Abstract
Red cell distribution width (RDW), routinely assessed as a component of a complete blood count (CBC), quantifies the variation in the size of red blood cells. It increases with age, and increased RDW predicts many aging-related diseases and mortality. However, whether it also predicts hip fracture is unknown. We prospectively evaluated the association between RDW and hip fracture using data from the Osteoporotic Fracture in Men (MrOS) study. RDW was measured in 3635 men (aged 71 to 99 years) along with bone mineral density (BMD) in MrOS. RDW ranged from 11.3% to 32.9% (median 14.0%; interquartile range 13.5% to 14.8%) and was categorized into four groups (≤13.0%, 13.1% to 14.0%, 14.1% to 15.0%, ≥15.1%). Study participants with a hemoglobin level <13.0 g/dL were classified as having anemia. During an average 8.1 years, 164 men suffered hip fractures. The risks of hip fractures increased with increase of RDW category. Furthermore, there was a significant interaction between anemia and RDW: An association between RDW and hip fractures was only observed in participants without anemia. In those without anemia, the relative hazard of hip fractures increased with increases in RDW category: Men in the highest RDW category had a 2.8 times higher risk of hip fractures than men in the lowest group (95% confidence interval 1.1 to 7.1). The risks of all-clinical fractures were also increased along with higher RDW values. Additionally, RDW was significantly associated with the risk of having a fall but not with femoral neck or total hip BMD. In conclusion, RDW and anemia defined by hemoglobin are widely available routine laboratory measurements that together could indicate increased risk of hip fracture, reflecting the neuromuscular effects of aging rather than lower hip BMD. © 2020 American Society for Bone and Mineral Research.
Keywords: AGING, ANEMIA, HIP FRACTURE, OSTEOPOROSIS, RED CELL DISTRIBUTION WIDTH
Introduction
Red cell distribution width (RDW) is routinely assessed with a complete blood count (CBC). It measures the variation in red blood cell (RBC) size.(1) Higher RDW value indicates greater heterogeneity in size.(2) Clinical use of RDW index had been traditionally limited in hematologic field, particularly in distinguishing causes of anemia. Interestingly, several studies have recently reported that RDW value is associated with adverse aging-related nonhematologic health outcomes, especially cardiovascular diseases, mortality, and poorer outcomes in critically ill patients.(3–5) It has been also reported that increased RDW value is associated with poor surgical outcomes or higher mortality rate in patients with osteoporotic fractures.(6–8) However, no studies have addressed whether RDW index is associated with the future risk of hip fractures or other clinical fractures.
RDW values can be influenced by nutritional deficiencies. However, the observed associations between RDW values and other negative health-related outcomes are independent of nutritional status.(9,10) RDW value increases with aging. Although biologic mechanisms are unclear, the RDW might reflect changes in hematopoiesis with aging.(11) In addition, based on its association with age and a wide range of aging-related conditions, increased RDW might reflect a fundamental process of cellular aging.(12,13)
Herein, we tested the hypothesis that increased RDW be associated with an increased risk of future hip fracture, a representative pathologic condition with aging. We also explored whether higher RDW was associated with an increased risk of fall or decreased BMD. Because anemia can contribute to higher risk of fracture(14–16) and also cause higher RDW values, the interaction between RDW and anemia was examined in the analyses. We tested these associations in the prospective Osteoporotic Fractures in Men (MrOS) cohort study.
Materials and Methods
Study population
Study participants were enrolled in the Osteoporotic Fractures in Men (MrOS) study, which was previously described.(17,18) A total of 5994 men who were ambulatory and community dwelling (≥65 years) were enrolled for a baseline evaluation from the six clinical sites across the Unites States between March 2000 and April 2002. Further information about the MrOS cohort can be found at the MrOS Online website (http://mrosdata.sfcc-cpmc.net). A third visit (visit 3) evaluation was conducted between March 2007 and March 2009, and 4681 participants (excluding 1043 participants who were deceased before visit 3, 101 participants who refused the visit, and 169 participants who left the study or were lost to follow-up) completed the visit 3 evaluation. Among them, 3790 participants completed the clinic visit, and 3635 men who had available RDW values composed the analytic sample.
Complete blood count (CBC)
Fasting blood samples were collected at the third clinic visit. CBC parameters, including RDW values, were measured at local Quest Diagnostic laboratories for five study sites and at Stanford Out-reach for one site. Measurements were standardized across laboratories. RDW values were prospectively categorized into four groups according to the absolute RDW values (≤13.0%, 13.1% to 14.0%, 14.1% to 15.0%, ≥15.1%).(19,20) Hemoglobin level was also measured and anemia was defined by a hemoglobin concentration of <13.0 g/dL according to the WHO criteria.(21)
Fracture
Participants were asked whether they had sustained any fractures at 4-month intervals using mailed questionnaires or phone follow-up. Fractures were centrally adjudicated by the physician’s review of the medical records. Participants were followed from the third visit to either date of first adjudicated fracture, date of death/termination, or the last contacted date up to February 2019, whichever came first. Analyses were separately performed for incident hip fractures and all fractures including clinical vertebral fractures.
Falls
Participants were asked whether they had fallen in the previous 4 months by follow-up postcards or phone calls at 4-month intervals. Falls reported on the first three tri-annual postcards returned after visit 3 were included in the analysis.
Clinical characteristics of the study participants
General demographic information, medical histories, and health behaviors were collected by standardized questionnaires or interviews. Prevalent fall in the past 12 months before visit 3 evaluation was assessed via self-reported questionnaire. Any past histories of heart attack, angina, and congestive heart failure was recoded as having cardiovascular diseases (CVD). Height (cm) was measured on Harpenden stadiometers, weight (kg) was measured on balance beams or digital scales, and body mass index (BMI) was calculated by weight (kg) divided by height-squared (m2).
Bone mineral density (BMD)
BMD of the proximal femur was measured at the third visit using dual-energy X-ray absorptiometry (DXA) on Hologic QDR 4500-W densitometers (Hologic, Waltham, MA, USA). To ensure reproducibility of DXA measurements at the six clinical sites, procedures for scanning were standardized, DXA operators were certified, and a central quality-control laboratory was used.(17)
Statistical analyses
Data were presented as mean ± standard deviation for continuous values or as numbers (percentages) for categorical values. RDW values were categorized into four groups and were not modeled as continuous variables because it was not normally distributed (Supplemental Fig. S1). Participant characteristics were compared across the RDW categories using ANOVA and chi-square test. Cochran-Armitage test or Cuzick’s test for trend was used to examine the linear trends in the proportions of fractures or the trends of mean values across the RDW groups. The associations between RDW and other continuous clinical parameters were analyzed by Pearson correlation coefficient. Kaplan–Meier estimate curves for the hip fractures and all clinical fractures including clinical vertebral fractures were plotted based on RDW category, whereas log-rank sum test was used for statistical comparison of the equality in the fractures across the groups. Hazard ratios (HRs) of hip fractures and all clinical fractures in each category were evaluated using a Cox proportional hazards model with the lowest RDW group (≤RDW 13.0%) as the referent group. Several types of anemia can cause increases in RDW values and anemia also affect tissue function. Therefore, we tested interaction between RDW and anemia. Because higher RDW values were associated with increased mortality, we used Fine and Gray competing risk regression models to estimate the effects of competing mortality on an individual’s probability of hip fracture.(22) Logistic regression analyses were applied for evaluating odds for incident falls within the first follow-up year across the RDW categories as the referent with lowest RDW group. Analyses were performed using Stata/MP version 15 (StataCorp, College Station, TX, USA), and p values <0.05 were considered statistically significant.
Results
Characteristics of the study participants
A total of 3635 men with RDW data at the third clinic visit were included in the current analysis. Mean age of the participants was 79.1 ± 5.1 years. RDW values were skewed toward higher values with a median value of 14.0% (interquartile range 13.5% to 14.8%) and range from 11.3% to 32.9% (Supplemental Fig. S1). RDW values were correlated positively with age and BMI and negatively with hemoglobin levels (p < 0.05, respectively) but not with femoral neck and total hip BMD (p = 0.29 and 0.69). Subjects with higher RDW values were more likely to be older, had lower hemoglobin levels, and were more likely to have had a previous fracture. The proportion who had histories of falls in the past year increased with increases of the RDW values. The rate of subjects who had previous histories of CVD was also greater with higher RDW values. However, there was no difference for the femoral neck and total hip BMDs among RDW categories (Table 1). {TBL 1}
Table 1.
Baseline Characteristics of Study Participants Total and According to the RDW Category
| Total | RDW (min-13.0%) | RDW (13.1%–14.0%) | RDW (14.1%–15.0%) | RDW (15.1%–max) | p Trend | |
|---|---|---|---|---|---|---|
| n | 3635 | 370 | 1449 | 1120 | 696 | |
| Age (years) | 79.1 (5.1) | 78.2 (5.0) | 78.8 (4.9) | 79.4 (5.2) | 79.9 (5.4) | <0.001 |
| BMI (kg/m2) | 27.1 (3.9) | 26.9 (3.6) | 27.1 (3.7) | 27.0 (3.9) | 27.5 (4.3) | 0.05 |
| Hemoglobin (g/dL) | 14.1 (1.4) | 14.4 (1.2) | 14.4 (1.2) | 14.1 (1.3) | 13.3 (1.6) | <0.001 |
| Femoral neck BMD (g/cm2) | 0.77 (0.13) | 0.77 (0.14) | 0.77 (0.12) | 0.77 (0.14) | 0.78 (0.15) | 0.57 |
| Total hip BMD (g/cm2) | 0.94 (0.15) | 0.95 (0.14) | 0.94 (0.14) | 0.94 (0.15) | 0.95 (0.16) | 0.44 |
| Prevalent fracture, n (%) | 342 (9.4%) | 25 (6.8%) | 113 (7.8%) | 136 (12.1%) | 68 (9.8%) | <0.001 |
| Falls in the past 12 months, n (%) | 1097 (30.2%) | 96 (25.9%) | 416 (28.7%) | 329 (29.4%) | 256 (36.8%) | 0.005 |
| History of CVD, n (%) | 853 (23.5%) | 75 (20.3%) | 289 (19.9%) | 268 (23.9%) | 221 (31.8%) | <0.001 |
RDW = red cell distribution width; BMI = body mass index; BMD = bone mineral density; CVD = cardiovascular diseases.
Data were shown as mean (SD) for continuous variables or n (%) for categorical variables, except RDW of median (min–max).
The p trend was analyzed by Cochran-Armitage test or Cuzick’s test.
RDW and hip fracture
During a median of 8.1 years follow-up (range 0 to 11.8 years), hip fractures occurred in 164 participants. The risk of hip fracture over the follow-up period increased with higher RDW values, and the highest RDW category conferred a 2.3-fold greater risk of hip fractures compared with the lowest RDW category (95% confidence interval [CI] 1.1–4.7, p < 0.05) (Supplemental Fig. S2). However, we found a significant interaction between anemia and RDW (p = 0.006). Therefore, subsequent analyses were stratified by the participants’ anemia status. In those without anemia, the risk of hip fracture increased with increasing RDW (p for trend = 0.02). These associations remained significant after adjustment for age and BMI (Table 2). {TBL 2} Further adjustment with histories of CVD also did not alter the results (data not shown). Compared with non-anemic group, the anemic group had greater risks of hip fracture (hazard ratio [HR] = 2.6, 95% CI 1.9–3.6). However, there was no further association between RDW category and risk of hip fracture in the group with anemia (Table 2). Resultingly, among four RDW groups without anemia and one group with anemia, there was a gradual increasing trend in risks of hip fracture (Fig. 1 {FIG1} and Supplemental Table S1). These associations were consistent after adjusting for age and BMI (Supplemental Table S1).
Table 2.
Hazard Ratios of RDW Category for Hip Fractures According to the Anemic Status
| n | HR | 95% CI | p trend | ||
|---|---|---|---|---|---|
| Participants without anemia | Unadjusted | ||||
| RDW (min–13.0%) | 326 | Referent | |||
| RDW (13.1%–14.0%) | 1289 | 2.1 | (0.9–4.8) | 0.02 | |
| RDW (14.1%–15.0%) | 898 | 2.6 | (1.1–6.2) | ||
| RDW (15.1%–max) | 407 | 2.8 | (1.1–7.1) | ||
| Age and BMI adjusted | |||||
| RDW (min–13.0%) | 326 | Referent | |||
| RDW (13.1%–14.0%) | 1289 | 1.9 | (0.8–4.4) | 0.04 | |
| RDW (14.1%–15.0%) | 898 | 2.3 | (1.0–5.4) | ||
| RDW (15.1%–max) | 407 | 2.5 | (1.0–6.3) | ||
| Participants with anemia | Unadjusted | ||||
| RDW (min–13.0%) | 44 | Referent | |||
| RDW (13.1%–14.0%) | 159 | 1.2 | (0.4–3.5) | 0.16 | |
| RDW (14.1%–15.0%) | 222 | 0.7 | (0.2–2.1) | ||
| RDW (15.1%–max) | 289 | 0.7 | (0.2–2.1) | ||
| Age and BMI adjusted | |||||
| RDW (min–13.0%) | 44 | Referent | |||
| RDW (13.1%–14.0%) | 159 | 1.4 | (0.5–4.1) | 0.20 | |
| RDW (14.1%–15.0%) | 222 | 0.8 | (0.3–2.4) | ||
| RDW (15.1%–max) | 289 | 0.8 | (0.3–2.4) | ||
RDW = red cell distribution width; HR = hazard ratio; CI = confidence interval; BMI = body mass index.
Fig. 1.

Kaplan–Meier plot for survival free risk of hip fractures during the follow-up period according to the red cell distribution index categories with the non-anemia and anemic groups.
To consider the probability of death as an alternative competing risk of hip fracture, we also analyzed the associations after accounting for a competing risk of mortality. The probability of hip fractures was attenuated for the anemia group (HR = 4.1; 1.7–9.5). However, the associations between RDW, anemia, and risk of hip fracture remained statistically significant (Supplemental Table S1). All the results were consistent after further adjusting for other conventional covariates of fracture, including alcohol, smoking, rheumatoid arthritis, corticosteroid, and femur neck BMD (data not shown).
All fractures by RDW category
A total of 701 participants suffered at least one clinical fracture (including clinical vertebral fractures) during the follow-up period. We did not observe an interaction between anemia and RDW with the risk of clinical fractures and, therefore, the data were analyzed for the entire group and without a term for anemia. The risk of fracture increases with higher RDW values (p for trend = 0.01) (Supplemental Fig. S3). The highest RDW category was associated with a 1.8-fold increased risk for all clinical fractures when compared with the lowest RDW category (95% CI 1.3–2.5). The differences were similar after excluding the hip fractures. Those significant differences across RDW category were also maintained after adjusting for age and BMI and other conventional risk factors (data not shown).
Falls
During the subsequent 12 months, at least one fall was reported by 29.4% of the participants. The risk of one fall also significantly increased with increasing RDW category (p for trend <0.001, Fig. 2). {FIG2} There was no interaction between RDW and anemic status for the risks of incident falls.
Fig. 2.
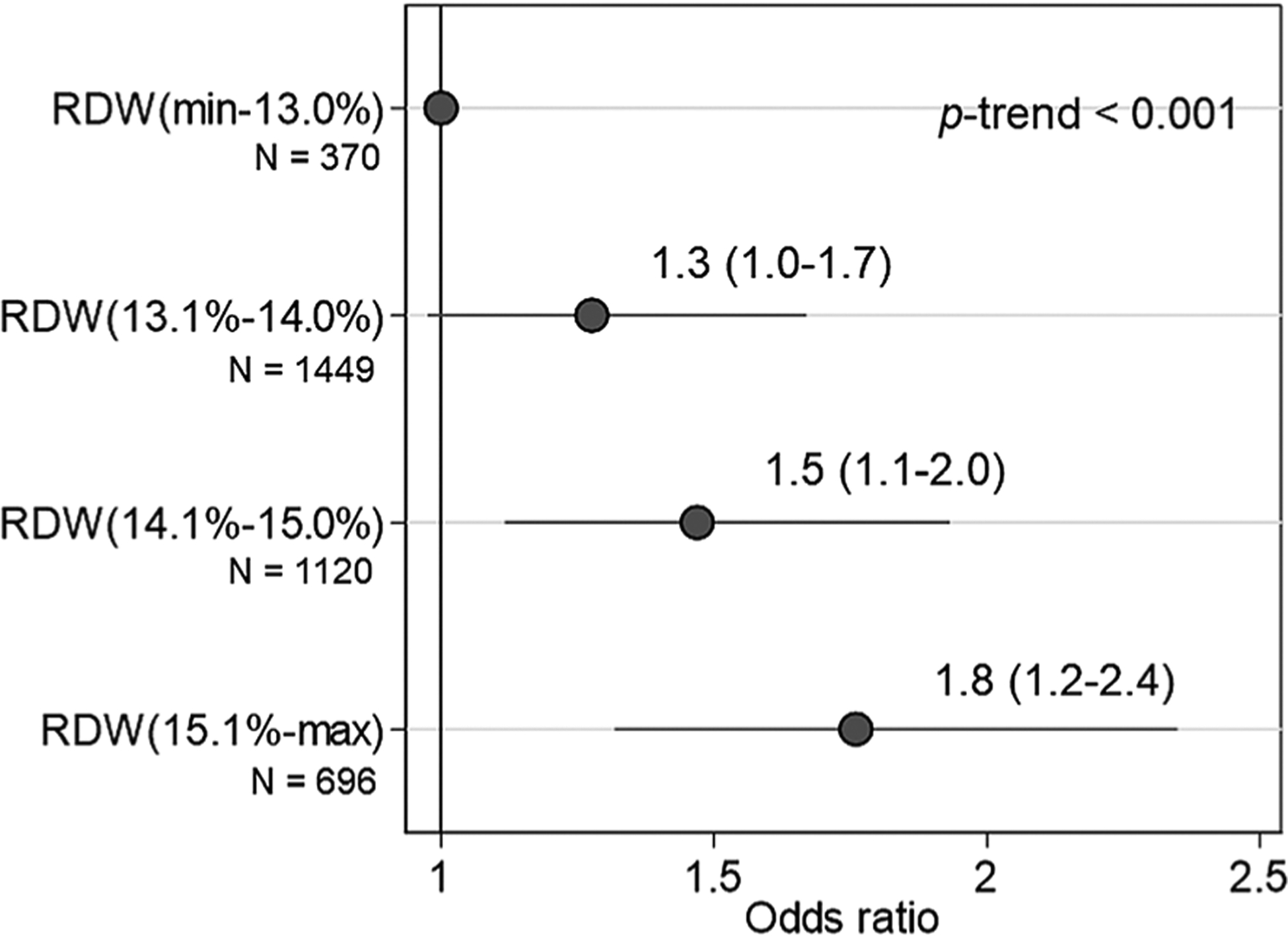
Odd ratios (with 95% CI) of falls within the first follow-up year according to the RDW category. *OR and 95% CI as the referent with the lowest RDW group (RDW ≤13.0%). RDW = red cell distribution index.
Discussion
We found that in community-dwelling older men, higher RDW is associated with an increased risk of hip fractures among men who do not have anemia. Those who have anemia have an ever greater risk of hip fracture than those with the highest value for RDW. Its association with falls, but not with BMD, suggests that RDW may reflect biological processes of aging that impair neuromuscular function that contributes to the greater risk of hip fracture. Our findings also revealed that RDW value was associated with an increased risk of all clinical fractures with or without inclusion of hip fracture. This is the first study to demonstrate that RDW level predicts hip and all clinical fractures.
Previous studies have recently reported that higher RDW values are related to poor health outcomes for several aging-related diseases in the elderly population, particularly cardiovascular disease, stroke, and mortality.(3,4,23) Few studies have also provided the associations between RDW values and conditions related to musculoskeletal diseases: Higher RDW values were related to poor postoperative outcomes of patients with hip fractures, and people with prevalent morphometric vertebral fractures have higher RDW levels compared with those without such fractures.(24,25) Together with previous other studies, our findings support that RDW could have clinical meaningful values in the associations with aging-related conditions, beyond its hematologic usages.
Although the biological mechanisms between RDW values and the relationship between nonhematological conditions are not clear yet, but several hypotheses have been suggested. Clonal hematopoiesis (CH) is a somatic mutation that occurs in hematopoietic stem cell and it increases with aging.(11,26) CH is known to be related with several aging-related diseases.(27) Because of disturbed hematopoiesis with CH, RBC size is varied with CH.(27) Therefore, higher RDW might reflect disturbed hematopoiesis with aging-related CH. Chronic inflammation suppresses bone marrow erythropoiesis and consequently affects the heterogeneity of RBC size.(12,28) Inflammation also affects BMD or risks of fracture, either directly or indirectly.(29–31) Therefore, common factors that could affect both higher RDW values and BMD may partially contribute to the association between RDW and fractures. However, in the present study, there was no significant association between RDW and BMD, and thus those common factors could not completely explain the biological mechanism between RDW and fracture risks. Nutritional status can also cause the higher RDW values, and several causes of anemia, including iron, folate, or vitamin B12 deficiency, increase RDW values.(32) Anemia is also related to the risk of fractures.(16,33) In the present study, however, the risk estimate for hip fracture was only significant in participants without anemia, whereas the associations between RDW and fracture risks were not observed in subjects with anemia. Although the reason for this interaction is unclear, other clinical factors that exacerbate the health status of anemic subjects might have a greater impact on fracture risks than the RDWs in the anemic group, whereas underlying aging process reflected by higher RDW values would make a relatively greater contribution to the risk of hip fracture in the non-anemia population. On the other hand, several previous studies have reported that hemoglobin level did not alter the association between RDW and other health outcomes.(24,34) Therefore, anemia cannot fully capture the relationships between RDW value and other health-related outcomes including fracture risks.
In the present study, the RDW values were significantly related to risks of incident falls. To determine whether a risk of hip fracture might be mediated in part by a risk of falls, we adjusted for the history of falls within the past 1 year before the visit, but the results were not changed (data not shown). The adjustment of incident falls for the following 12 months slightly weakened the associations but also did not alter the associations between RDW value and risks of hip fractures. Accordingly, the risks of falls might contribute to the risks of hip fracture, but the biological mechanisms behind the associations between RDW, fall, and fractures remain unclear.
A higher RDW is associated with an increased mortality rate, particularly in the elderly population.(23,35) After accounting for the competing risk of mortality, the associations between RDW and fracture risks remained similar and significant in the non-anemic group but were attenuated for participants with anemia.(36)
The strengths of this study include that MrOS is a prospective study with validation of fractures and standardized collection of falls. However, MrOS is a cohort of older males and largely white, which limits the generalizability of our results to younger adults, women, or other racial groups. Moreover, we used an international cut-off of 13.0 g/dL for defining anemia. However, lower values than 13.0 g/dL are usually used for clinical practice and that might confer greater risk for hip fractures. In the present analyses, other biochemical parameters, such as protein, albumin or creatinine clearance, were not assessed. Therefore, possibility of hematologic abnormality or renal insufficiency could not be completely excluded.
In conclusion, we have shown that the RDW value is significantly associated with risks of future hip fracture in older men without anemia. This association may reflect an increased risk of falls. We also confirm that anemia is a strong risk factor for hip fracture. As RDW and hemoglobin are part of CBC that is most commonly performed in health screening and medical care, the vast majority of adults will have RDW and hemoglobin values in their medical records. Therefore, future research is needed to determine the clinical utility of these simple measures in fracture prediction. For this, future analyses may consider to test whether RDW values and anemia, combined with other readily available data including age, BMI, and other risk factors for fracture, can improve the predictive value to models for prediction of fracture risk.
Supplementary Material
Acknowledgments
The Osteoporotic Fractures in Men (MrOS) Study is supported by the National Institute on Aging, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Center for Advancing Translational Sciences, and the National Institutes of Health (NIH) Roadmap for Medical Research under the following grants: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Disclosures
All authors state that they have no conflicts of interest.
References
- 1.Danese E, Lippi G, Montagnana M. Red blood cell distribution width and cardiovascular diseases. J Thorac Dis. 2015;7(10):E402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52(2):86–105. [DOI] [PubMed] [Google Scholar]
- 3.Perlstein TS, Weuve J, Pfeffer MA, Beckman JA. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med. 2009;169(6):588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osadnik T, Strzelczyk J, Hawranek M, et al. Red cell distribution width is associated with long-term prognosis in patients with stable coronary artery disease. BMC Cardiovasc Disord. 2013;13:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunziker S, Stevens J, Howell MD. Red cell distribution width and mortality in newly hospitalized patients. Am J Med. 2012;125(3):283–91. [DOI] [PubMed] [Google Scholar]
- 6.Yin P, Lv H, Li Y, et al. Hip fracture patients who experience a greater fluctuation in RDW during hospital course are at heightened risk for all-cause mortality: a prospective study with 2-year follow-up. Osteoporos Int. 2018;29(7):1559–67. [DOI] [PubMed] [Google Scholar]
- 7.Zehir S, Sipahioglu S, Ozdemir G, Sahin E, Yar U, Akgul T. Red cell distribution width and mortality in patients with hip fracture treated with partial prosthesis. Acta Orthop Traumatol Turc. 2014;48(2):141–6. [DOI] [PubMed] [Google Scholar]
- 8.Garbharran U, Chinthapalli S, Hopper I, George M, Back DL, Dockery F. Red cell distribution width is an independent predictor of mortality in hip fracture. Age Ageing. 2013;42(2):258–61. [DOI] [PubMed] [Google Scholar]
- 9.Forhecz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohaszka Z, Janoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. 2009;158(4):659–66. [DOI] [PubMed] [Google Scholar]
- 10.Aung N, Ling HZ, Cheng AS, et al. Expansion of the red cell distribution width and evolving iron deficiency as predictors of poor outcome in chronic heart failure. Int J Cardiol. 2013;168(3):1997–2002. [DOI] [PubMed] [Google Scholar]
- 11.Steensma DP. Clinical implications of clonal hematopoiesis. Mayo Clin Proc. 2018;93(8):1122–30. [DOI] [PubMed] [Google Scholar]
- 12.Lippi G, Turcato G, Cervellin G, Sanchis-Gomar F. Red blood cell distribution width in heart failure: a narrative review. World J Cardiol. 2018; 10(2):6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilling LC, Atkins JL, Duff MO, et al. Red blood cell distribution width: genetic evidence for aging pathways in 116,666 volunteers. PLoS One. 2017;12(9):e0185083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z, Thomson CA, Aickin M, et al. The relationship between incidence of fractures and anemia in older multiethnic women. J Am Geriatr Soc. 2010;58(12):2337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jorgensen L, Skjelbakken T, Lochen ML, et al. Anemia and the risk of non-vertebral fractures: the Tromso study. Osteoporos Int. 2010;21(10):1761–8. [DOI] [PubMed] [Google Scholar]
- 16.Valderrabano RJ, Lee J, Lui LY, et al. Older men with anemia have increased fracture risk independent of bone mineral density. J Clin Endocrinol Metab. 2017;102(7):2199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–85. [DOI] [PubMed] [Google Scholar]
- 18.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the Osteoporotic Fractures in Men Study (MrOS). Contemp Clin Trials. 2005;26(5):557–68. [DOI] [PubMed] [Google Scholar]
- 19.Evans TC, Jehle D. The red blood cell distribution width. J Emerg Med. 1991;9:71–4. [DOI] [PubMed] [Google Scholar]
- 20.May JE, Marques MB, Reddy VVB, Gangaraju R. Three neglected numbers in the CBC: the RDW, MPV, and NRBC count. Cleve Clin J Med. 2019;86(3):167–72. [DOI] [PubMed] [Google Scholar]
- 21.Izaks GJ, Westendorp RG, Knook DL. The definition of anemia in older persons. JAMA. 1999;281(18):1714–7. [DOI] [PubMed] [Google Scholar]
- 22.Harkonen R, Harju R, Alaranta H. Accuracy of the Jamar dynamometer. J Hand Ther. 1993;6(4):259–62. [DOI] [PubMed] [Google Scholar]
- 23.Patel KV, Ferrucci L, Ershler WB, Longo DL, Guralnik JM. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch Intern Med. 2009;169(5):515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong N, Kim CO, Youm Y, Choi JY, Kim HC, Rhee Y. Elevated red blood cell distribution width is associated with morphometric vertebral fracture in community-dwelling older adults, independent of anemia, inflammation, and nutritional status: the Korean Urban Rural Elderly (KURE) study. Calcif Tissue Int. 2019;104(1):26–33. [DOI] [PubMed] [Google Scholar]
- 25.Lv H, Zhang L, Long A, et al. Red cell distribution width as an independent predictor of long-term mortality in hip fracture patients: a prospective cohort study. J Bone Miner Res. 2016;31(1):223–33. [DOI] [PubMed] [Google Scholar]
- 26.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377(2): 111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133(4):628–32. [DOI] [PubMed] [Google Scholar]
- 29.Takayanagi H New developments in osteoimmunology. Nat Rev Rheumatol. 2012;8(11):684–9. [DOI] [PubMed] [Google Scholar]
- 30.Chandra A, Park SS, Pignolo RJ. Potential role of senescence in radiation-induced damage of the aged skeleton. Bone. 2019;120:423–31. [DOI] [PubMed] [Google Scholar]
- 31.Cauley JA, Barbour KE, Harrison SL, et al. Inflammatory markers and the risk of hip and vertebral fractures in men: the Osteoporotic Fractures in Men (MrOS). J Bone Miner Res. 2016;31(12):2129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng YF, Pan GG. Red blood cell distribution width predicts homo-cysteine levels in adult population without vitamin B12 and folate deficiencies. Int J Cardiol. 2017;227:8–10. [DOI] [PubMed] [Google Scholar]
- 33.Piriyakhuntorn P, Tantiworawit A, Rattanathammethee T, Chai-Adisaksopha C, Rattarittamrong E, Norasetthada L. The role of red cell distribution width in the differential diagnosis of iron deficiency anemia and non-transfusion dependent thalassemia patients. Hematol Rep. 2018;10(3):7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lappegard J, Ellingsen TS, Skjelbakken T, et al. Red cell distribution width is associated with future risk of incident stroke. The Tromso study. Thromb Haemost. 2016;115(1):126–34. [DOI] [PubMed] [Google Scholar]
- 35.Tonelli M, Wiebe N, James MT, et al. Red cell distribution width associations with clinical outcomes: a population-based cohort study. PLoS One. 2019;14(3):e0212374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buzkova P, Barzilay JI, Mukamal KJ. Assessing risk factors of non-fatal outcomes amid a competing risk of mortality: the example of hip fracture. Osteoporos Int. 2019;30(10):2073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


