Abstract
Marine leech Zeylanicobdella arugamensis (Piscicolidae), an economically important parasite is infesting predominantly cultured groupers, hybrid groupers and other fish in Southeast Asian countries. In this study, we tested the anti-parasitic potential of a medicinal plant Nephrolepis biserrata found in Sabah, East Malaysia against Z. arugamensis. Various concentrations of methanol extracts of the plant were tested experimentally against Z. arugamensis and disinfestation of the leech from its primary host hybrid groupers. The composition of methanol extract of N. biserrata was determined through LC-QTOF analysis. The significant anti-parasitic activity of 100% mortality of leeches was observed with the exposure of N. biserrata extracts. The average time to kill the leeches at concentrations of 25, 50 and 100 mg/ml was 25.11 ± 3.26, 11.91 ± 0.99, and 4.88 ± 0.50 min., respectively. Further, at various low concentrations of N. biserrata 2.5, 5 and 10 mg/ml, hybrid groupers were disinfested in an average time of 108.33 ± 12.65, 65.83 ± 9.70 and 29.16 ± 5.85 min., respectively. The tandem mass spectrometry data from LC-QTOF indicated some hits on useful bioactive compounds such as terpenoids (ivalin, isovelleral, brassinolide, and eschscholtzxanthin), flavonoids (alnustin, kaempferol 7,4′-dimethyl ether, and pachypodol), phenolics (piscidic acid, chlorogenic acid, and ankorine), and aromatic (3-hydroxycoumarin). Thus N. biserrata can act as a potential biocontrol agent.
Subject terms: Biological techniques, Biotechnology
Introduction
Groupers of the family Serranidae, are acknowledged as economically-valued marine fish in Asian countries1. Around 21 species of groupers are cultured in Asia, especially in China, Japan, Thailand, Indonesia, Philippines, and Malaysia for domestic consumption and overseas export2. Tiger grouper (Epinephelus fuscoguttatus) and giant grouper (Epinephelus lanceolatus) are having a high commercial value in Malaysia, the price of these groupers is between USD 12 and 90 per kilogram3. To speed up the growth of groupers in aquaculture industry hybrid groupers were started to produce and it demonstrated better growth rate under grow out conditions3,4. They utilized feed more efficiently, implies a reduction in the feed cost for commercial farming4,5.
A wide variety of parasitic organisms such as protozoans, monogeneans, didymozoid digeneans, nematodes, copepods, and leeches have been reported as causing significant harms to grouper aquaculture2,6,7. Leeches are ectoparasites with striated bodies, muscular body wall with two suckers used for feeding and movement. Zeylanicobdella arugamensis (Annelida: Hirudinea: Piscicolidae), a marine parasitic leech, distributed throughout the Indian Ocean due to global ecological changes8. It is considered as a harmful parasite and affects a large number of fish species, can represent stressors for fish and have been associated with reduced food intake, anti-predator behaviour and decreased growth9. They attach in great numbers on the fins, jaws, inside the mouth and eyes of the host fishes9. In addition to this, the infestation of ectoparasites also results in the secondary infection of the host by the pathogenic bacteria such as Vibrio alginolyticus which further leads to the mortality of the host in a very short period7,9,10. Thus, Z. arugamensis is a vital threat to the grouper and other species in the aquaculture industry.
Toxic chemicals have been used for the control, especially formalin which are harmful to both fish and human beings11–14. In addition, some other chemicals such as copper sulfate, potassium permanganate, and hydrogen peroxide, have also been used, they could affect the water quality and other parameters including health of human beings through accumulation of toxic contents in fish. Hence, researchers and farmers are looking for alternative approaches including the medicinal plants for the eco-friendly treatments13. The natural products such as medicinal plants are possessing anti-inflammatory and antipathogenic properties with minimal toxicity level to fish. To minimize the consumption of toxic chemicals plant derived extracts can be used as a natural control agent against parasitic infestation due to the presence of antiparasitic phytochemical compounds such as alkaloids, glycosides, terpenoids etc15–17. Hence, research on the development of safe and natural agent for controlling the infestation rate of this species is critical.
Nephrolepis biserrata is a perennial fern, belongs to the family Nephrolepidaceae18, locally known as “Paku pedang” 19. In addition to Malaysia, the plant is also distributed in other South-east Asian countries20,21. In this medicinal plant, phytochemical profiling via gas chromatography mass spectrometry (GCMS) and the hepatoprotective properties have been studied22,23. Besides, the plant possesses antibacterial and antifungal properties24. However, no data have so far been reported concerning the anti-parasitic properties of N. biserrata against Z. arugamensis. The main objectives of the present study were to evaluate the anti-parasitic activity of N. biserrata methanol extract against the leech Z. arugamensis, disinfestation of the hybrid groupers (Epinephelus fuscoguttatus x E.lanceolatus) and chemical composition by LC-QTOF.
Results
Anti-parasitic properties of N. biserrata
The mortality time of Z. arugamensis in formalin and extract-treated groups is indicated in Table 1. The plant treated groups showed the antiparasitic effect in a dose-dependent manner. The time taken for the mortality of the parasitic leeches was lesser in 100 mg/ml dose than 50 and 25 mg/ml of the methanol extract. No mortality was noticed in the normal control group throughout the 720 min observation.
Table 1.
Mortality time of the leeches at different concentrations of methanol extract of N. biserrata.
| No. | Group | Mortality time (min) Mean ± S. D |
Mortality (%) |
|---|---|---|---|
| 1 | Normal control | 720.00 ± 00 | 0 |
| 2 | Positive control (formalin 0.25%) (v/v) | 3.68 ± 0.46a | 100 |
| 3 | N. biserrata (25 mg/ml) | 25.11 ± 3.26a,b | 100 |
| 4 | N. biserrata (50 mg/ml) | 11.91 ± 0.99a,b,c | 100 |
| 5 | N. biserrata (100 mg/ml) | 4.88 ± 0.50a,c,d | 100 |
Each value represents the mean ± S.D of 6 parasitic leeches per group.
aSignificance at p < 0.05 compared with the normal control group.
bSignificance at p < 0.05 compared with the formalin treated group (0.25% v/v).
cSignificance at p < 0.05 compared with N. biserrata (25 mg/ml).
dSignificance at p < 0.05 compared with N. biserrata (50 mg/ml).
Fish disinfestation
The disinfestation time of fishes treated with formalin and methanol extract of N. biserrata is shown in Table 2. The normal control group is free of leeches. The negative control group indicated no disinfestation of the leeches until 720 min. The plant treated groups indicated the effect in a dose-dependent manner. The time taken for 100% disinfestation of the fishes at a dose of 10 mg/ml is less than 5 and 2.5 mg/ml of the methanol extracts.
Table 2.
Fish disinfestation time of leeches at different concentrations of methanol extract of N. biserrata.
| No. | Group | Disinfestation time (min) Mean ± S.D |
Disinfestation (%) |
|---|---|---|---|
| 1 | Normal control | – | – |
| 2 | Negative control | 720.00 ± 0.00 | 0 |
| 3 | Positive control (formalin 0.1%) (v/v) | 26.33 ± 1.52a | 100 |
| 4 | N. biserrata (2.5 mg/ml) | 108.33 ± 12.65a,b | 100 |
| 5 | N. biserrata (5 mg/ml) | 65.83 ± 9.70a,b,c | 100 |
| 6 | N. biserrata (10 mg/ml) | 29.16 ± 5.85a,c,d | 100 |
Each value represents mean ± S.D of 6 fishes per group.
aSignificance at p < 0.05 compared with the negative control group.
bSignificance at p < 0.05 compared with the formalin treated group (0.1%) (v/v).
cSignificance at p < 0.05 compared with N. biserrata (2.5 mg/ml).
dSignificance at p < 0.05 compared with N. biserrata (5.0 mg/ml).
Behaviour of Z. arugamensis
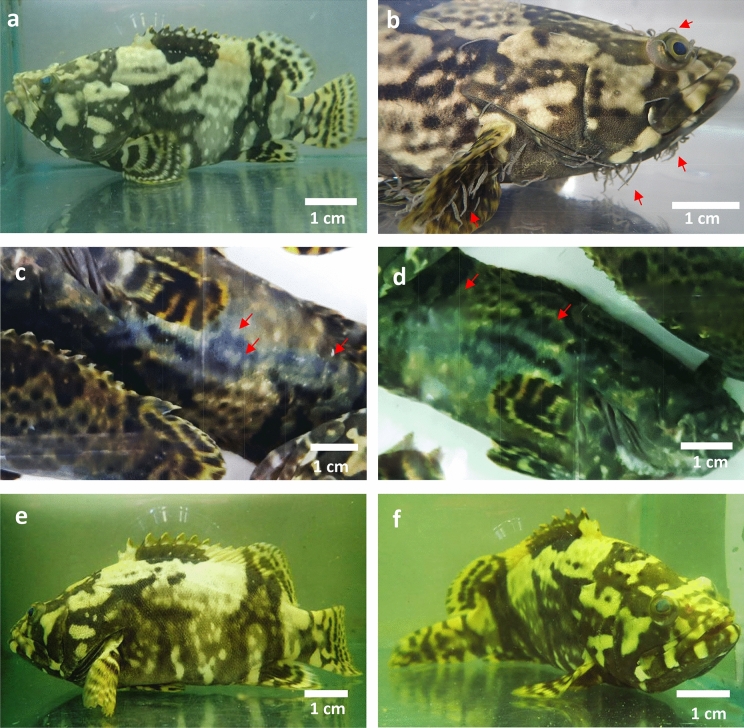
The leeches used their anterior and posterior suckers for locomotion and showed well-organized movement. In the normal control group, the leeches were attached firmly with suckers (Fig. 1a). In the case of formalin, the leeches showed strong swimming for 60 s and it declined sluggishly, after 3 min no movements were noticed. In the formalin treated group, some of the parasitic leeches were able to attach the anterior or posterior sucker to the bottom of the Petri dish before they were dead as compared to the extract-treated group (Fig. 1b). When the leeches were exposed to various concentrations of methanol extract of N. biserrata, the movement was not organized and the leeches were not able to use either posterior or anterior suckers for the movement. At 100 mg/ml, the leeches showed vigorous swimming in a zig-zag pattern from 30 to 60 s; then, the swimming gradually reduced; while after 3–4 min, the movement stopped, and the leeches were paralyzed and dead, indicated no movement after physical touch. At 50 mg/ml, fast-swimming was noticed for 30 s and it decreased slowly. After 7 min, no movements were noticed, and the leeches moved only with a physical touch before they were dead. At 25 mg/ml, strong movements were noticed for 30 s and it lessened gradually, after 18 to 25 min the movements were stopped (Fig. 1c, d).
Figure 1.
Zeylanicobdella arugamensis, (a) normal control, (b) formalin administered group, (c) and (d) Nephrolepis biserrata methanol extract administered group.
Behaviour of hybrid groupers
Fishes in the normal control group showed normal swimming activity (Fig. 2a) and in the negative control group they were weak (Fig. 2b). Fishes treated with 0.1% formalin showed extreme aggressive behaviour throughout the treatment. Around body areas of the formalin treated groups were found with wounds (Fig. 2c, d) as compared to the plant treated groups. However, active swimming was noticed in the group exposed to 10 mg/ml of the plant extract for the first 1 to 5 min later, they were relaxed. The fishes exposed to 5 mg/ml of the extract showed active swimming for 1 to 2 min while no active swimming was noticed in the fishes treated with 2.5 mg/ml of the plant extract. In the end, all the fishes treated with plant extracts were relaxed (Fig. 2e, f).
Figure 2.
Hybrid groupers (Epinephelus fuscoguttatus x E.lanceolatus) (a) normal control, (b) positive control, highly infested with parasitic leeches, the arrow indicates the prevalence of the leeches on the head, eyes and fins area, (c,d) formalin (0.1%) treated group, arrows indicate the appearance of wounds on the body surface due to the aggressive swimming and stress induced by the chemical, (e,f) Nephrolepis biserrata methanol extract administered group, no wounds or leeches were noticed on the surface of the fishes after the treatment.
Physicochemical parameters of leeches treated solutions
The water quality parameters of the control groups and methanol extract groups solutions applied for the anti-parasitic assays are indicated in Table 3. All parameters determined were remained constant except with a change in pH value of the extract solution as compared to the control solutions. The pH ranged from 4.4 to 4.15 in the methanol solutions of N. biserrata. The change in pH of the plant solution could be due to the presence of different bioactive compounds with acidic nature.
Table 3.
Water quality parameters of the solutions for the treatment of leeches.
| No. | Water parameters | Concentrations | ||||
|---|---|---|---|---|---|---|
| Groups | Normal control | Positive control (formalin 0.25%) (v/v) | N. biserrata (mg/ml) | |||
| (25) | (50) | (100) | ||||
| 1 | Temperature (°C) | 24.76 | 26.16 | 24.9 | 24.9 | 25.1 |
| 2 | pH | 7.89 | 7.24 | 4.31 | 4.20 | 4.15 |
| 3 | Salinity (ppt) | 30.9 | 30.9 | 30.9 | 30.9 | 30.9 |
| 4 | Dissolved oxygen (mg/l) | 5.50 | 5.00 | 5.28 | 5.40 | 5.50 |
Physicochemical parameters of hybrid groupers treated solutions
The water quality parameters of the normal, negative and positive control group solutions and methanol extract solutions applied for the fish disinfestation are indicated in Table 4. All parameters determined remained constant. The pH of the methanol extract solutions was maintained above 7 with the addition of sodium bicarbonate (I mg/ml) to provide an alkaline environment to the fishes.
Table 4.
Water quality parameters of the solutions applied for the fish treatment.
| No. | Water parameters | Concentrations | |||||
|---|---|---|---|---|---|---|---|
| Groups | Normal control | Negative control | Positive control (formalin 0.1%) (v/v) | N. biserrata (mg/ml) | |||
| (2.5) | (5) | (10) | |||||
| 1 | Temperature (°C) | 28.0 | 28.0 | 28.0 | 28.0 | 28.0 | 28.0 |
| 2 | pH | 7.13 | 7.36 | 7.68 | 7.04 | 8.04 | 7.15 |
| 3 | Salinity (ppt) | 30.29 | 33.34 | 30.51 | 33.03 | 33.03 | 33.39 |
| 4 | Dissolved oxygen (mg/l) | 5.16 | 5.56 | 4.91 | 5.09 | 5.63 | 5.87 |
LC-QTOF analysis and metabolites identification
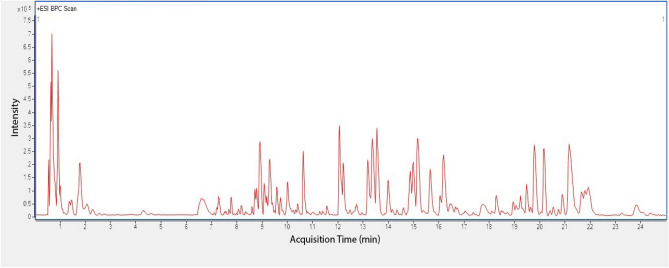
A total of 77 compound’s features were extracted and determined for their molecular formula by executing Molecular Feature Extraction (MFE) algorithm in Agilent MassHunter software. Among these 77 metabolites, 18 of them were successfully matched with the METLIN databases and previously reported in plant extract (Table 5; Fig. 3).
Table 5.
Matched metabolites in methanol extract of N. biserrata.
| Retention time (min) | Mass to charge ratio (m/z) | Formula | Mass error (ppm) | Matched metabolites | References |
|---|---|---|---|---|---|
| 0.766 | 236.1497 | C10H18O5 | − 1.91 | 2-Hydroxydecanedioic acid | 40 |
| 0.869 | 130.0501 | C5H7NO3 | − 1.71 | Pyroglutamic acid | 41 |
| 0.898 | 274.0927 | C11H12O7 | − 2.92 | Piscidic Acid | 42 |
| 7.357 | 355.1027 | C16H18O9 | − 1.25 | Chlorogenic Acid | 34 |
| 8.817 | 163.0388 | C9H6O3 | 0 | 3-Hydroxycoumarin | 35 |
| 9.112 | 197.1169 | C11H16O3 | 1.72 | Benzenemethanol, 2-(2-aminopropoxy)-3-methyl | 43 |
| 11.609 | 329.1029 | C18H16O6 | − 1.79 | Alnustin | 44 |
| 12.767 | 336.2172 | C19H29NO4 | − 1.2 | Ankorine | 45 |
| 13.378 | 315.0867 | C17H14O6 | − 1.26 | Kaempferol 7,4′-dimethyl ether | 46 |
| 13.437 | 345.0975 | C18H16O7 | − 1.57 | Pachypodol | 47 |
| 13.965 | 249.1489 | C15H20O3 | − 1.16 | Ivalin | 48 |
| 14.856 | 277.2163 | C18H28O2 | 0.1 | 9,13-Octadecadiynoic acid | 36 |
| 15.061 | 277.2164 | C18H28O2 | − 0.47 | 9,13-Octadecadiynoic acid | 49 |
| 15.216 | 233.1539 | C15H20O2 | − 1.14 | Isovelleral | 37 |
| 16.138 | 295.2266 | C18H30O3 | 0.09 | 17-Hydroxy-linolenic acid | 50 |
| 16.511 | 498.3794 | C28H48O6 | − 0.19 | Brassinolide | 51 |
| 19.427 | 282.2795 | C18H35NO | − 0.88 | Oleamide | 52 |
| 22.004 | 567.4204 | C40H54O2 | − 1.31 | Eschscholtzxanthin | 53 |
Figure 3.
Base peak chromatogram (BPC) of the methanol extract of Nephrolepis biserrata.
Discussion
Ectoparasitic leech (Z. arugamensis) provided a significant threat to the grouper aquaculture facilities in Malaysia8,25. To control the leech infestation, formalin and other chemicals have been utilized by fish farmers, which cannot provide a conducive environment for the eco-friendly aquaculture policy14. On the other hand, plant-based extracts can be used as a safe and natural treatment for the parasite infestations due to the presence of antiparasitic secondary metabolites16. In addition to this, the secondary metabolites present in the plant extract also play an important role in the enhancement of growth and immunity of cultured species. The olive oil leaf extract has been reported to boost the activation of intestinal digestive enzyme activity and the expression of genes related with growth in brain, liver, muscle and other vital tissues of common carp Cyprinus carpio26. In the present study, we determined the potential of antiparasitic activity of the local medicinal plant N. biserrata. The mortality time of the leeches after the exposure at various concentrations of the plant extracts in a dose-dependent manner has been elucidated (Table 1). Average time of 4.88 ± 0.50 min., was taken to achieve 100% mortality of the leeches in the group treated with 100 mg/ml of the plant extract followed by 11.91 ± 0.99 min. (50 mg/ml) and 25.11 ± 3.26 min. (25 mg/ml). Our results are in comparison with the recently published data regarding the anti-parasitic activity of Dillenia suffruticosa27. The methanol extract of D. suffruticosa showed antiparasitic activity against Z. arugamensis with 100% mortality at the concentrations of 100 mg/ml (14.39 ± 3.75 min), 50 mg/ml (32.97 ± 9.29 min) and 25 mg/ml (41.77 ± 5.40 min). In the present study, the anti-parasitic potential of the methanol extract of N. biserrata was almost 3 times faster than D. suffruticosa methanol extract28. In another study, the parasitic leech Piscicola geometra from the infested cobia fish exposed for 96 h to various dilutions of the extracts of Scutellaria baicalensis and Morinda citrifolia and obtained 100% and 80% mortalities, respectively28. Further P. geometra treated with roots and stem bark extract of Tetracera alnifolia plant and indicated a significant effect on the rates of the mortality of the leech after 24 h29. The effectiveness of various concentrations of Nicotiana spp., Zanthoxylum alatum, and Solanum khasianum extracts was tested against Hirudinaria manillensis leech. All leeches were dead at an average time of 2.11 ± 0.11 min. (Nicotiana spp.), 3.00 ± 0.33 min. (Z. alatum) and 24.89 ± 2.34 min. (S. khasianum)30.
Low and suitable concentrations such as 2.5, 5 and 10 mg/ml of the plant extracts were administered for fish disinfestation to avoid the fishes from stress. The disinfestation of fishes was obtained in a dose-dependent manner (Table 2). Groups of infested fishes treated with 10 mg/ml of the methanol extract were disinfested in less than 30 min. Similar results were noticed in the group treated with 5 and 2.5 mg/ml of the extract in the period of 65 and 108 min., respectively. The infested fishes treated with 0.1% formalin took about 26 min. to get rid of the parasitic leeches. According to statistical analysis, no significant differences were noticed in the disinfestation time of the formalin (0.1%) and plant treated group (10 mg/ml). This showed that the treatment effect of the higher concentration of the methanol extract of N. biserrata (10 mg/ml) was the same as that of formalin (0.1%) (v/v). Our results are in agreement with the anti-parasitic activity of solvent extract (petroleum ether, chloroform, ethyl acetate, methanol) and aqueous extracts of plants, Radix angelicae pubescentis, Fructus bruceae, Caulis spatholobi, Semen aesculi, and Semen pharbitidis against the monogenean Dactylogyrus intermedius in goldfish31. Among the experimental extracts, the methanol and aqueous extracts of S. aesculi were noticed to be more effective after 48 h of exposure, followed by methanol extracts of Fructus bruceae, Radix angelicae pubescentis, Caulis spatholobi, and Semen pharbitidis31.
In the present study, for the comparison of the anti-parasitic effect of the plant extracts, we used formalin as a positive control, since it is vastly utilized for the removal of parasites in aquaculture facilities14. Besides, other chemicals such as trichlorfon, hydrogen peroxide and copper sulfate have also been used as an anti-parasitic agent32. In Malaysia, other drugs like benzalkonium chloride, acriflavine, malachite green, hypochlorite and poly-vinyl pyrrolidine are also utilized for the removal of parasites in aquaculture facilities33. These chemicals are harmful to fish and their consumers14,33. However, the methanol extract of N. biserrata could be obtained from a natural source with no known side effects23.
In the current study, LC-QTOF was employed to profile the methanol extract of N. biserrata and detected some interesting metabolites (Table 5). Current tandem mass spectrometry data from LC-QTOF showed some hits on such as terpenoids (ivalin, isovelleral, brassinolide, and eschscholtzxanthin), flavonoids (alnustin, kaempferol 7,4′-dimethyl ether, and pachypodol), phenolics (piscidic acid, chlorogenic acid, and ankorine), and aromatic (3-hydroxycoumarin), etc. Some of these compounds were reported possessing anti-parasitic characteristics, such as chlorogenic acid34,35, ivalin36 and isovelleral37. The chlorogenic acid was reported to possess anti-parasitic activities on vertebrate parasites Trypanosoma brucei rhodesiense and Leishmania donovani with IC50 values of 18.9 µg/ml and 7.0 µg/ml, respectively34. Ivalin was also reported with stronger anti-parasitic activity on Trypanosoma brucei rhodesiense and Trypanosoma cruzi (IC50: 1.9 µg/ml and 6.2 µg/ml, respectively)36. On the other hand, isovelleral was found significantly inhibited germination of plant-parasitic fungus Alternaria brassicicola O-264 isolates conidial at as low as 0.05 ppm of vapour concentration37. However, anti-parasitic properties of the detected bioactive compounds in N. biserrata have not been reported on marine parasites hence their efficacies are pending for discovery. The strong antiparasitic effect of the methanol extracts of N. biserrata could be due to the presence of the above-mentioned metabolites34–37.
Our study proved that administration of the methanol extract of N. biserrata showed a strong anti-parasitic activity with 100% mortality against the marine leech Z. arugamensis parasitic on hybrid groupers in Malaysia. The exposure of the leech-infested hybrid groupers to methanol extracts of N. biserrata at a higher concentration (10 mg/ml) also resulted in the removal of parasitic leeches in less than 30 min. The tandem mass spectrometry data from LC-QTOF indicated some hits on terpenoids, flavonoids, phenolics and aromatic. The antiparasitic properties of N. biserrata could be due to the presence of various bioactive compounds. Some of the compounds have already been reported with strong anti-parasitic properties. It indicates that N. biserrata has a strong potential to act as a biocontrol agent against leech infestation in hybrid groupers. However, further study on the purification and isolation of the pure bioactive compounds responsible for the antiparasitic properties is imperative.
Materials and methods
Chemicals
Formalin (37% aqueous formaldehyde solution) and sodium bicarbonates were purchased from Sigma, Leica, Microsystem, and Germany. HPLC grade methanol was obtained from Merck (Darmstadt, Germany). LCMS-grade acetonitrile was purchased from J. T. Baker (Philipsburg, NJ, USA). Deionized water was acquired using Milli-Q system (Merck, Darmstadt, Germany) at resistivity of > 18.2 MΩ cm. Reference mass solution containing 5.0 mM of purine and 2.5 mM of Hexakis [1H,1H,3H-tetrafluoropropoxy] phosphazine, was procured from Agilent Technologies (Santa Clara, CA, USA). LCMS-grade formic acid (HCOOC) was acquired from Fisher Scientific (Fair Lawn, NJ, USA). Polyvinylidene fluoride (PVDF) syringe filters with 0.22 µm pore size and 13 mm diameter were purchased from Merck (Darmstadt, Germany).
Plant collection
The leaves and stem of the N. biserrata were obtained from lowlands of Papar (5.7346° N, 115.9319° E.), Kota Kinabalu, Sabah, Malaysia. The identification of the plant was carried out and a voucher specimen (MDS-001) was deposited at the herbarium of the Institute for Tropical Biology and Conservation, Universiti Malaysia Sabah, Kota Kinabalu, Malaysia.
Solvent extraction
The leaves and stem of the plant were washed with distilled water and oven-dried in an oven at 37 °C for 3 days. The dried plant was grounded to a fine powder using a mechanical grinder and stored in an airtight container. About 60 g of dry powder of the plant was extracted with HPLC grade methanol (300 ml) using an orbital shaker (25–30 °C) for 3 days. The supernatant was filtered through Whatman No. 41 filter paper by vacuum and the fresh solvent was added to the samples, which were extracted for another 3 days. The methanol residues were removed from the extract using a vacuum rotary evaporator (R-215, BUCHI, Switzerland). The sample was kept at − 80 °C for 24 h and then lyophilized using a freeze drier (Freezon 12, Labconco, USA). The freeze-dried sample (4.80 g) was then stored in the freezer for further studies.
Anti-parasitic bioassay
Parasitic leeches were collected from the aquaculture facilities of Universiti Malaysia Sabah. The leeches were identified based on their morphological features10. Adult leeches were selected, divided into 5 groups (6 leeches per group) and each group was provided with six leeches in a Petri dish.
Group 1: Normal control, treated with seawater only (Fig. 1a).
Group 2: Positive control, treated with formalin (0.25% v/v) solution (Fig. 1b).
Groups 3, 4 and 5: Treated with 25, 50 and 100 mg/ml of the methanol extracts of N. biserrata, respectively.
During the challenge, mortality time was recorded using a stopwatch for 720 min27. The experiment was performed in triplicate.
Observation on the behaviour of leeches
The changes in the behaviour of leeches were observed visually after exposure to formalin and different concentrations of plant extract as compared to the normal control group.
Fish disinfestation
Hybrid groupers (Epinephelus fuscoguttatus x E.lanceolatus) (TL: 15–20 cm) were obtained from the hatchery facilities of University Malaysia Sabah. All animals were treated humanely and well maintained under standard ethical principles as per university regulations (UMS/IP7.5/M3/4–2012). All experimental protocols were approved by the University Malaysia Sabah committee. They were acclimatized for 2 weeks before the start of the experiment. Later, all fishes except normal control group were infested with leeches for one week. Further, they were divided into the following groups (6 fishes per group).
Group 1: Normal control, treated with seawater only, no leech infestation (Fig. 2a).
Group 2: Negative control, infested with leeches and no treatment was applied (Fig. 2b).
Group 3: Positive control, infested + treated with formalin (0.1% v/v) solution.
Groups 4, 5 and 6: Infested + treated with 2.5, 5.0 and 10 mg/ml of the methanol extract of N. biserrata, respectively.
During the experiment, the time required for the complete removal of all the leeches (disinfestation time) from the treated fishes was recorded using a stopwatch.
Observation on the behaviour of hybrid groupers
The changes in the behaviour of the hybrid groupers were monitored after exposure to formalin and different concentrations of the plant extracts in comparison to the normal control group.
Liquid chromatography quadrupole time-of-flight (LC-QTOF) acquisition
The methanol extract of N. biserrata was analyzed using an Agilent 1290 Infinity LC system coupled with an Agilent 6520 QTOF mass spectrometry system. A 2.0 µl sample was injected into a reversed-phase column, namely Agilent Zorbax Eclipse XDB-C18 (narrow bore, 2.1 mm × 150 mm × 3.5 µm; Agilent Technologies, Santa Clara, CA, USA). The column was maintained at 25 °C at a flow rate of 500µL/min during analysis. The mobile phases were composed of solvent A (H2O—0.1% HCOOH) and solvent B (acetonitrile—0.1% HCOOH). The gradient elution program was initiated at 5% of solvent B for 5 min, then from 5 to 100% solvent B in 15 min. and kept for 5 min. Later, the column was conditioned as initial for 5 min. prior to next injection.
The mass spectrometry (MS) signals were acquired as described by Ling et al. (2018)38 with minor modifications. Briefly, voltage for positive electrospray ionization was changed to 4 kV, while the remaining settings for ion source were maintained. The MS was calibrated with Tuning Mix (Agilent Technologies, Santa Clara, CA, USA) before each batch analysis. Internal mass calibration standards, purine (m/z 121.0508) and hexakis-(1H, 1H, 3H-tetrafluoropropoxy)-phosphazine (m/z 922.0097), were introduced throughout the runs for automated mass correction39.
Identification of metabolites
Automated tandem mass spectrometry (auto-MSMS) was employed for metabolites matching, where precursor ions were selected consistently and fragmented throughout the data acquisition. Briefly, two major precursor ions were selected from each MS scan for fragmentation with nitrogen gas at a collision energy of 30 eV. Data acquisition for product ions was set between an m/z of 50 and 1500. Acquired data were processed and identified using Agilent MassHunter Qualitative Workflows software (version B.08.00), which is equipped with MassHunter METLIN Metabolite PCD/PCDL (Personal Compound Database and Personal Compound Database and Library).
Statistical analysis
Data analysis was carried out using IBM SPSS Statistics 25 Window package (IBM, Armonk, NY, US). Significant differences between groups were investigated using one-way analysis of variance (ANOVA) followed by Tukey’s test. All data points were shown as mean ± standard deviation (S.D.). p values < 0.05 were viewed as significant27.
Acknowledgements
This research work was supported by the Fundamental Research Grant Scheme, The Ministry of Education Malaysia, Grant/Award Number: FRG0486-2018, PRF No.0011-2019 to BAVM and JSPS Bilateral Joint Research Project No. JPJSBP120209924 to SO.
Author contributions
M.D.S., B.A.V.M.: Methodology, Writing the original draft; B.A.V.M.: Conceptualization; M.D.S., B.A.V.M., F.K.H.: Investigation, Formal Analysis; B.A.V.M., J.R., F.F.C., S.R.M.S., R.S., S.O.: Validation, Supervision, Reviewing the Original Draft & Editing; M.D.S., B.A.V.M., Y.S.Y.: Data curation; B.A.V.M., S.O.: Funding acquisition. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rimmer MA, Glamuzina B. A review of grouper (Family Serranidae: Subfamily Epinephelinae) aquaculture from a sustainability science perspective. Rev. Aquac. 2019;11:58–87. doi: 10.1111/raq.12226. [DOI] [Google Scholar]
- 2.Nagasawa, K. & Cruz-lacierda, E. R. Diseases of cultured Groupers (Southeast Asian Fisheries Development Center, Aquaculture Department Tigbauan, 2004)
- 3.Ching CL, Senoo S. Egg and larval development of a New hybrid grouper, tiger grouper Epinephelus fuscoguttatus giant grouper E. lanceolatus. Aquac. Sci. 2008;56:505–512. [Google Scholar]
- 4.James CM, Al-Thobaiti SA, Rasem BM, Carlos MH. Potential of grouper hybrid (Epinephelus fuscoguttatus x E. polyphekadion) for aquaculture. Naga ICLARM Q. 1999;22:19–23. [Google Scholar]
- 5.Shapawi R, Ching FF, Senoo S, Mustafa S. Nutrition, growth and resilience of tiger grouper (Epinephelus fuscoguttatus) × giant grouper (Epinephelus lanceolatus) hybrid—a review. Rev. Aquac. 2019;11:1285–1296. doi: 10.1111/raq.12292. [DOI] [Google Scholar]
- 6.Kleinertz S, Palm HW. Parasites of the grouper fish Epinephelus coioides (Serranidae) as potential environmental indicators in Indonesian coastal ecosystems. J. Helminthol. 2015;89:86–99. doi: 10.1017/S0022149X1300062X. [DOI] [PubMed] [Google Scholar]
- 7.Venmathi Maran BA, Leong TS, Ohtsuka S, Nagasawa K. Records of Caligus (Crustacea: Copepoda: Caligidae) from marine fish cultured in floating cages in Malaysia with a redescription of the male of Caligus longipedisBassett-Smith, 1898. Zool. Stud. 2009;48:797–807. [Google Scholar]
- 8.Kua BC, Choong FC, Leaw YY. Effect of salinity and temperature on marine leech, Zeylanicobdella arugamensis (De Silva) under laboratory conditions. J. Fish Dis. 2014;37:201–207. doi: 10.1111/jfd.12087. [DOI] [PubMed] [Google Scholar]
- 9.Cruz-Lacierda ER, Toledo JD, Tan-Fermin JD, Burreson EM. Marine leech (Zeylanicobdella arugamensis) infestation in cultured orange-spotted grouper Epinephelus coioides. Aquaculture. 2000;185:191–196. doi: 10.1016/S0044-8486(99)00356-7. [DOI] [Google Scholar]
- 10.Kua BC, Azmi MA, Hamid NKA. Life cycle of the marine leech (Zeylanicobdella arugamensis) isolated from sea bass (Lates calcarifer) under laboratory conditions. Aquaculture. 2010;302:153–157. doi: 10.1016/j.aquaculture.2010.02.029. [DOI] [Google Scholar]
- 11.Pitten FA, Kramer A, Herrmann K, Bremer J, Koch S. Formaldehyde neurotoxicity in animal experiments. Pathol. Res. Pract. 2000;196:193–198. doi: 10.1016/S0344-0338(00)80100-4. [DOI] [PubMed] [Google Scholar]
- 12.National Toxicology Program. NTP 12th Report on Carcinogens. Rep. Carcinog.12, iii–499 (2011). [PubMed]
- 13.Plumb JA. Disease control in aquaculture. In: Shariff M, Subasinghe RP, Arthur JR, editors. Diseases in Asian Aquaculture. Fish Health Section. Manila: Asian Fisheries Society; 1992. pp. 3–17. [Google Scholar]
- 14.Leal JF, Neves MGPMS, Santos EBH, Esteves VI. Use of formalin in intensive aquaculture: properties, application and effects on fish and water quality. Rev. Aquac. 2018;10:281–295. doi: 10.1111/raq.12160. [DOI] [Google Scholar]
- 15.Wunderlich, A. C., Zica, É. de O. P., Ayres, V. F. dos S., Guimarães, A. C. & Takeara, R. Plant-derived compounds as an alternative treatment against parasites in fish farming: a review. in Natural Remedies in the Fight Against Parasites (InTech, 2017). 10.5772/67668.
- 16.Wink M. Medicinal plants: A source of anti-parasitic secondary metabolites. Molecules. 2012;17:12771–12791. doi: 10.3390/molecules171112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isah, M. B. et al. Terpenoids as emerging therapeutic agents: cellular targets and mechanisms of action against protozoan parasites. in Studies in Natural Products Chemistry vol. 59 227–250 (Elsevier B.V., 2018).
- 18.Kulip J, et al. Medicinal plants in Maliau Basin, Sabah Malaysia. J. Trop. Biol. Conserv. 2010;6:21–33. [Google Scholar]
- 19.Langeland, K. A. Natural area weeds: distinguishing native and non-native " Boston ferns " and " Sword ferns ". http://edis.ifas.ufl.edu (2014).
- 20.Christensen, H. Uses of ferns in two indigenous communities in Sarawak, Malaysia. in Holttum Memorial Volume United Kingdom (ed Johns, R. J.) 177–192 (1997).
- 21.Boonkerd T, Chantanaorrapint S, Khwaiphan W. Pteridophyte diversity in the tropical lowland rainforest of Khao Nan national park, Nakhon Si Thammarat province Thailand. Trop. Nat. Hist. 2008;8:83–97. [Google Scholar]
- 22.Shah MD, Yong YS, Iqbal M. Phytochemical investigation and free radical scavenging activities of essential oil, methanol extract and methanol fractions of Nephrolepis biserrata. Int. J. Pharm. Pharm. Sci. 2014;6:269–277. [Google Scholar]
- 23.Shah MD, Gnanaraj C, Haque AE, Iqbal M. Antioxidative and chemopreventive effects of Nephrolepis biserrata against carbon tetrachloride (CCl4)-induced oxidative stress and hepatic dysfunction in rats. Pharm. Biol. 2015;53:31–39. doi: 10.3109/13880209.2014.909502. [DOI] [PubMed] [Google Scholar]
- 24.Dantu P, Rani D, Khare P. In vitro antibacterial and antifungal properties of aqueous and non-aqueous frond extracts of Psilotum nudum, Nephrolepis biserrata and Nephrolepis cordifolia. Indian J. Pharm. Sci. 2010;72:818. doi: 10.4103/0250-474X.84606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravi R, Shariman Yahaya Z. Zeylanicobdella arugamensis, the marine leech from cultured crimson snapper (Lutjanus erythropterus), Jerejak Island, Penang Malaysia. Asian Pac. J. Trop. Biomed. 2017;7:473–477. doi: 10.1016/j.apjtb.2017.01.018. [DOI] [Google Scholar]
- 26.Zemheri-Navruz F, Acar Ü, Yılmaz S. Dietary supplementation of olive leaf extract enhances growth performance, digestive enzyme activity and growth related genes expression in common carp Cyprinus carpio. Gen. Comp. Endocrinol. 2020;296:113541. doi: 10.1016/j.ygcen.2020.113541. [DOI] [PubMed] [Google Scholar]
- 27.Shah MD, et al. Antiparasitic activity of the medicinal plant Dillenia suffruticosa against the marine leech Zeylanicobdella arugamensis (Hirudinea) and its phytochemical composition. Aquac. Res. 2020;51:215–221. doi: 10.1111/are.14367. [DOI] [Google Scholar]
- 28.Rizky PN, Cheng TC, Nursyam H. Anti-leech activity of Scutellaria baicalensis and Morinda citrifolia extracts against Piscicola geometra. IOP Conf. Ser. Earth Environ. Sci. 2018;137:012031. doi: 10.1088/1755-1315/137/1/012031. [DOI] [Google Scholar]
- 29.Nwabueze AA. A preliminary investigation on the toxicity of Tetracera alnifolia on Piscicola geometra in fish culture. Agric. Sci. 2013;1:1–9. [Google Scholar]
- 30.Bam J, et al. In-vitro effect of some plant extracts on Buffalo leech Hirudinaria manillensis. Indian J. Anim. Sci. 2016;86:988–990. [Google Scholar]
- 31.Liu Y-T, et al. In vivo anthelmintic activity of crude extracts of Radix angelicae pubescentis, Fructus bruceae, Caulis spatholobi, Semen aesculiand Semen pharbitidis against Dactylogyrus intermedius (Monogenea) in goldfish (Carassius auratus) Parasitol. Res. 2010;106:1233–1239. doi: 10.1007/s00436-010-1799-9. [DOI] [PubMed] [Google Scholar]
- 32.Thing CY, Ransangan J, Hatai K. Antiparasitic effect of formalin, trichlorfon, hydrogen peroxide, and copper sulfate on the parasitic isopod Caecognathia coralliophila. Fish Pathol. 2016;51:125–127. doi: 10.3147/jsfp.51.125. [DOI] [Google Scholar]
- 33.Shariff, M., Nagaraj, G., Chua, F. H. . & Wang, Y. G. The use of chemicals in aquaculture in Malaysia and Singapore. in Use of Chemicals in Aquaculture in Asia : Proceedings of the Meeting on the Use of Chemicals in Aquaculture in Asia, 20–22 May 1996, Tigbauan, Iloilo, Philippines (eds Arthur, J. R. & Lavilla-Pitogo, C. R.) 127–140 (2000).
- 34.Kırmızıbekmez H, et al. Inhibiting activities of the secondary metabolites of Phlomis brunneogaleata against parasitic protozoa and plasmodial enoyl-acp reductase, a crucial enzyme in fatty acid biosynthesis. Planta Med. 2004;70:711–717. doi: 10.1055/s-2004-827200. [DOI] [PubMed] [Google Scholar]
- 35.Palmer-Young EC, Sadd BM, Stevenson PC, Irwin RE, Adler LS. Bumble bee parasite strains vary in resistance to phytochemicals. Sci. Rep. 2016;6:37087. doi: 10.1038/srep37087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt TJ, Brun R, Willuhn G, Khalid SA. Anti-trypanosomal activity of Helenalin and some structurally related sesquiterpene lactones. Planta Med. 2002;68:750–751. doi: 10.1055/s-2002-33799. [DOI] [PubMed] [Google Scholar]
- 37.Osaki-Oka K, et al. Antifungal activity of the volatile compound isovelleral produced by ectomycorrhizal Russula fungi against plant-pathogenic fungi. J. Gen. Plant Pathol. 2019;85:428–435. doi: 10.1007/s10327-019-00872-8. [DOI] [Google Scholar]
- 38.Ling YS, Lim LR, Yong YS, Tamin O, Puah PY. MS-based metabolomics revealing Bornean Sinularia sp. extract dysregulated lipids triggering programmed cell death in Hepatocellular carcinoma. Nat. Prod. Res. 2018 doi: 10.1080/14786419.2018.1531288. [DOI] [PubMed] [Google Scholar]
- 39.Yong Y-S, Chong ETJ, Chen H-C, Lee P-C, Ling YS. A Comparative study of Pentafluorophenyl and Octadecylsilane columns in high-throughput profiling of biological fluids. J. Chin. Chem. Soc. 2017;64:699–710. doi: 10.1002/jccs.201600873. [DOI] [Google Scholar]
- 40.Ferreira JPA, Miranda I, Sousa VB, Pereira H. Chemical composition of barks from Quercus faginea trees and characterization of their lipophilic and polar extracts. PLoS ONE. 2018;13:e0197135. doi: 10.1371/journal.pone.0197135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schilling S, Wasternack C, Demuth H-U. Glutaminyl cyclases from animals and plants: a case of functionally convergent protein evolution. Biol. Chem. 2008;389:983–991. doi: 10.1515/BC.2008.111. [DOI] [PubMed] [Google Scholar]
- 42.Takahira M, Kusano A, Shibano M, Kusano G, Miyase T. Piscidic acid and fukiic acid esters from Cimicifuga simplex. Phytochemistry. 1998;49:2115–2119. doi: 10.1016/S0031-9422(98)00407-5. [DOI] [Google Scholar]
- 43.Pinto da Silva L, Simkovitch R, Huppert D, Esteves da Silva JCG. Combined experimental and theoretical study of the photochemistry of 4- and 3-hydroxycoumarin. J. Photochem. Photobiol. A Chem. 2017;338:23–36. doi: 10.1016/j.jphotochem.2017.01.032. [DOI] [Google Scholar]
- 44.Hussein HJ, Hadi MY, Hameed IH. Study of chemical composition of Foeniculum vulgare using Fourier transform infrared spectrophotometer and gas chromatography - mass spectrometry. J. Pharmacogn. Phyther. 2016;8:60–89. [Google Scholar]
- 45.Mesquita AAL, Corrêa DDB, De Pádua AP, Guedes MLO, Gottlieb OR. Flavonoids from four Compositae species. Phytochemistry. 1986;25:1255–1256. doi: 10.1016/S0031-9422(00)81599-X. [DOI] [Google Scholar]
- 46.Panara K, et al. Importance of Alangium salviifolium and its pharmacological update. Eur. J. Med. Plants. 2016;12:1–15. doi: 10.9734/EJMP/2016/23899. [DOI] [Google Scholar]
- 47.Sudsai T, Wattanapiromsakul C, Tewtrakul S. Inhibition of nitric oxide production by compounds from Boesenbergia longiflora using lipopolysaccharide-stimulated RAW264.7 macrophage cells. Songklanakarin J. Sci. Technol. 2013;35:317–323. [Google Scholar]
- 48.Ali H-A, et al. Pachypodol, a flavonol from the leaves of Calycopteris floribunda, inhibits the growth of CaCo 2 colon cancer cell line in vitro. Phyther. Res. 2008;22:1684–1687. doi: 10.1002/ptr.2539. [DOI] [PubMed] [Google Scholar]
- 49.Wang L, Yuan Z. Research progress in polysaccharide compositions of Glehnia littoralis. Asian J. Tradit. Med. 2020;15:136–143. [Google Scholar]
- 50.Moradi P, Mahdavi A, Khoshkam M, Iriti M. Lipidomics unravels the role of leaf lipids in thyme plant response to drought stress. Int. J. Mol. Sci. 2017;18:2067. doi: 10.3390/ijms18102067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grove MD, et al. Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature. 1979;281:216–217. doi: 10.1038/281216a0. [DOI] [Google Scholar]
- 52.Munigunti R, et al. Identification of Oleamide in Guatteria recurvisepala by LC/MS-based Plasmodium falciparum thioredoxin reductase ligand binding method. Planta Med. 2011;77:1749–1753. doi: 10.1055/s-0030-1271080. [DOI] [PubMed] [Google Scholar]
- 53.Czeczuga B, Czeczuga-Semeniuk E, Morales Mèndez A, Lopèz-Rgueiras M. Carotenoids in the thalli of certain Venezuelan lichen species of the genera Pyxine and Ramalina. Fedde Repert. 2004;115:504–509. doi: 10.1002/fedr.200411049. [DOI] [Google Scholar]