Abstract
Purpose
This study investigated whether hypofractionated adjuvant radiotherapy (RT) increased breast-related complication(s) compared to conventional fractionated RT in reconstructed breast cancer patients.
Methods
We conducted a retrospective review including 349 breast cancer patients who underwent immediate breast reconstruction following mastectomy or breast-conserving surgery (BCS) between 2009 and 2018 at two institutions. All patients were treated with adjuvant RT via either a conventional fractionated or hypofractionated regimen. We defined a major breast complication as a breast-related toxic event requiring re-operation or re-hospitalization during the follow-up period after the end of RT.
Results
The median follow-up was 32.3 months (4.8–118.5 months); 126 patients had conventional fractionated RT, and 223 patients received hypofractionated RT. In patients with mastectomy, there was no significant difference in the occurrence of any or major breast-related complications between the two fractionation regimens. In patients undergoing BCS, incidence of any breast complication showed no difference between two RT groups and no major breast complication was reported as well. Hypofractionated RT did not increase major wound problem (infection and dehiscence) compared to conventional RT. Incidence of major contracture was significantly lower in hypofractionated RT.
Conclusions
There was no significant difference in the occurrence of any or major breast-related complications between the two different fractionation regimens, even in patients with mastectomy. Hypofractionated RT may be used comparable to conventional fractionated RT in terms of breast-related complications in reconstructed breast cancer patients. The prospective randomized trial would be necessary to clarify this issue.
Keywords: Breast cancer, Reconstruction, Hypofractionation, Radiotherapy
Abbreviations: RT, Radiotherapy; BCS, Breast-conserving surgery; 3-D CRT, 3-dimensional conformal radiotherapy; IMRT, Intensity-modulated radiation therapy; fx, fraction
Highlights
-
•
There was no significant difference in the occurrence of breast complications between the two fractionation regimens.
-
•
Hypofractionated RT may be used comparable to conventional fractionated RT in reconstructed breast cancer patients.
-
•
The prospective randomized trial would be necessary to clarify this issue.
1. Introduction
In breast cancer patients, breast reconstruction is performed to restore the breast mound, to create symmetry and balance with the contralateral side of breast after mastectomy [[1], [2], [3]] or breast-conserving surgery (BCS) as an oncoplastic surgery [[4], [5], [6]]. In addition to cosmetic satisfaction, it might relieve the psychosocial burden after the breast surgery. Several methods are available for breast reconstruction, depending on when it is performed (immediate vs. delayed) and what type of reconstruction is done (autologous vs. implant) [[7], [8], [9]]. These various ways of breast reconstruction are the main factors that affect postoperative complications [7]. Radiotherapy (RT) may also affect outcomes after breast reconstruction, depending on the type of RT technique (3-dimensional conformal RT [3-D CRT] vs. intensity-modulated radiation therapy [IMRT]), the timing of RT (proceeding vs. following reconstruction) and the size of fractionation (hypofractionation vs. conventional fractionation) [7].
In clinical practice, the fractionation of adjuvant RT following breast reconstruction is determined by the clinician’s judgement and preference between conventional fractionated and hypofractionated RT. The efficacy and safety of hypofractionated RT has been demonstrated in START A and START B trials [10,11], and a recent randomized, phase 3 study showed that hypofractionated RT after mastectomy is non-inferior to conventional fractionated RT in terms of locoregional control and toxicity [12]. However, little is known about how hypofractionated RT affects breast-related complications after breast reconstruction. Indeed, there has been no clear guideline on which fractionation regimen is better either hypofractionated or conventional fractionation in breast cancer patients with reconstruction. If hypofractionated RT has complication rates that are comparable to conventional fractionated RT, it may become the standard treatment for patients with reconstructed breast cancer. Hypofractionation can be beneficial for patients because it can reduce the number of visits and the total cost of treatment [13,14]. Therefore, this study aimed to identify any difference in breast-related complications between hypofractionated and conventional fractionated RT in breast cancer patients undergoing breast reconstruction. The hypothesis of study was that hypofractionated RT induced comparable reconstruction-related toxicities compared to conventional fractionated RT in reconstructed breast cancer patients.
2. Materials and methods
2.1. Study design and patients
After receiving institutional review board approval from each institution (B-2001/586-112, J-2001-009-1091), we conducted retrospective reviews of 349 breast cancer patients who underwent immediate breast reconstruction following mastectomy or BCS at Seoul National University Bundang Hospital and Seoul National University Hospital from January 2009 to December 2018. All patients were treated with post-operative adjuvant RT via either a conventional fractionation (1.8–2Gy/fraction (fx)) or a hypofractionation (2.4–2.7Gy/fx) regimen. Patients with delayed breast reconstruction or incomplete RT were excluded.
2.2. Definitions of breast-related complication
Our primary toxicity endpoint was breast-related complication, which included hematoma, wound infection, wound dehiscence, reconstructive flap necrosis, flap contracture, fat necrosis, capsular contracture, implant leakage/rupture/deflation, breast pain and breast lymphedema [15]. We classified these complications into ‘any breast-related complication’, which included all of these complications, and ‘major breast-related complication’, which was defined as an event that required re-operation or re-hospitalization [15].
Some complications (wound infection, wound dehiscence, hematoma and breast pain) were graded using Common Terminology Criteria for Adverse Events version 5.0. Capsular contracture was evaluated by the Baker Scale [16], and breast lymphedema was scored according to the standards of International Society of Lymphology [17]. For reconstructive flap necrosis, we referred to grading described in previous studies [18,19]. Other complications, such as fat necrosis, implant leakage/rupture/deflation, and flap contracture were graded according to the following established criteria: Grade 1, observation; Grade 2, outpatient intervention; and Grade 3, re-hospitalization or re-operation for intervention. All complications were described in detail and reviewed by both a plastic surgeon and radiation oncologist. In case of the grade of toxicity was not recorded in the files, the investigators graded the complication retrospectively based on the description medical records according to the predefined definition.
2.3. Breast reconstruction
All 349 patients had breast reconstruction surgery following mastectomy or BCS. Of the patients, 267 had mastectomy and 82 received BCS. All patients with BCS underwent breast reconstruction as an oncoplastic surgery. The reason why BCS followed by reconstruction accounted for 23.5% of total patients was approximately 20–30% patients with BCS showed unsatisfactory aesthetic appearance due to retraction or distortion [20]. In addition, Asian women have relatively small- or medium-sized breasts. Therefore, if the tumor size is large compared to the breast size, significant breast deformation is maintained even after BCS [21], so breast reconstruction surgery may be necessary for cosmetic purposes.
The timing of reconstruction was all ‘immediate’; reconstruction surgery was performed simultaneously with mastectomy or BCS. There were two types of reconstruction, autologous or implant, depending on the tissues used. Autologous tissue for breast reconstruction is transferred from any part of the body, including abdomen, infra-umbilical area, back, thigh, or buttocks [7]. The most commonly used autologous reconstruction types were latissimus dorsi myocutaneous-free flap and transverse rectus abdominis myocutaneous flap. Of the patients who had done mastectomy (n = 267), 147 patients received the autologous reconstruction and 120 did implant reconstruction. All the patients with BCS (n = 82) received autologous flap reconstructions. Reconstruction types were stated at the time of their initial visit to the outpatient office and depended on the preferences of the plastic surgeon and the patient.
2.4. Radiotherapy
Patients received RT within 5–6 weeks after surgery or 3–4 weeks after adjuvant chemotherapy. RT was administered with 6–15 MV photons from a linear accelerator using either 3-D CRT (n = 198) or IMRT (n = 151). Simulation computed tomography scans were performed with free breathing. IMRT was planned by forward planning Field in Field (FiF) technique to reduce the size of high-dose region and to improve homogeneity index. According to the fractionation schedule, patients received RT using either a conventional fractionated (1.8–2 Gy/fx) or hypofractionated (2.4–2.7 Gy/fx) schedule. Determination of fractionation was depended on preference of each radiation oncologist and individual patient. The two fractionation schedules may have different patients’ preferences because the number of hospital visits and treatment costs may vary. If the patient doesn’t reveal their preference, the opinion of radiation oncologist is mainly reflected. The median dose for whole breast or chest wall was 50.3Gy (range, 50–66Gy) in conventional RT and 44.3Gy (range, 40.5–48.6Gy) in hypofractionated RT. When indicated, regional nodal irradiation was performed at an equivalent dose of whole breast or chest wall. Tumor bed boost was sequentially delivered 5.4–16Gy in 3–8 fractions for conventional RT and 6–15Gy in 3–5 fractions for hypofractionated RT. Tumor bed boost was selectively delivered depending on the resection margin or high-risk tumors. In patients who underwent mastectomy, tumor bed boost was considered in tumors such as T4 or close/positive resection margin. Nearly all the patients who received BCS had radiotherapy including tumor bed boost. Tumor bed boost was delineated depending on the initial tumor location and margin status. Especially, in patients with mastectomy, tumor bed boost was contoured at near skin or chest wall. When delineating the tumor bed boost of patients with BCS, it was set through the postoperative lumpectomy cavity or the location of the surgical clip [22]. Of all, 37.3% of patients with conventional RT and 26.9% of hypofractionated RT received tumor bed boost. Finally, total median dose was 54.6Gy (range, 50–66Gy) in conventional RT and that of hypofractionated RT was 47.5Gy (range, 42.6–57.6Gy). The criteria for the use of bolus slightly differed depending on the radiation oncologists. Some of them used bolus if superficial margin was too close or positive for skin in patients with mastectomy with reconstruction. Meanwhile, others adapted bolus only if the patient was too lean to have enough chest wall tissue, or definitely, if a sufficient dose was not prescribed to chest wall following evaluation of each treatment planning. Of all the patients, 28.6% of conventional and 16.1% of hypofractionated RT group used bolus, respectively.
Dose constraints for 3-D CRT and IMRT were based on Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) published in 2010 or Radiation Therapy Oncology Group (RTOG) protocols. We ensured that 95% of the PTV should be covered by the 95% of the prescribed dose. The criterion was set to maximum point dose under 105–107% of prescribed dose. The volume of ipsilateral receiving 20Gy or more (V20) was stipulated under 20% and mean heart dose was mandated to be < 10 Gy regardless of right, left, or bilateral-sided breast cancer. In addition, a treatment planning was developed with the same heart dose limits applied to the patients treated including IMNs. When treating the left breast or regional nodes, the cardiac dose was relatively increased compared to right breast or no regional nodes. In the case of 3D-CRT, the heart dose was reduced by using a heart block in MLC, and in the case of IMRT, the cardiac dose was reduced as much as possible by balancing the correlation within the range that complied with the lung dose constraint (V20 < 20%).
2.5. Statistical analysis
Chi-square test was used for comparing patient characteristics between the two fractionated RT regimens (hypofractionated RT vs. conventional fractionated RT). To compare the incidence of complication rates considering a significant difference in follow-up period between two RT regimens, the Kaplan-Meier methodology was employed. A multivariable cox regression model was used to analyze factors that affected any and major breast complications. All statistical analyses were two-sided and performed using Stata/MP 15.0 (StataCorp, College Station, TX), with a significance level of <0.05.
3. Results
A total of 349 breast cancer patients who underwent mastectomy or BCS followed by immediate breast reconstruction were analyzed. The median age of patients was 45.1 years old (range, 22–74 years old). The median follow-up was 32.3 months (range, 4.8–118.5 months). There was a significant difference in follow-up period between two RT regimens (P < 0.001). The median follow-up time for conventional RT was 50.4 months (range, 5.1–118.5 months), but the time for hypofractionated RT was 25.4 months (range, 4.8–57.8 months).
Among these 349 patients, 126 patients (36.1%) had conventional fractionated RT, and 223 patients (63.9%) received hypofractionated RT. The demographics of the patients are shown in Table 1. There were no differences in age, body mass index, laterality, or histology between the conventional fractionated and hypofractionated RT groups. No difference was found in HER-2 targeted therapy or adjuvant chemotherapy between the two different RT groups, but patients who received hypofractionated RT had more neoadjuvant chemotherapy (P = 0.017). Meanwhile, there was no significant difference in type of operation (mastectomy or BCS) between the two RT groups. Compared with the conventional fractionated RT group, patients with hypofractionated RT had significantly more implant-based reconstruction (P < 0.001). Furthermore, hypofractionated RT was performed using the IMRT technique more often than conventional RT (P < 0.001), and patients treated with hypofractionated RT received more RT to internal mammary node (IMN) than those treated with hypofractionated RT (P < 0.001). Use of bolus was found significantly more in patients with conventional RT (P = 0.006).
Table 1.
Patient characteristics (n=349).
| Conventional RT (n=126) | Hypofractionated RT (n=223) | P-value | |
|---|---|---|---|
| Age (year) | |||
| < 45 | 69 (54.8%) | 122 (54.7%) | 0.992 |
| ≥ 45 | 57 (45.2%) | 101 (45.3%) | |
| Body mass index | |||
| <23 | 67 (53.2%) | 117 (52.5%) | 0.899 |
| ≥23 | 59 (46.8%) | 106 (47.5%) | |
| Laterality | |||
| Left | 62 (49.2%) | 114 (48.5%) | 0.753 |
| Right | 64 (50.8%) | 120 (51.1%) | |
| Bilateral | 0 (0.0%) | 1 (0.4%) | |
| Histology | |||
| IDC | 107 (84.9%) | 195 (87.4%) | 0.524 |
| Others+ | 19 (15.1%) | 29 (12.6%) | |
| Molecular type | |||
| Luminal A | 58 (46.1%) | 118 (52.9%) | 0.038 |
| Luminal B | 41 (32.5%) | 53 (23.7%) | |
| Her-2 enriched | 9 (7.1%) | 28 (12.6%) | |
| TNBC | 14 (11.1%) | 23 (10.3%) | |
| None | 4 (3.2%) | 1 (0.5%) | |
| Neoadjuvant chemotherapy | |||
| Yes | 40 (31.8%) | 100 (44.8%) | 0.017 |
| No | 86 (68.2%) | 123 (55.2%) | |
| Adjuvant chemotherapy | |||
| Yes | 69 (55.2%) | 102 (45.7%) | 0.090 |
| No | 56 (44.8%) | 121 (54.3%) | |
| Anti Her-2 therapy | |||
| Yes | 23 (18.3%) | 49 (22.0%) | 0.410 |
| No | 103 (81.7%) | 174 (78.0%) | |
| Endocrine therapy | |||
| Tamoxifen | 74 (58.7%) | 129 (57.9%) | 0.044 |
| Tamoxifen+zoladex | 9 (7.1%) | 31 (13.9%) | |
| Aromatase inhibitor | 13 (10.3%) | 31 (13.9%) | |
| No | 30 (23.8%) | 32 (14.3%) | |
| Operation type | |||
| Mastectomy | 91 (72.2%) | 176 (78.9%) | 0.156 |
| BCS | 35 (27.8%) | 47 (21.1%) | |
| Lymph node staging | |||
| SLNB | 44 (34.9%) | 101 (45.3%) | 0.082 |
| ALND | 80 (63.5%) | 122 (54.7%) | |
| None | 2 (1.6%) | 0 (0.0%) | |
| Reconstruction type | |||
| LD flap | 37 (29.4%) | 53 (23.8%) | <0.001 |
| TRAM | 69 (54.8%) | 68 (30.5%) | |
| Implant | 19 (15.1%) | 101 (45.3%) | |
| Others* | 1 (0.7%) | 1 (0.4%) | |
| RT technique | |||
| 3D | 119 (94.4%) | 79 (35.4%) | <0.001 |
| IMRT | 7 (5.6%) | 144 (64.6%) | |
| RT to SCL | |||
| Yes | 83 (65.9%) | 159 (71.3%) | 0.263 |
| No | 43 (34.1%) | 64 (28.7%) | |
| RT to IMN | |||
| Yes | 6 (4.8%) | 112 (50.2%) | <0.001 |
| No | 120 (95.2%) | 111 (49.8%) | |
| Tumor bed boost | |||
| Yes | 47 (37.3%) | 60 (26.9%) | 0.043 |
| No | 79 (62.7%) | 163 (73.1%) | |
| Bolus | |||
| Yes | 36 (28.6%) | 36 (16.1%) | 0.006 |
| No | 80 (71.4%) | 83 (83.9%) | |
P-value by chi-square test.
Others+, intralobular carcinoma, mucinous carcinoma, phyllodes tumor, invasive mammary carcinoma
Abbreviations: IDC, intraductal carcinoma; TNBC, triple negative breast cancer; BCS, breast-conserving surgery; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; LD flap. latissimus dorsi flap; TRAM, transverse rectus abdominis myocutaneous flap; 3D, 3-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; SCL, supraclavicular lymph node; IMN, internal mammary lymph node Others*, fat graft, advancement flap, free deep inferior epigastric artery perforator.
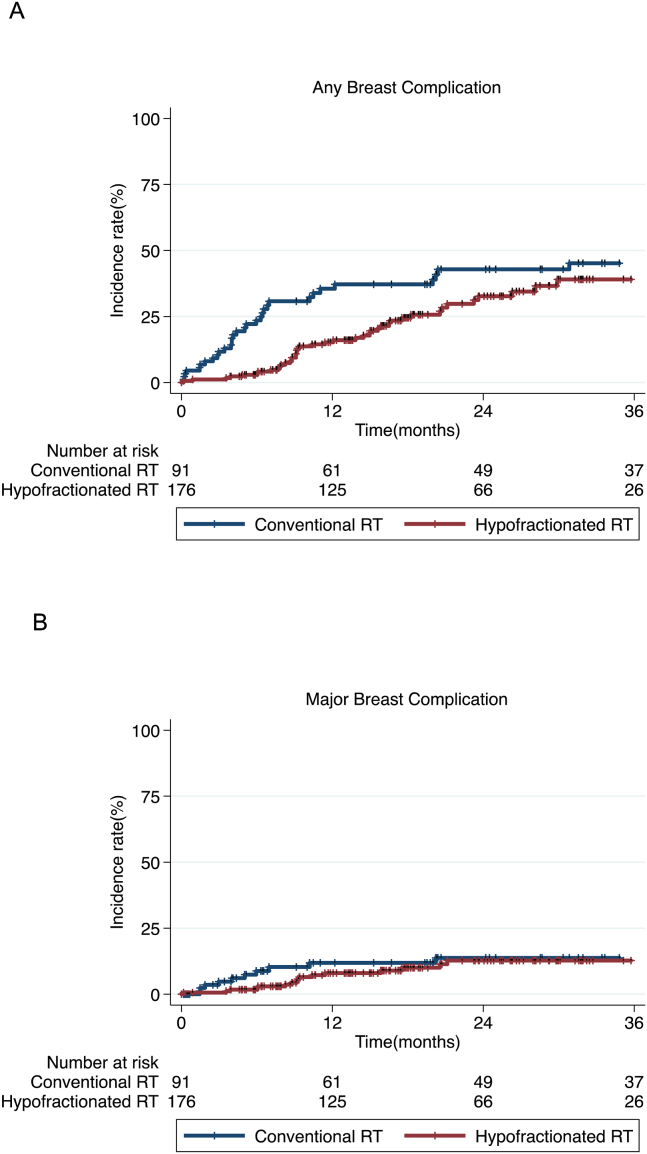
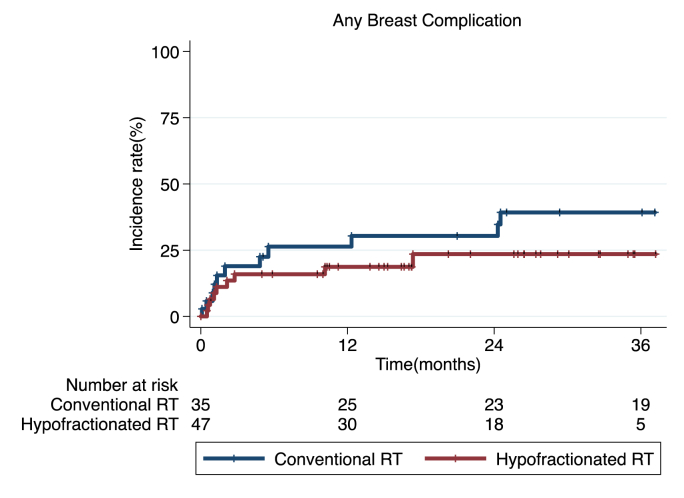
In patients with mastectomy, there was no significant difference in the occurrence of any or major breast-related complications between the two fractionation regimens (P = 0.064 and 0.420, respectively; Fig. 1A and B). In patients with BCS, incidence of any breast complication showed no difference between two RT groups (P = 0.301; Fig. 2), and no major breast complication was reported. In mastectomy patients, the 6-month, 1-year and 2-year incidence rates of any breast-related complications were 21.1%, 30.1% and 35.0% in conventional fractionated RT group; Any breast complications were reported in 2.8% by 6 months, in 14.2% by 1 year, and 28.0% by 2 years in hypofractionated RT group. In patients with BCS, the 6-month, 1-year and 2-year actuarial rates of any breast complications were 23.5%, 23.5%, and 26.6%, respectively; Those of hypofractionated RT group were 14.9%, 17.3% and 21.2%, respectively. In terms of major breast-related complication in patients with mastectomy, 6-month, 1-year and 2-year incidence rates were 8.5%, 11.2% and 12.9% in conventional RT group; 1.7%, 7.7% and 12.0% in hypofractionated RT group. Most of major breast complications in two RT regimens were reported before 20 months.
Fig. 1.
Incidence of any breast-related complications (A, P = 0.064) and major breast-related complications (B, P = 0.420) over time in patients who received mastectomy followed by conventional fractionated RT or hypofractionated RT.
Fig. 2.
Incidence of any breast-related complications (P = 0.301) over time in patients who received BCS followed by conventional fractionated RT or hypofractionated RT. (Note that no major breast complication in patients with BCS was reported).
The most common major breast-related complications were wound problems (infection and dehiscence) in both RT fractionation groups; 4.8% in conventional RT group and 4.4% in hypofractionated RT group. In the conventional RT group, major breast complications were reported in 20 cases: 4 cases of wound infection, 2 wound dehiscence, 4 fat necrosis, 2 implant leakage, 3 capsular contracture, 3 flap contracture, 1 lymphedema and 1 hematoma. On the other hand, hypofractionated RT group showed 14 cases of major breast complications: 7 cases of wound infection, 3 wound dehiscence, 2 fat necrosis, and 2 implant leakage (Table 2).
Table 2.
Summary of types of major breast complication in patients with mastectomy: Conventional RT vs. hypofractionated RT.
| Conventional RT n (%) | Hypofractionated RT n (%) | |||
|---|---|---|---|---|
| Hematoma | 1 (0.8%) | 0 (0.0%) | ||
| Wound Infection | 4 (3.2%) | 7 (3.1%) | ||
| Wound Dehiscence | 2 (1.6%) | 3 (1.3%) | ||
| Fat Necrosis | 4 (3.2%) | 2 (0.9%) | ||
| Capsular Contracture | 3 (2.4%) | 0 (0.0%) | ||
| Lymphedema | 1 (0.8%) | 0 (0.0%) | ||
| Implant Leakage/Rupture/Deflation | 2 (1.6%) | 2 (0.9%) | ||
| Flap contracture | 3 (2.4%) | 0 (0.0%) | ||
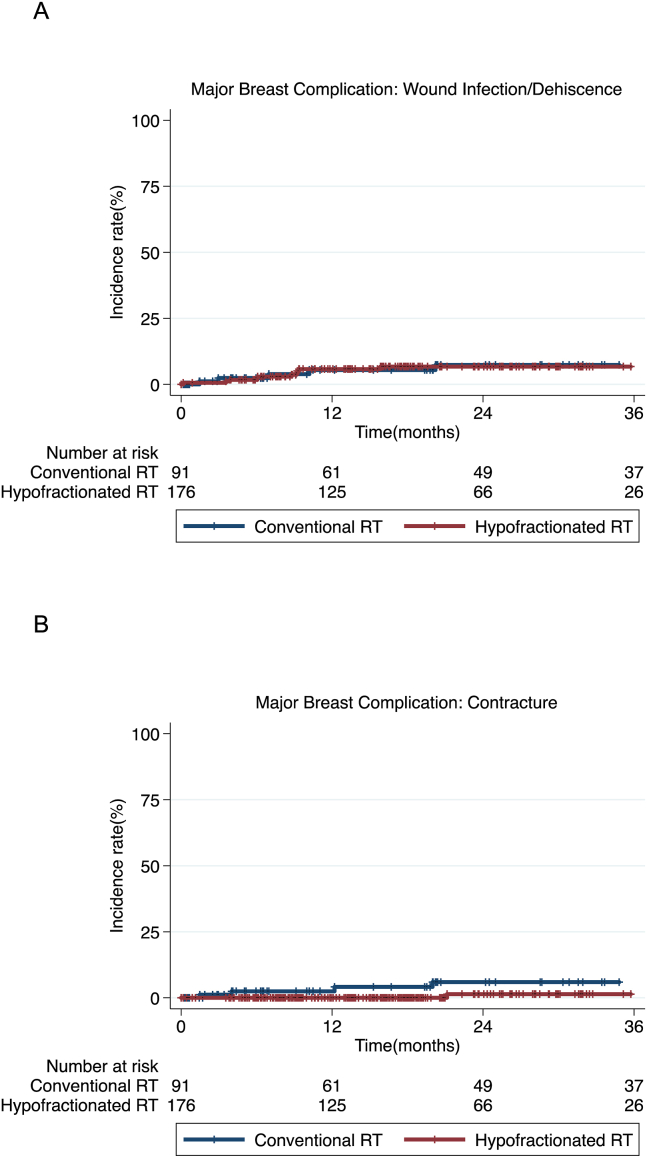
Hypofractionated RT did not increase major wound problems (infection and dehiscence) compared to conventional RT (P = 0.948, Fig. 3A). Two fractionation regimens showed similar incidence rates of major wound problems; 2.4% by 6 months, 5.3% by 1 year in patients with conventional RT and 1.7% by 6 months, 5.4% by 1 year in hypofractionated RT. Most of major adverse wound events occurred in the first year, and no more wound problems happened after 20 months in conventional RT and 15 months in hypofractionated RT. Major contracture was significantly less reported in hypofractionated RT (P = 0.033, Fig. 3B). At 2 year of follow-up, major contractures were reported 9.6% of conventional RT group and 1.4% of hypofractionated RT group, respectively. Patents with hypofractionated RT showed no contracture event until the first year, 1.4% at the second year.
Fig. 3.
Incidence of major wound infection/dehiscence (A, P = 0.948) and major contracture (B, P = 0.033) over time in patients who received mastectomy followed by conventional fractionated RT or hypofractionated RT.
Multivariate analyses revealed that implant reconstruction and age ≥45 years were significant predictive factors for higher incidence major breast complications in patients with mastectomy (P = 0.041 and P = 0.016) (Table 3). Hypofractionated RT was associated with lower risk of any breast complications in mastectomy patients (HR 0.58, P = 0.027), and did not affect the occurrence of major breast complications. There was no predictive factor which was related to occurrence of any breast complication in patients with BCS.
Table 3.
Prognostic factors for any breast complication and major breast complication in patients with mastectomy.
|
Variable |
Any breast complication |
Major breast complication |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
|||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Age | ||||||||||||
| ≥ 45 (vs. <45) | 1.63 | 1.04–2.54 | 0.032 | 1.46 | 0.93–2.29 | 0.099 | 2.46 | 1.26–5.21 | 0.019 | 2.56 | 1.18–5.52 | 0.016 |
| Body Mass Index | ||||||||||||
| ≥ 23 (vs. <23) | 1.55 | 0.99–2.43 | 0.055 | 1.41 | 0.89–2.23 | 0.147 | 2.08 | 0.98–4.42 | 0.057 | 1.98 | 0.91–4.34 | 0.087 |
| Reconstruction type | ||||||||||||
| Implant (vs. Autologous) | 1.24 | 0.80–1.94 | 0.337 | – | – | – | 1.84 | 0.88–3.87 | 0.106 | 2.23 | 1.03–4.81 | 0.041 |
| Neoadjuvant chemotherapy | ||||||||||||
| Yes (vs. No) | 0.82 | 0.53–1.29 | 0.391 | – | – | – | 0.95 | 0.46–1.97 | 0.892 | – | – | – |
| Adjuvant chemotherapy | ||||||||||||
| Yes (vs. No) | 1.41 | 0.90–2.20 | 0.134 | 1.21 | 0.77–1.92 | 0.408 | 1.06 | 0.51–2.20 | 0.879 | – | – | – |
| RT technique | ||||||||||||
| IMRT (vs. 3D) | 0.81 | 0.51–1.28 | 0.365 | – | – | – | 0.75 | 0.35–1.63 | 0.468 | – | – | – |
| RT Fractionation | ||||||||||||
| Hypofractionated RT (vs. Conventional RT) |
0.65 | 0.41–1.03 | 0.066 | 0.58 | 0.36–0.94 | 0.027 | 0.73 | 0.34–1.57 | 0.422 | – | – | – |
| Tumor Bed Boost | ||||||||||||
| Yes (vs. No) | 0.96 | 0.46–1.99 | 0.902 | – | – | – | 1.72 | 0.66–4.51 | 0.269 | – | – | – |
| Bolus | ||||||||||||
| Yes (vs. No) | 0.58 | 0.31–1.09 | 0.091 | 0.52 | 0.27–1.02 | 0.056 | 0.43 | 0.14–1.32 | 0.140 | 0.59 | 0.18–1.90 | 0.377 |
P-value by cox regression model.
Abbreviations: HR, hazard ratio; CI, confidence interval; IMRT, intensity-modulated radiotherapy; 3D, 3-dimensional conformal radiotherapy.
4. Discussion
About 40% of all breast cancer patients undergo breast reconstruction after mastectomy or BCS, and the overall rate of breast reconstruction has been increasing [25]. Breast reconstruction can be challenging when integrating with postmastectomy or postlumpectomy RT, which may compromise the skin and underlying tissue due to radiation-induced fibrosis [26,27]. Little is known, however, about the effects of hypofractionated RT on reconstructed breast following mastectomy or BCS. Therefore, we aimed to identify any difference in breast-related complications between hypofractionated and conventional fractionated RT in breast cancer patients undergoing reconstruction.
The present study analyzed 349 patients with immediate reconstructive breast cancer treated with either hypofractionated adjuvant RT or conventional fractionated RT. We found no significant difference in the occurrence of any breast-related complications between the two fractionation regimens in patients with both mastectomy and BCS, respectively. Major breast-related complications were only reported in patients with mastectomy, and RT fractionation didn’t affect the incidence of major breast complications as well. Hypofractionated RT did not increase major wound problems (infection and dehiscence) compared to conventional fractionated RT and rather lowered the incidence of major contracture.
Our findings could be helpful for physicians to use adjuvant hypofractionated RT for breast cancer patients with reconstruction in a real world. Although hypofractionated RT has been widely used lately, there are few studies investigated the effect of hypofractionated RT on reconstructed breast. Prior to 2016, conventional fractionated RT with daily 1.8–2.0 Gy was used as the standard RT regimen with a total dose of 50–60 Gy; however, the widespread use of the START A, B, and Canadian regimens led to the prevalence of the hypofractionated RT schedule in many institutions [[10], [23], [24], [25]]. Note that in clinical practice, these randomized trials using hypofractionated regimens were mostly for patients with BCS and not for patients undergoing breast reconstruction after mastectomy. As the efficacy and safety of hypofractionated RT after mastectomy was reported in a randomized controlled study from China [12], hypofractionated RT has become more prevalent. However, the superiority of the two fractionation schedules has not yet been identified in the reconstruction setting. Therefore, determination of RT fractionation was depended on preference of each radiation oncologist and individual patient for breast reconstruction. In this unclear situation, both conventional fractionated and hypofractionated RT regimens had been used together in actual clinical practice. However, concerns have been remained that hypofractionated RT regimen might lead to greater toxicity. Especially in the early days of hypofractionated RT, patients who had breast reconstruction after mastectomy tended to prefer conventional fractionated RT based on concerns about potential greater skin toxicity. Based on our findings, it was notable that hypofractionated RT did not increase major breast-related complications compared to conventional RT. In particular, hypofractionated RT had similar incidence in acute toxicity such as major wound problems, and late sequelae like flap contracture occurred rather less. In addition, in terms of convenience, hypofractionation resulted in higher patient satisfaction, due to the shorter overall duration and reduced cost of hypofractionated RT compared to conventional fractionated RT [13,14]. Therefore, hypofractionated RT has been gradually replacing conventional fractionated RT as a standard of care [11].Therefore, our results might support the selection of hypofractionated RT in the real world, even considering safety as well as cost-effectiveness.
However, the prospective randomized trials are definitely necessary to clarify this issue. Alliance A221505 is an ongoing randomized, phase 3 trial that seeks to evaluate whether the reconstruction-related complication rate at 24 months post-RT is non-inferior with hypofractionation compared to conventional fractionation [28]. This study eventually would answer the questions we addressed in this study; however, the estimated study completion is 2035, precluding conclusions about this issue in the near future. Therefore, our findings could be helpful in making decisions until the results of prospective study would be available.
This study, however, has several limitations. First, our study was retrospective in nature.
Second, types of RT technique (3D-CRT vs. IMRT) or IMN irradiation used between hypofractionated RT and conventional RT were significantly different. Hypofractionated RT was significantly more planned with IMRT technique. Third, the indication for use of bolus was not established uniformly. And lastly, the median follow-up time between two RT fractionation regimens was different. Though these limitations are inherent to our design, we tried to overcome those flaws to clarify the results. Firstly, since hypofractionated RT has been widely used after 2016, it was inevitable that IMRT technique was more adopted in hypofractionated RT. In addition, breast reconstruction surgery itself began to gradually increase as health insurance benefits have been applied since 2015 in Korea. These two points are presumed to be the reason why IMRT plans are more common in hypofractionated RT followed by breast reconstruction. Regarding IMNs, it was estimated that the difference in IMN RT between the two RT groups was due to the difference in stage. Though the stage was not significantly different between two groups (stage I/stage II/stage III 23.8%/45.2%/31.0% in conventional fractionated RT, 21.5%/41.3%/37.2% in hypofractionated RT, P = 0.499), the absolute value of stage 2 and 3 patients who were able to receive regional node irradiation including IMNs was higher in the hypofractionated RT group. In the case of treatment of regional nodes, this difference in IMN irradiation was thought to have occurred, considering that the inclusion of IMN might be different depending on the patient’s disease characteristics or the preference of individual radiation oncologist.
But we analyzed the results by correcting the factors representing statistical differences using a multivariable cox regression model. The use of bolus was also adjusted through the statistical analysis, but the result of using bolus was not a predictive factor for incidence of breast-related complication. Therefore, even if criteria for use of bolus had not been set, bolus could be used when necessary according to judgement of the radiation oncologists. Finally, the difference in follow-up period between two RTs was to be overcome by using the Kaplan-Meir method and cox regression model that considers the time course. Of course, a longer follow-up period would be necessary to confirm the accurate complication rates. Despite the several limitations, our results would be clinically meaningful, due to the inclusion of a relatively large number of patients from two institutions, allowing testing of statistical significance. In addition, as mentioned above, there have been few studies on complications of hypofractionated RT in breast reconstruction patients. Therefore, the results of this study suggest that clinicians might use hypofractionated RT comparably with conventional RT in breast cancer patients with reconstruction.
5. Conclusions
This study showed hypofractionated RT did not increase breast-related complications compared to conventional fractionated RT, even in patients with mastectomy followed by breast reconstruction. Hypofractionated RT may be used comparable to conventional fractionated RT in terms of breast-related complications in reconstructed breast cancer patients. The prospective randomized trial would be necessary to clarify this issue.
Funding
This work was supported by the grants from the Ministry of Science and Information & Communication Technology (#2020R1A2C005141) and SNUBH (#14-2018-003) to In Ah Kim. The funding sources had no involvement in the study.
Ethical approval
This study was approved by the institutional review board of each institution. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Because of the retrospective design of the analysis, requirement for obtaining informed consent of participants included in the study was exempted.
Data sharing statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Declaration of competing interest
None.
Acknowledgments
None.
References
- 1.Fracon S., Renzi N., Manara M. Patient satisfaction after breast reconstruction: implants vs. Autologous tissues. Acta Chir Plast. 2018;59(3–4):120–128. [PubMed] [Google Scholar]
- 2.Macadam S.A., Ho A.L., Lennox P.A. Patient-reported satisfaction and health-related quality of life following breast reconstruction: a comparison of shaped cohesive gel and round cohesive gel implant recipients. Plast Reconstr Surg. 2018;131(3):431–441. doi: 10.1097/PRS.0b013e31827c6d55. [DOI] [PubMed] [Google Scholar]
- 3.Metcalfe K.A., Zhong T., Narod S.A. A prospective study of mastectomy patients with and without delayed breast reconstruction: long-term psychosocial functioning in the breast cancer survivorship period. J Surg Oncol. 2015;111(3):258–264. doi: 10.1002/jso.23829. [DOI] [PubMed] [Google Scholar]
- 4.Hamdi Moustapha. Oncoplastic and reconstructive surgery of the breast. Breast. 2013;22:S100–S105. doi: 10.1016/j.breast.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Silverstein Melvin J. Oncoplastic breast conservation surgery: the new paradigm. J Surg Oncol. 2014;110(1):82–89. doi: 10.1002/jso.23641. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman Cary S. Increasing role of oncoplastic surgery for breast cancer. Curr Oncol Rep. 2019;21(12):111. doi: 10.1007/s11912-019-0860-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho A.Y., Hu Z.I., Mehrara B.J. Radiotherapy in the setting of breast reconstruction: types, techniques, and timing. Lancet Oncol. 2017;18(12):e742–e753. doi: 10.1016/S1470-2045(17)30617-4. [DOI] [PubMed] [Google Scholar]
- 8.Lee H.-H., Hou M.-F., Wei S.-Y. Comparison of long-term outcomes of postmastectomy radiotherapy between breast cancer patients with and without immediate flap reconstruction. PloS One. 2016;11(2) doi: 10.1371/journal.pone.0148318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliver J.D., Boczar D., Huayllani M.T. Postmastectomy radiation therapy (PMRT) before and after 2-stage expander-implant breast reconstruction: a systematic review. Medicina. 2019;55(6):226. doi: 10.3390/medicina55060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haviland J.S., Owen J.R., Dewar J.A. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11):1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 11.John Yarnold. Changes in radiotherapy fractionation — breast cancer. Br J Radiol. 2018;92(1093):20170849. doi: 10.1259/bjr.20170849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S.-L., Fang H., Song Y.-W. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2019;20(3):352–360. doi: 10.1016/S1470-2045(18)30813-1. [DOI] [PubMed] [Google Scholar]
- 13.Lievens Yolande. Hypofractionated breast radiotherapy: financial and economic consequences. Breast. 2010;19(3):192–197. doi: 10.1016/j.breast.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Irabor O.C., Swanson W., Shaukat F. Can the adoption of hypofractionation guidelines expand global radiotherapy access? An analysis for breast and prostate radiotherapy. JCO Global Oncology. 2020;6:667–678. doi: 10.1200/JGO.19.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jagsi R., Momoh A.O., Qi J. Impact of radiotherapy on complications and patient-reported outcomes after breast reconstruction. JNCI (J Natl Cancer Inst): Journal of the National Cancer Institute. 2017;110(2):157–165. doi: 10.1093/jnci/djx148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Headon Hannah, Kasem A. Capsular contracture after breast augmentation: an update for clinical practice. Arch Plast Surg. 2015;42(5):532. doi: 10.5999/aps.2015.42.5.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lymphology E.C.J. The diagnosis and treatment of peripheral lymphedema: 2016 consensus document of the International Society of Lymphology. Lymphology. 2016;49(4):170–184. [PubMed] [Google Scholar]
- 18.Gorai K., Inoue K., Saegusa N. Prediction of skin necrosis after mastectomy for breast cancer using indocyanine green angiography imaging. Plastic and Reconstructive Surgery Global Open. 2017;5(4) doi: 10.1097/GOX.0000000000001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lie K.H., Barker A.S., Ashton M.W. A classification system for partial and complete DIEP flap necrosis based on a review of 17,096 DIEP flaps in 693 articles including analysis of 152 total flap failures. Plast Reconstr Surg. 2013;132(6):1401–1408. doi: 10.1097/01.prs.0000434402.06564.bd. [DOI] [PubMed] [Google Scholar]
- 20.Clough Krishna B., Jerome Cumient, Alfred Fitoussi. Cosmetic sequelae after conservative treatment for breast cancer: classification and results of surgical correction. Ann Plast Surg. 1998:471–481. doi: 10.1097/00000637-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Clough Krishna B., Kroll Stephen S., Wermer Audretscj. An approach to the repair of partial mastectomy defects. Plast Reconstr Surg. 1999:409–420. doi: 10.1097/00006534-199908000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Alço, Igdem, Dincer Replacement of the tumor bed following oncoplastic breast-conserving surgery with immediate latissimus dorsi mini-flap. Mol Clin Oncol. 2016;5(4):365–371. doi: 10.3892/mco.2016.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunt Adrian Murray, Haviland, Joanne S., Wheatley Duncan A. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet Oncol. 2020 doi: 10.1016/S0140-6736(20)30932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whelan T.J., Pignol J.-P., Levine M.N. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362(6):513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 25.Alderman A.K., Wei Y., Birkmeyer J.D. Use of breast reconstruction after mastectomy following the women’s health and cancer rights act. J Am Med Assoc. 2006;295(4):383–388. doi: 10.1001/jama.295.4.387. [DOI] [PubMed] [Google Scholar]
- 26.Claßen J., Nitzsche S., Wallwiener D. Fibrotic changes after postmastectomy radiotherapy and reconstructive surgery in breast cancer. Strahlenther Onkol. 2010;186(11):630–636. doi: 10.1007/s00066-010-2158-6. [DOI] [PubMed] [Google Scholar]
- 27.Nava M., Benson J., Audretsch W. International multidisciplinary expert panel consensus on breast reconstruction and radiotherapy. Br J Surg. 2019;106(10):1327–1340. doi: 10.1002/bjs.11256. [DOI] [PubMed] [Google Scholar]
- 28.Khan A.J., Poppe M.M., Goyal S. Hypofractionated postmastectomy radiation therapy is safe and effective: first results from a prospective phase II trial. J Clin Oncol. 2017;35(18):2037. doi: 10.1200/JCO.2016.70.7158. [DOI] [PMC free article] [PubMed] [Google Scholar]