Highlights
-
•
Elevated soluble TNFR1 levels are predictive of liver toxicity among patients receiving radiation.
-
•
Soluble TNFR1 levels do not independently predict liver toxicity when included in models with ALBI and mean liver dose.
-
•
Data suggest that liver inflammation mediates toxicity after liver irradiation and that the TNFα axis is associated with this inflammation.
-
•
Future studies of should evaluate approaches that target pre-treatment inflammation to reduce the risk of toxicity.
Keywords: TNFα, Inflammation, Soluble tumor necrosis factor receptor 1, TNFR1, Hepatocellular cancer, Radiation therapy
Abbreviations: sTNFR1, soluble TNFα receptor 1
Abstract
Introduction
Radiation therapy for the management of intrahepatic malignancies can adversely affect liver function. Liver damage has been associated with increased levels of inflammatory cytokines, including tumor necrosis factor alpha (TNFα). We hypothesized that an inflammatory state, characterized by increased soluble TNFα receptor (sTNFR1), mediates sensitivity of the liver to radiation.
Materials/Methods
Plasma samples collected during 3 trials of liver radiation for liver malignancies were assayed for sTNFR1 level via enzyme-linked immunosorbent assay (ELISA). Univariate and multivariate logistic regression and longitudinal models were used to characterize associations between liver toxicity (defined as a ≥2-point increase in Child-Pugh [CP] score within 6 months of radiation treatment) and sTNFR1 levels, ALBI score, biocorrected mean liver dose (MLD), age, and baseline laboratory values.
Results
Samples from 78 patients given liver stereotactic body radiation therapy [SBRT] (92%) or hypofractionated radiation were examined. There was a significant association between liver toxicity and sTNFR1 levels, and higher values were associated with increased toxicity over a range of mean liver doses. When ALBI score and biocorrected dose were included in the model with sTNFR1, baseline ALBI score and change in ALBI (ΔALBI) were significantly associated with toxicity, but sTNFR1 was not. Baseline aminotransferase levels also predicted toxicity but not independently of ALBI score.
Conclusions
Elevated plasma sTNFR1 levels are associated with liver injury after liver radiation, suggesting that elevated inflammatory cytokine activity is a predictor of radiation-induced liver dysfunction. Future studies should determine whether administration of agents that decrease inflammation prior to treatment is warranted.
Introduction
Stereotactic body radiotherapy (SBRT) is a standard approach for management of hepatic malignancies [1,2]. The highly conformal dose distributions characteristic of this technique facilitate sparing of functional liver from prescription dose; however, steps must be taken to minimize toxicity risk given that mean liver dose (MLD) is predictive of radiation-induced liver disease (RILD) [3], [4]–5]. Normal tissue complication probability (NTCP) models that account for MLD have been developed in an effort to minimize risks of therapy, though these models are limited by the assumption that all liver tissue contributes equally to liver function [6,7]. Unfortunately, patients with primary hepatic malignancy typically suffer from reduced liver function due to underlying cirrhosis, diffuse intrahepatic tumor burden, and/or previous liver-directed therapies. As a result, NTCP models are less accurate for predicting toxicity risk, and those with larger tumors (>4 cm) or poor baseline liver function are much more likely to experience clinically significant liver injury [1,8].
Given the limitations of NTCP models, predictive measurements that assist with risk stratification and targetable biomarkers of activity by processes that mediate toxicity have been sought. Currently, predictive measurements include albumin-bilirubin score (ALBI), indocyanine green (ICG) clearance, and Child-Pugh (CP) score [9–14]. In some cases, these values have been used to adapt therapy to reduce risk [9], but these predictive measurements do not provide an avenue for risk reduction beyond guiding plan adaptation to reduce MLD. In contrast to these predictive measurements, potentially causative, targetable pathways involved in liver toxicity have also been sought through studies of cytokines that are positively or negatively associated with liver toxicity. Previous clinical and preclinical studies have found relationships between hepatic injury and each of the following cytokines: HGF (hepatocyte growth factor), CD40L, TGFβ, and TNFα [15], [16], [17], [18]–19]. Further study of these proteins may suggest potential clinical targets for prevention of liver damage after radiation.
Tumor necrosis factor alpha (TNFα) is a cytokine associated with immune system activation and inflammation, and increased levels of TNFα signaling have been strongly linked to both acute and chronic liver disease and liver injury [20], [21]–22]. Additionally, TNFα and soluble TNF receptor (e.g. sTNFR1) levels in circulation have been associated with disease activity and severity in many inflammatory conditions such as multiple sclerosis, lupus, and inflammatory arthritides [23–25]. Furthermore, inhibition of the TNFα signaling axis has led to improvements in patient outcomes in several inflammatory conditions including rheumatoid arthritis, juvenile arthritis, psoriatic arthritis, psoriasis, ankylosing spondylitis, ulcerative colitis, and Crohn's disease. Intervention within the TNFα signaling cascade with Etanercept or high dose corticosteroids in children with idiopathic pneumonia syndrome has resulted in improved clinical outcomes [26,27].
TNFα is unstable in serum, and its short half-life (approximately 20–70 min) makes it a suboptimal biomarker [28,29]. However, one of its major receptors, TNFR1, has a soluble form that is a stable marker for TNFα-mediated inflammation, and this receptor has been associated with numerous inflammatory conditions [24,25]. We hypothesized that liver inflammation, as assessed through measurement of sTNFR1, is a mediator of radiation-induced liver injury and explored this question through analysis of serum samples collected before and during radiation therapy for intrahepatic malignancy. If inflammation predicts toxicity, this finding would motivate future studies investigating the potential benefit from approaches designed to reduce inflammation prior to treatment.
Materials and methods
Specimen collection, preparation, and storage
Blood specimens were collected prospectively (at baseline, 1 month after treatment start [at the time of decisions regarding treatment adaptation] and then at 1, 3, and 6 months after completion of radiation) under a protocol approved by the Institutional Review Board (IRB) at the University of Michigan following Good Clinical Practice guidelines (IRB ID: HUM00029467, HUM00098022, and HUM00133653). All participants provided written informed consent prior to study enrollment. Whole blood samples were centrifuged for preparation of plasma and stored at −80 °C until the time of analysis. No patients were taking anti-TNF agents during treatment or follow up.
Clinical protocols
The specimens analyzed in this study were from patients with primary liver cancer or liver metastases and were procured during the course of three studies: a pair of prospective phase II trials examining adaptive liver radiation (Trial 1 – NCT01522937, Trial 2 – NCT02460835) and a prospective biomarker study (Trial 3 – HUM00133653; Table S1). In Trials 1 and 2, most of the radiation dose (60%) was delivered up front, with initial dose determined using a NTCP model (most patients received 10–12 Gy per fraction [30]). The remainder of the dose was delivered 1 month later with modification to dose based on ICG clearance [31]. Serum specimens were collected twice (baseline and 1 month after treatment start). Toxicity assessments were performed as described below at 1, 3, and 6 months after treatment completion. In Trial 2, only patients with hepatocellular carcinoma were included, and in the cases where a tumor was >5 cm, treatment was delivered over 20 fractions. In Trial 3, patients were treated with liver SBRT, and blood specimens were collected at baseline, after 60% of radiation was completed, and at 2–6 weeks after completing treatment.
Radiation therapy
Radiation therapy simulation included contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI). Where possible, breath hold techniques were employed. Internal target volumes (ITVs) were created from 4 dimensional CT (4DCT) to capture respiratory motion for free breathing patients. Gross tumor volumes (GTVs) and ITVs were typically expanded 5 mm axially and 5–8 mm craniocaudally to generate planning target volumes (PTVs). Over 3–5 fractions, patients received 18 – 60 Gy with dose/fractionation variability a product of the adaptive protocols described above. A few patients (8%) received fractionated image-guided radiation therapy (up to 60 Gy in 20 fractions) using conformal techniques with the 1-month break after the 12th fraction. Biocorrected MLD was calculated for each radiation plan. Biocorrected MLD was derived by calculating the mean of the linear quadratic model-corrected EQD2 values for each voxel of liver tissue outside of the GTV [32].
Laboratory values, liver function, and toxicity
Lab and clinical data acquired for each participant were used to prospectively calculate ALBI and CP scores at baseline, 1 month later at time of treatment adaptation, and then at 1, 3, and 6 months after completion of radiation. ALBI score [(log10 bilirubin in µmol/L × 0.66) + (albumin in g/L × −0.085)] was used to characterize liver function at each time point [33]. CP score was calculated from laboratory values [albumin, bilirubin, international normalized ratio (INR)] and clinical information (presence of ascites or hepatic encephalopathy) [14]. Other acquired lab data included white blood cell (WBC) count, absolute neutrophil count (ANC), absolute lymphocyte count, aspartate transaminase (AST), and alanine transaminase (ALT). In this study, liver toxicity was defined as an increase in CP score of ≥2 points in the 6 months following delivery of liver radiation.
sTNFR1 levels
Plasma samples were assayed for sTNFR1 using the Quantikine ELISA kit (Catalog #: DRT100) from R&D systems per the supplied manufacturer protocol. Briefly, each plasma sample was diluted 1 to 10 in RD60 Calibrator Diluent and assayed in duplicate. The samples were first incubated with assay diluent on the plate for 2 h at room temperature (RT) and then washed 3 times with the supplied wash buffer. Human TNFR1 conjugate was added to each well followed by another 2 h RT incubation. Following an additional 3 washes, the substrate and stop solutions were added, and the plate was read on a SpectraMax 384 plate reader (Molecular Devices, San Jose, CA, USA) per the manufacturer's protocol. Cytokine concentrations were calculated against a standard curve utilizing the TNFR1 protein standard supplied with the ELISA kit.
Statistical methods
To evaluate the association between sTNFR1 and toxicity, we assessed the relationship between both MLD and sTNFR1 and subsequent toxicity. Analysis was performed on log2-transformed plasma sTNFR1 levels scaled by the standard deviation of these values. We considered modeling with sTNFR1 measured at different time points: baseline, 1 month post-treatment, or the difference between those measures (defined as ΔsTNFR1). Toxicity was defined as an increase in CP score of 2 or more within six months of treatment for logistic regression models, and CP values were fitted directly to evaluate toxicity in longitudinal models. Logistic regression models were fitted to estimate the odds of toxicity for an increase in one standard deviation of sTNFR1 levels at baseline or 1 month post-treatment. Cross-validated AUC is used to evaluate the performance of the logistic regression models. Because some information may be lost by collapsing change in CP into a binary outcome, a longitudinal model was also fitted as a more efficient alternative [12]. Marginal R-squared was used to compare the performance of the longitudinal models. Models including both sTNFR1 and ALBI were fit to better understand if additional information was gained by including both sTNFR1 level and ALBI score. A t-test for slope was used to assess for relationship between sTNFR1 and ALBI. Additional models were fit to examine baseline and 1-month AST, ALT, WBC count, and neutrophil-to-lymphocyte ratio contributions to toxicity. In cases where a significant relationship was observed between toxicity and a specific lab value, the relationship between ALBI and the relevant value was further studied to assess for potential independent toxicity prediction in models including ALBI score.
Results
Plasma specimens from patients (n = 78) were analyzed (Table 1). Most patients had hepatocellular carcinoma [74 (95%)]; 2 patients had cholangiocarcinoma, and 2 patients had liver metastases. Most patients also had cirrhosis (85%), usually due to viral etiology (58%). A minority of patients had multiple lesions treated [22 (28%)], and a total of 106 lesions were treated. Most patients were treated with liver SBRT with 3 or 5 fractions [72 (92%)], while the remainder of patients [6 (8%)] were treated with hypofractionated image-guided radiation therapy and received 11, 12, or 20 fractions. Of the 78 subjects in the study, 22 experienced toxicity classified as a rise in CP score of 2 or more points within 6 months of treatment completion.
Table 1.
Patient, tumor, and treatment characteristics.
| Variable | Value |
|---|---|
| # Patients | 78 |
| Age [median (range)] | 64 (48–89) |
| Male | 58 (74%) |
| Diagnosis | |
| HCC | 74 (95%) |
| Other | 4 (5%) |
| Cirrhosis | 66 (85%) |
| Viral | 38 (58%) |
| NAFLD | 15 (23%) |
| Other | 13 (20%) |
| # prior liver therapies | |
| 0 | 33 (42%) |
| 1 | 13 (17%) |
| ≥2 | 32 (41%) |
| Treatment | |
| SBRT (3 or 5 fractions) | 72 (92%) |
| Fractionated (11, 12, or 20 fractions) | 6 (8%) |
| Number treatments adapted | 71 (91%) |
| MLD (Gy) [median (range)] * | 13.2 (3.9–33.1) |
| MELD [median (range)] ⁎⁎ | 9 (6–29) |
| CP Score [median (range)] | 6 (5–11) |
| sTNFR1 levels | |
| Baseline (pg/mL) [median (range)] | 2143 (1154–5448) |
| 1 month (pg/mL) [median (range)] | 2169 (140–8028) |
| # Tumors per patient | |
| 1 | 56 (72%) |
| 2 | 18 (23%) |
| 3 | 3 (4%) |
| 4 | 0 (0%) |
| 5 | 1 (1%) |
| Total | 106 |
| Tumor diameter (cm) [median (range)] | 3.0 (0.9–14.6) |
Mean liver dose delivered to the patient.
OPTN Policy 9.1 (1996).
Initial multivariate analyses including biocorrected MLD and sTNFR1 level noted an association between liver toxicity and baseline sTNFR1 (OR 1.62, p = 0.0573); however, this did not reach statistical significance. At the time point 1 month after treatment start, a significant association between sTNFR1 and toxicity was noted (OR 2.35, p = 0.0181). Biocorrected MLD was not associated with toxicity in either the baseline model or the 1 month model (baseline OR 1.03, p = 0.40; 1 month OR 1.05, p = 0.22). The change in sTNFR1 level from baseline to 1 month was not associated with toxicity. In a longitudinal model that includes biocorrected MLD and sTNFR1 baseline level as well as time from treatment, both time and sTNFR1 baseline values were predictive of CP score increase (time p-value = 0.002; sTNFR1 baseline p-value = 0.042). In a similar model, time from treatment and sTNFR1 levels at 1 month post-treatment start were also predictive of CP score increase (time p-value = 0.002; TNRF1 month p-value = 0.011).
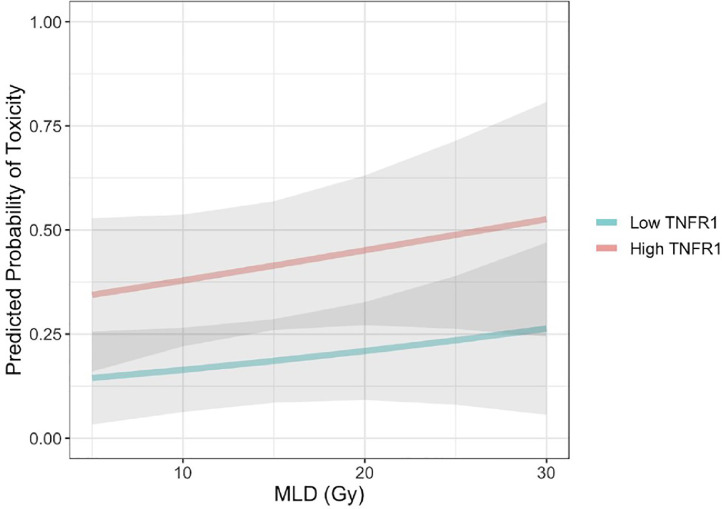
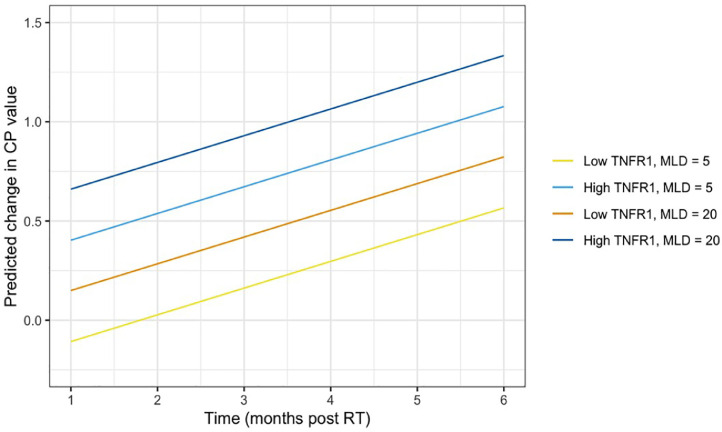
Having established a relationship between toxicity and sTNFR1 level, we sought to assess the impact of sTNFR1 level on the probability of liver toxicity over a range of potential liver radiation doses. For any given MLD, patients with higher baseline sTNFR1 values (90th percentile) had a greater chance of toxicity than patients who had lower baseline soluble TNFR1 values at all values for MLD (10th percentile; Fig. 1). Estimates of the predicted change in CP score over time after liver SBRT were generated, accounting for both MLD and sTNFR1 levels (Fig. 2). The difference in risk of toxicity in those with baseline sTNFR1 at the 90th percentile versus the 10th percentile was much greater than the impact of adding 15 Gy of MLD to patients with the same baseline sTNFR1 (Fig. 2).
Fig. 1.
Probability of toxicity after liver SBRT with increasing mean liver dose (MLD) for those with low (10th percentile) vs. high (90th percentile) baseline sTNFR1 levels. MLD data range: 3.85–33.13 Gy. Gray shaded area represents area included in 95% confidence bounds.
Fig. 2.
Predicted change in Child-Pugh (CP) score over time following liver SBRT for patients with low (10th percentile) baseline sTNFR1 vs. high (90th percentile) baseline sTNFR1 by low (5 Gy) MLD vs. high (20 Gy) MLD.
We then examined both liver-specific and systemic laboratory studies associated with inflammation, including AST, ALT, WBC count, and neutrophil-to-lymphocyte ratio. In models containing AST or ALT values at baseline along with MLD, baseline elevated AST (OR 1.012; p = 0.009) and ALT (OR 1.002; p = 0.019) were predictive of toxicity. Neither 1-month AST nor 1-month ALT was predictive of toxicity. No strong relationship was noted between sTNFR1 level and either AST or ALT (Fig. S1). In models containing MLD along with WBC count, WBC count did not predict toxicity at either baseline (p = 0.491) or 1 month (p = 0.752). The neutrophil-to-lymphocyte ratio at baseline or 1 month was included in models along with MLD, and this ratio did not predict toxicity (p = 0.456 and p = 0.623 at baseline or 1 month, respectively).
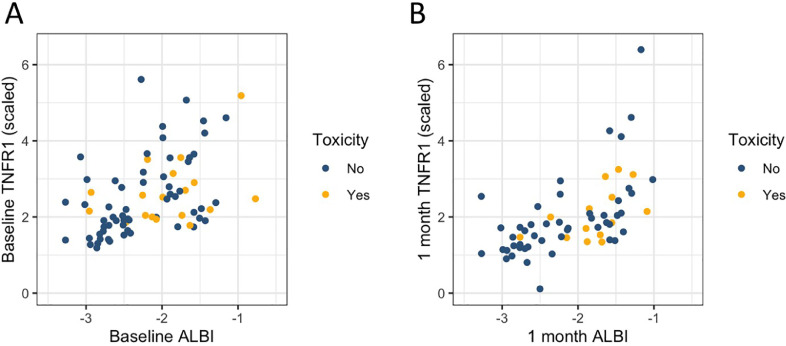
After noting the potential utility of sTNFR1 levels for prediction of liver toxicity, we sought to assess whether sTNFR1 levels predicted toxicity independently from ALBI score. Scatterplots of ALBI score and sTNFR1 levels at baseline and 1 month demonstrate strong associations (p<0.001 for baseline and 1 month) and weak linear correlations (R2 = 0.22 and 0.32 for baseline and 1 month, respectively) between these values (Fig. 3). When ALBI score and sTNFR1 levels were examined simultaneously with multivariate models to evaluate the relationship between ALBI score (at baseline and 1 month post-treatment start) and liver toxicity, there was no longer a significant effect of sTNFR1 level at either baseline or 1 month post-treatment start with regard to toxicity (Table 2). Given that ALBI score was a better predictor of toxicity than sTNFR1 level, a longitudinal model including ALBI score, time, and dose was constructed, revealing significant associations between toxicity and time (p=0.001), ALBI score (baseline; p = 0.005), and the change in ALBI over 1 month between the baseline and the value prior to delivering the last 40% of treatment (p<0.012; Table 3).
Fig. 3.
Scatterplot of scaled sTNFR1 level vs. ALBI score at baseline (A, prior to treatment start) and 1 month after start of treatment course (B). sTNFR1 values were scaled by the standard deviation of observed values.
Table 2.
Multivariate model of liver toxicity accounting for sTNFR1 and ALBI (AUC = 0.86).
| Variable | Estimate | Standard error | p-value |
|---|---|---|---|
| sTNFR1 (baseline) | −0.001 | 0.004 | 0.669 |
| ALBI (baseline) | 3.507 | 1.110 | 0.002 |
| ΔALBI | 5.990 | 2.079 | 0.004 |
| Mean dose | 0.128 | 0.066 | 0.053 |
Table 3.
Multivariate longitudinal model accounting for time, mean dose, and ALBI (R2=0.178).
| Variable | Estimate | Standard error | p-value |
|---|---|---|---|
| Time (months) | 0.116 | 0.035 | 0.001 |
| Mean dose | 0.023 | 0.014 | 0.102 |
| ALBI (baseline) | 0.512 | 0.174 | 0.005 |
| ΔALBI | 1.142 | 0.439 | 0.012 |
Discussion
In this study, we demonstrated that elevated sTNFR1 levels were predictive of liver toxicity among patients receiving radiation. This provides a linkage between TNFα, a cytokine long known to participate in liver inflammation, and radiation-induced liver toxicity. Second, elevated AST and ALT, markers of liver injury, were also associated with liver toxicity. These relatively specific markers of liver injury suggest that inflammation and ongoing damage within the liver prior to the start of radiation portend increased risk of toxicity. Third, general markers of global inflammation, WBC count and neutrophil-to-lymphocyte ratio, were not related to liver toxicity, underscoring the fact that liver injury from radiation is not related to systemic inflammatory toxicity. Fourth, this study found that ALBI score, a measure of liver function, proved superior to sTNFR1, AST, and ALT in predicting liver toxicity. This suggests that a measure of liver function is more complete in capturing susceptibility to toxicity than are individual markers of inflammation, although this may partially reflect our definition of toxicity (see below). Taken together, these findings suggest that liver inflammation mediates toxicity after liver irradiation and that the TNFα axis is associated with this inflammation.
Our results and those of other investigators support the role of inflammation in exacerbating radiation-induced liver injury. Specifically, inflammatory processes are closely linked to the progression of cirrhosis through apoptosis and fibrosis [34]. Inflammation may also be linked to portal hypertension in setting of cirrhosis via increased translocation of bacterial cell wall components [35]. In addition to cirrhosis, TNFα has also been shown to participate in multiple processes of acute liver pathology [20–22]. In cell culture experiments, hepatocytes treated with radiation in the setting of TNFα exposure experienced marked increases in apoptosis [19]. Also, antisense oligonucleotides against sTNFR1 protected cells from radiation-induced apoptosis [36]. In both of these studies, liver macrophages were shown to produce TNFα in response to radiation, suggesting a pathway in which radiation increases intrahepatic inflammatory signals. Therefore, data from the present study are in agreement with the work of others suggesting a link between toxicity and both TNFα and inflammation.
Cytokine biomarkers of liver toxicity need not be restricted to inflammatory pathways directly but have been linked to complex physiologic responses to liver injury. Beyond sTNFR1 and the TNFα axis, decreased serum CD40L and increased HGF levels have also been shown to predict liver toxicity [15,16]. It has been surmised that declines in CD40L, a platelet-derived cytokine, could represent reduced platelet numbers seen in advanced liver disease. Increased HGF levels are thought to be related to its function in promoting liver regeneration following liver injury [37,38]. A thorough understanding of the role of cytokines associated with liver toxicity is critical to the development of therapeutic interventions. Cytokines such as CD40L and HGF are not thought to be involved in the process of liver injury but may change through physiologic compensatory processes after liver damage occurs. As a result, these molecules would not be considered therapeutic targets based on present understanding.
Predictive measurements that have been investigated previously provide information on liver function and have distinct uses from potentially targetable biomarkers mentioned above. These measurements have been developed with specialized calculations, algorithms, and categories that are associated with disease outcomes and have been carefully validated. We do not propose this use for sTNFR1 as a predictive measurement but would defer to the use of previously developed measurements given the statistical findings of this report. The prognostic uses of CP score have been understood for decades. ICG clearance has been used to understand changes in liver function during treatment and as a guide for radiation treatment adaptation [9]. More recently, ALBI score has been shown to predict toxicity, and multiple groups are exploring ALBI for prediction of toxicity in the setting of liver irradiation [10], [11], [12]–13]. ALBI was the best predictor of toxicity in our study population. This is not surprising given that ALBI score is calculated from albumin and bilirubin, two of the five factors included in our toxicity read out (CP score increase ≥ 2). Though ALBI has great prognostic utility, it is not targetable with the goal of reducing toxicity. However, the potential involvement of the TNFα signaling axis in liver toxicity would present a targetable mechanism of pathology.
There are several weaknesses of this study that should be noted. This is a retrospective study and is therefore subject to potential bias. Also, the sample size of 78 individuals treated at a single institution may not capture the full breadth of scenarios seen in liver patients receiving SBRT. Lastly, the sample is not exclusively made up of individuals with HCC. There could be differences in tumor microenvironment in addition to known differences in baseline liver function associated with HCC vs. other malignancies that might impact susceptibility to radiation. It is important to consider these weaknesses when interpreting the data described in this report.
Our findings demonstrate the importance of inflammation in toxicity following liver radiation. Targeting inflammation both specifically and generally has proven to be a successful approach in other disease entities, dramatically altering outcomes. Etanercept has been approved for management of many chronic inflammatory conditions and has also been used successfully to treat idiopathic pneumonia syndrome [26,39]. Anti TNF therapies have been studied for management of multiple hepatitides with promising results, though researchers have cautioned about potential hepatic toxicity from these agents [40]. In a broader sense, steroids are used in the management of a host of processes mediated by inflammation, including entities caused by radiation, such as radiation pneumonitis and CNS radionecrosis [27,41]. Our findings and the findings of others support the development of a clinical trial to determine whether medical approaches for management of inflammation can alter the toxicity profile of liver radiation.
Conclusions
TNFα activity as measured by sTNFR1 plasma protein level is a predictor of toxicity following radiation, but this relationship is not independent of previously identified clinical predictors of toxicity as measured by ALBI score. We propose that TNFα is one component of inflammation that mediates radiation-induced liver toxicity. This consideration motivates a potential clinical trial to evaluate prednisone or Etanercept for reduction of risk of radiation-induced liver injury in patients receiving radiation for hepatic malignancy. Our data also support the use of ALBI score for prediction of liver toxicity and treatment adaptation.
CRediT authorship contribution statement
Matthew M. Cousins: Data curation, Visualization, Writing - original draft, Writing - review & editing. Emily Morris: Formal analysis, Visualization, Writing - review & editing. Christopher Maurino: Data curation. Theresa P. Devasia: Writing - review & editing. David Karnak: Investigation, Resources. Dipankar Ray: Writing - review & editing. Neehar D. Parikh: Writing - review & editing. Dawn Owen: Writing - review & editing. Randall K. Ten Haken: Writing - review & editing. Matthew J. Schipper: Formal analysis. Theodore S. Lawrence: Methodology, Conceptualization, Funding acquisition, Writing - review & editing. Kyle C. Cuneo: Methodology, Conceptualization, Supervision, Project administration, Writing - review & editing.
Declaration of Competing Interest
Dr. Parikh reports personal fees from Bristol Myers-Squibb, personal fees from Eisai, personal fees from Exelixis, grants and personal fees from Exact Sciences, grants and personal fees from Bayer, personal fees from Freenome, personal fees from Eli Lilly, and personal fees from Wako/Fujifilm, outside the submitted work. Dr. Schipper reports personal fees from Innovative Analytics, outside the submitted work. Dr. Ten Haken reports grants from NIH, during the conduct of the study; non-financial support from Varian Medical Systems, outside the submitted work. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Funding: This work was supported by the National Institutes of Health [grant numbers P01CA059827,P30CA046592]. The content is solely the responsibility of the authors.
Role of Sponsor: The sponsor did not participate in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2020.100950.
Appendix. Supplementary materials
References
- 1.Culleton S., Jiang H., Haddad C.R., Kim J., Brierley J., Brade A. Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma. Radiother. Oncol. 2014;111:412–417. doi: 10.1016/j.radonc.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 2.McPartlin A.J., Dawson L.A. Stereotactic body radiotherapy for hepatocellular carcinoma. Cancer J. 2016;22:296–301. doi: 10.1097/PPO.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 3.Cao Y., Platt J.F., Francis I.R., Balter J.M., Pan C., Normolle D. The prediction of radiation-induced liver dysfunction using a local dose and regional venous perfusion model. Med. Phys. 2007;34:604–612. doi: 10.1118/1.2431081. [DOI] [PubMed] [Google Scholar]
- 4.Ten Haken R.K., Lawrence T.S., Dawson L.A. Prediction of radiation-induced liver disease by Lyman normal-tissue complication probability model in three-dimensional conformal radiation therapy for primary liver carcinoma: in regards to Xu et al. (Int J Radiat Oncol Biol Phys 2006;65:189-195) Int. J. Radiat. Oncol. Biol. Phys. 2006;66:1272. doi: 10.1016/j.ijrobp.2006.08.003. author reply -3. [DOI] [PubMed] [Google Scholar]
- 5.Dawson L.A., Normolle D., Balter J.M., McGinn C.J., Lawrence T.S., Ten Haken R.K. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int. J. Radiat. Oncol. Biol. Phys. 2002;53:810–821. doi: 10.1016/s0360-3016(02)02846-8. [DOI] [PubMed] [Google Scholar]
- 6.McGinn C.J., Ten Haken R.K., Ensminger W.D., Walker S., Wang S., Lawrence T.S. Treatment of intrahepatic cancers with radiation doses based on a normal tissue complication probability model. J. Clin. Oncol. 1998;16:2246–2252. doi: 10.1200/JCO.1998.16.6.2246. [DOI] [PubMed] [Google Scholar]
- 7.Jackson A., Ten Haken R.K., Robertson J.M., Kessler M.L., Kutcher G.J., Lawrence T.S. Analysis of clinical complication data for radiation hepatitis using a parallel architecture model. Int. J. Radiat. Oncol. Biol. Phys. 1995;31:883–891. doi: 10.1016/0360-3016(94)00471-4. [DOI] [PubMed] [Google Scholar]
- 8.Feng M., Ben-Josef E. Radiation therapy for hepatocellular carcinoma. Semin. Radiat. Oncol. 2011;21:271–277. doi: 10.1016/j.semradonc.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Suresh K., Owen D., Bazzi L., Jackson W., Ten Haken R.K., Cuneo K. Using indocyanine green extraction to predict liver function after stereotactic body radiation therapy for hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2018;100:131–137. doi: 10.1016/j.ijrobp.2017.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray L.J., Sykes J., Brierley J., Kim J.J., Wong R.K.S., Ringash J. Baseline Albumin-Bilirubin (ALBI) score in western patients with hepatocellular carcinoma treated with stereotactic body radiation therapy (SBRT) Int. J. Radiat. Oncol. Biol. Phys. 2018;101:900–909. doi: 10.1016/j.ijrobp.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Toesca D.A.S., Ibragimov B., Koong A.J., Xing L., Koong A.C., Chang D.T. Strategies for prediction and mitigation of radiation-induced liver toxicity. J. Radiat. Res. 2018;59:i40–ii9. doi: 10.1093/jrr/rrx104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson W.C., Tang M., Maurino C., Mendiratta-Lala M., Parikh N.D., Matuszak M.M. Individualized adaptive radiation therapy allows for safe treatment of hepatocellular carcinoma in patients with Child-Turcotte-Pugh B liver disease. Int. J. Radiat. Oncol. Biol. Phys. 2020 doi: 10.1016/j.ijrobp.2020.08.046. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nabavizadeh N., Waller J.G., Fain R., 3rd., Chen Y., Degnin C.R., Elliott D.A. Safety and efficacy of accelerated hypofractionation and stereotactic body radiation therapy for hepatocellular carcinoma patients with varying degrees of hepatic impairment. Int. J. Radiat. Oncol. Biol. Phys. 2018;100:577–585. doi: 10.1016/j.ijrobp.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 14.Pugh R.N., Murray-Lyon I.M., Dawson J.L., Pietroni M.C., Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 15.Cuneo K.C., Devasia T., Sun Y., Schipper M.J., Karnak D., Davis M.A. Serum levels of hepatocyte growth factor and CD40 ligand predict radiation-induced liver injury. Transl. Oncol. 2019;12:889–894. doi: 10.1016/j.tranon.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong T.S., Grassberger C., Yeap B.Y., Jiang W., Wo J.Y., Goyal L. Pretreatment plasma HGF as potential biomarker for susceptibility to radiation-induced liver dysfunction after radiotherapy. NPJ Precis. Oncol. 2018;2:22. doi: 10.1038/s41698-018-0065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anscher M.S., Crocker I.R., Jirtle R.L. Transforming growth factor-beta 1 expression in irradiated liver. Radiat. Res. 1990;122:77–85. [PubMed] [Google Scholar]
- 18.Anscher M.S., Peters W.P., Reisenbichler H., Petros W.P., Jirtle R.L. Transforming growth factor β as a predictor of liver and lung fibrosis after autologous bone marrow transplantation for advanced breast cancer. NEJM. 1993;328:1592–1598. doi: 10.1056/NEJM199306033282203. [DOI] [PubMed] [Google Scholar]
- 19.Christiansen H., Saile B., Neubauer-Saile K., Tippelt S., Rave-Frank M., Hermann R.M. Irradiation leads to susceptibility of hepatocytes to TNF-alpha mediated apoptosis. Radiother. Oncol. 2004;72:291–296. doi: 10.1016/j.radonc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Kusters S., Tiegs G., Alexopoulou L., Pasparakis M., Douni E., Kunstle G. In vivo evidence for a functional role of both tumor necrosis factor (TNF) receptors and transmembrane TNF in experimental hepatitis. Eur. J. Immunol. 1997;27:2870–2875. doi: 10.1002/eji.1830271119. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y.M., Seki E. TNFα in liver fibrosis. Curr. Pathobiol. Rep. 2015;3:253–261. doi: 10.1007/s40139-015-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heyninck K., Wullaert A., Beyaert R. Nuclear factor-kappa B plays a central role in tumour necrosis factor-mediated liver disease. Biochem. Pharmacol. 2003;66:1409–1415. doi: 10.1016/s0006-2952(03)00491-x. [DOI] [PubMed] [Google Scholar]
- 23.Khoury S.J., Orav E.J., Guttmann C.R., Kikinis R., Jolesz F.A., Weiner H.L. Changes in serum levels of ICAM and TNF-R correlate with disease activity in multiple sclerosis. Neurology. 1999;53:758–764. doi: 10.1212/wnl.53.4.758. [DOI] [PubMed] [Google Scholar]
- 24.Cope A.P., Aderka D., Doherty M., Engelmann H., Gibbons D., Jones A.C. Increased levels of soluble tumor necrosis factor receptors in the sera and synovial fluid of patients with rheumatic diseases. Arthritis Rheum. 1992;35:1160–1169. doi: 10.1002/art.1780351008. [DOI] [PubMed] [Google Scholar]
- 25.Aderka D., Wysenbeek A., Engelmann H., Cope A.P., Brennan F., Molad Y. Correlation between serum levels of soluble tumor necrosis factor receptor and disease activity in systemic lupus erythematosus. Arthritis Rheum. 1993;36:1111–1120. doi: 10.1002/art.1780360812. [DOI] [PubMed] [Google Scholar]
- 26.Yanik G.A., Grupp S.A., Pulsipher M.A., Levine J.E., Schultz K.R., Wall D.A. TNF-receptor inhibitor therapy for the treatment of children with idiopathic pneumonia syndrome. A joint Pediatric Blood and Marrow Transplant Consortium and Children's Oncology Group Study (ASCT0521) Biol. Blood Marrow Transplant. 2015;21:67–73. doi: 10.1016/j.bbmt.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bledsoe T.J., Nath S.K., Decker R.H. Radiation Pneumonitis. Clin. Chest Med. 2017;38:201–208. doi: 10.1016/j.ccm.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J. Exp. Med. 1989;169:333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliver J.C., Bland L.A., Oettinger C.W., Arduino M.J., McAllister S.K., Aguero S.M. Cytokine kinetics in an in vitro whole blood model following an endotoxin challenge. Lymphokine Cytokine Res. 1993;12:115–120. [PubMed] [Google Scholar]
- 30.Feng M., Suresh K., Schipper M.J., Bazzi L., Ben-Josef E., Matuszak M.M. Individualized adaptive stereotactic body radiotherapy for liver tumors in patients at high risk for liver damage: a phase 2 clinical trial. JAMA Oncol. 2018;4:40–47. doi: 10.1001/jamaoncol.2017.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stenmark M.H., Cao Y., Wang H., Jackson A., Ben-Josef E., Ten Haken R.K. Estimating functional liver reserve following hepatic irradiation: adaptive normal tissue response models. Radiother. Oncol. 2014;111:418–423. doi: 10.1016/j.radonc.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joiner M.C., Bentzen S.M. Fractionation: the linear-quadratic approach. In: Joiner M.C., van der Kogel A.J., editors. Basic Clinical Radiobiology. 5th ed. Taylor and Francis Group; Boca Raton: 2019. pp. 99–111. [Google Scholar]
- 33.Johnson P.J., Berhane S., Kagebayashi C., Satomura S., Teng M., Reeves H.L. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Del Campo J.A., Gallego P., Grande L. Role of inflammatory response in liver diseases: therapeutic strategies. World J. Hepatol. 2018;10:1–7. doi: 10.4254/wjh.v10.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seo Y.S., Shah V.H. The role of gut-liver axis in the pathogenesis of liver cirrhosis and portal hypertension. Clin. Mol. Hepatol. 2012;18:337–346. doi: 10.3350/cmh.2012.18.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang X.W., Yang J., Dragovic A.F., Zhang H., Lawrence T.S., Zhang M. Antisense oligonucleotide inhibition of tumor necrosis factor receptor 1 protects the liver from radiation-induced apoptosis. Clin. Cancer Res. 2006;12:2849–2855. doi: 10.1158/1078-0432.CCR-06-0360. [DOI] [PubMed] [Google Scholar]
- 37.Ishiki Y., Ohnishi H., Muto Y., Matsumoto K., Nakamura T. Direct evidence that hepatocyte growth factor is a hepatotrophic factor for liver regeneration and has a potent antihepatitis effect in vivo. Hepatology (Baltimore, Md) 1992;16:1227–1235. [PubMed] [Google Scholar]
- 38.Matsumoto K., Nakamura T. Hepatocyte growth factor: molecular structure, roles in liver regeneration, and other biological functions. Crit. Rev. Oncog. 1992;3:27–54. [PubMed] [Google Scholar]
- 39.Chiang G.T., Glaser R.L. FDA; 2016. Clinical Review: GP2015 (proposed biosimilar to US-licensed Enbrel) [Google Scholar]
- 40.Lopetuso L.R., Mocci G., Marzo M., D'Aversa F., Rapaccini G.L., Guidi L. Harmful effects and potential benefits of anti-tumor necrosis factor (TNF)-α on the liver. Int. J. Mol. Sci. 2018;19:2199. doi: 10.3390/ijms19082199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vellayappan B., Tan C.L., Yong C., Khor L.K., Koh W.Y., Yeo T.T. Diagnosis and management of radiation necrosis in patients with brain metastases. Front. Oncol. 2018;8:395. doi: 10.3389/fonc.2018.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.