The differences in the frequency and immune differentiation potential of HSCs in BM between young donors and older donors may partly explain the different outcomes of allo‐HSCT.
Keywords: graft‐versus‐host disease, haematopoietic stem cells, immune cells, reactive oxygen species, young donors
Summary
Young donors are reported to be associated with better transplant outcomes than older donors in allogeneic hematopoietic stem cell transplantation (allo‐HSCT), but the mechanism is still unclear. The current study compared the different subsets of haematopoietic stem cells (HSCs) and their progenitors as well as immune cells in bone marrow (BM) between young and older donors. The frequencies of HSCs, multipotent progenitors (MPPs) and myeloid progenitors, including common myeloid progenitors (CMPs) and megakaryocyte–erythroid progenitors (MEPs), were decreased, whereas those of lymphoid progenitors, including multi‐potent lymphoid progenitors (MLPs) and common lymphoid progenitors (CLPs), were increased in the BM of young donors compared with in that of older donors. Lower reactive oxygen species (ROS) levels were observed in BM HSCs and six progenitor lines in young donors. Furthermore, young donors demonstrated higher frequencies of naive T cells and immune suppressor cells, such as alternative macrophages (M2) and lower frequencies of memory T cells and immune effectors, including T helper‐1 and T cytotoxic‐1 cells, in BM than older donors. Multivariate analysis demonstrated that donor age was independently correlated with BM HSC frequency. Although further validation is required, our results suggest that the differences in the frequency and immune differentiation potential of HSCs in BM between young donors and older donors may partly explain the different outcomes of allo‐HSCT.
Introduction
Allogeneic haematopoietic stem cell transplantation (allo‐HSCT) provides a potentially curative method for the majority of malignant haematologic diseases. Patients may have more than one donor for both human leukocyte antigen (HLA)‐haploidentical allo‐HSCT and HLA‐matched allo‐HSCT [1, 2]. The European Society for Blood and Marrow Transplantation Consensus for HLA‐haploidentical allo‐HSCT and the British Society for Histocompatibility and Immunogenetics Guideline for HLA‐matched allo‐HSCT recommended that younger donors should be preferentially selected [3, 4]. Our previous study including 1210 patients undergoing HLA‐mismatched allo‐HSCT reported that patients receiving HSCs from donors aged <30 years demonstrated significantly less grade 2‐4 acute graft‐versus‐host disease (aGVHD), lower non‐relapse mortality and better survival than those receiving HSCs from donors aged ≥30 years [5]. Kollman et al. found less grade 3‐4 aGVHD and higher overall survival in patients receiving HSCs from donors aged 18‐30 years compared to those from donors aged 30‐45 years and over 45 years in HLA‐matched allo‐HSCT [2]. These studies suggested that HSCT outcomes from young donors were generally superior to those from older donors, but the underlying mechanism is still uncertain.
Successful allo‐HSCT relies on the rapid reconstitution of donor‐derived haematopoietic and immune systems in the recipient [6]. Therefore, characterizing the differences in percentages of HSCs and progenitors and immune cell subtypes between young and older donors may help explain the disparities in transplant outcomes. HSCs, which reside in bone marrow (BM), are able to self‐renew and differentiate into various lineages of blood cells via haematopoietic progenitor cells [7, 8]. In humans, HSCs give rise to multipotent progenitors (MPPs) that further segregate into either common myeloid progenitors (CMPs) or multipotent lymphoid progenitors (MLPs), which in turn segregate into either common lymphoid progenitors (CLPs) or granulocyte‐macrophage progenitors (GMPs) [9, 10, 11, 12]. CLPs give rise to B cells, T cells and natural killer cells, while CMPs further segregate into either megakaryocyte‐erythroid progenitor MEPs, which can differentiate into erythrocytes and platelets, or GMPs, which can produce macrophages, eosinophils, basophils, neutrophils and mast cells [9, 10, 11, 12]. Few human studies have reported the differences in the frequencies of BM HSCs and progenitors in healthy young and old adults [13, 14]. Pang et al. suggested an increase in the percentage of HSCs and a decrease in CLP frequency in older adults [13], whereas Kuranda et al. showed that HSC frequency persisted at the same level with age and MLP frequency increased in older adults [14]. Therefore, differences in the frequencies of HSCs and their progenitors between young and older adults remain uncertain and need to be precisely identified.
Donor immune cells play a critical role in the efficacy of engraftment [15]. Moreover, aGVHD is generally considered to be associated with increased percentages of donor T helper‐1 (Th1) and T cytotoxic‐1 (Tc1) cells and decreased percentages of T helper‐2 (Th2) and T cytotoxic‐2 (Tc2) cells [16, 17, 18, 19]. Macrophages were involved in aGVHD, poor graft function and some other complications post allo‐HSCT [20, 21, 22, 23]. However, little is known about the differences in the percentages of cytokine‐producing T cell subsets and macrophage subsets in BM between young and older donors.
Therefore, the current study was performed to evaluate the different subsets of HSCs and their progenitors and immune cells among young (aged <30 years), middle‐aged (aged 30‐45 years), and old donors (aged >45 years). Moreover, we analysed the association between donor characteristics and HSC frequency. Our study may help identify the potential mechanism underlying younger donors being preferred in allo‐HSCT.
Materials and methods
Healthy donors
In this prospective study, a total of 60 healthy adult donors, including 20 young donors, 20 middle‐aged donors, and 20 old donors who underwent physical examination for allo‐HSCT, were enrolled between January 1, 2019, and January 1, 2020, at Peking University Institute of Hematology. The donor characteristics of young, middle‐aged and old donors are summarized in Table 1.
Table 1.
Characteristics of different‐aged donors
| Characteristics | Young donor (N = 20) | Middle‐aged donor (N = 20) | Old donor (N = 20) | P * | P † | P ‡ |
|---|---|---|---|---|---|---|
| Physical variables | ||||||
| Age (years) a | 24 (18–29) | 38 (30–45) | 53 (46–62) | < 0·0001* | < 0·0001* | < 0·0001* |
| Gender, female versus male | 10/10 | 4/16 | 4/16 | |||
| BMI (kg/m2) a | 21·48 (16·89–31·12) | 25·69 (21·26–35·25) | 23·89 (16·42–30·02) | 0·01* | 0·51 | 0·03* |
| Weight (kg) a | 65 (30–97·5) | 73 (54–85) | 75 (55–82) | 0·22 | 0·74 | 0·09 |
| Height (m) a | 168 (150–187) | 169 (158–188) | 172 (155–183) | 0·93 | 0·86 | 0·75 |
| Blood cell counts | ||||||
| WBC count (×109/l) a | 5·79 (4·10–9·90) | 6·30 (4·90–8·39) | 5·89 (4·09–9·11) | 0·31 | 0·47 | 0·94 |
| Neutrophil (×109/l) a | 3·4 (1·77–7·22) | 3·59 (2·47–6·00) | 3·45 (1·98–6·10) | 0·37 | 0·57 | 0·9 |
| Lymphocyte (×109/l) a | 1·93 (1·27–2·96) | 2·1 (1·18–3·09) | 1·9 (1·12–3·27) | 0·54 | 0·91 | 0·91 |
| Monocytes (×109/l) a | 0·37 (0·23–0·62) | 0·43 (0·30–0·63) | 0·38 (0·27–0·72) | 0·05 | 0·05 | 0·75 |
| Haemoglobin (g/l) a | 141 (110–170) | 151 (104–166) | 155 (103–170) | 0·36 | 0·15 | 0·18 |
| RBC (×1012/l) a | 4·74 (3·95–5·88) | 5·04 (4·51–5·41) | 5·27 (3·99–6·45) | 0·26 | 0·49 | 0·12 |
| Platelet (×109/l) a | 219 (139–377) | 215 (128–357) | 225 (117–335) | 0·73 | 0·6 | 0·95 |
| Eosinophil (×109/l) a | 0·13 (0·03–0·41) | 0·09 (0·04–0·21) | 0·12 (0·03–0·32) | 0·28 | 0·49 | 0·63 |
| Basophil (×109/l) a | 0·03 (0–0·07) | 0·03 (0·01–0·1) | 0·03 (0·01–0·07) | 0·75 | 0·93 | 0·85 |
Data are reported as median (range).
The P‐values present the comparison between the young donor and middle‐aged donor groups.
The P‐values present the comparison between the middle‐aged donor and old donor groups.
The P‐values present the comparison between the young donor and old donor groups.
The study was approved by the Ethics Committee of Peking University People’s Hospital, and written informed consent was obtained from all subjects before study entry, in accordance with the Declaration of Helsinki.
Characterization of HSCs and progenitors in BM
BM mononuclear cells (BMMNCs) were separated with lymphocyte separation medium (HaoYang, Tianjin, China). HSCs and their progenitors were quantified by flow cytometry using the following directly conjugated mouse anti‐human monoclonal antibodies (BD Biosciences, San Jose, CA): anti‐CD34‐PE/Cy7, anti‐CD38‐BV421, anti‐CD45RA‐BUV395, anti‐CD90‐BV510, anti‐CD7‐BV711, anti‐CD10‐BUV737, and anti‐CD135‐PE. As previously reported [11, 24, 25], HSCs, MPPs, and MLPs were identified as CD34+CD38−CD90+CD45RA−, CD34+CD38−CD90−CD45RA−, and CD34+CD38−CD45RA+, respectively. CLPs, GMPs, CMPs, and MEPs were defined as CD34+CD38+CD7−CD10+CD45RA+, CD34+CD38+CD7−CD10−CD45RA+, CD34+CD38+CD7−CD10−CD135+CD45RA−, and CD34+CD38+CD7−CD10−CD135−CD45RA−, respectively. The relative frequencies of these HSCs and progenitors were expressed as a fraction of standard CD34+ cells. Multiparameter flow cytometric analyses were performed using a BD LSRFortessa cell analyser (BD Biosciences), and the data were analysed using BD LSRFortessa software (BD Biosciences).
Measurement of ROS levels via flow cytometry
As previously reported [26, 27, 28, 29, 30], the cells stained with the aforementioned HSCs and their progenitor markers were incubated with 10 μM 2′,7′‐dichlorofluorescence diacetate (Beyotime, Beijing, China) at 37°C for 15 minutes. The mean fluorescence intensity of intracellular ROS was measured on a BD LSRFortessa (BD Biosciences).
Analysis of T cell subsets
Lymphocyte subpopulations were quantified via flow cytometry using the following directly conjugated mouse anti‐human monoclonal antibodies: anti‐CD3‐PE/Cy7, anti‐CD4‐APC/H7, anti‐CD8‐V500, anti‐CD45RA‐BV605, and anti‐CCR7‐BUV395 (BD Biosciences). As previously described [31], effector T cells, naïve T cells, effector memory T cells and central memory T cells were identified as CD45RA+CCR7−, CD45RA+CCR7+, CD45RA−CCR7−, and CD45RA−CCR7+, respectively, in CD3+CD4+CD8− cells and CD3+CD4−CD8+ cells.
As previously described [31, 32, 33, 34], cellular cytokine secretions were determined after incubating the cells with phorbol myristate acetate (100 ng/mL) and ionomycin (2 μg/mL, both from Sigma‐Aldrich, St. Louis, MO, USA) for 4 hours to stimulate maximal IFN‐γ and IL‐4 production. GolgiStop (0.7 μL/mL) was added to the samples during this 4‐hour incubation to sequester proteins in the cytoplasm. The conjugated mouse anti‐human monoclonal antibodies were anti‐CD3‐APC‐H7, anti‐CD8‐eFluor450, anti‐IL‐4‐PE (BD Biosciences) and anti‐IFN‐γ‐Percp/Cy5.5 (eBioscience, San Diego, CA, USA). Th1, Th2, Tc1 and Tc2 cells were identified as CD3+CD8−IFN‐γ+, CD3+CD8−IL‐4+, CD3+CD8+IFN‐γ+ and CD3+CD8+IL‐4+, respectively.
Analysis of macrophage subsets
Macrophages can be referred to as either classical macrophages (M1) or alternative macrophages (M2) [35, 36]. As previously reported [21, 22] for analysis of M1 and M2, BMMNCs were stained with anti‐CD14‐PE, anti‐CD68‐FITC, anti‐CCR2‐BV421, anti‐CX3CR1‐PerCP/Cy5.5 and anti‐CD163‐PE/Cy7 (BioLegend, San Diego, CA, USA). M1 and M2 were identified as CD14+CCR2+CD68+ and CD14+CX3CR1+CD163+, respectively. The relative frequencies of these macrophage subsets are expressed as a fraction of standard monocytes.
Statistical analysis
Comparisons among three groups were made using one‐way ANOVA, and comparisons between two groups were performed using the Mann‐Whitney U test. All donor characteristics were analysed as dichotomous with the median as the cut‐off point. Multivariate analyses were performed using logistic regression with a forward selection procedure to determine the independent donor factors involved in donor dichotomous variables selected from the univariate analysis. The parameters with P<0.10 according to the univariate analysis were entered into a multivariate model. Statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA). Univariate and multivariate analyses were performed using SPSS (IBM Corporation, Armonk, NY, USA) version 24 software. P‐values <0.05 were considered statistically significant.
Results
Donor characteristics
The current cohort enrolled 42 males and 18 females, and the median age of the donors was 37 years, with a range from 18 to 62 years old. The donor characteristics of the young, middle‐aged and old donors were compared in Table 1. Young donors had a lower BMI than middle‐aged donors (22.31kg/m2 ± 0.88 kg/m2 versus 26.01kg/m2 ± 1.07 kg/m2, P = 0.01) and old donors (22.31kg/m2 ± 0.88 kg/m2 versus 24.48kg/m2 ± 0.81 kg/m2, P = 0.03). No significant differences were found in blood cell counts among the three donor age groups.
Reduced frequency of HSCs and increased frequencies of MLPs and CLPs in the BM of young donors
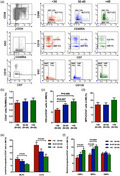
To determine the differences in the frequencies of HSCs and progenitors in donors of different ages, HSCs and their subpopulations were quantified by flow cytometry in young, middle‐aged and old donors. The representative gating strategy for HSCs and progenitors from the three age groups is shown in Fig. 1A. Compared with old donors, young donors had lower frequencies of HSCs (Fig. 1C, 6.11% ± 0.65% versus 10.09% ± 1.16%, P = 0.006) and MPPs (Fig. 1D, 11.91% ± 1.62% versus 15.17% ± 1.49%, P = 0.07), remarkably higher frequencies of MLPs (Fig. 1E, 10.18% ± 1.36% versus 6.00% ± 0.55%, P = 0.04) and CLPs (Fig. 1E, 21.05% ± 1.32% versus 12.02% ± 2.94%, P = 0.003), and significantly lower frequencies of CMPs (Fig. 1F, 8.46% ± 1.28% versus 13.20% ± 1.22%, P = 0.007) and MEPs (Fig. 1F, 10.34% ± 0.93% versus 15.55% ± 0.89%, P = 0.0004). Similarly, young donors had lower frequencies of HSCs (Fig. 1C, 6.11% ± 0.65% versus 8.51% ± 0.61%, P = 0.007) and MEPs (Fig. 1F, 10.34% ± 0.93% versus 13.93% ± 0.76%, P = 0.004) than middle‐aged donors. There were no significant differences in the percentages of CD34+ cells (Fig. 1B) and GMPs (Fig. 1F) among the three donor age groups.
Fig. 1.
Reduced frequency of haematopoietic stem cells (HSCs) and increased frequencies of multi‐potent lymphoid progenitors (MLPs) and common lymphoid progenitors (CLPs) in the bone marrow (BM) of young donors compared with older donors. Representative gating strategy for BM HSCs (CD34+CD38−CD90+CD45RA−), multi‐potent progenitors (MPPs) (CD34+CD38−CD90−CD45RA−), MLPs (CD34+CD38−CD45RA+), CLPs (CD34+CD38+CD7−CD10+CD45RA+), granulocyte–macrophage progenitors (GMPs) (CD34+CD38+CD7−CD10−CD45RA+), CMPs (CD34+CD38+CD7−CD10−CD135+CD45RA+) and MEPs (CD34+CD38+CD7−CD10−CD135−CD45RA−) from young, middle‐aged and old donors (a). Frequencies of BM CD34+ cells (b), HSCs (c), MPPs (d), lymphoid progenitors (e) and myeloid progenitors (f) among young, middle‐aged and old donors. Data are expressed as the mean ± standard error of the mean (s.e.m.). All P‐values < 0·05 were considered significant and are provided in the figure.
Decreased ROS levels in BM haematopoietic progenitors in young donors
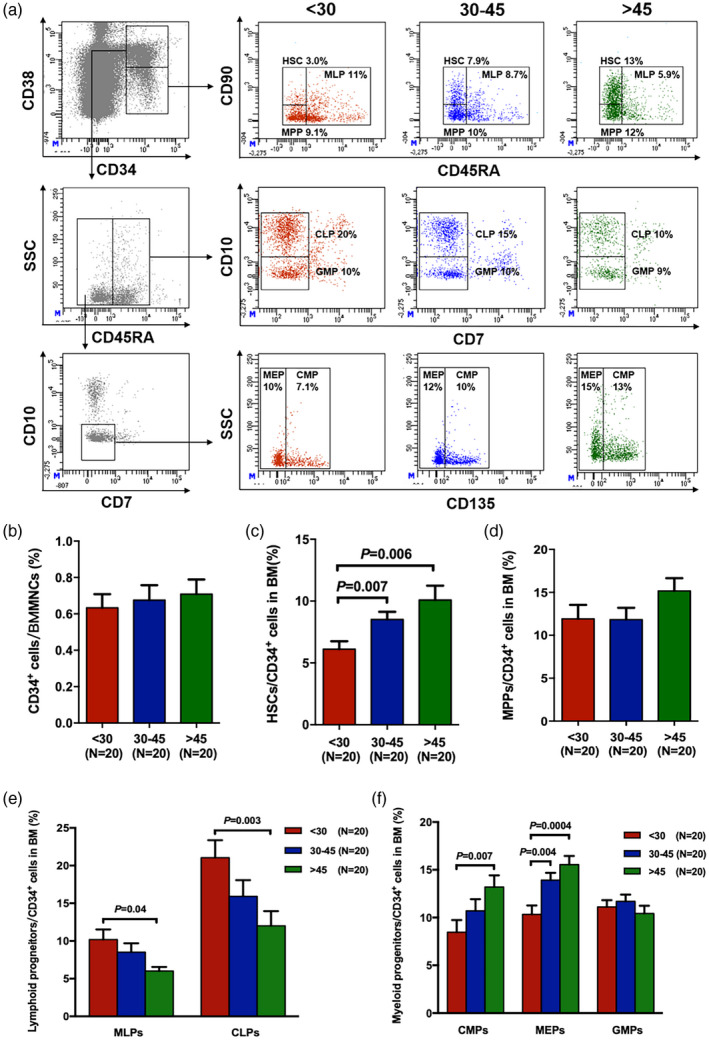
ROS levels in BM HSCs and progenitors were compared among young, middle‐aged and old donors (Fig. 2). Young donors had notably lower ROS levels in HSCs (Fig. 2A, 2888 ± 211 versus 3577 ± 251, P = 0.01), CD34+ BM cells (Fig. 2B, 2194 ± 170 versus 2980 ± 264, P = 0.03), MLPs (Fig. 2D, 3417 ± 186 versus 4946 ± 218, P = 0.0001) and CLPs (Fig. 2D, 1378 ± 98 versus 1792 ± 123, P = 0.02) than old donors. In addition, the ROS levels in CMPs (Fig. 2E, 2275 ± 207 versus 2805 ± 289, P = 0.19), MEPs (Fig. 2E, 2499 ± 226 versus 2994 ± 252, P = 0.30) and GMPs (Fig. 2E, 2107 ± 160 versus 2704 ± 222, P = 0.10) were lower in young donors than in old donors, although the results were not statistically significant. There were no significant differences in the ROS levels in MPPs (Fig. 2C) among the three donor age groups.
Fig. 2.
Decreased reactive oxygen species (ROS) levels in the bone marrow (BM) of haematopoietic progenitors from young donors compared with those from older donors. Intercellular ROS levels in BM haematopoietic stem cells (HSCs) (a), CD34+ cells (b) and multi‐potent progenitors (MPPs) (c) among young, middle‐aged and old donors. ROS levels in BM lymphoid progenitors (d) and myeloid progenitors (e) in the three donor groups. Data are expressed as the mean ± standard error of the mean (s.e.m.). All P‐values < 0·05 were considered significant and are provided in the figure.
Higher naive T cell frequency but lower memory T cell frequency in the BM of young donors
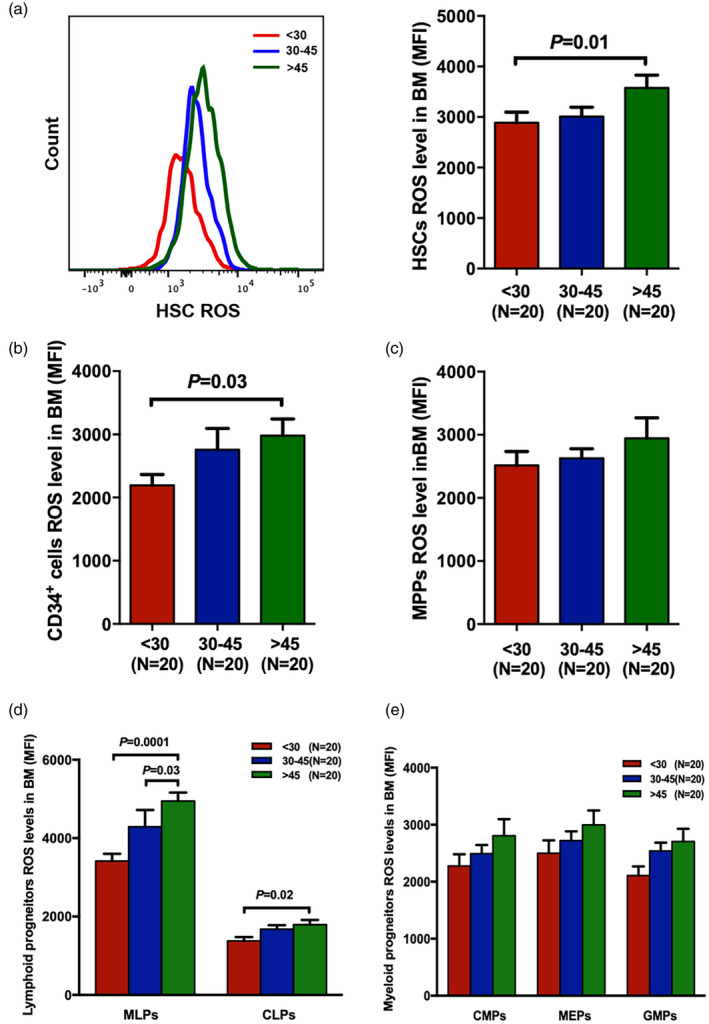
To investigate the differences in the differentiation potential from HSCs to immune cells among the three donor age groups, T cell subsets were explored via flow cytometry. The representative gating strategy is indicated in Fig. 3A. Significantly lower frequencies of CD3+CD4+CD8− T cells (Fig. 3B, 43.90% ± 2.06% versus 51.44% ± 1.80%, P = 0.02; 44.20% ± 1.58% versus 51.44% ± 1.80%, P = 0.003, respectively), but higher percentages of CD3+CD4−CD8+ T cells (Fig. 3C, 46.96% ± 1.64% versus 41.34% ± 1.53%, P = 0.04; 47.41% ± 1.59% versus 41.34% ± 1.53%, P = 0.01, respectively), were revealed in the BM of young and middle‐aged donors than in those of old donors. As a result, the ratio of CD4+/CD8+ T cells was significantly lower in young donors (Fig. 3D, 1.00 ± 0.08 versus 1.31 ± 0.09, P = 0.03) and middle‐aged donors (Fig. 3D, 0.97 ± 0.06 versus 1.31 ± 0.09, P = 0.005) than in old donors.
Fig. 3.
Higher frequency of naive T cells but lower memory T cells in the bone marrow (BM) of young donors. The gating strategy was used to identify effector T cells (CD45RA+CCR7−), naive T cells (CD45RA+CCR7+), effector memory T cells (CD45RA−CCR7−) and central memory T cells (CD45RA−CCR7+) from the three donor age groups (a). The percentages of BM CD3+CD4+ CD8− cells (b), CD3+CD4−CD8+ cells (c) and the ratio of CD4+/CD8+ T cells (d) in young, middle‐aged and older donors. Percentages of effector T cells, naive T cells, effector memory T cells and central memory T cells among CD4+ T cells (e) and CD8+ T cells (f) in the BM of the three donor age groups. The results are expressed as mean ± standard error of the mean (s.e.m.). P‐values < 0·05 were considered statistically significant and are provided in the figure.
Furthermore, young donors and middle‐aged donors had higher levels of CD4+ naïve T cells (Fig. 3E, 40.00% ± 2.12% versus 33.82% ± 2.14%, P = 0.04; 39.51% ± 1.56% versus 33.82% ± 2.14%, P = 0.047, respectively), and CD8+ naïve T cells (Fig. 3F, 31.69% ± 2.58% versus 20.22% ± 2.26%, P = 0.002; 29.99% ± 2.96% versus 20.22% ± 2.26%, P = 0.02, respectively), but fewer CD8+ effector memory T cells (Fig. 3F, 35.39% ± 3.14% versus 43.77% ± 2.68%, P = 0.04; 35.68% ± 2.91% versus 43.77% ± 2.68%, P = 0.04, respectively), and CD8+ central memory T cells (Fig. 3F, 1.83% ± 0.16% versus 4.26% ± 0.76%, P = 0.0002; 2.21% ± 0.27% versus 4.26% ± 0.76%, P = 0.003, respectively), than old donors. The percentages of CD4+ effector memory T cells (Fig. 3E) and CD8+ effector T cells (Fig. 3F) showed no significant differences among the three donor age groups.
Reduced frequencies of Th1 and Tc1 cells in the BM of young donors
To further investigate the potential of HSCs to differentiate into cytokine‐producing T cells in donors of different ages, flow cytometry was used to analyse the intracellular cytokines. The characteristics of the Th1, Tc1, Th2 and Tc2 cells in the BM of young, middle‐aged and old donors are shown in Fig. 4A. The percentages of Th1 cells (Fig. 4B, 8.45% ± 1.50% versus 13.74% ± 1.96%, P = 0.04) and Tc1 cells (Fig. 4E, 21.07% ± 4.08% versus 32.15% ± 3.48%, P = 0.02) were significantly lower in young donors than in old donors. The percentages of Th2 (Fig. 4C) and Tc2 cells (Fig. 4F) between young and old donors were comparable. The ratios of Th1/Th2 cells (Fig. 4D, 9.18 ± 2.19 versus 21.27 ± 4.56, P = 0.04) and Tc1/Tc2 cells (Fig. 4G, 20.2 ± 5.78 versus 33.66 ± 8.53, P = 0.14) were lower in young donors than in old donors.
Fig. 4.
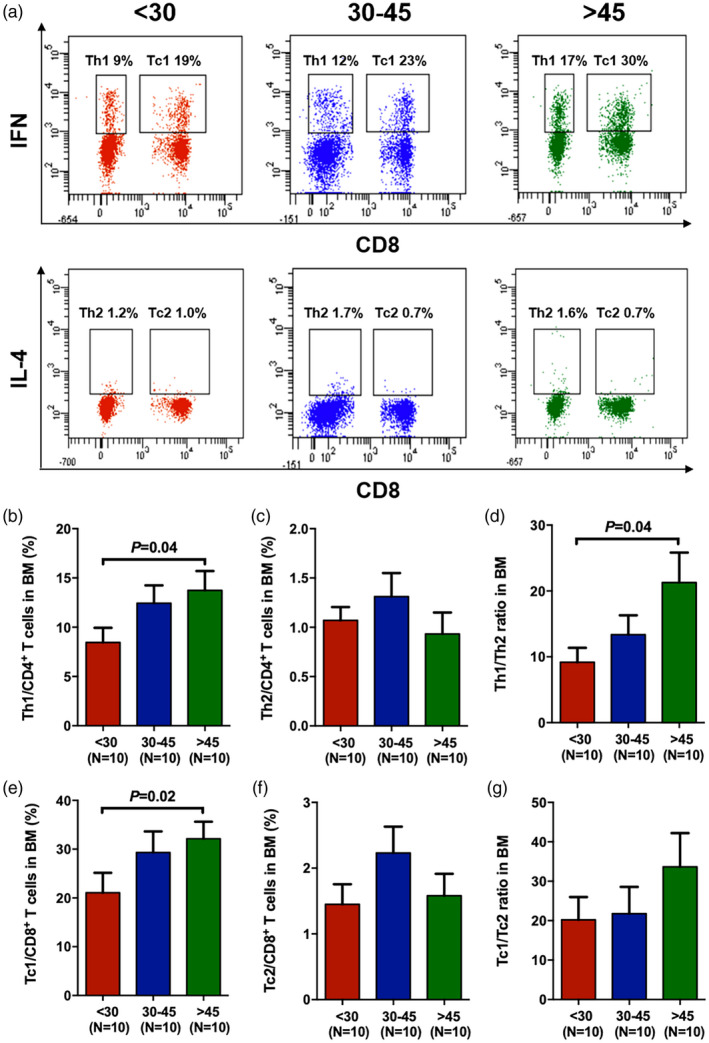
Reduced frequencies of T helper type 1 (Th1) and T cytotoxic type 2 (Tc1) cells in the bone marrow (BM) of young donors. Representative flow cytometry analyses of Th1 [CD3+CD8−interferon (IFN)‐γ+], Tc1 (CD3+CD8+IFN‐γ+), Th2 [CD3+CD8−interleukin (IL)‐4+] and Tc2 (CD3+CD8+IL‐4+) cells from the three donor age groups (a). The percentages of BM Th1 (b) and Th2 (c) cells among CD4+ T cells and Tc1 (e) and Tc2 (f) cells among CD8+ T cells. The ratios of Th1/Th2 (d) and Tc1/Tc2 (g) in the BM of the three donor age groups. The results are expressed as the mean ± standard error of the mean (s.e.m.). All P‐values < 0·05 were considered significant and are provided in the figure.
Higher frequency of M2 and lower ratio of M1/M2 in the BM of young donors
The percentages of BM M1 and M2 macrophages were analysed using flow cytometry to explore the differences in the potential of HSCs to develop into immune cells in the three donor age groups, following the gating strategies depicted in Fig. 5A. The baseline percentage of BM M1 in young donors was lower (Fig. 5B, 0.17% ± 0.02% versus 0.24% ± 0.04%, P = 0.12) than that in old donors, although the difference was not statistically significant. The percentage of BM M2 was significantly higher (Fig. 5C, 29.49% ± 3.24% versus 19.71% ± 2.40%, P = 0.03) in young donors than in old donors. As a result, a significantly reduced M1/M2 ratio (Fig. 5D, 0.008 ± 0.002 versus 0.014 ± 0.002, P = 0.02) was indicated in the BM of young donors compared with in that of old donors.
Fig. 5.
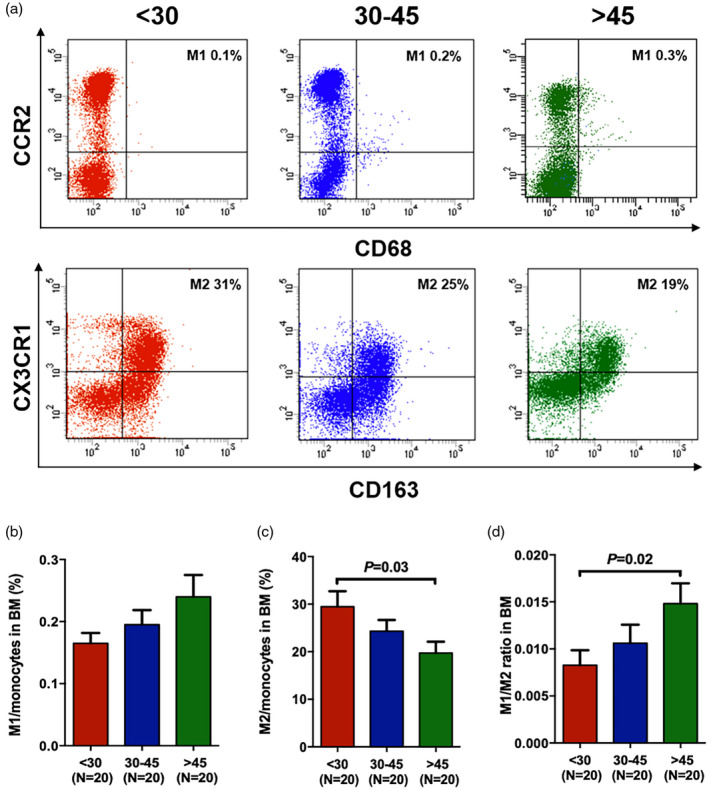
Higher M2 frequency in the bone marrow (BM) of young donors. The representative gating strategy (A) was used to identify M1 (CD14+CD68+CCR2+) and alternative macrophage (M2) (CD14+CX3CR1+CD163+) cells. The relative frequencies of these macrophage subsets are expressed as a fraction of standard monocytes. Classical macrophage (M1) percentage (b), M2 percentage (c) and M1/M2 ratio (d) in the BM of three donor age groups. The results are expressed as mean ± standard error of the mean (s.e.m.). All P‐values < 0·05 were considered significant and are provided in the figure.
Donor age was independently correlated with BM HSC frequency
To explore the factors that can influence the percentage of HSCs in CD34+ cells, the association of donor characteristics with the frequency of HSCs was analysed by univariate and multivariate analysis (Table 2). In univariate analysis, donor age (P = 0.04) was positively associated with the frequency of HSCs. Furthermore, multivariate analysis indicated that age≥37 (hazard ratio (HR), 2.98; 95% confidence interval (CI), 1.04‐8.53, P = 0.04) was independently correlated with a higher frequency of HSCs.
Table 2.
Univariate and multivariate analysis of independent influencing factors for HSC frequency a
| Factors | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| P‐value | HR | 95% CI | P‐value | |
| Donor age (years) < 37 versus ≥ 37 | 0·04* | 2·98 | 1·04–8·53 | 0·04* |
| Donor gender, female versus male | 0·82 | |||
| Donor BMI (kg/m2) < 23·5 versus ≥ 23·5 | 0·12 | |||
| WBC count (×109/l) < 5·91 versus ≥ 5·91 | 0·51 | |||
| Neutrophil (×109/l) < 3·46 versus ≥ 3·46 | 0·87 | |||
| Lymphocytes (×109/l) < 1·94 versus ≥ 1·94 | 0·14 | |||
| Monocytes (×109/l) < 0·39 versus ≥ 0·39 | 0·47 | |||
| Haemoglobin (g/l) < 152 versus ≥ 152 | 0·47 | |||
| Platelet (×109/l) < 221 versus ≥ 221 | 0·59 | |||
| RBC (×1012/l) < 5·04 versus ≥ 5·04 | 0·41 | |||
| Eosinophil (×109/l) < 0·11 versus ≥ 0·11 | 0·44 | |||
| Basophilia (×109/l) < 0·03 versus ≥ 0·03 | 0·88 | |||
BMI = body mass index; CI = confidence interval; HR = hazard ratio.
To avoid potential confounding factors, logistic regression was assessed for interaction terms with covariates. The variables included in the logistic regression analyses exhibited P < 0·10 after univariate analyses. The final multivariate models were constructed using a forward stepwise selection approach.
Discussion
In the current study, we systematically characterized the differences in haematopoietic cells and immune cell subsets in young, middle‐aged and old donors. We found that the frequencies of HSCs, MPPs and myeloid progenitors, including CMPs and MEPs were lower and lymphoid progenitors including MLPs and CLPs were higher in the BM of young donors than in that of older donors. Lower levels of ROS in HSCs and progenitors were observed in young donors than in the other group donors. Moreover, a lower CD4+/CD8+ T cell ratio, lower frequencies of memory T cells, Th1 and Tc1, and higher frequencies of naïve T cells and M2 were found in the BM of young donors than in that of older donors. Furthermore, multivariate analysis indicated that donor age was independently correlated with BM HSC frequency.
Emerging clinical evidence has shown that young donors have better transplant outcomes than older donors [2, 3, 4, 5], however, the mechanism is unclear. We observed that HSCs from young donors had a more balanced potential to differentiate into myeloid and lymphoid lineages. In ageing mice, enhanced ROS levels in HSCs have been demonstrated to be a significant cause of HSC differentiation, low homing and engraftment efficiency [37, 38, 39]. Our study first showed lower ROS levels in BM HSCs and progenitors in young donors, which may indicate better function of HSCs and progenitors in these donors. Several studies using engraftment of immune‐deficient mice with human HSCs and in vivo dynamic tracking of the human progenitor hierarchy have explained that HSCs could produce all immune cells through the process of haematopoiesis differentiation [40, 41, 42]. Therefore, changes in HSCs and progenitors with age may play an important role in the differences observed in immune cell subsets between young and older donors.
The occurrence of aGVHD was reported to be associated with the proliferation of donor immune effector cells, including Th1, Th2 and M2 [16, 17, 18, 19, 21]. Our data showed that young donors had more immune suppressor cells, such as M2, but fewer immune effector cells, including Th1 and Th2, in BM, which may partly explain the lower incidence of aGVHD in transplants from young donors. The correlation of CD34+ cells with the occurrence of aGVHD remains controversial [43, 44, 45, 46]. Remberger et al. demonstrated that patients transplanted with high CD34+ cell doses (>17×106/kg) had an increased incidence of grade 2‐4 aGVHD in allo‐HSCT [45]. Gaziev et al. showed a positive correlation between CD34+ cell number (>4×106/kg) and aGVHD in children receiving sibling allo‐HSCT [46]. In addition, preceding studies showed that HSCs could sense external factors and produce relevant immune cell subtypes in response to an infection [47, 48]. A recent mouse study provided evidence that HSCs could establish long‐term memory in response to acute pathogen exposure and direct an improved immune response and myeloid differentiation to secondary infection by epigenetic changes [49]. Compared to old adults, young adults frequently present with less chronic low‐grade inflammation, characterized by lower levels of proinflammatory cytokines such as IL‐1, IL‐6, IL‐8, TNF‐α and C‐reactive protein [50]. Based on these studies, we speculate that under allo‐HSCT stress, HSCs from young donors may decrease the occurrence of aGVHD by differentiating into fewer immune effector cells and more immune suppressor cells in patients after allo‐HSCT.
We are aware that this is only an early stage study and further definite characterization is required to determine whether the frequency and function of HSCs in allografts directly participate in the occurrence of aGVHD after allo‐HSCT. Moreover, single‐cell sequencing in purified HSCs from different age donors may be helpful to better understand the molecular regulatory networks underlying different immune differentiation potential of young and old HSCs.
In summary, the current study showed that BM HSCs from young donors exhibited a lower frequency, more balanced myeloid‐lymphoid differentiation potential and lower ROS levels and produced more naïve T cells and immune suppressors, including M2, and fewer memory T cells and immune effector cells, such as Th1 and Tc1, than those from older donors. In addition, donor age might be a good predictor of HSC frequency. Although further validation is required, our results may provide laboratory evidence to better understand why young donors are preferred for allo‐HSCT.
Disclosure
The authors declare that they have no competing interests.
Author contributions
Y. K. designed the study and supervised the manuscript preparation. W. L. Y., Q. W. and H. Y. Z. performed the research and analysed the data. W. L. Y., Y. K. and Q. W. wrote the manuscript. All other authors participated in the collection of patient data. All the authors agreed to submit the final manuscript.
Acknowledgements
This work was supported by the National Key Research and Development Program (2017YFA0104500 & 2019YFC0840606), the National Natural Science Foundation of China (82070188, 81870139, 81530046 and 81930004), the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (81621001) and the Peking University Clinical Scientist Program (BMU2019LCKXJ003). The authors thank all the core facilities at Peking University Institute of Hematology for patient care and sample collection. American Journal Experts (www.journalexperts.com) provided editorial assistance to the authors during the preparation of the manuscript. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Chang YJ, Luznik L, Fuchs EJ, Huang XJ. How do we choose the best donor for T‐cell‐replete, HLA‐haploidentical transplantation? J Hematol Oncol 2016; 9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kollman C, Howe CW, Anasetti C et al Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood 2001; 98:2043–51. [DOI] [PubMed] [Google Scholar]
- 3. Ciurea SO, Al Malki MM, Kongtim P et al The European Society for Blood and Marrow Transplantation (EBMT) consensus recommendations for donor selection in haploidentical hematopoietic cell transplantation. Bone Marrow Transplant 2020; 55:12–24. [DOI] [PubMed] [Google Scholar]
- 4. Little AM, Green A, Harvey J et al BSHI guideline: HLA matching and donor selection for haematopoietic progenitor cell transplantation. Int J Immunogenet 2016; 43:263–86. [DOI] [PubMed] [Google Scholar]
- 5. Wang Y, Chang YJ, Xu LP et al Who is the best donor for a related HLA haplotype‐mismatched transplant? Blood 2014; 124:843–50. [DOI] [PubMed] [Google Scholar]
- 6. Ratajczak MZ, Suszynska M. Emerging strategies to enhance homing and engraftment of hematopoietic stem cells. Stem Cell Rev Rep 2016; 12:121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol 2001; 17:387–403. [DOI] [PubMed] [Google Scholar]
- 8. Laurenti E, Göttgens B. From haematopoietic stem cells to complex differentiation landscapes. Nature 2018; 553:418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Velten L, Haas SF, Raffel S et al Human haematopoietic stem cell lineage commitment is a continuous process. Nat Cell Biol 2017; 19:271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dzierzak E, Philipsen S. Erythropoiesis: development and differentiation. Cold Spring Harb Perspect Med 2013; 3:a011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farlik M, Halbritter F, Muller F et al DNA methylation dynamics of human hematopoietic stem cell differentiation. Cell Stem Cell 2016; 19:808–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen L, Kostadima M, Martens JHA et al Transcriptional diversity during lineage commitment of human blood progenitors. Science 2014; 345:1251033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pang WW, Price EA, Sahoo D et al Human bone marrow hematopoietic stem cells are increased in frequency and myeloid‐biased with age. Proc Natl Acad Sci USA 2011; 108:20012–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuranda K, Vargaftig J, de la Rochere P et al Age‐related changes in human hematopoietic stem/progenitor cells. Aging Cell 2011; 10:542–6. [DOI] [PubMed] [Google Scholar]
- 15. Chang YJ, Zhao XY, Huang XJ. Immune reconstitution after haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2014; 20:440–9. [DOI] [PubMed] [Google Scholar]
- 16. Blazar BR, Murphy WJ, Abedi M. Advances in graft‐versus‐host disease biology and therapy. Nat Rev Immunol 2012; 12:443–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zeiser R, Socie G, Blazar BR. Pathogenesis of acute graft‐versus‐host disease: from intestinal microbiota alterations to donor T cell activation. Br J Haematol 2016; 175:191–207. [DOI] [PubMed] [Google Scholar]
- 18. Zeiser R, Blazar BR. Acute graft‐versus‐host disease – biologic process, prevention, and therapy. N Engl J Med 2017; 377:2167–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tawara I, Maeda Y, Sun Y et al Combined Th2 cytokine deficiency in donor T cells aggravates experimental acute graft‐versus‐host disease. Exp Hematol 2008; 36:988–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matte CC, Liu J, Cormier J et al Donor APCs are required for maximal GVHD but not for GVL. Nat Med 2004; 10:987–92. [DOI] [PubMed] [Google Scholar]
- 21. Wen Q, Kong Y, Zhao HY et al G‐CSF‐induced macrophage polarization and mobilization may prevent acute graft‐versus‐host disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2019; 54:1419–33. [DOI] [PubMed] [Google Scholar]
- 22. Zhao HY, Lyu ZS, Duan CW et al An unbalanced monocyte macrophage polarization in the bone marrow microenvironment of patients with poor graft function after allogeneic haematopoietic stem cell transplantation. Br J Haematol 2018; 182:679–92. [DOI] [PubMed] [Google Scholar]
- 23. Ito M, Fujino M. Macrophage‐mediated complications after stem cell transplantation. Pathol Int 2019; 69:679–87. [DOI] [PubMed] [Google Scholar]
- 24. Shlush LI, Zandi S, Mitchell A et al Identification of pre‐leukaemic haematopoietic stem cells in acute leukaemia. Nature 2014; 506:328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shlush LI, Mitchell A, Heisler L et al Tracing the origins of relapse in acute myeloid leukaemia to stem cells. Nature 2017; 547:104–8. [DOI] [PubMed] [Google Scholar]
- 26. Kong Y, Cao XN, Zhang XH et al Atorvastatin enhances bone marrow endothelial cell function in corticosteroid‐resistant immune thrombocytopenia patients. Blood 2018; 131:1219–33. [DOI] [PubMed] [Google Scholar]
- 27. Kong Y, Shi MM, Zhang YY et al N‐acetyl‐L‐cysteine improves bone marrow endothelial progenitor cells in prolonged isolated thrombocytopenia patients post allogeneic hematopoietic stem cell transplantation. Am J Hematol 2018; 93:931–42. [DOI] [PubMed] [Google Scholar]
- 28. Cao XN, Kong Y, Song Y et al Impairment of bone marrow endothelial progenitor cells in acute graft‐versus‐host disease patients after allotransplant. Br J Haematol 2018; 182:870–86. [DOI] [PubMed] [Google Scholar]
- 29. Kong Y, Wang Y, Zhang YY et al Prophylactic oral NAC reduced poor hematopoietic reconstitution by improving endothelial cells after haploidentical transplantation. Blood Adv 2019; 3:1303–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kong Y, Song Y, Tang FF et al N‐acetyl‐L‐cysteine improves mesenchymal stem cell function in prolonged isolated thrombocytopenia post‐allotransplant. Br J Haematol 2018; 180:863–78. [DOI] [PubMed] [Google Scholar]
- 31. Song Y, Shi MM, Zhang YY et al Abnormalities of the bone marrow immune microenvironment in patients with prolonged isolated thrombocytopenia after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2017; 23:906–12. [DOI] [PubMed] [Google Scholar]
- 32. Song Y, Wang YT, Huang XJ, Kong Y. Abnormalities of the bone marrow immune microenvironment in patients with immune thrombocytopenia. Ann Hematol 2016; 95:959–65. [DOI] [PubMed] [Google Scholar]
- 33. Wang YT, Kong Y, Song Y et al Increased type 1 immune response in the bone marrow immune microenvironment of patients with poor graft function after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2016; 22:1376–82. [DOI] [PubMed] [Google Scholar]
- 34. Kong Y, Wang YT, Cao XN et al Aberrant T cell responses in the bone marrow microenvironment of patients with poor graft function after allogeneic hematopoietic stem cell transplantation. J Transl Med 2017; 15:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity 2014; 41:36–48. [DOI] [PubMed] [Google Scholar]
- 36. Murray PJ, Allen JE, Biswas SK et al Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014; 41:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hu L, Zhang Y, Miao W, Cheng T. Reactive oxygen species and Nrf2: functional and transcriptional regulators of hematopoiesis. Oxid Med Cell Longev 2019; 2019:5153268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rönn RE, Guibentif C, Saxena S, Woods NB. Reactive oxygen species impair the function of CD90(+) hematopoietic progenitors generated from human pluripotent stem cells. Stem Cells 2017; 35:197–206. [DOI] [PubMed] [Google Scholar]
- 39. Ito K, Hirao A, Arai F et al Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med 2006; 12:446–51. [DOI] [PubMed] [Google Scholar]
- 40. Kamel‐Reid S, Dick JE. Engraftment of immune‐deficient mice with human hematopoietic stem cells. Science 1988; 242:1706–9. [DOI] [PubMed] [Google Scholar]
- 41. Scala S, Basso‐Ricci L, Dionisio F et al Dynamics of genetically engineered hematopoietic stem and progenitor cells after autologous transplantation in humans. Nat Med 2018; 24:1683–90. [DOI] [PubMed] [Google Scholar]
- 42. Doulatov S, Notta F, Laurenti E, Dick JE. Hematopoiesis: a human perspective. Cell Stem Cell 2012; 10:120–36. [DOI] [PubMed] [Google Scholar]
- 43. Reisner Y, Martelli MF. Transplantation tolerance induced by ‘mega dose’ CD34+ cell transplants. Exp Hematol 2000; 28:119–27. [DOI] [PubMed] [Google Scholar]
- 44. Ophir E, Reisner Y. The use of donor‐derived veto cells in hematopoietic stem cell transplantation. Front Immunol 2012; 3:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Remberger M, Mattsson J, Hassan Z et al Risk factors for acute graft‐versus‐host disease grades II‐IV after reduced intensity conditioning allogeneic stem cell transplantation with unrelated donors: a single centre study. Bone Marrow Transplant 2008; 41:399–405. [DOI] [PubMed] [Google Scholar]
- 46. Gaziev J, Isgro A, Marziali M et al Higher CD3(+) and CD34(+) cell doses in the graft increase the incidence of acute GVHD in children receiving BMT for thalassemia. Bone Marrow Transplant 2012; 47:107–14. [DOI] [PubMed] [Google Scholar]
- 47. Takizawa H, Boettcher S, Manz MG. Demand‐adapted regulation of early hematopoiesis in infection and inflammation. Blood 2012; 119:2991–3002. [DOI] [PubMed] [Google Scholar]
- 48. King KY, Goodell MA. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol 2011; 11:685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de Laval B, Maurizio J, Kandalla PK et al C/EBPbeta‐dependent epigenetic memory induces trained immunity in hematopoietic stem cells. Cell Stem Cell 2020; 26:657–74.e8. [DOI] [PubMed] [Google Scholar]
- 50. Ferrucci L, Corsi A, Lauretani F et al The origins of age‐related proinflammatory state. Blood 2005; 105:2294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


