Abstract
Introduction
Persistent sciatic artery is a rare vascular anomaly. The occurrence of infected persistent sciatic artery aneurysm (PSAA) is extremely rare.
Report
An 84 year old woman who was under observation for a massive thrombosed right PSAA since the age of 74 presented with severe pain in her right lower limb. The patient was diagnosed with the infected PSAA by computed tomography and laboratory test. The condition was treated with antibiotics as well as drainage and removal of the infected thrombus with a small incision. Subsequently, the patient's symptoms improved, and she was discharged ambulatory. Sixteen months after the surgery, her condition remained good, with no evidence of recurrent infection.
Conclusion
Extensive debridement requires a large muscle incision and carries with it a risk of sciatic nerve injury. However, a thrombosed aneurysm has little risk of haemorrhage. Therefore, drainage and removal of the thrombus via a small incision, which is less invasive, was considered effective for this infected thrombosed PSAA.
Keywords: Drainage, Infected aneurysm, Persistent sciatic artery
Highlights
-
•
Infected persistent sciatic artery aneurysm (PSAA) is extremely rare.
-
•
Primary treatment is extensive resection of the PSAA and surrounding tissues.
-
•
Extensive debridement requiring a large incision poses a risk of sciatic nerve injury.
-
•
PSAA was treated by drainage and removal of the thrombus via a small incision.
Introduction
Persistent sciatic artery (PSA) is a remnant of the sciatic artery, which naturally regresses in the embryonic period. It is a rare vascular anomaly, and aneurysm formation is seen in 50% of patients with PSA.1 The proportion of individuals with an infected PSA aneurysm (PSAA) is extremely low.2,3 The general treatment strategy for infected aneurysms comprises extensive resection of both the aneurysm and surrounding tissues.4 In the case reported herein, the infected PSAA was extremely large but chronically thrombosed, therefore it was treated by drainage and removal of the thrombus via a small incision to prevent damage to the muscles and sciatic nerve.
Case report
An 84 year old woman with a thrombosed right PSAA had been under observation since the age of 74 years. The PSAA was unilateral and the complete type. The popliteal and distal pulses in the right leg were not palpable and without ischaemic symptoms.
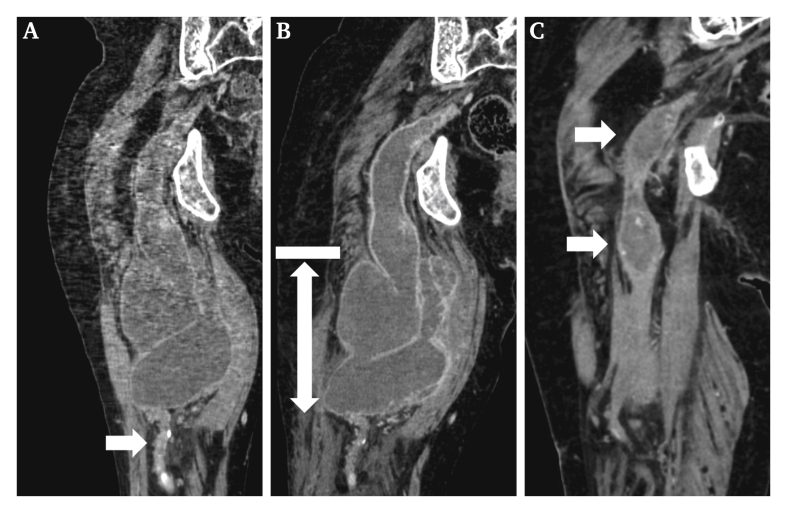
The patient presented as she had lost the ability to walk due to worsening of the pain in her right leg. Laboratory data revealed a white blood cell count of 11 400/μL and a C reactive protein level of 19.0 mg/dL. Computed tomography (CT) revealed an enlarged multilobulated PSAA and increased fat density (Fig. 1A and B). Purulent liquid was aspirated from the PSAA via ultrasound guided exploratory puncture, and Peptostreptococcus micros was detected in the bacterial culture. The patient was therefore diagnosed with an infected PSAA and was admitted to hospital.
Figure 1.
Multiplanar reconstruction images of computed tomography scan. (A) Persistent sciatic artery aneurysm (PSAA) in the right thigh three years before surgery. The maximum diameter of the PSAA was 78 mm. The PSAA was thrombosed, and its distal artery was occluded and scarred (arrow). (B) On admission. The size of the PSAA had increased, and it had become multilobulated. There was no blood flow in the PSAA, and the fat density around it had increased. The drainage area is represented by a solid arrow. The thrombus in the proximal portion of the aneurysm was organised and hard (bar). (C) Sixteen months after the surgery. The PSAA had shrunk, and there was no evidence of the recurrent infection. The proximal dilated portion of the PSAA also had shrunk slightly, and two small thrombosed aneurysms remained (arrow).
The patient had a temperature of 37.6ºC and a blood pressure of 150/90 mmHg. There was significant tenderness in the right thigh. The ankle brachial pressure indices were 0.88 in the right side and 1.10 in the left side, respectively, and there were no remarkable changes compared with before. Sulbactam/ampicillin (SBT/ABPC) was administered. Incisional drainage was performed on the third day of admission. The procedure was performed in the prone position. A 7 cm skin incision was made on the posterolateral side of the thigh, where the PSAA was nearest to the skin on ultrasound, to prevent damage to the muscles and sciatic nerve. After incising the PSAA wall, the infected thrombus between the organised structure of the proximal side of the PSAA and the distal scarred vessel was removed (Fig. 1B). After lavage, two drains without irrigation were placed in the PSAA. The wound was then closed.
Pain in the lower limb improved remarkably after the surgery. The inflammation improved gradually. SBT/ABPC was changed to oral amoxicillin on post-operative day 12. After rehabilitation, the patient was discharged ambulatory on post-operative day 44. Amoxicillin was administered continuously for 12 months. Sixteen months after the surgery, the patient's condition remained good and CT revealed that the size of the PSAA had decreased, and there was no recurrent infection (Fig. 1C).
Discussion
In this case, the cause of the infected PSAA was unclear, because it was thrombosed and the patient had no history of injury. Peptostreptococcus micros, which was the causative bacterium in this case, is a Gram positive coccus and generally exists in the oral cavity. It triggers odontitis, sepsis, endocarditis, and absess.5 Therefore, it was possible that the PSAA infection arouse from septic embolisation through the vasa vasorum into the arterial intima as a result of oral diseases such as odontitis.5,6
The general treatment principles of infected arterial aneurysms involve extensive resection of both the aneurysm and the surrounding infected and necrotic tissues with or without revascularisation.4 In this case, a large skin and muscle incision would have been required to completely remove the aneurysm because of its size. In addition, PSAA excision has a risk of sciatic nerve injury.2 Dissection of the sciatic nerve from the aneurysm wall would have been challenging owing to inflammatory adhesion.2 The PSAA in this case was chronically thrombosed with little risk of bleeding. Therefore, the PSAA was treated by drainage and removal of the infected thrombus via the small incision made on the posterolateral side of the thigh to avoid muscle and nerve injuries. However, this approach could result in insufficient debridement of the infected aneurysm wall and surrounding tissues, possibly resulting in poor control of the infection.
Recently, the efficacy of the additional percutaneous drainage after endovascular aneurysm repair, has been considered the treatment of choice for infected aortic aneurysms.7,8 Although percutaneous abscess drainage does not remove the infected aortic wall and peri-aortic tissue, sufficient drainage and long term administration of antibiotic agents can control infection.8 In this case, the aneurysm was massive and had a complicated structure. Therefore, the incisional approach was chosen instead of percutaneous drainage.
Antibiotic therapy is essential for the treatment of infected arterial aneurysms. However, the appropriate duration of post-operative antibiotic therapy has not been determined, and the treatment was based on the pathological characteristics of the disease, such as location, organism virulence, and antibiotic sensitivity. To manage an infected aortic aneurysm successfully, antibiotic therapy for more than six months should be recommended.7,8 In this case, although the drained part of the aneurysm shrank on CT, the small proximal aneurysm with an organised structure was still observed. Thus, amoxicillin was administered continuously for 12 months. Sixteen months after the surgery, there were no signs of recurrence. However, recurrence of infection must be cautiously monitored.
Herein, a case of an infected thrombosed PSAA that was successfully managed by incisional drainage and removal of the infected thrombus is presented. In similar massive, infected thrombosed PSAA that do not require revascularisation, incisional drainage, which is a less invasive procedure, is considered effective, even if the aneurysm wall is not completely resected.
Funding
None.
Conflicts of interest
None.
References
- 1.Van Hooft I.M., Zeebregts C.J., van Sterkenburg S.M., De Vries W.R., Reijnen M.M. The persistent sciatic artery. Eur J Vasc Endovasc Surg. 2009;37:585–591. doi: 10.1016/j.ejvs.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Ito S., Kowatari R., Konishi T., Imazuru T., Matsuzaki K., Jikuya T. Thrombosed infectious aneurysm of the left persistent sciatic artery. Jpn J Vasc Surg. 2013;22:73–76. [Google Scholar]
- 3.Hikone M., Ariyada K., Kobayashi K.I. Mycotic aneurysm of a persistent sciatic artery. Intern Med. 2017;56:239. doi: 10.2169/internalmedicine.56.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu R.B., Chen R.J., Wang S.S., Chu S.H. Infected aortic aneurysms: clinical outcome and risk factor analysis. J Vasc Surg. 2004;40:30–35. doi: 10.1016/j.jvs.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Takigawa T., Baba H., Hisahara M., Ando Y., Ochiai Y., Tokunaga S. Use of computed tomography-guided biopsy to detect Peptostreptococcus micros-induced mycotic abdominal aortic aneurysm after endovascular repair. J Vasc Surg Cases Innov Tech. 2019;5:477–480. doi: 10.1016/j.jvscit.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laohapensang K., Rutherford R.B., Arworn S. Infected aneurysm. Ann Vasc Dis. 2010;3:16–23. doi: 10.3400/avd.AVDctiia09002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chino S., Kato N., Noda Y., Oue K., Tanaka S., Hashimoto T. Treatment of infected aneurysms of the abdominal aorta and iliac artery with endovascular aneurysm repair and percutaneous drainage. Ann Vasc Surg. 2016;36:289. doi: 10.1016/j.avsg.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Nakajima K., Kato N., Hashimoto T., Chino S., Higashigawa T., Ouchi T. Treatment of infected aneurysm with combined endovascular aneurysm repair and abscess drainage. J Vasc Interv Radiol. 2018;29:188–193. doi: 10.1016/j.jvir.2017.09.014. [DOI] [PubMed] [Google Scholar]