | F‐1956 | M‐1981 | F‐1939 | F‐1945 | F‐1977 | F‐1988 |
|---|---|---|---|---|---|---|---|---|
| Year of SLE diagnosis | 2008 | 1999 | 1983 | 2002 | 2001 | 2008 | 2017 | 1994 |
| Year of sampling | 2008 | 2010 | 2008 | 2009 | 2009 | 2009 | 2017 | 2010 |
| Year of AILD diagnosis | PBC (2013) | PBC (2010) | PBC (2002) | PBC (2013) | AIH (2008) | AIH (2008) | AIH (2017) | AIH (1994) |
| Liver biopsy | No | Yes | Yes | Yes | No | No | Yes | Yes |
| Liver enzymes | Normal | Hepatocellular | Cholestatic | Cholestatic | Cholestatic | Normal | Normal | Normal |
| SMA | − | − | − | − | positive | − | positive | − |
| M2/M4 | − | positive | − | positive | − | − | − | − |
| LKM | − | − | − | − | − | − | − | − |
| AMA‐M2 | ++ | +++ | +++ | +++ | − | − | − | − |
| 3E (BPO) | + | +++ | +++ | +++ | − | − | − | − |
| Sp100 | − | +++ | − | − | − | − | − | − |
| gp210 | +++ | − | − | − | − | − | − | − |
| SLA/LP | − | + | − | − | +++ | − | ++ | − |
| Ro52/SSA | + | +++ | − | +++ | + | +++ | − | +++ |
| IF‐ANA | AC‐4 | AC‐4 | AC‐3 | AC‐1+4 | AC‐4 | AC‐1 | AC‐1 | AC‐1+4 |
SLE = systemic lupus erythematosus; AILD = autoimmune liver disease; SMA = smooth muscle antibodies; LKM = liver kidney microsomal antigen; AMA = anti‐mitochondrial antibodies; SLA/LP = soluble liver antigen/liver pancreas antigen; ANA = anti‐nuclear antibodies; PBC = primary biliary cholangitis; AIH = autoimmune hepatitis.
Among the pSS cases, three were diagnosed with AIH type 1 (2·6%) and confirmed by histopathology. Only one of them had AIH‐associated autoantibodies (i.e. anti‐SLA/LP and SMA) (Table 4). At the time‐point of sampling, all three were IF‐ANA‐positive and all were on immunosuppressive therapy due to extra‐glandular manifestations of pSS.
Table 4.
Characteristics of the three pSS patients with previously identified autoimmune liver disease (AILD).
| Sex/year of birth | F‐1939 | F‐1957 | F‐1978 |
|---|---|---|---|
| Year of pSS diagnosis | 2002 | 2012 | 2012 |
| Year of sampling | 2014 | 2014 | 2014 |
| Year of AILD diagnosis | AIH (2013) | AIH (2017) | AIH (2017) |
| Liver biopsy | Yes | Yes | Yes |
| Liver enzymes | Hepatocellular | Normal | Cholestatic |
| SMA | − | (+) | − |
| M2/M4 | − | − | − |
| LKM | − | − | − |
| AMA‐M2 | − | − | − |
| 3E (BPO) | − | − | − |
| Sp100 | − | − | − |
| gp210 | − | − | − |
| SLA/LP | − | +++ | − |
| Ro52/SSA | +++ | +++ | +++ |
| IF‐ANA (pattern) | AC‐1+4 | AC‐1+8 | AC‐5 |
F = female; M = male; − = negative; +++ = strongly positive; (+) = weakly positive; pSS = primary Sjögren’s syndrome; AILD = autoimmune liver disease; SMA = smooth muscle antibodies; LKM = liver kidney microsomal antigen; AMA = anti‐mitochondrial antibodies; SLA/LP = soluble liver antigen/liver pancreas antigen; ANA = anti‐nuclear antibodies; AIH = autoimmune hepatitis.
SLE
Of the remaining cases with SLE (n = 272), AILD‐associated autoantibodies were detected in 42 (15%). PBC‐associated autoantibodies were most frequent and detected in 26 (9·6%) cases, whereas AIH‐associated autoantibodies were found in 15 (5·5%) (Fig. 1). In addition, one patient had serological overlap with both PBC‐ and AIH‐associated autoantibodies (Sp100, LC‐1 and SLA/LP). In patients with PBC‐associated autoantibodies, including the one with serological overlap, laboratory signs of cholestasis were found in two (7·4%). One of these was weakly positive for AMA‐M2, meeting the diagnostic criteria for PBC, whereas the other one was only IF‐ANA‐positive with multiple nuclear dots pattern (AC‐6) and strongly positive for anti‐Sp100. Another two patients (7·4%) had elevated aminotransferases and displayed isolated AMA‐M2. One (3·7%) with isolated elevated GGT was AMA‐M2‐positive. The remaining 22 (81%) cases with PBC‐associated autoantibodies had liver enzymes within reference limits (Fig. 2). The most frequent PBC‐associated autoantibody was AMA‐M2, detected by immunoblot in sera from 20 of 27 patients (74·1%), but all were negative using the IF‐technique. Of the 20 AMA‐M2‐positive sera, six (30%) were also M2‐3E (BPO)‐positive; all were negative with IF‐microscopy in serum dilution 1 : 40. In addition, two patients without laboratory signs of cholestasis had AMA detected by IF, but were negative with immunoblot.
Fig. 1.
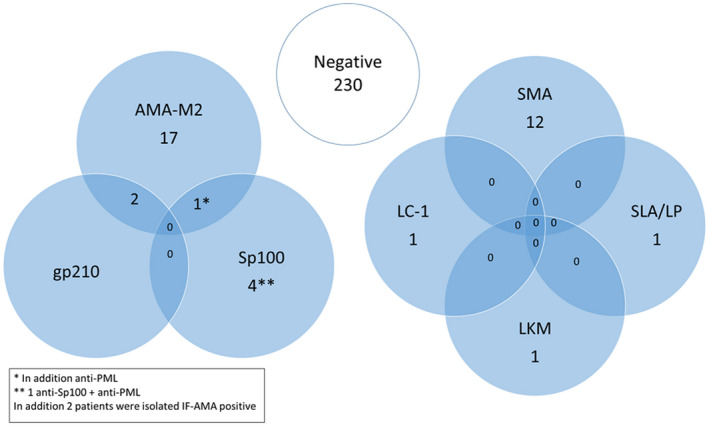
The left Venn diagram illustrates the number of individuals with primary biliary cholangitis (PBC)‐associated autoantibodies and the right diagram demonstrates the number of individuals with autoimmune liver disease (AIH)‐associated autoantibodies among the 272 systemic lupus erythematosus (SLE) patients.
Fig. 2.
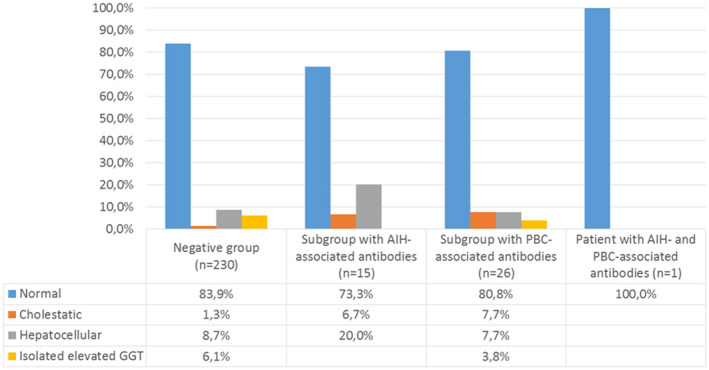
Distribution of different liver enzyme patterns in relation to autoimmune liver disease (AILD)‐associated autoantibody findings among the 272 included SLE cases without established AILD prior to sampling.
Among cases with PBC‐associated autoantibodies, two (7·4%) were diagnosed with secondary SS (both with liver enzymes within reference limits) compared to 60 (24·5%) with secondary SS among PBC‐autoantibody negative SLE cases, which was not significantly different.
Including the patient with overlap serology, AIH‐associated autoantibodies were found in 16 (5·9%) SLE cases, with SMA being the most frequent, detected in 12 cases (4·4%) (Fig. 1). Of the 12 SMA‐positive cases, hepatocellular liver values were slightly elevated in three (25%), while another (8·3%) had laboratory signs of cholestasis. The remaining eight (67%) with positive SMA displayed liver enzymes within reference limits. In addition, three patients had other isolated AIH‐associated autoantibodies. One of these was a 74‐year‐old woman with anti‐LKM detected by IF‐microscopy (rat tissue), but specificity for LKM‐1 could not be verified by immunoblot. The other two were females with isolated anti‐SLA/LP and anti‐LC‐1, respectively. None of them had laboratory signs of liver affection. Figure 2 illustrates the distribution of different liver enzyme patterns of the entire positive AILD‐associated autoantibody subgroup; the case with overlap serology is shown separately.
Among SLE cases with AIH‐associated antibodies, four (25%) were diagnosed with secondary Sjögren’s syndrome compared to 62 (24·2%) with secondary SS among AIH‐autoantibody‐negative SLE cases (not significant).
Overall among SLE cases, 230 of 272 (84·6%) patients tested negative for all liver‐associated autoantibodies (excluding anti‐Ro52/SSA or positive IF‐ANA without PBC‐associated staining patterns).
pSS
Among pSS cases without known AILD (n = 111), AILD‐associated autoantibodies were detected in 23 (20·7%) (Fig. 3). PBC‐associated autoantibodies were detected in nine (8·1%) patients, whereas AIH‐associated autoantibodies were found in 12 (10·8%). In addition, two (1·8%) patients had serological overlap with SMA and AMA‐M2, and both had normal liver enzymes.
Fig. 3.
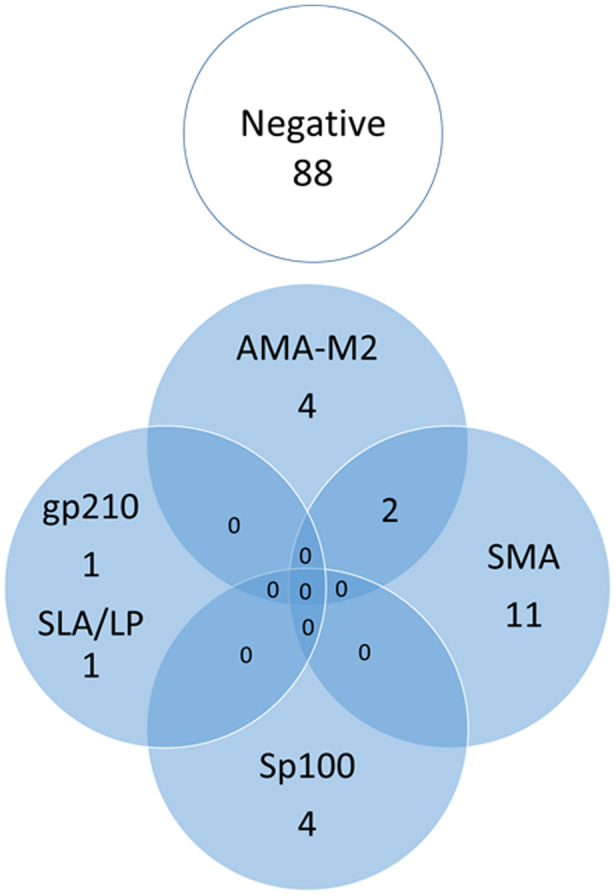
Venn diagram illustrating the number of individuals with autoimmune liver disease (AILD)‐associated autoantibodies among the 111 primary Sjögren’s syndrome (pSS) patients.
Liver enzymes within reference limits were also observed among the nine cases with PBC‐associated antibodies, except for one with elevated GGT and anti‐sp100. The most frequent PBC‐associated autoantibody was AMA‐M2 (n = 6); only one could be verified using IF‐technique, and three of the six were also positive for anti‐BPO. Another four cases were isolated positive regarding anti‐Sp100 and one regarding anti‐gp210 (Fig. 3).
AIH‐associated antibodies were found in 14 of 111 cases (12·6%), 13 of which had SMA and one had isolated positive anti‐SLA/LP. All these patients showed liver enzymes within reference limits.
ANA in SLE and pSS
In contrast to the time‐point of SLE onset, when 277 of 280 SLE cases were IF‐ANA positive, 202 (72·1%) tested positive in this cross‐sectional analysis and PBC‐associated IF‐ANA patterns were found in only two (0·7%). They both had multiple nuclear dots (AC‐6) and were also anti‐Sp100‐positive. No IF‐ANA with nuclear lamins pattern (AC‐12) was observed. Two patients (0·7%) showed centromere‐ANA (AC‐3), and one of these had an established diagnosis of PBC with characteristic AMA‐M2 positivity. The final patient with centromere‐ANA (AC‐3) was not diagnosed with AILD, had liver enzymes within reference limits, but tested anti‐SLA/LP‐positive.
IF‐ANA was positive in 84 (73·7%) of the 114 pSS cases. No PBC‐associated IF‐ANA‐pattern was observed. One patient (0·8%) without established AILD showed centromere‐ANA (AC‐3), but no PBC‐associated autoantibodies were detected with immunoblot technique.
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Discussion
The main objective of the present study was to evaluate the frequencies of AILD‐associated autoantibodies in cases with well‐characterized SLE. pSS was included as a comparator group, as the association of pSS with PBC is established [12, 13, 14]. In addition, pSS shares several clinical features with SLE as well as an enhanced activation of the type I interferon system [15]. Although AIH may be regarded as part of the autoimmune umbrella of SLE, we were surprised of the scarce literature on AILD‐associated autoantibodies, especially with emphasis on the multitude of different autoantibodies described and frequently detected in SLE [39].
SLE with concomitant AIH/lupus hepatitis has a reported prevalence of 2–4% [18]. To differentiate between AIH and lupus hepatitis remains challenging both clinically and by histopathology, and we cannot entirely exclude that any of the SLE/pSS cases diagnosed with AIH in fact had lupus hepatitis. A thorough review by Shizuma described only 34 observed cases of SLE with concomitant PBC in English and Japanese scientific literature [7]. However, elevated liver enzymes are commonly found during follow‐up of patients with SLE. As the participants herein took part of prospective follow‐up programmes at the rheumatology clinic, we had ethical permission to carefully review their medical records, including laboratories with a focus on liver disease. Thus, although the study was cross‐sectional with respect to autoantibody testing it could be regarded as longitudinal, as laboratory data were obtained from up to 5 years before AILD autoantibody testing and/or at least 2 years following testing.
Our findings indicate that autoantibodies associated with PBC were found to a similar extent in SLE and pSS, whereas the frequency of the AIH‐marker SMA was higher in pSS (9·9%). In fact, the prevalence of SMA among the SLE cases (4·4%) was comparable to the prevalence in sera from healthy blood donors (n = 200) which were used to set the 95th percentile cut‐off of the assay at our laboratory. Of particular interest was the somewhat high frequency of PBC‐associated autoantibodies in SLE (27 of 272 cases, 9·9%) which, to our knowledge, has not previously been reported. However, it is worth noting that most of these autoantibodies were detected by the immunoblot test, and that the findings of AMA‐M2 with immunoblot could not be verified by IF‐microscopy on sections of rat tissue as antigen source, not even when a cut‐off corresponding to 20 IU AMA was used. The M2‐antigen in the immunoblot is composed of the E2‐subunit of purified PDC, which is the most frequent target for AMA in PBC. The blot test also includes the recombinant protein construct BPO which, in addition to PDC‐E2, contains the E2‐subunits of two other mitochondrial enzymes recognized by AMA [40]. Even though the IF‐microscopy method is known to have limitations regarding both sensitivity and specificity, a question concerning specificity of the blot tests may also be raised. Similar issues were recently highlighted regarding myositis autoantibodies detected by immunoblot [41]. A problem with blotting‐based assays is that relevant epitopes can be lost due to conformational changes or by the use of recombinant antigens, and thereby autoantibodies may not recognize their target. In addition, irrelevant epitopes can be exposed and confer less specificity. Conversely, these tests have advantages, as they are technically easy to perform and simultaneously can detect multiple specificities which explain why they are currently widely used. Awareness of the advantages and the disadvantages with these techniques is of great importance for clinicians. However, with IF‐microscopy the laboratory must also be aware of the methodological limitations. Detection of AMA‐M2 was initially based on IF‐microscopy, but even though the microscopy techniques have evolved over time the sensitivity is still considered suboptimal [42]. The presence of secondary SS, which applied to approximately 20% of SLE cases, was not associated with a higher likelihood of being positive for PBC‐ or AIH‐associated antibodies. Even more important from a clinical perspective was the fact that presence of AILD‐associated autoantibodies correlated poorly with laboratory signs of liver disease in pSS, as well as in SLE. Thus, our data do not support screening with AILD‐associated autoantibodies in the absence of elevated liver enzymes. In addition, abnormal liver enzymes in our patients with SLE and pSS were much more often attributed to other conditions (or side‐effects of ongoing medication) than to AILD per se.
In several autoimmune conditions, the relation between onset of disease and epitope spreading with increasing numbers of specificities preceding clinical diagnosis has been subject to intense research [43, 44, 45]. From our data, it is not possible to judge whether the observed AILD autoantibodies in SLE/pSS cases without established AILD or elevated liver enzymes: (i) will precede clinical liver disease, (ii) only reflect a B cell hyperactivity with a general tendency to produce autoantibodies targeting a broad repertoire or (iii) simply reflect an epiphenomenon. The relatively long follow‐up of most included patients does not support the first alternative. Conversely, their autoimmune disease could later have affected organ systems (i.e. renal involvement), which required potent immunosuppressive therapy targeting B cells that may have hindered the development of AILD [46]. To scrutinize this further and evaluate potential pathogenic roles of these AILD‐associated autoantibodies, special focus should include strength of autoantibody binding and glycosylation analysis of Fc portions [47, 48, 49, 50].
The study has some limitations that should be mentioned. During the review of medical records ALT and AST were the most frequently assessed liver enzymes, whereas results of ALP and GGT were less frequently available (approximately 70%). In addition, as our data are entirely observational, liver enzymes were analysed more frequently in individuals who were on regular treatment with immunosuppressants, had a suspected liver disease, experienced side‐effects from therapies or had multiple co‐morbidities. Subsequently, cases with mild pSS/SLE who presented with elevated liver enzymes were not assessed as frequently. Nevertheless, the well‐characterized patient cohorts with longitudinal laboratory data sets, combined with reliable autoantibody analyses from the accredited Clinical Immunology Routine Laboratory in Linköping, constitute major strengths.
To conclude, this study demonstrates presence of AILD‐associated autoantibodies in a considerable proportion of well‐characterized patients with pSS and SLE without established or laboratory signs of liver disease. We are not aware that PBC‐associated autoantibodies have been reported in a substantial percentage of cases with SLE previously. However, the association between autoantibody findings and AILD was poor, as no significant difference in liver enzymes was observed between autoantibody‐negative and ‐positive cases.
Disclosures
The authors declare that they have no disclosures or competing interests related to this manuscript.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. A. A. and C.S. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study conception and design: A. A., S. K., C. D., C. S. Acquisition of data: A. A., R. H., P. E., L. W., S. K., C. D., C. S. Analysis and interpretation of data: A. A., R. H., S. K., C. D., C. S.
Acknowledgements
We thank Marianne Petersson for biobank administration, all the clinicians for their efforts and the staff at the Clinical Immunology laboratory in Linköping. This work was supported by grants from the Swedish Rheumatism Association, the Region Östergötland (ALF grants), the Swedish Society of Medicine, the King Gustaf V’s 80‐year Anniversary foundation and the King Gustaf V and Queen Victoria’s Freemasons foundation.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Bengtsson AA, Rönnblom L. Systemic lupus erythematosus: still a challenge for physicians. J Intern Med 2017; 281:52–64. [DOI] [PubMed] [Google Scholar]
- 2. Tzioufas AG. The clinical relevance of antibodies to ribosomal‐P common epitope in two targeted systemic lupus erythematosus populations: a large cohort of consecutive patients and patients with active central nervous system disease. Ann Rheum Dis 2000; 59:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sjӧwall C, Bentow C, Aure MA, Mahler M. Two‐Parametric immunological score development for assessing renal involvement and disease activity in systemic lupus erythematosus. J Immunol Res 2018; 2018:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mummert E, Fritzler MJ, Sjöwall C, Bentow C, Mahler M. The clinical utility of anti‐double‐stranded DNA antibodies and the challenges of their determination. J Immunol Methods 2018; 459:11–9. [DOI] [PubMed] [Google Scholar]
- 5. Mathsson L, Åhlin E, Sjöwall C, Skogh T, Rönnelid J. Cytokine induction by circulating immune complexes and signs of in‐vivo complement activation in systemic lupus erythematosus are associated with the occurrence of anti‐Sjögren’s syndrome A antibodies. Clin Exp Immunol 2007; 147:513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frodlund M, Wetterö J, Dahle C et al Longitudinal anti‐nuclear antibody (ANA) seroconversion in systemic lupus erythematosus: a prospective study of Swedish cases with recent‐onset disease. Clin Exp Immunol 2020; 199:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shizuma T. Clinical characteristics of concomitant systemic lupus erythematosus and primary biliary cirrhosis: a literature review. J Immunol Res 2015; 2015:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beisel C. Association of autoimmune hepatitis and systemic lupus erythematodes: a case series and review of the literature. World J Gastroenterol 2014; 20:12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choi MY, Fritzler MJ. Challenges and advances in SLE autoantibody detection and interpretation. Curr Treat Options Rheumatol 2019; 5:147–67. [Google Scholar]
- 10. Adiga A, Nugent K. Lupus hepatitis and autoimmune hepatitis (lupoid hepatitis). Am J Med Sci 2017; 353:329–35. [DOI] [PubMed] [Google Scholar]
- 11. Grover S, Rastogi A, Singh J, Rajbongshi A, Bihari C. Spectrum of histomorphologic findings in liver in patients with SLE: a review. Hepat Res Treat 2014; 2014:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsianos EV, Hoofnagle JH, Fox PC et al Sjögren’s syndrome in patients with primary biliary cirrhosis. Hepatology 1990; 11:730–4. [DOI] [PubMed] [Google Scholar]
- 13. Hatzis GS, Fragoulis GE, Karatzaferis A, Delladetsima I, Barbatis C. Prevalence and longterm course of primary biliary cirrhosis in primary Sjögren’s syndrome. J Rheumatol 2008; 35:2012–6. [PubMed] [Google Scholar]
- 14. Zeron PB, Retamozo S, Bové A, Kostov BA, Sisó A, Ramos‐Casals M. Diagnosis of liver involvement in primary Sjögren syndrome. J Clin Transl Hepatol 2013; 1:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ruacho G, Kvarnström M, Zickert A et al Sjögren syndrome in systemic lupus erythematosus: a subset characterized by a systemic inflammatory state. J Rheumatol 2020; 47:865–75. [DOI] [PubMed] [Google Scholar]
- 16. Piga M, Vacca A, Porru G, Cauli A, Mathieu A. Liver involvement in systemic lupus erythematosus: incidence, clinical course and outcome of lupus hepatitis. Clin Exp Rheumatol 2010;28: 504–10. [PubMed] [Google Scholar]
- 17. Shumyak S, Yang L‐J, Han S, Zhuang H, Reeves WH. ‘Lupoid hepatitis’ in SLE patients and mice with experimental lupus. Clin Immunol 2016; 172:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. González‐Regueiro JA, Cruz‐Contreras M, Merayo‐Chalico J et al Hepatic manifestations in systemic lupus erythematosus. Lupus 2020; 9:813–24. [DOI] [PubMed] [Google Scholar]
- 19. Kaw R, Gota C, Bennett A, Barnes D, Calabrese L. Lupus‐related hepatitis: complication of lupus or autoimmune association? Case report and review of the literature. Dig Dis Sci 2006; 51:813–8. [DOI] [PubMed] [Google Scholar]
- 20. Lai W‐T, Cho W‐H, Eng H‐L, Kuo M‐H, Huang F‐C. Overlap syndrome involving systemic lupus erythematosus and autoimmune hepatitis in children: a case report and literature review. Front Pediatr 2019; 31:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Michielsen P, Francque A, Vonghia C, Ramon D. Epidemiology and treatment of autoimmune hepatitis. Hepatic Med Evid Res 2012; 4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liberal R, Grant CR, Mieli‐Vergani G, Vergani D. Autoimmune hepatitis: A comprehensive review. J Autoimmun 2013; 41:126–39. [DOI] [PubMed] [Google Scholar]
- 23. Washington MK. Autoimmune liver disease: overlap and outliers. Mod Pathol 2007; 20(Suppl 1):15–30. [DOI] [PubMed] [Google Scholar]
- 24. Lohse AW. Diagnostic criteria for autoimmune hepatitis: scores and more. Dig Dis 2015; 33:47–52. [DOI] [PubMed] [Google Scholar]
- 25. Heneghan MA, Yeoman AD, Verma S, Smith AD, Longhi MS. Autoimmune hepatitis. Lancet 2013; 382:1433–44. [DOI] [PubMed] [Google Scholar]
- 26. Gurung A, Assis DN, McCarty TR, Mitchell KA, Boyer JL, Jain D. Histologic features of autoimmune hepatitis: a critical appraisal. Hum Pathol 2018; 82:51–60. [DOI] [PubMed] [Google Scholar]
- 27. Terracciano LM, Patzina RA, Lehmann FS et al A spectrum of histopathologic findings in autoimmune liver disease. Am J Clin Pathol 2000; 114:705–11. [DOI] [PubMed] [Google Scholar]
- 28. Makol A, Watt KD, Chowdhary VR. Autoimmune hepatitis: a review of current diagnosis and treatment. Hepat Res Treat 2011; 2011:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet 2015; 386:1565–75. [DOI] [PubMed] [Google Scholar]
- 30. Tanaka A, Leung PSC, Gershwin ME. Pathogen infections and primary biliary cholangitis: pathogen infections and primary biliary cholangitis. Clin Exp Immunol 2019; 195:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Joshita S, Umemura T, Tanaka E, Ota M. Genetics and epigenetics in the pathogenesis of primary biliary cholangitis. Clin J Gastroenterol 2018; 11:11–8. [DOI] [PubMed] [Google Scholar]
- 32. Invernizzi P, Floreani A, Carbone M et al Primary biliary cholangitis: advances in management and treatment of the disease. Dig Liver Dis 2017; 49:841–6. [DOI] [PubMed] [Google Scholar]
- 33. Fries JF, Holman HR. Systemic lupus erythematosus: a clinical analysis: Volume VI In: Smith LH., Jr ed. Major problems in internal medicine. Philadelphia: W.B. Saunders, 1975:8–20. [PubMed] [Google Scholar]
- 34. Tan EM, Cohen AS, Fries JF et al The 1982 revised criteria for the classification of systemic lupus erythematosus: revised criteria for SLE. Arthritis Rheum 1982; 25:1271–7. [DOI] [PubMed] [Google Scholar]
- 35. Ighe A, Dahlström Ö, Skogh T, Sjöwall C. Application of the 2012 Systemic Lupus International Collaborating Clinics classification criteria to patients in a regional Swedish systemic lupus erythematosus register. Arthritis Res Ther 2015; 17:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sjöwall C, Ernerudh J, Bengtsson AA, Sturfelt G, Skogh T. Reduced anti‐TNFα autoantibody levels coincide with flare in systemic lupus erythematosus. J Autoimmun 2004; 22:315–23. [DOI] [PubMed] [Google Scholar]
- 37. Vitali C, Bombardieri S, Jonsson R, et al Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann Rheum Dis 2002; 61:554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Agmon‐Levin N, Damoiseaux J, Kallenberg C et al International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti‐nuclear antibodies. Ann Rheum Dis 2014; 73:17–23. [DOI] [PubMed] [Google Scholar]
- 39. Yaniv G, Twig G, Shor DB‐A et al A volcanic explosion of autoantibodies in systemic lupus erythematosus: A diversity of 180 different antibodies found in SLE patients. Autoimmun Rev 2015; 14:75–9. [DOI] [PubMed] [Google Scholar]
- 40. Leung PSC, Choi J, Yang G, Woo E, Kenny TP, Gershwin ME. A contemporary perspective on the molecular characteristics of mitochondrial autoantigens and diagnosis in primary biliary cholangitis. Expert Rev Mol Diagn 2016; 16:697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tansley SL, Snowball J, Pauling JD et al, International Myositis Assessment and Clinical Studies (IMACS) Group and Myositis Autoantibody Scientific Interest Group. The promise, perceptions, and pitfalls of immunoassays for autoantibody testing in myositis. Arthritis Res Ther 2020; 22:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bowlus CL, Gershwin ME. The diagnosis of primary biliary cirrhosis. Autoimmun Rev 2014; 13:441–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rantapää‐Dahlqvist S, de Jong BAW, Berglin E et al Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis: anti‐CCP antibody and IgA‐RF predict RA. Arthritis Rheum 2003; 48:2741–9. [DOI] [PubMed] [Google Scholar]
- 44. Arbuckle MR, McClain MT, Rubertone MV et al Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 2003; 349:1526–33. [DOI] [PubMed] [Google Scholar]
- 45. Knip M, Korhonen S, Kulmala P et al Prediction of type 1 diabetes in the general population. Diabetes Care 2010; 33:1206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parodis I, Stockfelt M, Sjöwall C. B cell therapy in systemic lupus erythematous: from rationale to clinical practice. Front Med 2020; 7:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Anthony RM, Nimmerjahn F. The role of differential IgG glycosylation in the interaction of antibodies with FcγRs in vivo . Curr Opin Organ Transplant 2011; 16:7–14. [DOI] [PubMed] [Google Scholar]
- 48. Chen S, Lu C, Gu H, et al Aleuria aurantia lectin (AAL)‐reactive immunoglobulin G rapidly appears in sera of animals following antigen exposure. PLOS ONE 2012; 7:e44422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sjöwall C, Zapf J, von Löhneysen S et al Altered glycosylation of complexed native IgG molecules is associated with disease activity of systemic lupus erythematosus. Lupus 2015; 24:569–81. [DOI] [PubMed] [Google Scholar]
- 50. Stümer J, Biermann MHC, Knopf J et al Altered glycan accessibility on native immunoglobulin G complexes in early rheumatoid arthritis and its changes during therapy: altered glycosylation of IgG complexes in RA. Clin Exp Immunol 2017; 189:372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Miyakis S, Lockshin MD, Atsumi T et al International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


