Secretion of bacterial and host proteases in the lungs of CF patients strongly contribute to exacerbation of the inflammatory response and lung damage. Local treatment with matrix metalloprotease inhibitors Marimastat and Ilomastat can reduce the IL‐8‐mediated lung inflammation induced by Pseudomonas aeruginosa acute lung infection in CF mice.
Keywords: cystic fibrosis, in‐vivo imaging, lung inflammation, protease inhibitors, Pseudomonas aeruginosa
Summary
Pseudomonas aeruginosa is the major respiratory pathogen in patients with cystic fibrosis (CF). P. aeruginosa‐secreted proteases, in addition to host proteases, degrade lung tissue and interfere with immune processes. In this study, we aimed at evaluating the possible anti‐inflammatory effects of protease inhibitors Marimastat and Ilomastat in the treatment of P. aeruginosa infection. Lung infection with the P. aeruginosa PAO1 strain was established in wild‐type and cystic fibrosis transmembrane conductance regulator (CFTR) knock‐out C57BL/6 mice expressing a luciferase gene under control of bovine interleukin (IL)‐8 promoter. After intratracheal instillation with 150 µM Marimastat and Ilomastat, lung inflammation was monitored by in‐vivo bioluminescence imaging and bacterial load in the lungs was assessed. In vitro, the effects of protease inhibitors on PAO1 growth and viability were evaluated. Acute lung infection was established in both wild‐type and CFTR knock‐out mice. After 24 h, the infection induced IL‐8‐dependent bioluminescence emission, indicating lung inflammation. In infected mice with ongoing inflammation, intratracheal treatment with 150 µM Marimastat and Ilomastat reduced the bioluminescence signal in comparison to untreated, infected animals. Bacterial load in the lungs was not affected by the treatment, and in vitro the same dose of Marimastat and Ilomastat did not affect PAO1 growth and viability, confirming that these molecules have no additional anti‐bacterial activity. Our results show that inhibition of protease activity elicits anti‐inflammatory effects in cystic fibrosis (CF) mice with acute P. aeruginosa lung infection. Thus, Marimastat and Ilomastat represent candidate molecules for the treatment of CF patients, encouraging further studies on protease inhibitors and their application in inflammatory diseases.
Introduction
Cystic fibrosis (CF) is a common life‐threatening, recessively inherited genetic disease characterized by chronic pulmonary involvement [1]. Pseudomonas aeruginosa is considered the main airway pathogen in CF, affecting more than 50% of patients, particularly adults [2]. Especially during early colonization, P. aeruginosa releases a multitude of virulence factors which contribute to lung damage and trigger airways immune defenses, causing an excessive and prolonged inflammatory response characterized by intense recruitment of neutrophils [3]. Microbial persistence eventually develops into chronic infection. Progressive inflammation and chronic infection lead to lung deterioration and ultimate lung failure, which is the first cause of death for CF patients [4].
Excessive and dysregulated secretion of host and bacterial proteases in the CF lung strongly contribute to exacerbation of the inflammatory response and lung damage. Neutrophil elastase can disrupt lung tissue, contribute to mucociliary impairment, support excessive neutrophils influx, hinder phagocytosis and efferocytosis and trigger the senescence of bronchial epithelial cells which secrete large amount of proinflammatory cytokines and matrix metalloproteases [5, 6, 7, 8, 9, 10, 11]. Neutrophil elastase also degrades many components of innate anti‐microbial defenses, indirectly promoting P. aeruginosa infection [12]. Although the main source of protease activity in CF lungs is thought to be activated neutrophils, it has become evident that P. aeruginosa secreted proteases disrupt key host processes by several means, such as activation of cascade pathways, disruption of cytokine signaling, inactivation of cell receptors and host protease inhibitors, degradation of host complement factors, mucins, surfactant and disruption of tight junctions between epithelial cells, thus strongly contributing to lung disease [13, 14].
Protease‐inhibiting molecules could target the damaging effects of proteases present in CF airways, limiting inflammatory response and lung injury. Within this category of therapeutic molecules, various hydroxamate‐based broad‐spectrum matrix metalloprotease inhibitors (MMPIs) have already entered clinical trials as cancer therapeutics, although with disappointing results regarding their effectiveness in delaying cancer progression [15, 16]. In particular, MMPIs Marimastat and Ilomastat are attractive candidates, as they have previously been reported to inhibit both neutrophil elastase and P. aeruginosa‐secreted proteases [17, 18, 19, 20]. By targeting both host and bacterial proteases involved in lung infection processes, such as dysregulation of the inflammatory response, they could be a useful addition to CF therapeutic treatments. Furthermore, unlike traditional antibiotics which target fundamental processes, thereby creating enormous selection pressures, anti‐protease therapy should be less likely to result in the generation of resistant pathogens.
In this study, we aimed at evaluating the possible anti‐inflammatory effects of MMPIs Marimastat and Ilomastat in the treatment of P. aeruginosa acute infection using an in‐vivo imaging approach.
Materials and methods
Bacterial strain
P. aeruginosa laboratory strain PAO1 was used in this study. The strain was stored in MicrobankTM (Biolife Italiana, cat. no. 17PL170M; Biolife Italiana, Monza, Italy) at −80°C.
MMPIs
Marimastat (Tocris Bioscience, Bristol, UK) and Ilomastat (Galardin, GM6001; Tocris Bioscience) were diluted in dimethylsulfoxide (DMSO) following the manufacturers’ instructions and stored in aliquots at −20°C. For each experiment an aliquot was diluted in saline solution (1 : 100 dilution) to the appropriate concentration (150 µM). MMPIs’ inhibitory effect on protease activity exerted by PAO1 strain was confirmed in vitro (Supporting information, Fig. S1).
Experimental animals
Female congenic C57BL/6J wild‐type (WT) and gut‐corrected cystic fibrosis transmembrane conductance regulator (CFTR)tm1UNC (KO) mice (12 weeks old) were purchased from the Cystic Fibrosis Animal Core facility (San Raffaele Hospital, Milan, Italy). Animals were maintained under conventional housing conditions. Prior to use, animals were acclimatized for at least 5–7 days to the local vivarium conditions, having free access to standard rodent chow and tap water. Animal experiments were conducted in compliance with national (Legislative Decree 26/2014, authorization no. 953/2017‐PR) and international laws and policies (Guide for the Care and Use of Laboratory Animals).
Reporter construct
Experimental animals were transfected with the bovine interleukin‐8 promoter/luciferase (bIL‐8‐Luc) construct containing a luciferase gene under the control of the bovine IL‐8 promoter (kindly provided by Professor Gaetano Donofrio, University of Parma, Italy) [21]. Competent Escherichia coli DH5α cells were transformed by heat shock and the plasmid was purified by Qiagen Plasmid Plus Mega Kit (Qiagen, cat. no. 12981; Qiagen, Valencia, CA, USA). Plasmid concentration and purity were evaluated using NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific, Fremont, CA, USA).
In‐vivo gene delivery
In‐vivo JetPEI (Polyplus Transfection, cat. no. 201‐50G; Polyplus Transfection, Illkirch‐Graffenstaden, France) was used as carrier for delivering bIL‐8‐Luc construct to lung tissue. As previously described [22], DNA and JetPEI were mixed with a final nitrogen/phosphate (N/P) ratio of 7–7·5 following the manufacturer’s instructions. Briefly, 38–42 µg DNA and 5·3‐6·3 µl JetPEI were separately diluted in 200 µl 5% glucose, mixed and incubated at room temperature for 15 min; 400 µl of the mixture was intravenously injected through the tail vein after warming the animals for 5 min under a heating lamp. The expression of the IL‐8 reporter can be temporarily verified after transfection: activation and inactivation of the reporter were monitored by in‐vivo bioluminescence imaging after 24 h and 7 days, respectively. Only mice presenting a measurable bioluminescence (confirming expression of the transgene) at 24 h after transfection and no emission after 7 days continued in the study. Bioluminescence emission in representative WT and KO animals at 24 h after transfection is shown in Supporting information, Fig. S2.
Bacterial preparation for lung challenge
PAO1 strain was plated onto Luria–Bertani (LB) agar and grown at 37°C for 24 h. A single colony was inoculated in LB medium and grown for 16 h at 37°C shaking. Bacteria were washed twice and resuspended in saline solution. OD600 was measured, and bacterial cells were diluted to the appropriate load for lung challenge. An aliquot of the bacterial suspension was plated on LB agar, incubated at 37°C for 24 h and colony‐forming units (CFU) were counted.
Intratracheal instillation
BIL‐8‐Luc transgenic mice were intratracheally challenged with 3–5 × 105 PAO1 cells for development of acute lung infection. As previously described [22], mice were anesthetized with 2·5% isoflurane and placed on an intubation platform, hanging by their incisor teeth. After visualization of the opening of the trachea using a laryngoscope, 50 µl of bacterial suspension were instilled by an intubation tube connected to a pressure control system. After 4, 24 and 48 h, reporter activation was monitored by in‐vivo imaging. Control non‐infected mice were intratracheally instilled with saline solution. For evaluation of MMPI anti‐inflammatory effect, 20 h after instillation with PAO1 cells, mice were intratracheally instilled with 50 µl of 150 µM Marimastat and Ilomastat. Four and 24 h after treatment with MMPIs (corresponding to 24 and 48 h after infection), reporter activation was monitored by in‐vivo imaging. Control infected mice were not treated with MMPIs.
In‐vivo bioluminescence imaging
Bioluminescence imaging of experimental animals was performed as previously described [22], using IVIS Spectrum imaging system (PerkinElmer, Waltham, MA, USA). Ten min prior to bioluminescence recording, mice were anesthetized with 2·5% isoflurane and intraperitoneally injected with 150 mg/kg D‐luciferin (PerkinElmer). After 5 min‐long luminescence recording, photons emitted from the chest region were quantified using Living Image software (PerkinElmer).
Lung recovery and CFU count
Mice were euthanized by cervical dislocation and lungs were excised, placed in 2 ml ice‐cold sterile saline solution and homogenized. Homogenate was plated on LB agar. Plates were incubated at 37°C for 24 h and CFU were counted.
Bacterial growth curves
PAO1 strain was plated on LB agar plates and incubated at 37°C for 24 h; one to two colonies were inoculated in 10 ml LB medium shaking at 37°C overnight. OD600 was measured, culture was diluted to 0·05 optical density (OD)/ml in 50 ml LB medium with/without Marimastat or Ilomastat (150 µM) and incubated at 37°C shaking. OD600 was measured every hour for 7 h and growth rate was calculated using GraphPad Prism version 7.0 software. After 24 h, cultures were serially diluted and plated on LB agar plates. Plates were incubated at 37°C for 24 h and CFU were counted.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 7.0 software. Mice bioluminescence emission was analyzed by two‐way analysis of variance (anova) followed by Dunnett’s multiple comparison test.
Results
In‐vivo imaging of lung inflammation induced by acute infection with P. aeruginosa
To evaluate in vivo the possible anti‐inflammatory effects associated with inhibition of proteases, we used a transgenic mouse model that expresses a luciferase gene under control of bovine IL‐8 promoter allowing monitoring of lung inflammation by in‐vivo bioluminescence imaging [22]. The model was previously validated for evaluating inflammation induced by simple proinflammatory stimuli such as bacterial lipopolysaccharide and culture supernatant [23]. Thus, we first verified that the model was suitable to monitor lung inflammation induced by an infectious process. Transgenic WT and CFTR‐KO mice were intratracheally challenged with P. aeruginosa PAO1 strain, and IL‐8‐dependent bioluminescence emission was monitored up to 48 h. Intratracheal challenge with PAO1 induced IL‐8‐dependent bioluminescence emission after 24 h in both WT and KO mice (Fig. 1a). The increase in the emission was statistically significant only in KO mice (P = 0·043), indicating a stronger inflammatory response associated with the CF phenotype. Instillation of saline solution in control mice also caused a light inflammatory reaction, probably due to the instillation procedure itself; no difference between control WT and KO mice was observed. The establishment of an acute infection was confirmed by recovery of 1–2 × 106 CFU/mouse from the lungs of both WT and KO mice at 24 h after intratracheal challenge (Fig. 1b); there was no statistically significant difference between the two mouse strains. Survival of the animals in the first 48 h after the challenge allowed monitoring of the inflammation – and evaluation of MMPIs’ anti‐inflammatory properties – during this time‐frame.
Fig. 1.
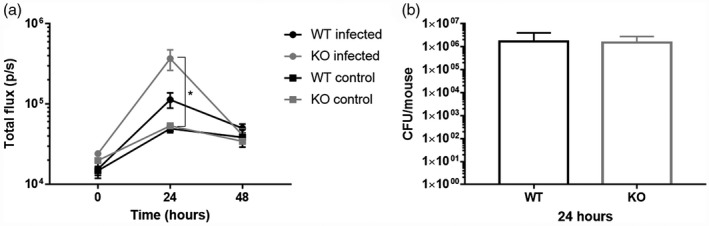
Interleukin (IL)‐8‐dependent bioluminescence emission in wild‐type (WT) and knock‐out (KO) mice with PAO1 acute lung infection. (a) Photon emission was measured before challenge with bacteria (indicated as 0 h) and after 24 and 48 h. Photon emission is expressed as total flux (p/s). Each value represents the mean ± standard error of the mean (s.e.m.) of four animals (biological replicates) per group; *P < 0·05. (b) Bacterial load recovered from mice lungs at 24 h after intratracheal instillation with PAO1 cells. Each value represents the mean ± standard deviation (s.d.) of four animals (biological replicates) per group.
Local treatment with Marimastat and Ilomastat reduces lung inflammation in infected mice
At 20 h after intratracheal challenge with the PAO1 strain to allow the prior establishment of acute lung infection, intratracheal treatment with 150 µM protease inhibitors Marimastat and Ilomastat was performed. The IL‐8‐dependent bioluminescence emission was monitored before treatment with protease inhibitors (20 h after infection) to verify the presence of an inflammatory condition and after 4 and 24 h (corresponding to 24 and 48 hours after infection). In both WT and KO mice with ongoing inflammation, as confirmed by IL‐8‐dependent bioluminescence emission, a reduction of the bioluminescence signal was observed at 4 h after intratracheal treatment with both MMPIs (corresponding to 24 h after infection) in comparison to untreated, infected animals (Fig. 2). As shown in Fig. 2d, in KO mice Marimastat and Ilomastat produced a reduction of the emission by 63 and 78%, respectively, which was statistically significant in both cases (P = 0·025 and P = 0·001, respectively).
Fig. 2.
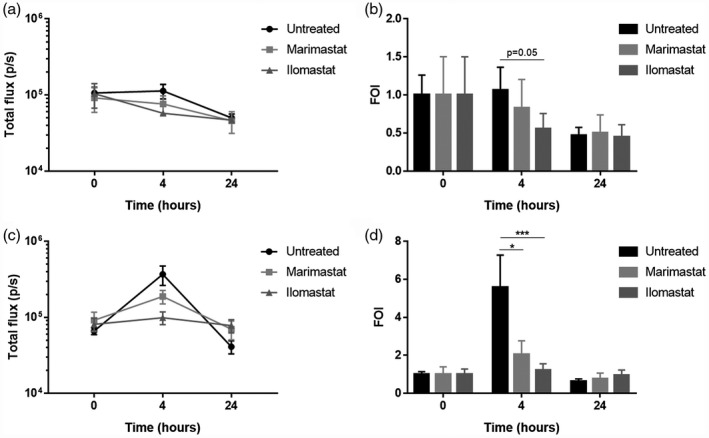
Interleukin (IL)‐8‐dependent bioluminescence emission in mice with PAO1 acute lung infection treated with Marimastat and Ilomastat. Photon emission was measured before treatment with matrix metalloprotease inhibitors (MMPIs) (indicated as 0 h) and after 4 and 24 h. Photon emission is expressed as total flux (p/s) (left panels) and as folds of induction (FOI) versus baseline (right panels) in wild‐type (WT) (a,b) and knock‐out (KO) (c,d) mice. Each value represents the mean ± standard error of the mean (s.e.m.) of eight animals (biological replicates) per group; *P < 0·05, ***P < 0·001.
Reduction of inflammation in infected mice is not due to anti‐bacterial effects
A group of mice was euthanized 4 h after treatment with MMPIs (corresponding to 24 h after infection) to evaluate the bacterial load in their lungs. No significant inhibitory effect was observed on the bacterial load compared to untreated, infected animals (Fig. 3), supporting that the observed anti‐inflammatory effect of MMPIs is not related to additional anti‐bacterial activity.
Fig. 3.
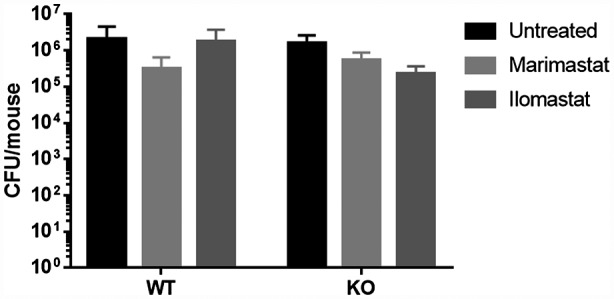
Effects of protease inhibitors Marimastat and Ilomastat on PAO1 load in mice lungs. Bacteria were recovered from the lungs of wild‐type (WT) and knock‐out (KO) mice with PAO1 acute lung infection at 4 h after treatment with 150 µM Marimastat and Ilomastat (corresponding to 24 h after infection). Untreated infected mice were used as control. Each value represents the mean ± standard deviation (s.d.) of four animals (biological replicates) per group.
To further verify this hypothesis, PAO1 strain was cultured in the presence of 150 µM Marimastat and Ilomastat. Optical density was recorded every hour to calculate the bacterial growth rate and cultures were plated for CFU count. The presence of protease inhibitors did not affect bacterial growth or viability compared to the untreated cultures (Fig. 4), confirming that the MMPIs have no bactericidal or bacteriostatic effects. Thus, the anti‐inflammatory effects observed in mice are probably mainly due to their anti‐protease activity.
Fig. 4.
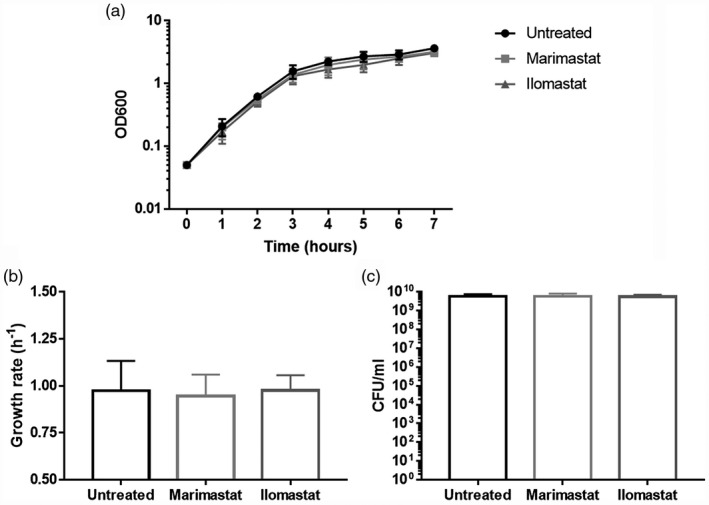
Growth curves, growth rate and viability of PAO1 grown in presence of Marimastat and Ilomastat. (a) For growth curves, absorbance at 600 nm was measured every hour for the first 7 h. (b) Growth rate was calculated from exponential phase of growth curves. (c) Colony‐forming units (CFU) were counted after plating serial dilutions of 24‐h‐long cultures. PAO1 grown without protease inhibitors was used as control. Each value represents the mean ± standard deviation (s.d.) of three experiments.
Discussion
P. aeruginosa is the main airway pathogen affecting CF patients. Especially during early colonization, it releases a multitude of virulence factors that trigger the production of proinflammatory mediators. In particular, P. aeruginosa challenge of human tracheobronchial epithelial cell cultures is associated with IL‐8 secretion [24, 25]. IL‐8 is a strong promoter of neutrophil recruitment, and its concentration in bronchoalveolar lavage fluid has been shown to often correlate with the concentration of neutrophils and their products [26, 27]. We previously showed that the challenge with P. aeruginosa secreted virulence factors and lipopolysaccharide induces an IL‐8‐dependent bioluminescence emission from the lungs of KO and WT mice, associated with increased recruitment of white blood cells (WBC) and neutrophils, up‐regulation of proinflammatory cytokines [IL‐1β, IL‐5, keratinocyte‐derived chemokine (KC), IL‐6, granulocyte–colony‐stimulatory factor (G‐CSF), interferon (IFN), macrophage inflammatory protein (MIP)‐1α, tumor necrosis factor (TNF)‐α] and lung injury characterized by neutrophil accumulation and alveolar wall thickening [23]. This IL‐8‐mediated inflammatory response could be reduced by pre‐inhibiting bacterial proteases with Marimastat and Ilomastat [20]. However, during the P. aeruginosa infection the anti‐protease defenses of the CF lung are most probably overwhelmed by a combination of both bacterial and endogenous proteases. This complex situation requires to be studied in an appropriate model of infection where all these proteolytic forces are probably contemporarily activated. To this purpose, in the present study we optimized a transgenic CF mouse model to perform in‐vivo imaging of IL‐8‐mediated inflammation during P. aeruginosa lung infection. The correct experimental conditions were identified in order to develop an acute infection able to induce IL‐8‐dependent bioluminescence emission as well as allowing survival of the animals. In these conditions, we could then confirm that local treatment with Marimastat and Ilomastat can reduce the IL‐8‐mediated lung inflammation induced by P. aeruginosa infection by 63 and 78%, respectively.
We previously demonstrated that Marimastat can elicit a stronger anti‐inflammatory action in mice challenged with bacterial proteases [20]; however, in the present study Ilomastat demonstrated a higher effect in reducing the IL‐8‐dependent bioluminescence. This difference is probably due to the diverse models used in the two studies: the previous results were based on lung challenge with bacterial exoproducts, while in the present study an acute infection was developed. The higher complexity of the infection model used here enables a more complete evaluation of P. aeruginosa virulence. Not only the secreted virulence factors, but also exotoxins and membrane‐bound factors such as flagella, pili and lipopolysaccharide, play an important role in P. aeruginosa pathogenicity and contribute to the induction of the inflammatory response characterized by excessive release of various host proteases. Additionally, Ilomastat is known as the most potent compound within the family of the hydroxamate‐based MMPIs, as it can inhibit a wider range of MMPs at lower concentrations compared to other members of this drug’s family. For instance, it has been reported to inhibit MMP‐2, MMP‐8 and MMP‐9 – all known to potentiate the inflammatory response in CF lung disease [28] – while Marimastat has no activity against MMP‐8 [29]. Moreover, Ilomastat previously showed stronger inhibition of P. aeruginosa proteases compared to other MMPIs such as Marimastat and Batimastat [20]. As both Marimastat and Ilomastat can inhibit bacterial and host proteases and have been reported to interact with host inflammatory processes, such as neutrophil and macrophage recruitment [30, 31], the combination of various mechanisms – rather than a specific mechanism – is likely to be responsible for their anti‐inflammatory effects. Although in this study we did not directly address the link between protease inhibition and inflammatory response, a comprehensive study specifically targeting this subject is needed to unravel the mechanisms responsible for the observed down‐regulation of lung inflammation in response to treatment with MMPIs.
P. aeruginosa infection induced a significant increase of IL‐8‐dependent bioluminescence in KO mice but not in WT animals, differently from what was previously observed using bacterial exoproducts as proinflammatory stimulus [20]. However, in our previous work mice were intratracheally challenged with ×10‐concentrated culture supernatant, probably containing a number of proteases and other virulence factors much higher than in a physiological situation, where bacteria produce and secrete them during growth, as in the infectious process. The anti‐inflammatory effect of MMPIs is better appreciated in KO mice, which show a higher response than WT animals in terms of IL‐8‐dependent bioluminescence emission caused by P. aeruginosa infection. Although no CF mouse model develops spontaneous lung disease, as confirmed by various studies reporting no significant differences at baseline between the lungs of WT and KO mice in terms of inflammatory markers [32, 33, 34], significant pulmonary inflammation and associated pathology can be induced in CFTRtm1UNC mice by mimicking lung infection through intranasal or intratracheal challenge with P. aeruginosa or other pathogens. While WT and CFTRtm1UNC mice have been reported to develop bronchopneumonia at similar levels after induced lung infection with P. aeruginosa, the KO mice also demonstrated defective clearance of bacteria from the lungs and higher mortality [35]. Enhanced susceptibility to lung infection was observed already at 6–8 weeks of age, as reported by Sajjan and colleagues [36]. Our results confirm the higher degree of sensitivity to lung infection observed in CF mice and highlight the potential anti‐inflammatory action of MMPIs – particularly Ilomastat – in the CF inflammatory phenotype.
Both the acute and the chronic lung infection models are considered powerful tools to study the host response to P. aeruginosa infection. The acute infection model is of particular interest for the initial phases of lung infection, while the chronic infection model can more effectively mimic the long‐term lung situation [37]. However, the latter also poses technical, analytical and ethical challenges that had to be considered when choosing the most appropriate infection model for this study. Chronic infection is induced using bacterial cells embedded within agar beads, which usually require invasive surgical procedures for intratracheal injection [33, 38, 39]. This implies a necessary post‐surgical recovery of the mice and a higher risk of occurrence of clinical complications that may impact the animals’ symptoms and even survival. Moreover, the consequently enhanced inflammation associated with the healing process could influence the susceptibility of mice to infection. Considering the early stage of our evaluation on the anti‐inflammatory action of MMPIs, we opted in favor of a moderately invasive model still able to provide highly relevant information. In light of the current results from the acute infection model, the adoption of a chronic infection model will be considered for further evaluation of these molecules. In particular, Guilbault and colleagues developed a moderately invasive model of chronic lung infection that represents an interesting alternative to the previously mentioned surgical models [40]. The chronic infection model will probably enable study of the inflammatory reaction for a longer time, while our study was limited to the initial 48 h of the infection, when the inflammatory response was detectable through the IL‐8‐dependent bioluminescence emission.
Together with inflammation and infection, a prolonged endobronchial protease activity due to local protease–antiprotease imbalance has been observed to establish very early in the airways of CF patients (by the age of 1 year) and is sustained chronically thereafter [41]. This dysregulated protease activity results in up‐regulation of proinflammatory mediators, increased recruitment of inflammatory cells to the lung, impaired phagocytosis, increased mucin production and inactivation of important innate and anti‐microbial proteins resulting in sustained inflammation and predisposition to infection [42]. In this scenario, inhibition of protease activity might help to reduce not only the infection‐induced inflammation but also the endogenous protease‐mediated damaging effects, especially in the setting of acute episodes such as pulmonary exacerbations. However, long‐term inhibition of constitutive proteases could also cause undesired effects. Hence, developing rational therapeutic regimens is crucial for the clinical administration of MMPIs. Alternatively, the identification and targeting of specific host/bacterial key proteases central to the inflammatory process may be sufficient to lessen the protease‐mediated inflammation in CF airways.
In conclusion, our study showed that Marimastat and Ilomastat elicits anti‐inflammatory effects in CF mice with acute P. aeruginosa lung infection. Thus, these MMPIs might be potential candidate molecules for the treatment of CF patients. In particular, local inhalable treatment would be preferable to avoid undesired systemic effects and target only the airways. To our knowledge, an inhalable formulation of Ilomastat has been proposed [30], encouraging the possibility of a future local therapy for CF lung disease as well as for other respiratory inflammatory diseases.
Disclosures
The authors declare that they have no conflicts of interest.
Author contributions
A. S., F. B. and M. B. designed the study; A. S. and F. B. performed data acquisition and analysis; A. S., F. B. and M. L. wrote the paper; C. S. and M. B. revised the paper.
Supporting information
Fig. S1. Protease activity in culture supernatant collected from PAO1 strain measured in presence of increasing concentrations of MMPIs Marimastat and Ilomastat. Culture supernatant was collected by centrifugation after overnight growth at 37°C in tryptic soy broth. Protease activity was determined by degradation of azocasein substrate and measurement of the azo dye absorbance at 450 nm (Sandri et al. 2018). One protease unit was calculated as the amount of enzyme producing an increase of 1 OD unit per minute. Protease activity is expressed as enzymatic units per ml of reaction volume (U/ml). Statistical analysis was performed by one‐way ANOVA test and Dunnett’s multiple comparisons test against non‐treated supernatant (0 µM MMPIs); **p < 0.01, ****p < 0.0001.
Fig. S2. IL‐8‐dependent bioluminescence emission in representative WT and KO mice at 24 hours after transfection. The activation of the IL‐8 reporter was verified by in vivo bioluminescence imaging at 24 hours after intravenous injection. Each point represents the bioluminescence emission of one animal. The mean ± SEM of representative WT (n = 8) and KO (n = 7) animals is shown. Photon emission is expressed as total flux (p/s). Statistical analysis was performed by t test.
Acknowledgements
We thank Professor Gaetano Donofrio (Department of Veterinary Science, University of Parma, Italy) for bIL‐8‐Luc plasmid; Eleonora Cremonini (Department of Diagnostic and Public Health, University of Verona, Italy) for technical support; and the Interdepartmental Centre for Experimental Research using Laboratory Animals (CIRSAL) of the University of Verona for housing and care of the laboratory animals; the Technological Platforms Center (CPT) of the University of Verona for imaging data acquisition. This study was funded by the Italian Cystic Fibrosis Research Foundation, project FFC#21/2017, adopted by Delegazione FFC di Boschi Sant’Anna Minerbe ‘Alla fine esce sempre il sole’ and Delegazione FFC di Massafra. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 2005; 352:1992–2001. [DOI] [PubMed] [Google Scholar]
- 2. Vonberg RP, Gastmeier P. Isolation of infectious cystic fibrosis patients: results of a systematic review. Infect Control Hosp Epidemiol 2005; 26:401–9. [DOI] [PubMed] [Google Scholar]
- 3. Hogardt M, Heesemann J. Adaptation of Pseudomonas aeruginosa during persistence in the cystic fibrosis lung. Int J Med Microbiol 2010; 300:557–62. [DOI] [PubMed] [Google Scholar]
- 4. Pittman JE, Cutting G, Davis SD, Ferkol T, Boucher R. Cystic fibrosis: NHLBI Workshop on the Primary Prevention of Chronic Lung Diseases. Ann Am Thorac Soc 2014; 11(Suppl 3):S161–S168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park JA, He F, Martin LD, Li Y, Chorley BN, Adler KB. Human neutrophil elastase induces hypersecretion of mucin from well‐differentiated human bronchial epithelial cells in vitro via a protein kinase C{delta}‐mediated mechanism. Am J Pathol 2005; 167:651‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caldwell RA, Boucher RC, Stutts MJ. Neutrophil elastase activates near‐silent epithelial Na+ channels and increases airway epithelial Na+ transport. Am J Physiol Lung Cell Mol Physiol 2005; 288:L813–L819. [DOI] [PubMed] [Google Scholar]
- 7. Nakamura H, Yoshimura K, McElvaney NG, Crystal RG. Neutrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces interleukin‐8 gene expression in a human bronchial epithelial cell line. J Clin Invest 1992; 89:1478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tosi MF, Zakem H, Berger M. Neutrophil elastase cleaves C3bi on opsonized pseudomonas as well as CR1 on neutrophils to create a functionally important opsonin receptor mismatch. J Clin Invest 1990; 86:300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vandivier RW, Fadok VA, Hoffmann PR et al Elastase‐mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J Clin Invest 2002; 109:661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence exacerbates pulmonary inflammation in patients with chronic obstructive pulmonary disease. Respiration 2010; 80:59‐70. [DOI] [PubMed] [Google Scholar]
- 11. Gifford AM, Chalmers JD. The role of neutrophils in cystic fibrosis. Curr Opin Hematol 2014; 21:16–22. [DOI] [PubMed] [Google Scholar]
- 12. Nordin SL, Jovic S, Kurut A et al High expression of midkine in the airways of patients with cystic fibrosis. Am J Respir Cell Mol Biol 2013; 49:935–42. [DOI] [PubMed] [Google Scholar]
- 13. Travis J, Potempa J. Bacterial proteinases as targets for the development of second‐generation antibiotics. Biochim Biophys Acta 2000; 1477:35–50. [DOI] [PubMed] [Google Scholar]
- 14. Kipnis E, Sawa T, Wiener‐Kronish J. Targeting mechanisms of Pseudomonas aeruginosa pathogenesis. Med Mal Infect 2006; 36 78–91. [DOI] [PubMed] [Google Scholar]
- 15. Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J 2011; 278:16–27. [DOI] [PubMed] [Google Scholar]
- 16. Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 2002; 295:2387–92. [DOI] [PubMed] [Google Scholar]
- 17. Antonelli PJ, Schultz GS, Kim KM et al Alpha 1‐antitrypsin and ilomastat inhibit inflammatory proteases present in human middle ear effusions. Laryngoscope 2003; 113:1347–51. [DOI] [PubMed] [Google Scholar]
- 18. Kok K, Curciarello R, Biancheri P, Langmead L, MacDonald T. The role of human neutrophil elastase and its inhibitor elafin in ulcerative colitis. Gut 2013; 62:A165. [Google Scholar]
- 19. Grobelny D, Poncz L, Galardy RE. Inhibition of human skin fibroblast collagenase, thermolysin, and Pseudomonas aeruginosa elastase by peptide hydroxamic acids. Biochemistry 1992; 31:7152–4. [DOI] [PubMed] [Google Scholar]
- 20. Sandri A, Ortombina A, Boschi F et al Inhibition of Pseudomonas aeruginosa secreted virulence factors reduces lung inflammation in CF mice. Virulence 2018; 9:1008–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stellari FF, Franceschi V, Capocefalo A et al In vivo imaging of transiently transgenized mice with a bovine interleukin 8 (CXCL8) promoter/luciferase reporter construct. PLOS ONE 2012; 7:e39716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bergamini G, Stellari F, Sandri A et al An IL‐8 transiently transgenized mouse model for the in vivo long‐term monitoring of inflammatory responses. J Vis Exp 2017; 124:5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stellari F, Bergamini G, Ruscitti F et al In vivo monitoring of lung inflammation in CFTR‐deficient mice. J Transl Med 2016; 14:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu Q, Lu Z, Verghese MW, Randell SH. Airway epithelial cell tolerance to Pseudomonas aeruginosa . Respir Res 2005; 6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Delgado MA, Poschet JF, Deretic V. Nonclassical pathway of Pseudomonas aeruginosa DNA‐induced interleukin‐8 secretion in cystic fibrosis airway epithelial cells. Infect Immun 2006; 74:2975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med 1995; 151:1075–82. [DOI] [PubMed] [Google Scholar]
- 27. Tan HL, Rosenthal M. IL‐17 in lung disease: friend or foe? Thorax 2013; 68:788–90. [DOI] [PubMed] [Google Scholar]
- 28. Jojas‐Quintero J, Owen C. Matrix metalloproteinases in cystic fibrosis: pathophysiologic and therapeutic perspectives. Metalloproteinases Med 2016; 3:49–62. [Google Scholar]
- 29. Liu J, Khalil RA. Matrix metalloproteinase inhibitors as investigational and therapeutic tools in unrestrained tissue remodeling and pathological disorders. Prog Mol Biol Transl Sci 2017; 148:355–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pemberton PA, Cantwell JS, Kim KM et al An inhaled matrix metalloprotease inhibitor prevents cigarette smoke‐induced emphysema in the mouse. COPD 2005; 2:303–10. [DOI] [PubMed] [Google Scholar]
- 31. Nenan S, Lagente V, Planquois JM et al Metalloelastase (MMP‐12) induced inflammatory response in mice airways: effects of dexamethasone, rolipram and marimastat. Eur J Pharmacol 2007; 559:75–81. [DOI] [PubMed] [Google Scholar]
- 32. Snouwaert JN, Brigman KK, Latour AM et al A murine model of cystic fibrosis. Am J Respir Crit Care Med 1995; 151:S59–64. [DOI] [PubMed] [Google Scholar]
- 33. van Heeckeren AM, Schluchter MD. Murine models of chronic Pseudomonas aeruginosa lung infection. Lab Anim 2002; 36:291–312. [DOI] [PubMed] [Google Scholar]
- 34. Guilbault C, Saeed Z, Downey GP, Radzioch D. Cystic fibrosis mouse models. Am J Respir Cell Mol Biol 2007; 36:1–7. [DOI] [PubMed] [Google Scholar]
- 35. Gosselin D, Stevenson MM, Cowley EA et al Impaired ability of CFTR knockout mice to control lung infection with Pseudomonas aeruginosa . Am J Respir Crit Care Med 1998; 157:1253–62. [DOI] [PubMed] [Google Scholar]
- 36. Sajjan U, Thanassoulis G, Cherapanov V et al Enhanced susceptibility to pulmonary infection with Burkholderia cepacia in CFTR(–/–) mice. Infect Immun 2001; 69:5138–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kukavica‐Ibrulj I, Facchini M, Cigana C, Levesque RC, Bragonzi A. Assessing Pseudomonas aeruginosa virulence and the host response using murine models of acute and chronic lung infection. Methods Mol Biol 2014; 1149:757–71. [DOI] [PubMed] [Google Scholar]
- 38. Facchini M, De Fino I, Riva C, Bragonzi A. Long term chronic Pseudomonas aeruginosa airway infection in mice. J Vis Exp 2014; 85:e51019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bayes HK, Ritchie N, Irvine S, Evans TJ. A murine model of early Pseudomonas aeruginosa lung disease with transition to chronic infection. Sci Rep 2016; 6:35838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guilbault C, Martin P, Houle D et al Cystic fibrosis lung disease following infection with Pseudomonas aeruginosa in CFTR knockout mice using novel non‐invasive direct pulmonary infection technique. Lab Anim 2005; 39:336–52. [DOI] [PubMed] [Google Scholar]
- 41. Griese M, Kappler M, Gaggar A, Hartl D. Inhibition of airway proteases in cystic fibrosis lung disease. Eur Respir J 2008; 32:783–95. [DOI] [PubMed] [Google Scholar]
- 42. Quinn DJ, Weldon S, Taggart CC. Antiproteases as therapeutics to target inflammation in cystic fibrosis. Open Respir Med J 2010; 4:20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Protease activity in culture supernatant collected from PAO1 strain measured in presence of increasing concentrations of MMPIs Marimastat and Ilomastat. Culture supernatant was collected by centrifugation after overnight growth at 37°C in tryptic soy broth. Protease activity was determined by degradation of azocasein substrate and measurement of the azo dye absorbance at 450 nm (Sandri et al. 2018). One protease unit was calculated as the amount of enzyme producing an increase of 1 OD unit per minute. Protease activity is expressed as enzymatic units per ml of reaction volume (U/ml). Statistical analysis was performed by one‐way ANOVA test and Dunnett’s multiple comparisons test against non‐treated supernatant (0 µM MMPIs); **p < 0.01, ****p < 0.0001.
Fig. S2. IL‐8‐dependent bioluminescence emission in representative WT and KO mice at 24 hours after transfection. The activation of the IL‐8 reporter was verified by in vivo bioluminescence imaging at 24 hours after intravenous injection. Each point represents the bioluminescence emission of one animal. The mean ± SEM of representative WT (n = 8) and KO (n = 7) animals is shown. Photon emission is expressed as total flux (p/s). Statistical analysis was performed by t test.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


