Abstract
Purpose
The present study aimed to evaluate the significance of Pleuroparenchymal fibroelastosis (PPFE)-like lesions in predicting prognosis in patients with chronic interstitial pneumonia (IP).
Method
The present study enrolled 207 patients with IP in whom surgical lung biopsy was performed. Among the patients enrolled in the present study, 77 had idiopathic pulmonary fibrosis (IPF), 15 had nonspecific interstitial pneumonia (NSIP), 13 had chronic hypersensitivity pneumonitis (CHP), 41 had connective tissue disease (CTD), three had PPFE, and 58 had unclassifiable diagnosis. The incidence, characteristics, and thickness of PPFE-like lesions were evaluated in each patient with IP. Additionally, the influence of PPFE-like lesions on the prognosis was also determined.
Results
Of 207 patients, 160 (77.3 %) showed PPFE-like lesions. The frequency of PPFE-like lesions was similar in patients with IPF, NSIP, CHP, CTD, and unclassifiable diagnosis (79.5 %, 79.5 %, 73.2 %, 65.9 %, and 81 %, respectively); however, PPFE-like lesions were present in all patients with PPFE (p = 0.42). Consequently, there was no significant difference in the characteristics of PPFE-like lesions among patients with all forms of IP, except PPFE. PPFE-like lesions were not a significant predictor of prognosis (hazard ratio [HR], 1.16; 95 % confidence interval [CI], 0.64–2.10, p = 0.62); however, patients with PPFE-like lesions under the aortic arch had significantly poorer prognoses (HR, 2.70; 95 % CI, 1.66–4.39, p < 0.001). For craniocaudal extent comparison, patients with IPF with PPFE-like lesions below the level of the carina had significantly poorer prognoses than those without PPFE-like lesions (p = 0.001, overall survival 53.1 and 80.6, respectively).
Conclusion
PPFE-like lesions are common in patients with IP, and their characteristics were not significantly different among all forms of IP, except idiopathic PPFE. The broad extent of PPFE-like lesions is an important predictor of prognosis in patients with IPF.
Abbreviations: IPF, idiopathic pulmonary fibrosis; NSIP, nonspecific interstitial pneumonia; CHP, chronic hypersensitivity pneumonitis; CTD, connective tissue disease; UIP, usual interstitial pneumonia; PPFE, pleuroparenchymal fibroelastosis; IP, interstitial pneumonia; HR, hazard ratio; 95 % CI, 95 % confidence interval; HRCT, high-resolution computed tomography; FVC, forced vital capacity; FEV1.0, forced expiratory volume in 1 s; RV, residual volume; TLC, total lung capacity; DLCO, diffusing capacity of the lungs for carbon monoxide; %DLCO, % predicted diffusing capacity of the lungs for carbon monoxide; %DLCO/VA, % predicted diffusing capacity of the lungs for carbon monoxide per liter alveolar volume
Keywords: Interstitial pneumonia, Pleuroparencymal fibroelastosis, CT, Lung, Prognosis
1. Introduction
Idiopathic pleuroparenchymal fibroelastosis (PPFE) is one of the rare forms of idiopathic interstitial pneumonia (IP) and characterized by fibrosis, mainly involving the subpleural lung parenchyma, predominantly in the upper lobes [1]. Amitani et al. described idiopathic upper-lobe fibrosis in 1992 [2], and Frankel et al. presented these upper-lobe fibroses, known as PPFE, in 2004 [3]. High-resolution computed tomography (HRCT) of PPFE showed intense subpleural fibrosis with pleural thickening [[3], [4], [5], [6], [7]], and these findings were similar to those of apical cap fibrosis that was observed on lung autopsy and chest radiography [8]. These findings, including PPFE and apical cap fibrosis were collectively called PPFE-like lesions in this study because it was difficult to differentiate between PPFE and apical cap in one CT study. PPFE-like lesions were often observed in daily clinical chest CT, especially in patients with IP [9]. Moreover, idiopathic PPFE in the upper lobes of the lung and other forms of IP, such as usual interstitial pneumonia (UIP) and nonspecific interstitial pneumonia (NSIP), in the lower lobes sometimes coexist [6,10,11]. The significance of the coexistence of PPFE and IP in the lower lobes of the lung is still unclear; however, it would be an important feature. Some studies have reported that patients with idiopathic PPFE with a lower UIP pattern have poor prognoses [[12], [13], [14]]. Oda et al. revealed that survival time tended to be shorter in patients with idiopathic pulmonary fibrosis (IPF) with PPFE [10]. In other studies, PPFE was common in patients with hypersensitivity pneumonitis and connective tissue disease (CTD)-related interstitial lung diseases and independently associated with increased mortality [15,16]. PPFE-like lesions in patients with IP were an important predictor of prognosis; however, the difference in the characteristics of PPFE-like lesions among patients with various forms of IP and the effect of the characteristics of PPFE-like lesions on the prognosis were not evaluated.
The present study aimed to evaluate the frequency, features, and significance of PPFE-like lesions in patients with IP.
2. Materials and methods
2.1. Patients and diagnoses
The ethical review board of the authors’ institution approved this retrospective study and waived the requirement of informed consent (No. 1612069194). The present study enrolled patients who underwent surgical biopsy from 2010 to 2014 at one institution because of suspected IP. A total of 252 patients underwent surgical biopsy, and 32 patients whose CT data could not be obtained were excluded. Furthermore, four patients did not have sufficient clinical data. The remaining 216 patients were evaluated (Fig. 1).
Fig. 1.
Schematic illustration of the patient selection criteria.
The diagnoses of all cases were determined by a multidisciplinary discussion in the institution. IPF (n = 77), NSIP (n = 15), chronic hypersensitivity pneumonitis (CHP) (n = 13), CTD (n = 41), PPFE (n = 3), unclassifiable IP (n = 58), and others (n = 9) were diagnosed. The diseases under others were eosinophilic pneumonia (n = 2), cryptogenic organizing pneumonia (n = 1), organizing pneumonia after infection (n = 1), sarcoidosis (n = 1), granulomatosis with polyangiitis (n = 1), drug-induced pneumonia (n = 1), IgG4-related disease (n = 1), and pulmonary alveolar proteinosis (n = 1). There were extremely few patients with each disease in others; therefore, they were excluded from the present study.
2.2. CT images and review
CT examinations were performed using several scanners, and all patients were scanned at end inspiration and in supine position. The CT images were reconstructed by 0.5 mm thickness volume images with high-frequency spatial algorithm. The PPFE-like lesions of all cases were independently reviewed by two chest radiologists with 18 and 23 years of experience, respectively. The discordance of the two radiologists was resolved by a third radiologist with 19 years of experience. Consequently, the extent of abnormalities in the entire lung was reviewed by two other radiologists with 32 and 27 years of experience. Thereafter, the results of pulmonary extent were the average of the two readers.
PPFE-like lesions were defined as subpleural consolidation associated with fibrosis in the upper lobe of the lung [5,10]. The radiologists evaluated the location, laterality, characteristics, surrounding features, and thicknesses of the PPFE-like lesions using the same methods as in the previous studies [9]. Craniocaudal and axial positions were determined as locations of the PPFE-like lesions. The most caudal extent was classified into the following five areas: (a) there was no PPFE-like lesion; (b) the upper area was the area above the level of the sternoclavicular joint; (c) the middle-upper area was the area below the level of the sternoclavicular joint and above the level of the aortic arch; (d) the middle-lower area was the area below the level of the aortic arch and above the level of the carina; and (e) the lower area was the area below the level of the carina. The axial position was classified into four zones on axial images. The medial zone was the plane adjacent to the mediastinum. The other plane was divided into the following three equal zones: dorsal, lateral, and ventral zones. The laterality of the lesions was categorized into the following five scales: (1) right only, (2) right predominant, (3) bilateral, (4) left predominant, and (5) left only. The characteristics and surrounding features of the PPFE-like lesions, that is, the presence of air bronchogram in PPFE-like lesion, nodular opacity, interlobular septa, consolidation along the bronchus, and bronchiectasis around the PPFE-like lesions, were evaluated. The thickness of the PPFE-like lesions was measured using the thickest part of the axial images.
In the findings of the entire lung, ground-glass opacity, ground-glass opacity with traction bronchiectasis, air-space consolidation, honeycombing, reticular opacity, emphysema, cyst, and area of traction bronchiectasis were evaluated. The definition of each finding adhered to the Glossary of Terms by Fleischner Society [17]. The abnormal findings of the entire lung, except for traction bronchiectasis, were evaluated by the percentage of the involved pulmonary parenchyma. The six zones (upper, middle, and lower zones on both sides) of the lungs were evaluated separately. The border of the upper, middle, and lower lung zones was indicated from the level of the tracheal carina to the inferior pulmonary vein. The extent of lung involvement in each lung zone was scored by obtaining the percentage of the involved pulmonary parenchyma and estimated to the nearest 10 % of parenchymal involvement. The overall percentage of lung involvement was calculated by obtaining the average of the six lung zones. The area of traction bronchiectasis was evaluated by counting the number of segments in each lobe.
2.3. Clinical data
The pulmonologists in the institution reviewed the clinical features and pulmonary function data from the medical records. The pulmonary function tests within 6 months following CT examinations were included in the present study. The CT data of 10 patients could not be retrieved, and two patients had missing CT data. These patients were excluded from the analysis.
2.4. Statistical analysis
All statistical analyses were conducted using statistical software (IBM SPSS Statistics, 2017; IBM, USA). Interobserver variability with respect to the presence and characteristics of PPFE-like lesions was evaluated using Cohen’s kappa (κ) coefficient. Interobserver agreement was classified as follows: poor (κ = 0–0.20), fair (κ = 0.21–0.40), moderate (κ = 0.41–0.60), good (κ = 0.61–0.80), and excellent (κ = 0.81–1.00). Consequently, interobserver variability of all pulmonary abnormal findings was evaluated using Spearman’s rank correlation coefficient.
Differences in the CT characteristics of PPFE-like lesions among the diseases were evaluated using univariate analysis. P values of <0.05 were considered statistically significant. Correlations between the presence, location, and features of PPFE-like lesions were analyzed using a chi-square test. The thickness of PPFE-like lesions was evaluated using the Kruskal–Wallis test. Abnormalities of the entire lung, except PPFE-like lesions, were compared between those with and without PPFE-like lesions using the Mann–Whitney U test
Univariate and multivariate Cox proportional hazards regression models were used to identify independent CT predictors for PPFE-like lesions. In the multivariate analysis, variables were selected using a stepwise procedure. Findings that contributed to the power of the regression equation (p < 0.10) were retained. Differences in survival among patients with PPFE-like lesions in the craniocaudal position were presented using Kaplan–Meier curves and evaluated using the log-rank test in all patients.
3. Results
3.1. Interobserver’s variability
The interobserver agreements regarding the presence and characteristics of PPFE-like lesions were moderate (κ = 0.41). The interobserver agreements as regards the location and findings were poor to good (κ = 0.17–0.64), and regarding the thickness, the interobserver agreement was good (r = 0.67). The interobserver variability of all lung findings was poor to good (r = 0.25–0.74).
3.2. Clinical featues
The clinical features of the patients are shown in Table 1. A total of 113 men and 94 women were included in the present study, and the mean age was 62.6 years (range, 31–76 years). Patients with PPFE-like lesions had significantly lower body mass indices (BMIs) (mean BMI, 22.6 kg/m2) than those without PPFE-like lesions (mean BMI, 25.7 kg/m2 (p < 0.001). Pulmonary function tests showed that %residual volume (RV) and RV/total lung capacity (TLC) between patients with and without PPFE-like lesions had significant differences (p = 0.04 and <0.001, respectively). Additionally, higher %forced vital capacity (FVC), higher forced expiratory volume in 1 s/FVC (FEV1.0/FVC), lower %TLC, and higher %predicted diffusing capacity of the lungs for carbon monoxide per liter alveolar volume were noted in patients with PPFE-like lesions under the aortic arch (p = 0.004, <0.001, 0.001, and 0.006, respectively).
Table 1.
Clinical features and CT findings in comparison with the presence of PPFE-like lesions.
| All | With PPFE-like lesion | Without PPFE-like lesions | P value | With PPF-like lesions below the aortic arch | Without PPFE-like lesions below the aortic arch | P value | |
|---|---|---|---|---|---|---|---|
| Sex (M/F) | 113/94 | 87/73 | 26/21 | 0.91 | 38/27 | 75/67 | 0.45 |
| Age | 62.6 ± 8.3 | 63.0 ± 7.6 | 61.2 ± 10.5 | 0.61 | 63.9 ± 7.2 | 62.0 ± 8.8 | 0.18 |
| Smoking history (never/former and current) | 84/123 | 69/91 | 15/32 | 0.17 | 30/35 | 54/88 | 0.27 |
| BMI | 23.3 ± 3.5 | 22.6 ± 3.2 | 25.7 ± 3.6 | <0.001 | 22.2 ± 3.6 | 23.8 ± 3.4 | 0.004 |
| FVC, %predicted | 88.8 ± 47.0 | 88.5 ± 52.5 | 89.9 ± 18.7 | 0.72 | 89.3 ± 78.9 | 88.6 ± 18.4 | 0.004 |
| FEV1, %predicted | 91.9 ± 18.0 | 91.5 ± 18.1 | 93.4 ± 17.7 | 0.12 | 89.8 ± 18.4 | 92.9 ± 17.7 | 0.39 |
| FEV1/FVC ratio | 83.5 ± 8.1 | 84.1 ± 8.3 | 81.4 ± 6.3 | 0.39 | 86.9 ± 9.1 | 81.9 ± 7.0 | <0.001 |
| RV | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.2 ± 0.4 | 0.93 | 1.1 ± 0.4 | 1.2 ± 0.3 | 0.20 |
| TLC, %predicted | 78.8 ± 18.9 | 78.2 ± 18.4 | 81.0 ± 20.6 | 0.58 | 73.5 ± 16.6 | 81.4 ± 19.4 | 0.001 |
| RV/TLC | 32.0 ± 7.5 | 32.4 ± 7.8 | 30.7 ± 6.3 | <0.001 | 32.8 ± 9.3 | 31.7 ± 6.5 | 0.95 |
| DLCO, %predicted | 64.8 ± 19.4 | 66.0 ± 19.3 | 60.8 ± 19.4 | 0.14 | 67.4 ± 20.1 | 63.6 ± 18.9 | 0.21 |
| DLCO/VA, %predicted | 80.7 ± 26.2 | 80.4 ± 27.3 | 81.6 ± 22.3 | 0.53 | 87.2 ± 25.8 | 77.6 ± 26.0 | 0.006 |
Values are mean ± SD.
3.3. CT findings
PPFE-like lesions were observed in 160 of 207 patients (77.3 %). In patients with IPF, NSIP, CHP, CTD, unclassifiable IP, and PPFE, 62 (80.5 %), 11 (73.3 %), 10 (76.9 %), 27 (65.9 %), 47 (81.0 %), and three (1.0 %) had PPFE-like lesions, respectively (Fig. 2, Fig. 3). There was no significant difference among the types of IPs (p = 0.42). The correlation between the extent and characteristics and surrounding features of PPFE-like lesions is shown in Table 2. The mean thickness of the PPFE-like lesions was 7.7 mm (range, 3–26 mm and median 7 mm), and that of idiopathic PPFE was the thickest (mean thickness, 18 mm). There was no significant difference in thickness among the types of IP, including PPFE (p = 0.12). In the craniocaudal position, 54 (33.8 %) of 160 patients had lesions in the upper, 41 (25.6 %) in the middle-upper, 43 (26.9 %) in the middle-lower, and 22 (13.8 %) in the lower zones. Regarding the location of the axis, the dorsal location was the most common (n = 149; 93.7 %), and the ventral location was the least common (n = 95; 59.4 %). Comparing both lungs, most patients showed both lesions equally (n = 109; 68.1 %), and right side predominat was subsequently predominant (n = 37; 23.1 %).
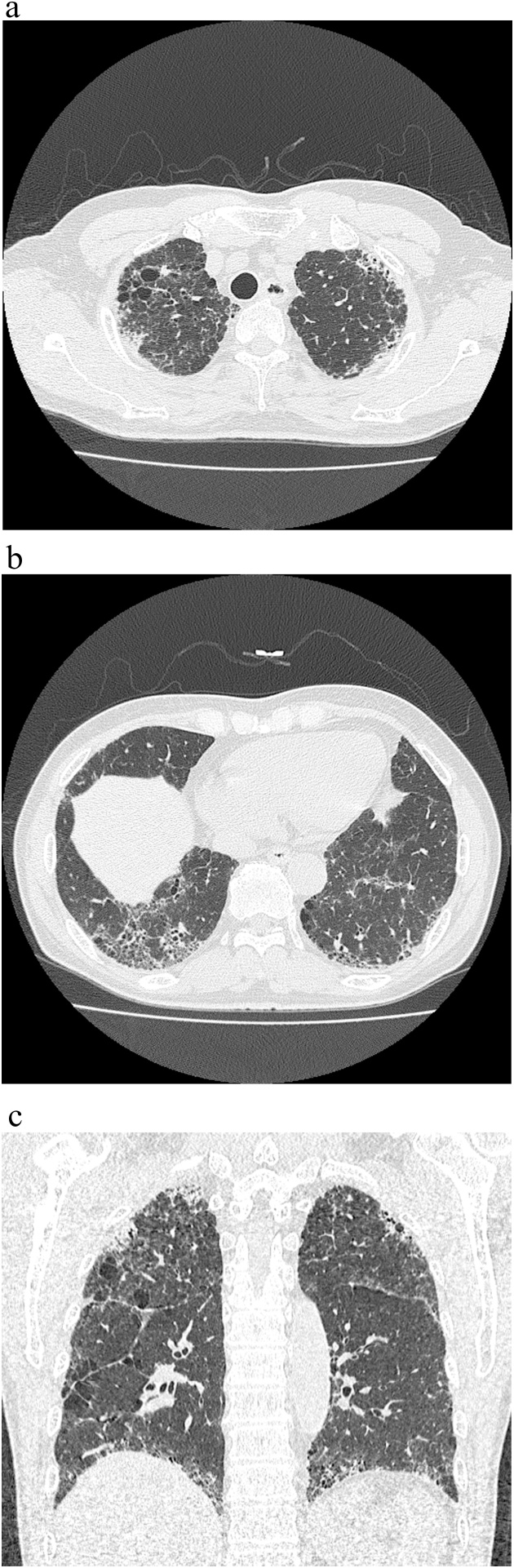
Fig. 2.
CT radiographs of a 70 year-old woman with IPF. (a) There are PPFE-like lesions in the bilateral upper subpleural areas. (b) There are UIP patterns in the lower lobe.
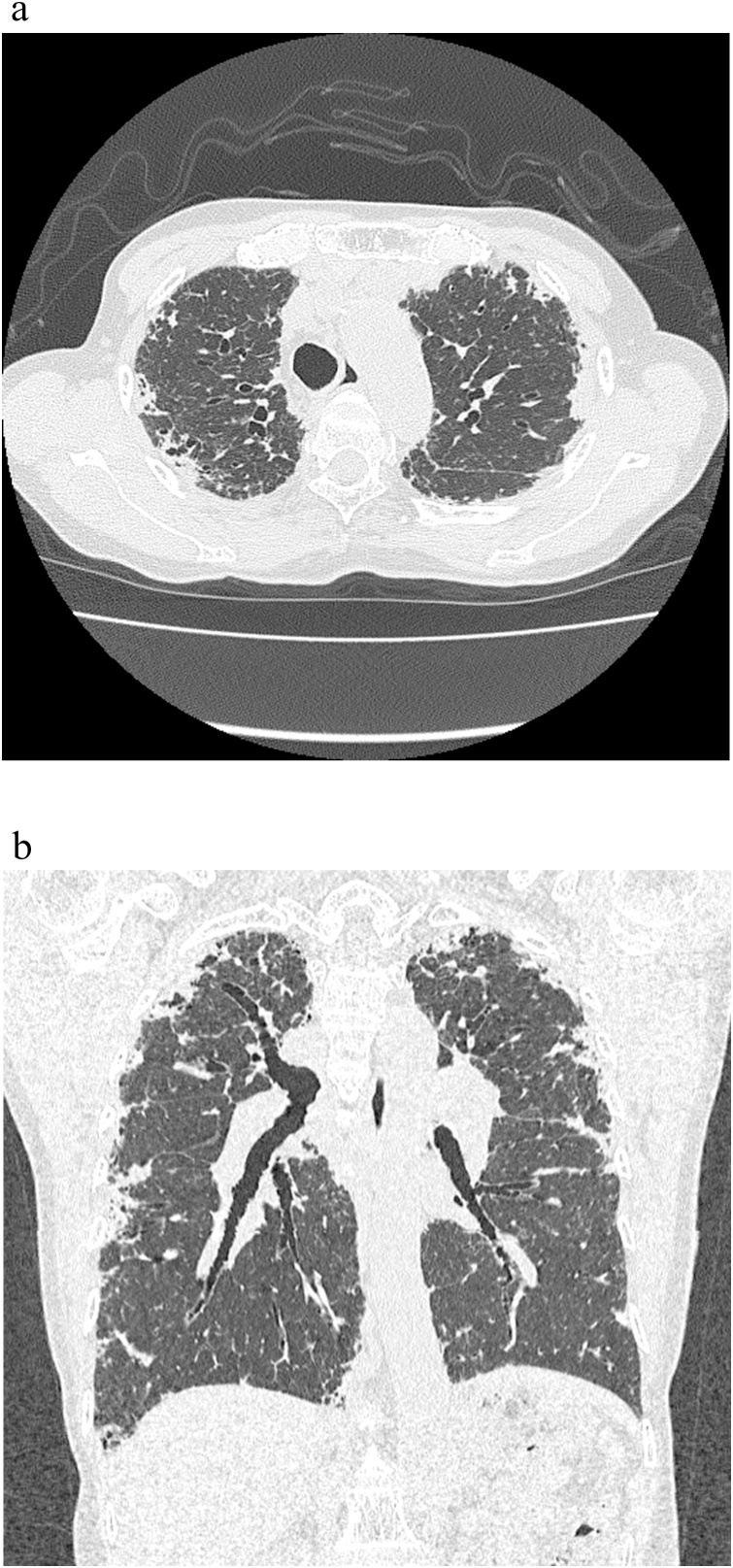
Fig. 3.
CT radiographs of a 72 year-old man with idiopathic PPFE. (a) There were diffuse subpleural pleuroparenchymal opacities with volume loss. (b) There were broad subpleural lesions beyond the carina in the coronal plane.
Table 2.
Pattern of interstitial pneumonia and PPFE-like lesions.
| IPF (N = 77) | NSIP (N = 15) | CHP (N = 13) | CTD-IP (N = 41) | Unclassifiable (N = 58) |
PPFE (N = 3) | P value | P value without PPFE | |
|---|---|---|---|---|---|---|---|---|
| PPFE-like lesions | 62 (80.5 %) | 11 (73.3 %) | 10 (76.9 %) | 27 (65.9 %) | 47 (81.0 %) | 3 (100 %) | 0.42 | 0.40 |
| Upper | 17 (27.4 %) | 2 (18.2 %) | 4 (40 %) | 9 (33.3 %) | 22 (46.8 %) | 0 (0 %) | 0.46 | 0.44 |
| Upper middle | 13 (21 %) | 5 (45.5 %) | 4 (40 %) | 8 (29.6 %) | 11 (23.4 %) | 0 (0 %) | ||
| Lower middle | 23 (37.1 %) | 2 (18.2 %) | 1 (10 %) | 7 (25.9 %) | 10 (21.3 %) | 0 (0 %) | ||
| Lower | 9 (14.5 %) | 2 (18.2 %) | 1 (10 %) | 3 (11.1 %) | 4 (8.5 %) | 3 (1.0 %) | ||
| Inner | 39 (62.9 %) | 8 (72.7 %) | 6 (60 %) | 17 (63.0 %) | 30 (63.8 %) | 3 (100 %) | 0.83 | 0.98 |
| Dorsal | 57 (91.9 %) | 11 (1.0 %) | 9 (1.0 %) | 27 (1.0 %) | 42 (89.4 %) | 3 (1.0 %) | 0.58 | 0.48 |
| Outer | 58 (93.5 %) | 10 (90.9 %) | 10 (90.9 %) | 25 (92.6 %) | 42 (89.4 %) | 3 (100 %) | 0.87 | 0.81 |
| Ventral | 41 (66.1 %) | 8 (72.7 %) | 6 (60 %) | 10 (37.0 %) | 27 (57.4 %) | 3 (100 %) | 0.08 | 0.11 |
| Lateralitya | 1/11/46/4/0 | 0/6/5/0/0 | 1/2/7/0/0 | 0/4/19/3/0 | 1/13/29/3/1 | 0/1/2/0/0 | 0.62 | 0.38 |
| Air bronchogram | 38 (61 %) | 7 (64 %) | 5 (50 %) | 14 (52 %) | 19 (40 %) | 3 (1.0 %) | 0.16 | 0.26 |
| Nodules | 38 (61 %) | 6 (55 %) | 6 (60 %) | 14 (52 %) | 16 (34 %) | 3 (100 %) | 0.046 | 0.08 |
| Interlobular septal thickening | 34 (55 %) | 6 (55 %) | 6 (60 %) | 21 (78 %) | 26 (57 %) | 3 (100 %) | 0.25 | 0.33 |
| Consolidation along the bronchus | 24 (39 %) | 2 (18 %) | 3 (30 %) | 9 (33 %) | 12 (27 %) | 3 (100 %) | 0.12 | 0.58 |
| Bronchiectasis around PPFE-like lesions | 9 (15 %) | 2 (18 %) | 2 (20 %) | 4 (15 %) | 3 (6.4 %) | 3 (100 %) | 0.001 | 0.60 |
| Thickness (mm) | 7.9 ± 3.9 | 7.3 ± 2.5 | 8.2 ± 6.5 | 7.2 ± 3.9 | 7.2 ± 3.8 | 18 ± 6 | 0.13 | 0.87 |
Laterality (right only/right predominant/bilateral/left predominant/left only).
The correlations between the abnormal findings in the entire lung and PPFE-like lesions were not significantly different (Table 3). When the PPFE-like lesions below the aortic arch were evaluated, consolidation, reticular opacity, and area of traction bronchiectasis were more commonly observed (p = 0.04, 0.04, and 0.001, respectively), and emphysema was less significantly noted (p = 0.009).
Table 3.
Correlation between CT findings and PPFE-like lesions.
| All | With PPFE-like lesion | Without PPFE-like lesions | P value | With PPFE-like lesions below the aortic arch | Without PPFE-like lesions below the aortic arch | P value | |
|---|---|---|---|---|---|---|---|
| GGA | 11.4 ± 12.2 | 11.1 ± 12.3 | 12.3 ± 11.7 | 0.43 | 9.6 ± 9.8 | 12.2 ± 13 | 0.18 |
| GGA + TB | 12.7 ± 10.5 | 12.3 ± 10.2 | 14.0 ± 11.3 | 0.40 | 13.0 ± 9.5 | 12.6 ± 10.9 | 0.43 |
| Consolidation | 3.0 ± 5.5 | 2.9 ± 5.2 | 3.0 ± 6.3 | 0.21 | 3.5 ± 4.9 | 2.7 ± 5.7 | 0.04 |
| Honeycombing | 1.7 ± 3.8 | 1.7 ± 3.9 | 2.0 ± 3.8 | 0.70 | 1.9 ± 3.7 | 1.7 ± 3.9 | 0.11 |
| Reticular opacity | 11.2 ± 8.6 | 11.3 ± 8.9 | 10.6 ± 7.6 | 0.64 | 13.2 ± 10.7 | 10.2 ± 7.3 | 0.04 |
| Emphysema | 2.9 ± 7.7 | 2.6 ± 7.5 | 3.7 ± 8.5 | 0.31 | 0.9 ± 2.7 | 3.7 ± 9.0 | 0.009 |
| Cyst | 1.3 ± 3.3 | 1.3 ± 3.4 | 1.4 ± 2.7 | 0.95 | 1.4 ± 2.8 | 1.3 ± 3.5 | 0.22 |
| TB | 11.6 ± 5.2 | 11.5 ± 5.5 | 11.9 ± 4.0 | 0.90 | 13.1 ± 4.6 | 10.9 ± 5.3 | 0.001 |
Values are mean ± SD.
3.4. Predictors of mortality
The results of the Cox proportional hazards regression analysis of the relationship between the characteristics of PPFE-like lesions are shown in Table 4. In the univariate analysis, the craniocaudal location under aortic arch, ventral distribution, air bronchogram, nodules, consolidation along the bronchus around the PPFE-like lesions, bronchiectasis around the PPFE-like lesions and thickness were significant predictors (hazard ratios [HRs], 2.70, 2.51, 3.98, 2.36, 1.93, 3.56, and 1.11, respectively). In the multivariate analysis, the presence of air bronchogram (HR, 2.60; 95 % confidence interval [CI], 1.23–5.51), bronchiectasis around the PPFE-like lesions (odds ratio [OR], 2.60; 95 % CI, 1.23–5.51), and extent of the PPFE-like lesions under aortic arch (OR, 1.93; 95 % CI, 1.02–3.64) were significantly correlated with poor prognosis.
Table 4.
Predictor values of PPFE-like lesions.
| All |
IPF |
IP forms except for IPF and PPFE |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95 % CI | P value | HR | 95 % CI | P value | HR | 95 % CI | P value | |
| PPFE-like lesions | 1.16 | 0.64–2.10 | 0.62 | 0.37 | 0.082–1.68 | 0.20 | 0.76 | 0.34–1.69 | 0.51 |
| Craniocaudal distribution under the aortic arch | 2.70 | 1.66–4.39 | <0.001 | 3.44 | 1.73–6.84 | <0.001 | 1.42 | 0.62–3.24 | 0.41 |
| Inner distribution | 1.08 | 0.60–1.92 | 0.81 | 0.54 | 0.08–3.85 | 0.54 | 0.92 | 0.36–2.33 | 0.85 |
| Dorsal distribution | 0.88 | 0.27–2.82 | 0.82 | 0.19 | 0.02–1.83 | 0.15 | 0.78 | 0.10–5.87 | 0.81 |
| Outer distribution | 1.48 | 0.46–4.76 | 0.51 | 0.32 | 0.03–3.05 | 0.32 | 1.75 | 0.23–13.1 | 0.59 |
| Ventral distribution | 2.51 | 1.33–4.71 | 0.004 | 0.75 | 0.17–3.35 | 0.70 | 1.62 | 0.64–4.13 | 0.31 |
| Laterality | 1.41 | 0.89–2.23 | 0.14 | 1.71 | 0.17–16.8 | 0.65 | 1.46 | 0.73–2.94 | 0.29 |
| Air bronchogram | 3.98 | 2.04–7.77 | <0.001 | 3.62 | 0.38–34.8 | 0.27 | 3.49 | 1.26–9.73 | 0.02 |
| Nodules | 2.36 | 1.31–4.28 | 0.004 | 1.85 | 0.84–4.04 | 0.13 | 2.23 | 0.88–5.67 | 0.09 |
| Interlobular septal thickening | 1.29 | 0.72–2.29 | 0.39 | 1.09 | 0.53–2.25 | 0.82 | 1.94 | 0.69–5.47 | 0.21 |
| Consolidation along the bronchus | 1.93 | 1.11–3.36 | 0.02 | 1.74 | 0.84–3.57 | 0.13 | 1.62 | 0.64–4.12 | 0.31 |
| Bronchiectasis around PPFE-like lesions | 3.56 | 1.90–6.65 | <0.001 | 4.68 | 1.88–11.64 | 0.001 | 3.45 | 1.22–9.71 | 0.02 |
| Thickness | 1.11 | 1.05–1.17 | <0.001 | 1.10 | 1.02–1.20 | 0.02 | 1.12 | 1.01–1.23 | 0.03 |
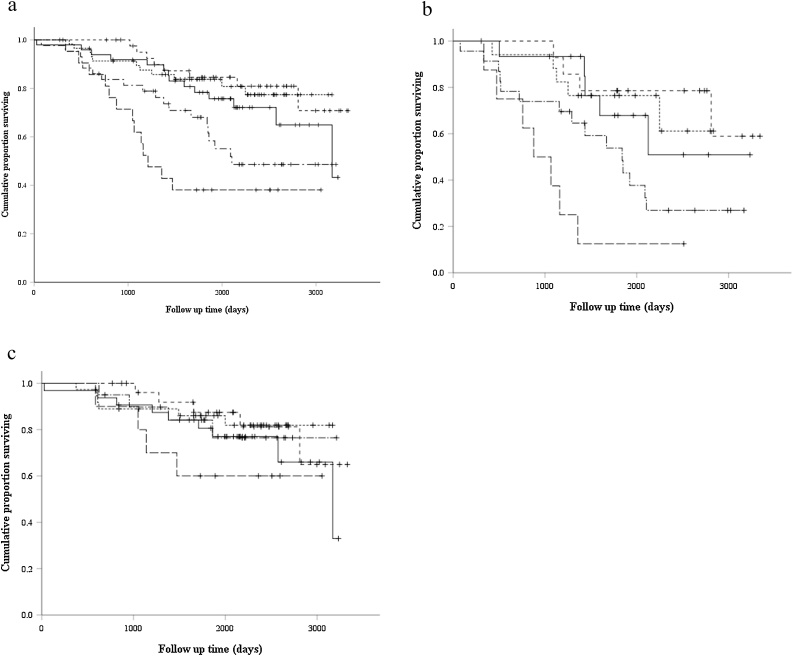
The mean follow-up duration was 1869 days (27–3342 days). The correlation between prognosis and craniocaudal position is shown in Fig. 4. The mean overall survival of the groups without PPFE-likelesions, with PPFE-like lesions in the upper, the middle upper, the middle lower and the lower area was 86.1, 89.1, 96.9, 73.8 and 58.3 months, respectively. In comparison of the patients with extent of the PPFE-like lesions under aortic arch (middle lower and lower) and others, overall survivals were 69 and 92.2 months, respectively. Patients with PPFE-like lesions in the lower area had significantly poorer prognoses than those without PPFE-like lesions (p = 0.001). Patients with PPFE-like lesions in the middle-lower area had lower prognoses than those without PPFE-like lesions; however, there was no significant difference (p = 0.08). There was no significant difference in prognoses between patients with PPFE-like lesions in the upper area and those without PPFE-like lesions (p = 0.22). In patients with IPF, the mean overall survival of the groups without PPFE-likelesions, with PPFE-like lesions in the upper, the middle upper, the middle lower and the lower area was 80.6, 75.1, 92.8, 58.4 and 35.6 months, respectively. In comparison of the patients with extent of the PPFE-like lesions under aortic arch (middle lower and lower) and others, overall survivals were 53.1 and 88.1 months, respectively. In patients with IPF, those with the PPFE-like lesions in the lower area had significantly poorer prognoses than those without PPFE-like lesions (p = 0.001). Patients with PPPFE-like lesions in the middle-lower area had lower prognoses than those without PPFE-like lesions; however, no significant difference was noted (p = 0.11). In patients with IP without IPF, the mean overall survival of the groups without PPFE-likelesions, with PPFE-like lesions in the upper, the middle upper, the middle lower and the lower area was 87.3, 92.3, 97.4, 91.9 and 75.2 months, respectively. In comparison of the patients with extent of the PPFE-like lesions under aortic arch (middle lower and lower) and others, overall survivals were 87.3 and 93.7 months, respectively. In patients with IP without IPF and PPFE, there was no significant difference in prognoses among patients with PPFE-like lesions in all craniocaudal positions.
Fig. 4.
Kaplan–Meier survival curves for the groups with the most caudal extent of PPFE-like lesions in (a) all cases, (b) IPF, and (c) IP without IPF and PPFE. The group without PPFE-likelesions (solid line), that of the area above the level of the sternoclavicular joint (dotted line), that of the area of above the level of the aortic arch (short dashed line), that of the area below the level of the aortic arch and above the level of the carina (dashed and dotted line), and that of the area below the level of the carina (long dashed line) were shown.
4. Discussion
PPFE is a new entity, and many characteristics of this disease are still unknown. The correlation between PPFE with IP at the lower lobe of the lung and IP with PPFE-like lesions at the upper lobe was unclear. However, the combination of PPFE and IP at the lower lobe of the lung was reported as a predictor of poor prognosis in some studies [10,13,15,16]. Moreover, IP in the lower lobe was an important progressive factor of PPFE-like lesions [9]. In the present study, PPFE-like lesions, including small lesions, were observed in several patients with IP (77.3 %). The frequency of these lesions was higher than that shown in a previous study (42.9 %) [9]. This might be the case because the progression of IP was different among these studies. The present study included patients with advanced IP because all patients underwent surgical lung biopsy and there were few asymptomatic patients. Conversely, the previous study included patients with mild IP with slight interstitial lung abnormalities because of daily chest CT. As IP was associated with the progression of PPFE-like lesions, increased extent of IP showed increased frequency of PPFE-like lesions [9]. However, the correlation of PPFE-like lesions and progression of IP was unclear, and further investigation was needed.
There was no significant difference in the presence of PPFE-like lesions among the types of IP, except for idiopathic PPFE. Furthermore, the thickness of the PPFE-like lesions was not different. The PPFE-like lesions were observed in patients with every form of IP and would not be an important feature for differential diagnosis, except for PPFE. Alternatively, idiopathic PPFE had the thickest PPFE-like lesions among the forms of IP, which would be useful in the diagnosis of PPFE. However, in the present study, the number of patients with idiopathic PPFE was extremely small, requiring further investigation.
Moreover, in the present study, the broad extent of PPFE-like lesions was an important predictor of prognosis; however, the small extent of these lesions was an insignificant predictor. Oda et al. reported that the survival time tended to be shorter in patients with PPFE with UIP; however, no significant difference was noted [10]. This might be because patients with both small and large PPFE-like lesions were included and not classified by extent of PPFE-like lesions in the previous study. Furthermore, PPFE-like lesions in patients with IP except for IPF and PPFE were a less important predictor than those in patients with IPF in the present study. In previous studies, the presence of PPFE-like lesions in patients with hypersensitivity pneumonitis and CTD was independently associated with increased mortality [15,16]. The broad extent of PPFE-like lesions in patients with IP, except for IPF and PPFE, could not show significant predictors in the Kaplan–Myer curves; however, some features of PPFE-like lesions, such as thickness, were significant predictors in the present study. The PPFE-like lesions were also important predictors in the patients with IP, except for IPF. The weak effect as a prognostic factor in patients with IP, except for IPF, would be associated with fewer total deaths in the present study.
Comparing the abnormal findings in the entire lung and PPFE-like lesions, patients with PPFE-like lesions under the aortic arch showed more consolidation and reticular opacity, larger area of traction bronchiectasis, and less emphysema. More reticular opacity and traction bronchiectasis indicate progressive IP. Poor prognoses in patients with PPFE-like lesions would be influenced by the progression of IP. Further investigation was needed on the correlation between PPFE-like lesions and extent of IP.
The present study has some limitations. First, this was a retrospective study. Second, the number of patients was small. The number of patients of each type of IP, especially PPFE, was too small for the evaluation. Third, PPFE-like lesions were diagnosed using CT only, and histological diagnoses were not determined. All patients underwent surgical biopsy; however, lower lung fields were resected to diagnose IP in the lower lobes. Pathological examination on the PPFE-like lesions in the upper lobe was insufficient. Fourth, the interobserver agreement of PPFE-like lesions was not so good. Small PPFE-like lesions were difficult to differentiate from other lesions of IP.
5. Conclusion
PPFE-like lesions are common in patients with IP, and their characteristics were not significantly different among all forms of IP, except idiopathic PPFE. The broad extent of PPFE-like lesions is an important predictor of prognosis in patients with IPF.
Funding sources
None.
Ethical approval details
Osaka International Cancer Institute 2016 No. 1612069194.
CRediT authorship contribution statement
Hiromitsu Sumikawa: Conceptualization, Methodology, Writing - original draft, Data curation, Formal analysis. Takeshi Johkoh: Conceptualization, Supervision, Writing - review & editing. Ryoko Egashira: Investigation. Hiroaki Sugiura: Investigation. Yasuhiko Yamano: Investigation, Resources. Kensuke Kataoka: Investigation, Resources. Yasuhiro Kondoh: Writing - review & editing, Investigation, Resources. Hiroaki Arakawa: Investigation. Masahisa Nakamura: Resources. Akihiro Kuriu: Resources. Katsuyuki Nakanishi: Writing - review & editing, Supervision. Noriyuki Tomiyama: Writing - review & editing, Project administration.
Declaration of Competing Interest
Dr Kondoh reports consulting fees and lecture fees from Asahi Kasei Pharma Corp., Boehringer Ingelheim Co., Ltd., Janssen Pharmaceutical K.K., and Shionogi & Co. Ltd., consulting fees from Chugai Pharmaceutical Co., Ltd., Healios K.K., and Roche, and lecturel fees from Eisai inc., KYORIN Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma, and Novartis Pharma K.K., outside the presentation work.
References
- 1.Travis W.D., Costabel U., Hansell D.M., King T.E., Jr., Lynch D.A., Nicholson A.G., Ryerson C.J., Ryu J.H., Selman M., Wells A.U., Behr J., Bouros D., Brown K.K., Colby T.V., Collard H.R., Cordeiro C.R., Cottin V., Crestani B., Drent M., Dudden R.F., Egan J., Flaherty K., Hogaboam C., Inoue Y., Johkoh T., Kim D.S., Kitaichi M., Loyd J., Martinez F.J., Myers J., Protzko S., Raghu G., Richeldi L., Sverzellati N., Swigris J., Valeyre D. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013;188(6):733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amitani R., Niimi A., Kuze F. Idiopathic pulmonary upper lobe fibrosis (IPUF) Kokyu. 1992;11:693–699. [Google Scholar]
- 3.Frankel S.K., Cool C.D., Lynch D.A., Brown K.K. Idiopathic pleuroparenchymal fibroelastosis: description of a novel clinicopathologic entity. Chest. 2004;126(6):2007–2013. doi: 10.1378/chest.126.6.2007. [DOI] [PubMed] [Google Scholar]
- 4.Piciucchi S., Tomassetti S., Casoni G., Sverzellati N., Carloni A., Dubini A., Gavelli G., Cavazza A., Chilosi M., Poletti V. High resolution CT and histological findings in idiopathic pleuroparenchymal fibroelastosis: features and differential diagnosis. Respir. Res. 2011;12:111. doi: 10.1186/1465-9921-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kusagaya H., Nakamura Y., Kono M., Kaida Y., Kuroishi S., Enomoto N., Fujisawa T., Koshimizu N., Yokomura K., Inui N., Suda T., Colby T.V., Chida K. Idiopathic pleuroparenchymal fibroelastosis: consideration of a clinicopathological entity in a series of Japanese patients. BMC Pulm. Med. 2012;12:72. doi: 10.1186/1471-2466-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy T.L., Tominaga M., Hansell D.M., von der Thusen J., Rassl D., Parfrey H., Guy S., Twentyman O., Rice A., Maher T.M., Renzoni E.A., Wells A.U., Nicholson A.G. Pleuroparenchymal fibroelastosis: a spectrum of histopathological and imaging phenotypes. Eur. Respir. J. 2012;40(2):377–385. doi: 10.1183/09031936.00165111. [DOI] [PubMed] [Google Scholar]
- 7.Enomoto N., Kusagaya H., Oyama Y., Kono M., Kaida Y., Kuroishi S., Hashimoto D., Fujisawa T., Yokomura K., Inui N., Nakamura Y., Suda T. Quantitative analysis of lung elastic fibers in idiopathic pleuroparenchymal fibroelastosis (IPPFE): comparison of clinical, radiological, and pathological findings with those of idiopathic pulmonary fibrosis (IPF) BMC Pulm. Med. 2014;14:91. doi: 10.1186/1471-2466-14-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renner R.R., Markarian B., Pernice N.J., Heitzman E.R. The apical cap. Radiology. 1974;110(3):569–573. doi: 10.1148/110.3.569. [DOI] [PubMed] [Google Scholar]
- 9.Sumikawa H., Johkoh T., Iwasawa T., Nakanishi K., Tomiyama N. Pleuroparenchymal fibroelastosis-like lesions on chest computed tomography in routine clinical practice. J. Radiol. 2019;37(3):230–236. doi: 10.1007/s11604-018-0805-5. [DOI] [PubMed] [Google Scholar]
- 10.Oda T., Ogura T., Kitamura H., Hagiwara E., Baba T., Enomoto Y., Iwasawa T., Okudela K., Takemura T., Sakai F., Hasegawa Y. Distinct characteristics of pleuroparenchymal fibroelastosis with usual interstitial pneumonia compared with idiopathic pulmonary fibrosis. Chest. 2014;146(5):1248–1255. doi: 10.1378/chest.13-2866. [DOI] [PubMed] [Google Scholar]
- 11.Nakatani T., Arai T., Kitaichi M., Akira M., Tachibana K., Sugimoto C., Hirooka A., Tsuji T., Minomo S., Hayashi S., Inoue Y. Pleuroparenchymal fibroelastosis from a consecutive database: a rare disease entity? Eur. Respir. J. 2015;45(4):1183–1186. doi: 10.1183/09031936.00214714. [DOI] [PubMed] [Google Scholar]
- 12.Kato M., Sasaki S., Kurokawa K., Nakamura T., Yamada T., Sasano H., Arano N., Komura M., Ihara H., Nagashima O., Shiota S., Takahashi F., Takahashi K. Usual interstitial pneumonia pattern in the lower lung lobes as a prognostic factor in idiopathic pleuroparenchymal fibroelastosis. Respiration. 2019;97(4):319–328. doi: 10.1159/000494061. [DOI] [PubMed] [Google Scholar]
- 13.Kono M., Fujita Y., Takeda K., Miyashita K., Tsutsumi A., Kobayashi T., Miki Y., Hashimoto D., Enomoto N., Nakamura Y., Suda T., Nakamura H. Clinical significance of lower-lobe interstitial lung disease on high-resolution computed tomography in patients with idiopathic pleuroparenchymal fibroelastosis. Respir. Med. 2019;154:122–126. doi: 10.1016/j.rmed.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Enomoto Y., Nakamura Y., Satake Y., Sumikawa H., Johkoh T., Colby T.V., Yasui H., Hozumi H., Karayama M., Suzuki Y., Furuhashi K., Fujisawa T., Enomoto N., Inui N., Iwashita T., Kuroishi S., Yokomura K., Koshimizu N., Toyoshima M., Imokawa S., Yamada T., Shirai T., Hayakawa H., Suda T. Clinical diagnosis of idiopathic pleuroparenchymal fibroelastosis: a retrospective multicenter study. Respir. Med. 2017;133:1–5. doi: 10.1016/j.rmed.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Jacob J., Odink A., Brun A.L., Macaluso C., de Lauretis A., Kokosi M., Devaraj A., Desai S., Renzoni E., Wells A.U. Functional associations of pleuroparenchymal fibroelastosis and emphysema with hypersensitivity pneumonitis. Respir. Med. 2018;138:95–101. doi: 10.1016/j.rmed.2018.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enomoto Y., Nakamura Y., Colby T.V., Johkoh T., Sumikawa H., Nishimoto K., Yoshimura K., Matsushima S., Oyama Y., Hozumi H., Kono M., Fujisawa T., Enomoto N., Inui N., Iwashita T., Suda T. Radiologic pleuroparenchymal fibroelastosis-like lesion in connective tissue disease-related interstitial lung disease. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0180283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Müller N.L., Remy J. Fleischner society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]