Abstract
Emerging evidence suggests that redox-active chemicals perturb pancreatic islet development. To better understand potential mechanisms for this, we used zebrafish (Danio rerio) embryos to investigate roles of glutathione (GSH; predominant cellular redox buffer) and the transcription factor Nrf2a (Nfe2l2a; zebrafish Nrf2 co-ortholog) in islet morphogenesis. We delineated critical windows of susceptibility to redox disruption of β-cell morphogenesis, interrogating embryos at 24, 48 and 72 h post fertilization (hpf) and visualized Nrf2a expression in the pancreas using whole-mount immunohistochemistry at 96 hpf. Chemical GSH modulation at 48 hpf induced significant islet morphology changes at 96 hpf. Pro-oxidant exposures to tert-butylhydroperoxide (77.6 μM; 10-min at 48 hpf) or tert-butylhydroquinone (1 μM; 48-56 hpf) decreased β-cell cluster area at 96 hpf. Conversely, exposures to antioxidant N-acetylcysteine (bolsters GSH pools; 100 μM; 48-72 hpf) or sulforaphane (activates Nrf2a; 20 μM; 48-72 hpf) significantly increased islet areas. Nrf2a was also stabilized in β-cells: 10-min exposures to 77.6 μM tert-butylhydroperoxide significantly increased Nrf2a protein compared to control islet cells that largely lack stabilized Nrf2a; 10-min exposures to higher (776 μM) tert-butylhydroperoxide concentration stabilized Nrf2a throughout the pancreas. Using biotinylated-GSH to visualize in situ protein glutathionylation, islet cells displayed high protein glutathionylation, indicating oxidized GSH pools. The 10-min high (776 μM) tert-butylhydroperoxide exposure (induced Nrf2a globally) decreased global protein glutathionylation at 96 hpf. Mutant fish expressing inactive Nrf2a were protected against tert-butylhydroperoxide-induced abnormal islet morphology. Our data indicate that disrupted redox homeostasis and Nrf2a stabilization during pancreatic β-cell development impact morphogenesis, with implications for disease states at later life stages. Our work identifies a potential molecular target (Nrf2) that mediates abnormal β-cell morphology in response to redox disruptions. Moreover, our findings imply that developmental exposure to exogenous stressors at distinct windows of susceptibility could diminish the reserve redox capacity of β-cells, rendering them vulnerable to later-life stresses and disease.
Keywords: Pancreas, Nfe2l2, Antioxidant defenses, Islet, Redox, Embryonic development
Abbreviations: BioGEE, Biotinylated Glutathione Ethyl Ester; DMSO, Dimethyl sulfoxide; Eh, Redox Potential; GSH, Glutathione; GSSG, Glutathione-disulfide; GR, Glutathione-disulfide Reductase; GST, Glutathione S-transferase; IHC, Immunohistochemistry; Keap1, Kelch-like ECH-associated protein 1; NAC, N-acetyl-l-cysteine; Nrf2/NFE2L2, Nuclear Factor Erythroid 2-like 2; ROS, Reactive Oxygen Species; SFN, Sulforaphane; tBOOH, tert-Butylhydroperoxide; tBHQ, tert-Butylhydroquinone
1. Introduction
With 100 million patients, and an annual cost of $327 billion in the United States alone, diabetes is a pressing healthcare challenge [1]. Although diabetes is a complex multifactorial group of diseases, pancreas dysfunction is a hallmark of all forms. The pancreas is largely comprised of exocrine tissue that secretes enzymes to digest biomacromolecules; the endocrine islets of Langerhans occupy approximately 2% of the total pancreas by volume, with pancreatic β-cells and α-cells secreting insulin and glucagon, respectively, to regulate blood glucose homeostasis [2]. Pancreas development in the D. rerio embryo has been shown to be impaired by environmental conditions including exposure to toxic chemicals, resulting in shortened pancreatic tail lengths, reduced β-cell mass, or aberrant morphologies of islet structure [[3], [4], [5], [6]], but the mechanisms involved are not understood. Pancreatic β-cells in particular appear to be especially sensitive to chemicals that cause redox disruptions, likely related to the fact that these cells rely on reactive oxygen species (ROS) as cellular signals during differentiation and to stimulate insulin secretion [7,8]. Although there is some dichotomy in literature about this [9], there is a preponderance of evidence supporting the notion that to facilitate these functions, β-cells possess inherently lower antioxidant capacities [7,8,10,11]. Therefore, chemical-induced redox stress, particularly during the sensitive period of embryonic development, could lead to disruptions in pancreatic morphogenesis and functional consequences related to glucose homeostasis and pancreatic insufficiency. However, thus far our understanding of the redox signaling of pancreatic β-cells in the embryo is limited.
Of the many redox signaling systems employed by the developing vertebrate embryo [12], glutathione (GSH), in tandem with its oxidized form glutathione disulfide (GSSG), provides the predominant redox buffering system [13,14]. Disruptions of the GSH system during organogenesis are known to induce adverse outcomes such as cell migration defects, premature differentiation, apoptosis and organ agenesis [12,15,16]. In the zebrafish (Danio rerio), GSH levels in the developing GI tract and pancreas are especially susceptible to redox modulators when compared to other developing organ systems [17]. Between 24 and 48 h post fertilization (hpf), the zebrafish go through the pharyngula stage – a period of rapid organogenesis [18]. During this time of heightened differentiation, the GSH redox potential, defined by the ratio of reduced GSH to oxidized GSSG, shifts from reducing at 24 hpf to oxidizing at 48 hpf [19]. The endocrine and exocrine pancreas both differentiate during this time, with the ventral and dorsal pancreatic ducts fusing around 48 hpf (Fig. 1) [20]. Given the specific, spatiotemporal changes in GSH levels of the developing embryo [17,19], coupled with intricate developmental processes that dictate pancreatogenesis, we hypothesized that there is differential sensitivity of the pancreas to redox disruptions at distinct developmental timepoints.
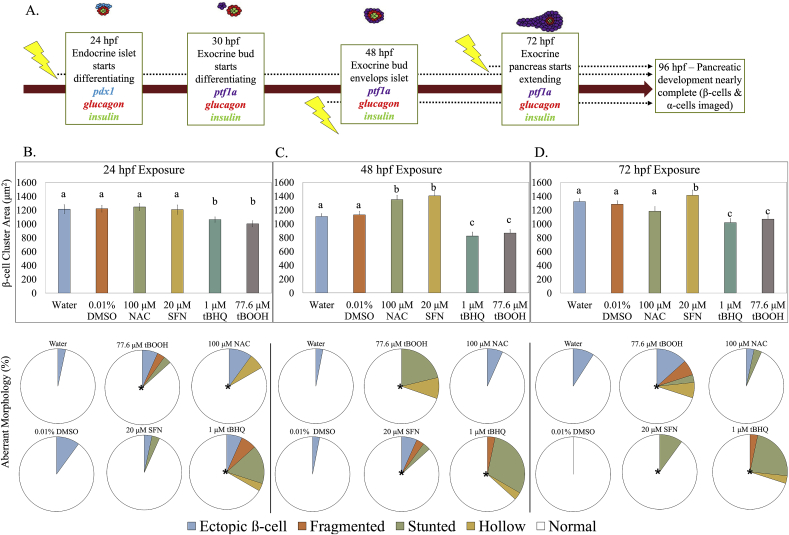
Fig. 1.
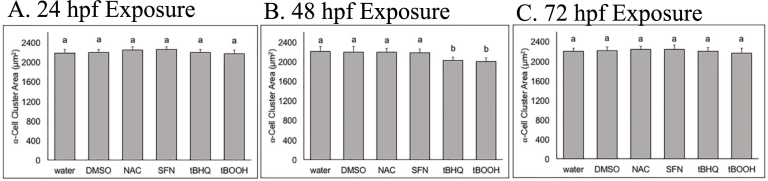
β-cell cluster area is impacted by redox modulation at critical windows of exposure. (A) Schematic of zebrafish pancreatic development and exposure paradigm. [pdx1 – transcription factor in pancreatic progenitor cells; glucagon – hormone secreted by α-cells; insulin – hormone secreted by β-cells; ptf1a – transcription factor present in exocrine pancreas] (B) β-cell cluster area in Tg(insa:eGFP) zebrafish at 96 hpf following a short exposure to the stated redox modulating chemical at 24 hpf then raised in clean conditions (n = 30 fish). Below, classification of aberrant islet morphologies. (C) β-cell cluster area at 96 hpf following exposure to the stated redox modulating chemical at 48 hpf (n = 37 fish). Below, classification of aberrant islet morphologies. (D) β-cell cluster area at 96 hpf following exposure to the stated redox modulating chemical at 72 hpf (n = 34 fish). Below, classification of aberrant islet morphologies. Different letters represent significant differences from the control. Different letters indicate significant differences (p < 0.05) as determined by a ONE-WAY ANOVA followed by a Fisher's LSD Post-Hoc Test. *p < 0.05 as determined by a Chi-square test.
In addition to driving the cellular redox potential, the pool of reduced GSH serves as the substrate for glutathione-S-transferase (GST) enzymes, which glutathionylate proteins and xenobiotics to regulate metabolism. Protein S-glutathionylation, a reversible post-translational modification that can activate or inactivate functional domains, provides cells with a powerful control over homeostasis and can also serve as a GSH storage system [[21], [22], [23]]. Induction of GST gene expression, along with numerous other antioxidant defense genes, is largely regulated by the transcription factor Nuclear Factor Erythroid 2-like 2 (NFE2L2 or Nrf2) [24].
A growing body of literature has established that normal Nrf2 signaling plays important roles in embryonic development [[25], [26], [27], [28]]. A multitude of redox stressors – endogenous metabolic byproduct ROS and exogenous xenobiotics – can deplete GSH, stabilize Nrf2 protein, and thus activate Nrf2 nuclear translocation and target gene expression [25,29]. Since the developing GI tract, including the pancreas, is susceptible to GSH depletion [17], it is a sensitive target of redox stress. Moreover, redox dysfunction is implicated as a mechanism of pancreatic β-cell dysfunction underlying diabetes, with Nrf2 activation protecting against the onset of diabetes [[30], [31], [32]]. Despite evidence for redox dysfunction being fundamental to endocrine pancreatic disruption across multiple model systems, the molecular pathways underlying this remain unknown. We hypothesized that glutathione depletion disrupts normal Nrf2 signaling in embryonic pancreatic β-cells, leading to aberrant growth and morphologies.
Here, we use gain-of-function and loss-of-function experiments to demonstrate the sufficiency and necessity of Nrf2 disruption for aberrant endocrine pancreatic development in the zebrafish (Danio rerio) embryo. Zebrafish have high fecundity and their embryos demonstrate rapid external development, serving as an excellent model for this study. Moreover, pancreatic function and development is conserved among zebrafish and humans, and the glutathione redox trajectory during concordant developmental stages between the mouse and zebrafish are also similar [25]. The zebrafish Nrf2 co-ortholog to human Nrf2 is Nrf2a [17,27,33]. We identify distinct temporal windows of redox susceptibility governing pancreatic organogenesis in vivo and demonstrate the lowered antioxidant defenses of the endocrine pancreas in situ. We also present a novel application of biotinylated glutathione ethyl ester (BioGEE) to probe protein glutathionylation levels in whole zebrafish (Danio rerio) embryos in situ, allowing--for the first time--the spatial resolution of an important post-translational modification at the whole organism level.
2. Materials and methods
2.1. Chemicals and reagents
Paraformaldehyde (PFA; Catalog # AAJ19943K2), Phosphate Buffered Saline (PBS; Catalog #AAJ75889AE), Methanol (Catalog #A412-4), Tween-20 (Catalog #BP337-100), and Dimethyl Sulfoxide (DMSO; Catalog #BP231-1) were purchased from Fisher Scientific (Pittsburgh, PA, USA). Tert-Butyl hydroperoxide (tBOOH; Catalog #A13926AP) and N-acetyl-l-cysteine (NAC; Catalog #A1540914) were purchased from Alfa Aesar (Ward Hill, MA, USA). Tert-butyl hydroquinone (tBHQ; Catalog #AC150820050) was purchased from Acros Organics (NJ, USA). dl-Sulforaphane (SFN; Catalog #S4441) was purchased from Millipore-Sigma (Burlington, MA, USA). Vectashield Antifade Mounting Medium with DAPI (Catalog #H-1200) was purchased from Vector Laboratories (Burlingame, CA, USA). Chicken Anti-Rabbit IgG AlexaFluor 594 (Catalog # A-21442), AlexaFluor 594 tagged Streptavidin (Catalog #S32356) and Biotinylated Glutathione Ethyl Ester (BioGEE; Catalog #G36000) were purchased from Invitrogen (Carlsbad, CA, USA).
2.2. Fish husbandry and embryo sampling
Tg(insa:eGFP) [34] and Tg(gcga:eGFP) [35] transgenic zebrafish on wild type (AB) background were used for in vivo observation of the β-cells and α -cells, respectively. Homozygous wild type (nrf2a+/+) and nrf2afh318/fh318 [36] crossed with Tg(insa:eGFP) zebrafish embryos on an AB strain background were used to investigate the Nrf2 pathway in the developing β-cells. All breeding adults were maintained on an automated Aquaneering (San Diego, CA, USA) system in accordance with the Guide for the Care and the Use of Laboratory Animals of the National Institutes of Health and with approval from the University of Massachusetts Amherst Institutional Animal Care and Use Committee (Animal Welfare Assurance Number A3551-01). The fish were housed at 28.5 °C on a 14 h light, 10 h dark cycle, and fed GEMMA Micro 300 (Skretting, Westbrook, ME, USA) twice daily. Large breeding tanks were setup with approximately 20 adult female and 10 adult male fish. Embryos were collected 1 h post fertilization, washed and screened for fertilization status and staged according to Kimmel et al. [18]. The embryos were dechorionated at 24 hpf and reared in borosilicate glass scintillation vials with 1 ml 0.3x Danieau's per embryo.
2.3. Chemical exposures
To investigate how the timing of pro-oxidant and antioxidant exposures impacts the development of the endocrine pancreas at either 24, 48, or 72 hpf, zebrafish eleutheroembryos (hatched and older than 72 hpf) were exposed to the following chemicals (concentrations and time of exposure indicated in parentheses): NAC (100 μM; 24 h), SFN (20 μM; 24 h), tBOOH (77.6 μM or 776 μM; 10minutes), tBHQ (1 μM; 6 h). Water (solvent for NAC and tBOOH) or DMSO (0.01%; solvent for SFN and tBHQ). Vehicle control exposures were run concurrently. The exposure durations were selected based on the known modes of action of these chemicals. Embryos were exposed to SFN and tBHQ for different time periods based on prior studies demonstrating these compounds required different amounts of time to induce Nrf2-mediated transcriptional response in zebrafish embryos [37,38]. All eleutheroembryos were manually dechorionated using watchmakers forceps at 24 hpf. Following exposures, fish were thoroughly washed and maintained in 0.3x Danieaus (http://cshprotocols.cshlp.org/content/2011/7/pdb.rec12467) for microscopy or immunohistochemistry at 96 hpf.
2.4. Immunohistochemistry
Embryos were fixed in 4% PFA in PBS for 24 h at 4 °C at a ratio of 15 embryos/ml PFA. Embryos were rinsed in 0.1% PBS-Tween-20 (PBST), and stored in 100% methanol at – 20 °C overnight. Samples were rehydrated using methanol-PBST gradients. Heat antigen retrieval was done by heating the rehydrated samples at 70 °C for 20 min. Immediately following heat retrieval, samples were permeabilized using ice-cold acetone for 8 min. Samples were blocked using 5% Sheep's serum in PBST for 2 h at room temperature, followed by the previously characterized anti-Nrf2a antibody (1:1000 in block) for 48 h at 4 °C [6]. Samples were placed in Alexa-594 tagged anti-rabbit antibody (1:5000 in block) overnight. Samples were washed with PBST and stored in Vectashield containing DAPI at 4 °C until imaging. A subset of embryos were run through the entire immunohistochemistry (IHC) procedure, without using any primary antibody as a control for non-specific binding of secondary antibody. The most robust response for Nrf2a, seen with a 10-min exposure to 77.6 μM tBOOH, was included as a positive control in every IHC procedure experiment.
For BioGEE samples, embryos were exposed to 100 μM BioGEE for 2 h prior to fixation. Following fixation, all steps were identical until blocking. Post-blocking, samples were placed in AlexaFluor 594 tagged Streptavidin (1:5000) for 1 h at room temperature. Samples were then washed with PBST and stored in Vectashield containing DAPI at 4 °C until imaging. A subset of embryos were run through the entire immunohistochemistry procedure, without exposing them to BioGEE as a control for non-specific binding of AlexaFluor 594 tagged Streptavidin.
2.5. Microscopy
Larvae were imaged at 96 hpf to observe the effects of exposure on the development of the primary islet, using an upright Olympus compound fluorescence microscope equipped with a Zeiss Axiocam 503 camera and Zen analysis software (Zeiss, USA). Fish were briefly anesthetized in 0.3x Danieaus containing MS-222 and staged laterally in 3% methylcellulose to optimize visualization of the endocrine islet. Images were captured using monochrome and GFP fluorescence filters at 2×, 5×, and 10× magnification to assess gross morphology and islet structure. Images were blinded, then analyzed using Zen (Zeiss) analysis software. Islet area was determined by tracing the perimeter of the cell cluster on the 10× GFP images, and the area was calculated by the software. Islet morphology was also assessed to quantify the frequency of islet variants such as hypomorphic, fragmented, stunted, hollow islets, or ectopic endocrine cells [39].
Larvae with immunohistochemistry for Nrf2a or BioGEE were imaged using a Nikon A1 Spectral Detector Confocal microscope equipped with 405 nm, 488 nm, 561 nm and 640 nm laser lines. Z-stacks were taken through the entire pancreas, using a 40× objective, 0.3 pixels equated 1 μm when imaging the whole pancreas, scale bars represent 20 μm. Sequence scanning was used to eliminate cross-channel fluorescence overlap. Images presented here are collapsed max-intensity projections, flipped horizontally to reflect the biological orientation of the pancreas. Structure outlines were drawn using the DAPI channel images; higher magnification confocal images cannot be obtained due to the physical limitations of the objective working distance that whole-organism mounts presented. Images were graded in a blinded manner for expression patterns based on OECD guidelines [40,41].
2.6. Statistics
To assess statistical significance of islet morphology variants reported in Fig. 1, we used Analysis of Variance (One-Way ANOVA) tests, followed by a Fishers Least Significant Differences (LSD) Post-Hoc Test. In Fig. 6, a One-Way ANOVA followed by a Tukey-Kramer post-hoc was used to assess tBOOH induced morphology changes. All statistical tests were performed in JMP Pro 14. All experiments were repeated for 5-7 biological replicates (total n > 30 fish). To assess statistical significance of immunohistochemistry expression patterns, χ2 tests were carried out using Excel, statistical significance was set to a p-value of 0.05. All experiments were repeated for 4-5 biological replicates (total n ≥ 12 fish); meeting or exceeding OECD guidelines [40,41].
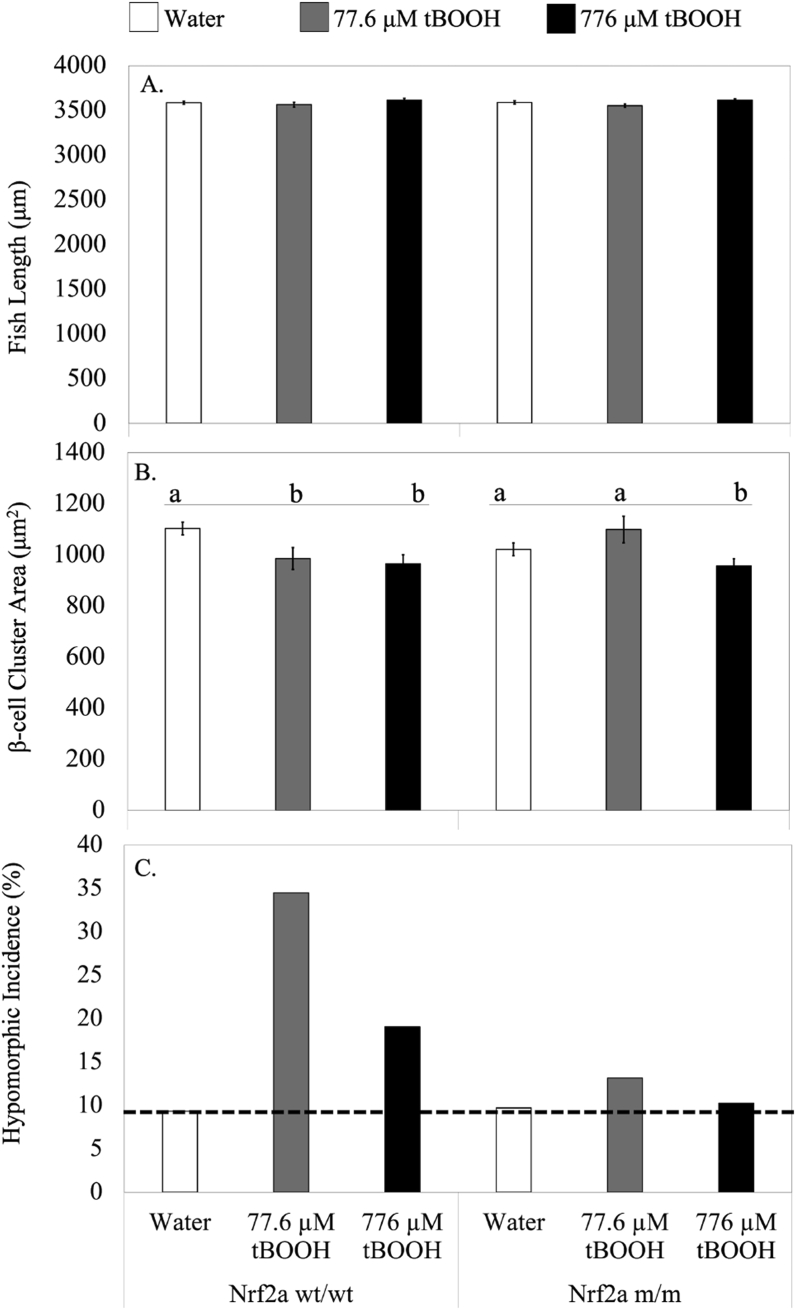
Fig. 6.
Nrf2a is essential for redox dependent disruption of β-cell morphology. Nrf2a wt/wt or Nrf2a m/m fish were exposed to water (n = 70 fish), 77.6 μM tBOOH (n = 32 fish) or 776 μM tBOOH (n = 35 fish) for 10 min at 48 hpf and their length (A), β-cell cluster area (B) and incidence of hypomorphic islets (cutoff represented by dashed line) (C) were measured at 96 hpf. Different letters indicate significantly different means across indicated group. p < 0.05 compared to respective control as determined by a 1-way ANOVA followed by a Tukey-Kramer post-hoc.
3. Results
3.1. Endocrine pancreatic organogenesis is governed by distinct spatiotemporal windows of redox susceptibility
To delineate critical windows of endocrine pancreatic susceptibility to redox disruption, we exposed zebrafish embryos to small molecules with either pro-oxidant or antioxidant activities for a brief period starting at either 24, 48 or 72 hpf, then raised the embryos in clean conditions and imaged the primary islet at 96 hpf. The redox modulators used were N-acetylcysteine (NAC) – which bolsters cellular GSH pools, tert-butylhydroperoxide (tBOOH) – a ROS generator that depletes cellular GSH, and sulforaphane (SFN) and tert-butylhydroquinone (tBHQ) – both Nrf2a activators [42,43]. Concentrations were selected to be below those causing lethality, overt morphological deformities, or impacts on growth, but sufficient to affect either the glutathione redox potential or Nrf2 activation.
The 24 hpf exposure window was most sensitive to the two pro-oxidants, tBHQ and tBOOH, which both resulted in significant decreases in the β-cell cluster area at 96 hpf and also increased occurrence of aberrant β-cell arrangement morphology. Exposure to tBOOH (77.6 μM) decreased β-cell cluster area by 18% (p < 0.05), and tBHQ exposure (1 μM) decreased β-cell area by 16% (p < 0.05). β-cell arrangements in the islet were significantly impacted by three of the four chemicals; tBHQ exposure increased occurrence of atypical arrangements of β-cells by 900% (p < 0.05) (Fig. 1) including fragmented islets and ectopic β-cells as previously described [5], and tBOOH exposure increased occurrence by 400%. Exposure to NAC or SFN at 24 hpf did not induce significant changes in either β-cell (Fig. 1) or α-cell areas (Fig. S1) when analyzed at 96 hpf, although exposure to NAC resulted in a significant increase in abnormal islet morphology (Fig. 1).
The islet was most sensitive to redox modulation at 48 hpf, with all four chemical exposures inducing significant changes in β-cell area and three of the four chemicals producing significant incidences of aberrant morphologies (Fig. 1). The antioxidant NAC and SFN induced a 20% and 25% increase in β-cell area, respectively (p < 0.05). The two pro-oxidants each produced a ~25% decrease in β-cell area when measured at 96 hpf, along with ~1000% increase in the incidence of variant islet morphologies (Fig. 1; p < 0.05). tBOOH and tBHQ at 48 hpf were the only treatments where the α-cells showed a response; there was a 5% reduction in α-cell area upon these exposures (Supp. Fig. S1). This reduction in α-cell area likely reflects the 25% reduction in β-cell area, as β-cells form the core of the spherical islet.
Redox modulation at 72 hpf produced changes in β-cell cluster area for three of the four chemicals, with the antioxidant SFN resulting in a significant increase, and the two pro-oxidants tBHQ and tBOOH resulting in a decrease in area. Each of these chemical exposures also resulted significant increases in aberrant islet morphology, with the two Nrf2 activating chemicals (SFN and tBHQ) resulting in an increase in stunted β-cell clusters. (Fig. 1).
3.2. Endocrine pancreas has low endogenous antioxidant defenses during development
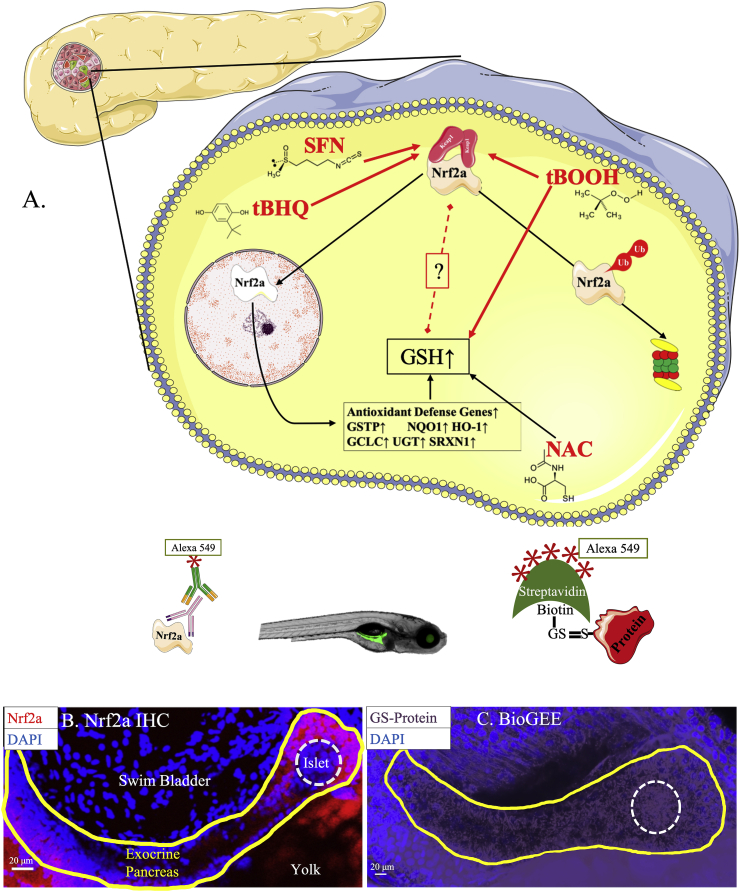
We characterized antioxidant defense levels of the endocrine pancreas using anti-Nrf2a antibodies and BioGEE; the latter is an indicator of protein-GSH mixed disulfides formed during oxidative stress [44]. None of the fish had Nrf2a expression in the islet (p < 0.05), while 100% had detectable protein glutathionylation (p < 0.05) (Fig. 2). In contrast, Nrf2a was robustly detected in the exocrine pancreas, indicating the effectiveness of the Nrf2a antibody and IHC procedure. Moreover, the robust expression of Nrf2a protein in the exocrine pancreata in all embryos suggests that the decreased Nrf2a levels in the endocrine islet are indicative of lower endogenous antioxidant defenses in these cells.
Fig. 2.
Endocrine pancreas has lower antioxidant defenses. (A) Schematic of all the Nrf2a redox modulators used in this study. (B) Nrf2a expression in pancreatic head and neck of 96 hpf zebrafish embryos (n = 23 fish). (C) Protein glutathionylation in pancreata of 96 hpf zebrafish embryos (n = 13 fish). Solid lines denote exocrine pancreas, dashed lines denote endocrine pancreas.
3.3. Peroxide-induced GSH depletion activates Nrf2a and impacts protein glutathionylation in the pancreas
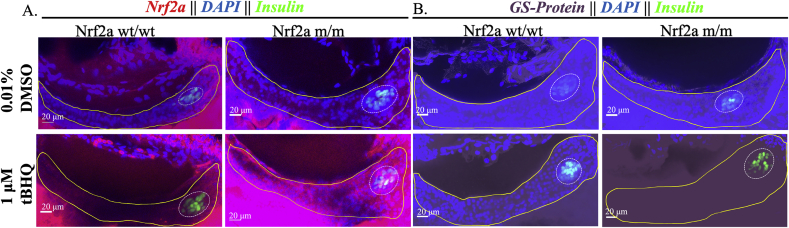
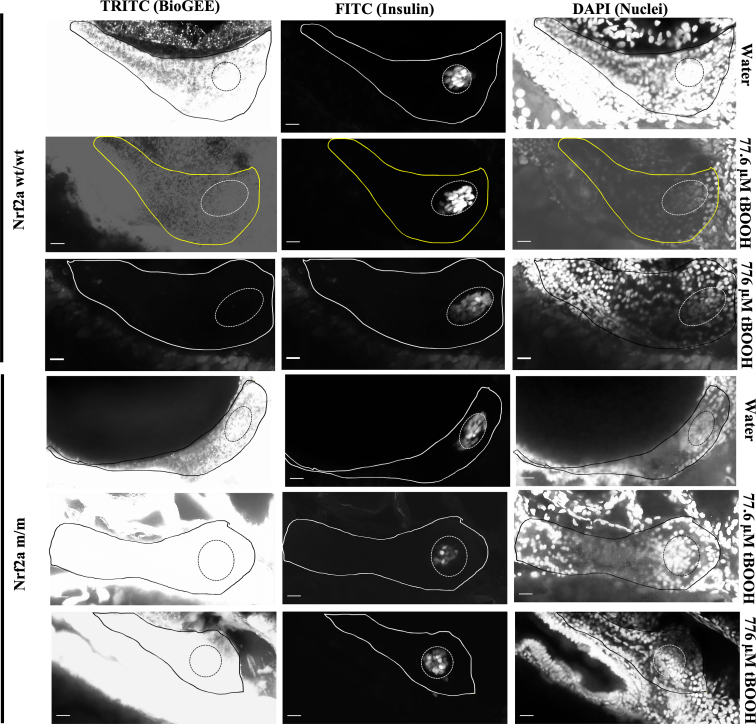
Given the heightened sensitivity of the endocrine pancreas to tBOOH exposure at 48 hpf, we investigated Nrf2a dynamics and protein glutathionylation in response to tBOOH treatment. Exposure to tBOOH (77.6 μM) at 48 hpf resulted in 100% of the larvae having stabilized Nrf2a in pancreatic β-cells at 96 hpf (p < 0.05; Fig. 3; Table 1) and protein glutathionylation in the endocrine and exocrine pancreata (p < 0.05; Fig. 3; Table 1). In this study, we conservatively refer to visible Nrf2a protein as being stabilized, i.e. bound to Keap1 or not degraded by proteasome since we have not measured actual Nrf2a protein expression levels, and, the exact mechanics of the Nrf2a pathway remain a subject of debate in literature [24].
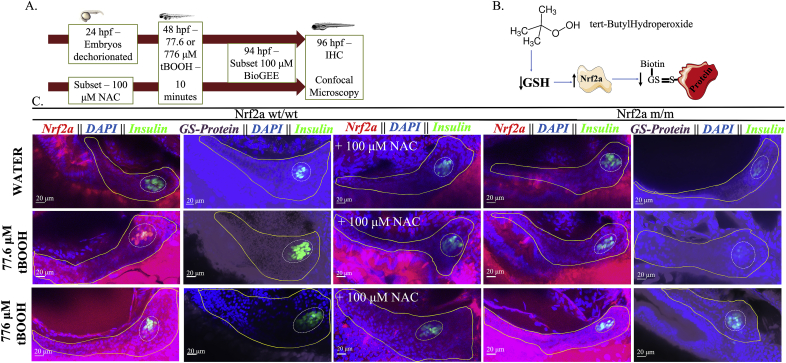
Fig. 3.
Pancreatic Nrf2a expression and protein glutathionylation patterns in response to tBOOH exposure. (A) Exposure paradigm. (B) Potential mechanism for tBOOH-mediated changes in Nrf2a and protein glutathionylation. (C) Nrf2a and protein glutathionylation patterns in pancreata of fish exposed to stated treatments. Solid lines denote exocrine pancreas, dashed lines denote endocrine pancreas; representative images from 10 to 14 fish. Scale bars represent 20 μm. Grayscale split channel images of the Nrf2a immunohistochemistry are provided in Supplementary Fig. 2, and BioGEE immunohistochemistry in Supplementary Fig. 3.
Table 1.
Nrf2a and BioGEE status of islets treated with tBOOH. *p < 0.05 as determined by a Chi-Square test.
| Genotype | Treatment | Nrf2a Positive Islets (%) | BioGEE Positive Islets (%) |
|---|---|---|---|
| Nrf2a wt/wt | Untreated Controls | 0 (n = 12 fish) | 100 (n = 12 fish) |
| Nrf2a wt/wt | 77.6 μM tBOOH | 100* (n = 13 fish) | 0* (n = 10 fish) |
| Nrf2a wt/wt | 776 μM tBOOH | 100* (n = 13 fish) | 0* (n = 13 fish) |
| Nrf2a wt/wt | 100 μM NAC | 0 (n = 12 fish) | – |
| Nrf2a wt/wt | 77.6 μM tBOOH + 100 μM NAC | 0 (n = 12 fish) | – |
| Nrf2a wt/wt | 776 μM tBOOH + 100 μM NAC | 0 (n = 12 fish) | – |
| Nrf2a m/m | Untreated Controls | 0 (n = 12 fish) | 100 (n = 12 fish) |
| Nrf2a m/m | 77.6 μM tBOOH | 0 (n = 14 fish) | 100 (n = 10 fish) |
| Nrf2a m/m | 776 μM tBOOH | 100 (n = 12 fish) | 100 (n = 13 fish) |
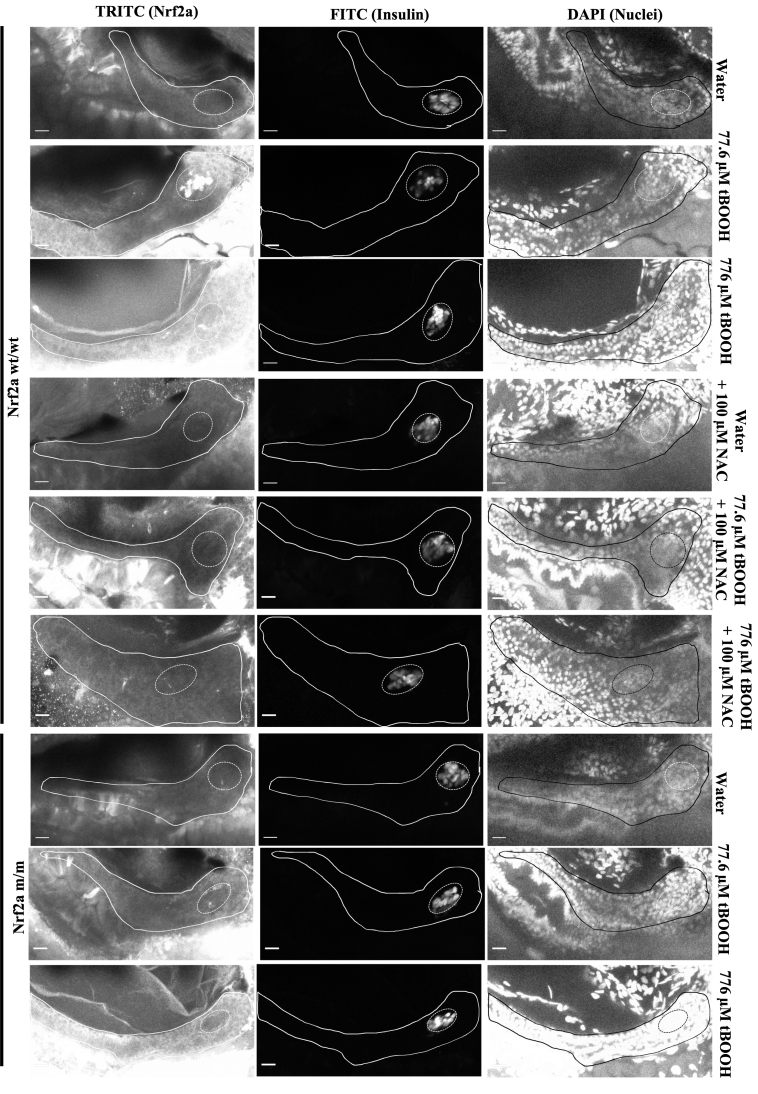
Exposure to a higher tBOOH concentration (776 μM) at 48 hpf resulted in the entire pancreas showing elevated levels of Nrf2a, accompanied by decreased global protein glutathionylation (p < 0.05; Fig. 3; Table 1), indicating Nrf2a stabilization ameliorates oxidative stress. Both 77.6 and 776 μM tBOOH are sublethal concentrations with no overt toxic or growth effects in the zebrafish embryo [17,26]. A 24-h pretreatment with 100 μM NAC, which bolsters cellular GSH pools, prevented the altered Nrf2a expression effects seen with both 77.6 μM and 776 μM tBOOH in 100% of the fish, indicating that GSH can protect against Nrf2a stabilization in pancreatic β-cells (Fig. 3; Table 1). In Nrf2a mutant (m/m) fish, which carry a mutation in the DNA-binding domain of Nrf2a, allowing for Nrf2a expression but hampering Nrf2a transcriptional activity [36], 77.6 μM tBOOH did not stabilize Nrf2a (Fig. 3; Table 1), suggesting that the stabilization of Nrf2a to mild redox challenges involves its own de novo upregulation. This is in contrast to the finding with the 10-fold higher, 776 μM tBOOH treatment, which stabilized Nrf2a in the mutant fish in a pattern similar to that seen in the Nrf2a wildtype fish (Fig. 3; Table 1).
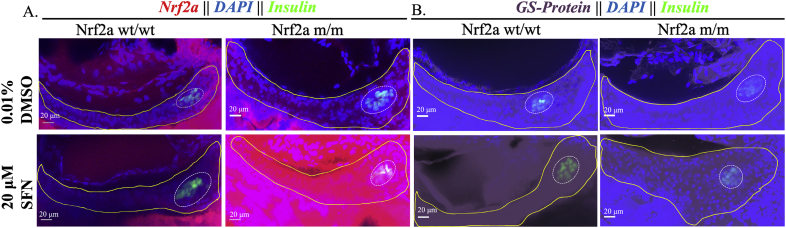
3.4. SFN and tBHQ impact Nrf2a expression protein levels and protein glutathionylation
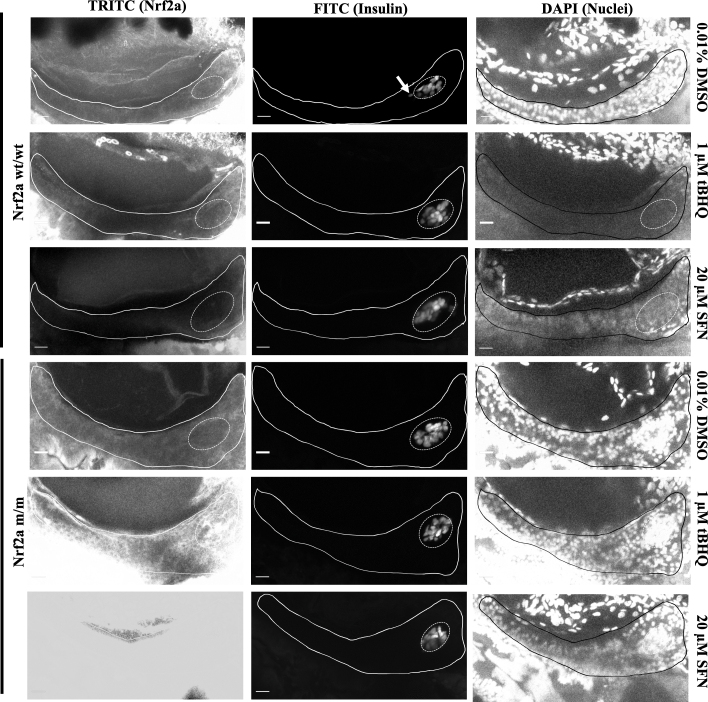
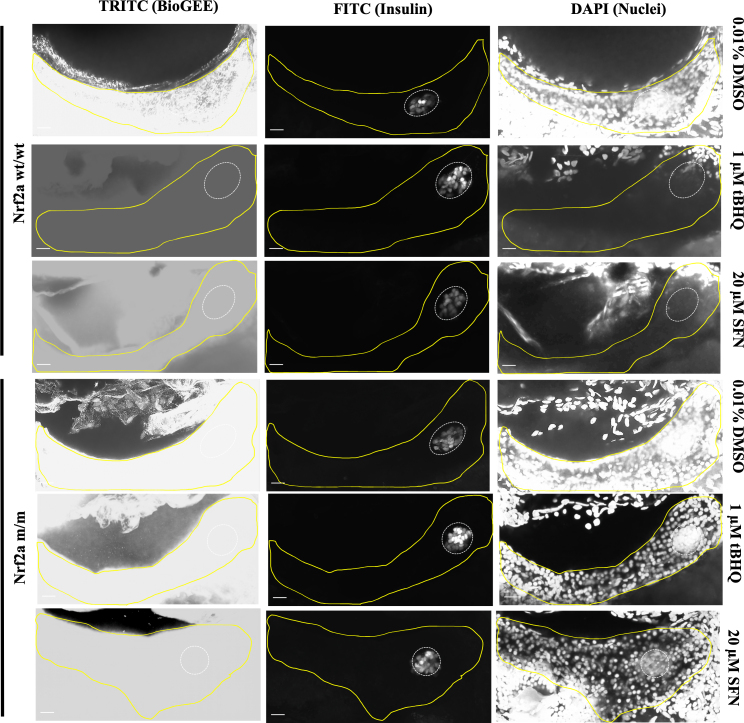
Upon exposure to the Nrf2a activators tBHQ and SFN, 100% of the Nrf2a wt/wt embryos demonstrated a near total absence of Nrf2a expression in both the endocrine and exocrine pancreas (p < 0.05; Fig. 4, Fig. 5; Table 2, Table 3). Protein glutathionylation levels were not altered by treatments (p < 0.05; Fig. 4, Fig. 5; Table 2, Table 3). In contrast, Nrf2a m/m fish showed increased stabilization of Nrf2a (p < 0.05; Fig. 4, Fig. 5 Table 2, Table 3).
Fig. 4.
Pancreatic Nrf2a expression and protein glutathionylation patterns in response to tBHQ exposure in zebrafish embryos. (A) Nrf2a protein and (B) protein glutathionylation patterns in pancreata of fish exposed to 1 μM tBHQ from 48 to 54 hpf. Solid lines denote exocrine pancreas, dashed lines denote endocrine pancreas; representative images from 11 to 14 fish. Scale bars represent 20 μm. Grayscale split channel images of the Nrf2a immunohistochemistry are provided in Supplementary Fig. 4, and BioGEE immunohistochemistry in Supplementary Fig. 5.
Fig. 5.
Pancreatic Nrf2a expression and protein glutathionylation patterns in response to SFN exposure in zebrafish embryos. (A) Nrf2a protein and (B) protein glutathionylation patterns in pancreata of fish exposed to 20 μM SFN from 24 to 48 hpf. Solid lines denote exocrine pancreas, dashed lines denote endocrine pancreas; representative images from 11 to 13 fish. Scale bars represent 20 μm. Grayscale split channel images of the Nrf2a immunohistochemistry are provided in Supplementary Fig. 4, and BioGEE immunohistochemistry in Supplementary Fig. 5.
Table 2.
Nrf2a and BioGEE status of islets treated with tBHQ. *p < 0.05 as determined by a Chi-Square test.
| Genotype | Treatment | Nrf2a Positive Islets (%) | BioGEE Positive Islets (%) |
|---|---|---|---|
| Nrf2a wt/wt | 0.01% DMSO | 0 (n = 12 fish) | 100 (n = 11 fish) |
| Nrf2a wt/wt | 1 μM tBHQ | 0 (n = 13 fish) | 100 (n = 14 fish) |
| Nrf2a m/m | 0.01% DMSO | 0 (n = 12 fish) | 100 (n = 12 fish) |
| Nrf2a m/m | 1 μM tBHQ | 100* (n = 12 fish) | 100 (n = 13 fish) |
Table 3.
Nrf2a and BioGEE status of islets treated with SFN. *p < 0.05 as determined by a Chi-Square test.
| Genotype | Treatment | Nrf2a Positive Islets (%) | BioGEE Positive Islets (%) |
|---|---|---|---|
| Nrf2a wt/wt | 0.01% DMSO | 0 (n = 12 fish) | 100 (n = 11 fish) |
| Nrf2a wt/wt | 20 μM SFN | 0 (n = 13 fish) | 100 (n = 11 fish) |
| Nrf2a m/m | 0.01% DMSO | 0 (n = 12 fish) | 100 (n = 12 fish) |
| Nrf2a m/m | 20 μM SFN | 100* (n = 12 fish) | 100 (n = 12 fish) |
3.5. Nrf2a m/m fish are protected against aberrant islet morphologies
To determine the contribution of Nrf2a in the development of aberrant islet morphologies, we exposed Nrf2a wt/wt and Nrf2a m/m embryos to tBOOH (77.6 μM) at 48 hpf and measured fish length and β-cell area. Incidence of hypomorphic islets was calculated as previously described [39]; briefly, the 10th percentile of the control measurements was calculated, and the numbers of individuals in each treatment group that fell below were considered hypomorphic; this is presented as the percent of the samples that met that criteria.
Fish length was unaltered, indicating the observed changes were β-cell specific, as opposed to overall decreased growth (Fig. 6). We found that wildtype embryos were more susceptible to decreases in both β-cell area and the incidence of hypomorphic islets (Fig. 6). Nrf2a wt/wt embryos exposed to 77.6 μM tBOOH showed a 13% reduction in β-cell area (p < 0.05) and a 300% increase in hypomorphic islets; embryos exposed to 776 μM showed an 18% reduction in β-cell area (p < 0.05) and a 100% increase in hypomorphic islets. Nrf2a m/m fish, on the other hand, showed no effect of tBOOH at a dose of 77.6 μM on either of these parameters (Fig. 6). Although at the 10-fold higher 776 μM dose of tBOOH the Nrf2a m/m embryos did show a decrease pf 4% (p < 0.05) in islet area, this was much smaller than the 18% decrease observed in wildtype embryos. This demonstrates that Nrf2a plays a role in tBOOH-mediated changes in β-cell morphology.
4. Discussion
Pancreatic development utilizes a dynamic network of redox-sensitive signaling pathways that dictate organ morphogenesis [30,31]. Interrupting these pathways can induce abnormal pancreatic morphologies, potentially predisposing individuals to adverse health outcomes [[3], [4], [5], [6]]. In this study, we characterize, for the first time, the glutathione redox conditions and Nrf2a protein levels in the developing endocrine pancreas. We demonstrate that exposure to redox-active xenobiotics at specific developmental timepoints can cause prolonged disruptions in Nrf2 protein expression in pancreatic β-cells. Our findings are indicative of a peroxide-mediated decrease in the reserve redox capacity of β-cells, with potential implications for β-cell function later in life.
We found distinct windows of pancreatic β-cell susceptibility to redox modulation, with embryos aged 48 hpf being most susceptible to abnormal endocrine pancreas morphologies. In the zebrafish embryo, the redox potential is oxidizing and GSH levels in the developing gut decrease at 48 hpf, providing a possible explanation for the significance of this timepoint (Fig. 1) [17,19]. Of all the parameters measured, β-cell cluster area is most impacted, whereas fish length is not affected and α-cell cluster area decreases only slightly, indicating the inherent susceptibility of β-cells to redox disruptions (Fig. 1; Fig. 6; Supp. Fig. 1). The redox sensitivity of pancreatic β-cells is further characterized by undetectable levels of Nrf2a and the higher levels of protein glutathionylation in the pancreatic islet, as compared to the exocrine pancreas (Fig. 2). Elevated glutathionylation is a marker of oxidative stress, and an oxidizing GSH redox potential [21]. Oxidizing redox potentials equate to lower levels of reduced GSH, and consequently, lower buffering capacity against oxidative insults.
The pro-oxidant tBOOH induced the most dramatic changes in β-cell morphology, with a short 10-min treatment at 48 hpf inducing changes sustained two days later at 96 hpf (Fig. 1). Previously, we showed that a similar 10-min tBOOH treatment at 48 hpf depletes 50% of the GSH pool in the zebrafish embryo, and the GSH pools within the embryo start to recover about 1.5 h post treatment [17]. We found that Nrf2a stabilization increases post tBOOH treatment, and two days later at 96 hpf it is still stabilized specifically in β-cells (Supp Fig. 1; Fig. 3; Table 1).
It should be noted that exposure to tBHQ and sulforaphane induced opposite changes in β-cell morphology, with sulforaphane inducing an increase in β-cell mass and tBHQ inducing a decrease in β-cell mass. This may be attributed to the different modes of action of the two chemicals. While SFN is considered a antioxidant, its function as such is mediated through upregulation of innate antioxidant defense mechanisms via activation of Nrf2 [45]. Thus, SFN protects against oxidative damage by arming the cell with its own defense systems. On the other hand, tBHQ has been classified as a potent Nrf2 activator through oxidative modification of Keap1 [46]. Thus, tBHQ acts as a weak pro-oxidant, in addition to activating Nrf2, similar to tBOOH.
The exocrine pancreata of fish exposed to 77.6 μM tBOOH lack the increased levels of Nrf2a protein whereas exposure to 776 μM tBOOH, a 10-fold higher dose, results in global Nrf2a protein expression (Fig. 3; Table 1). Additionally, a 100 μM NAC pretreatment, which bolsters cellular GSH, prevents the increase in Nrf2a expression at both doses of tBOOH. Thus, artificially increasing GSH levels in β-cells increases the inherent buffering capacity, requiring greater GSH depletion for pro-oxidant-mediated Nrf2a stabilization.
Exposing Nrf2a m/m embryos, which carry a mutation in the DNA-binding domain of Nrf2a, to 77.6 μM tBOOH does not elicit an increase in Nrf2a protein in the islet. In contrast, the 10-fold higher 776 μM tBOOH exposure does induce global Nrf2a protein expression in the pancreas (Fig. 3; Table 1). This suggests Nrf2a may self-regulate its de novo upregulation and subsequent stabilization in response to mild tBOOH mediated GSH depletion until a threshold is reached. However, once GSH drops below this internal setpoint, Nrf2a is triggered; in the zebrafish, this could potentially be activated by Nrf2b, a co-ortholog of Nrf2a [26,33] or perhaps other members of the Nrf family. Nrf2a protein increases are accompanied by decreased protein glutathionylation, dependent upon the magnitude of redox stress (Fig. 3; Table 1). This is readily apparent in embryos exposed to 776 μM tBOOH, where the global increase in Nrf2a expression led to a global decrease in protein glutathionylation levels (Fig. 3; Table 1).
Another possibility for the decreased protein glutathionylation levels seen following 776 μM tBOOH exposure is that the labile GSH pools are depleted in these embryos to combat the higher levels of ROS. Thus, these embryos might display increased levels of protein glutathionylation later in time, as the GSH pools recover.
One potential explanation for the sustained high levels of Nrf2a seen in the β-cells in response to tBOOH exposure could be the mechanism by which tBOOH is detoxified. tBOOH serves as a substrate for glutathione peroxidase 1 and glutathione peroxidase 4 [47]. In pancreatic β-cells Gpx expression levels are very low, and slower tBOOH catalysis likely leads to sustained secondary oxidative stress [10]. Gpx4 overexpression in β-cells has been demonstrated to reduce the generation of lipid hydroperoxides (including tBOOH), and reverse diabetic phenotypes [48,49]. Gpx expression can also be triggered by Nrf2a, with Gpx reporters being activated by the canonical Nrf2a activators tBHQ and SFN [50]. Thus, the elevated Nrf2a expression is likely a response by the embryo to try and return to homeostatic conditions by neutralizing tBOOH. In the exocrine pancreas, the innate antioxidant defenses coupled with Nrf2a are sufficient to neutralize tBOOH; in the endocrine pancreas the lower antioxidant defenses delay this recovery, leading to elevated Nrf2a expression levels two days later.
Interestingly, tBHQ and SFN, both activators of Nrf2a caused a near total absence of Nrf2a expression in both the endocrine and exocrine pancreas of exposed Nrf2a wt/wt fish (Fig. 4, Fig. 5). Despite the absence of Nrf2a expression, significantly elevated levels of protein glutathionylation were observed following tBHQ and SFN treatment. These results are congruent with our findings with 776 μM tBOOH in Nrf2a wt/wt fish, where increased Nrf2a was accompanied by decreased protein glutathionylation. In Nrf2a m/m fish, we saw high levels of Nrf2a expression upon tBHQ and SFN exposure, but this did not impact protein glutathionylation levels, which remained high. Thus, Nrf2a activity appears necessary for changes in protein glutathionylation in the pancreas.
Nrf2a mutant embryos showed increased Nrf2a stabilization levels upon tBHQ and SFN exposures (Fig. 4, Fig. 5). This implies that the lack of Nrf2a expression seen in Nrf2a wildtype embryos upon these exposures is mediated by Nrf2a or a downstream target of Nrf2a. In the zebrafish embryo, exposure to a ten-fold higher dose of tBHQ (10 μM) than the dose we used (1 μM), is insufficient to induce Nrf2a expression in 48 hpf, 72 hpf and 96 hpf embryos [38]. Genes downstream of Nrf2a, especially those involved in GSH synthesis are induced by 300%, but Nrf2a itself is not induced [38]. Among the highest induced genes are the heat shock proteins Hsp70 and Hsp90, the induction of Hsp70 in response to tBHQ mediated Nrf2a activation has been confirmed in multiple studies [26,33,38]. Both these proteins help stabilize protein folding, by either delaying completion of protein folding, or, accelerating proteasomal degradation of misfolded proteins [51]. Thus, changes in the total protein levels of Nrf2a might be masked by overexpression of Hsp70 and Hsp90. Given the increase in protein glutathionylation, it is apparent that tBHQ and SFN induce high levels of oxidative stress, arising at least in part due to decreased Nrf2a levels (Fig. 4, Fig. 5; Table 2, Table 3). It is possible that wildtype embryos recover Nrf2a levels sometime post 96 hpf; this merits further investigation.
Nrf2a mutant embryos were protected against the abnormal islet morphologies seen with 77.6 μM tBOOH, whereas wild-type embryos expressing functional Nrf2a showed a 13% decrease in islet area and a 300% increase in incidence of hypomorphic islets. Although the 10-fold higher 776 μM tBOOH exposure, induced decreases in both wild type and mutant fish, the decrease in islet area was significantly greater in Nrf2a wt/wt fish (18%) as compared to Nrf2a m/m fish (4%). Fish length was unaffected, indicating no overt toxicity due to exposures (Fig. 6). Both Nrf2a wt/wt and Nrf2a m/m embryos exposed to 77.6 μM tBOOH presented a slightly greater number of hypomorphic islets than embryos exposed to 776 μM tBOOH (Fig. 6). This may be due to the large increase in Nrf2a stabilization observed only in the islet following exposure to 77.6 μM tBOOH (Fig. 3; Table 1).
Given that Nrf2a mutant embryos are protected against altered Nrf2a expression patterns in response to redox stresses (Fig. 3, Fig. 4, Fig. 5), altered redox signaling during development appears to be important for disrupted endocrine pancreatogenesis. A possible mechanism for this is Nrf2a-mediated changes in glutathionylation, supported by the fact that 776 μM tBOOH triggered Nrf2a expression in Nrf2a m/m embryos but did not decrease protein glutathionylation as seen in Nrf2a wt/wt embryos (Fig. 3; Table 1). Differential glutathionylation is crucial from a developmental perspective, as protein glutathionylation is a well-characterized mechanism of transcriptional and functional regulation of proteins [22]. Within pancreatic β-cells, reversible glutathionylation of the inner mitochondrial membrane protein, Uncoupling Protein – 2, is necessary for glucose stimulated insulin secretion, and consequently, β-cell function [52]. Moreover glutathionylation of Kelch Like ECH Associated Protein 1 (Keap1), the cytosolic repressor of Nrf2, increases cellular Nrf2 protein levels [53]. This glutathionylation is catalyzed by GSTπ, a downstream target of Nrf2a; previously, we have shown altered GSTπ activity in the developing zebrafish pancreas upon exposure to multiple redox stressors including tBOOH [17]. GSTπ also silences the JNK signaling pathway via protein glutathionylation [54]. This is notable in the context of pancreatic β-cells, as the pancreatic and duodenal homeobox-1 (PDX1) transcription factor is known to be regulated by JNK signaling. PDX1 is essential for the differentiation of pancreatic β-cells, and, controls the expression of insulin [20,55]. Oxidative stress induced disruption of PDX1 signaling, a hallmark of diabetes, can be rescued by restoration of normal JNK signaling [56].
Nrf2a protein expression is highly consistent in the developing zebrafish pancreas, with 23 embryos displaying similar expression patterns (Fig. 2). We find that multiple redox modulators induce sustained disruptions to these expression patterns (Fig. 3, Fig. 4, Fig. 5). This sustained departure from normal signaling could have severe ramifications for the development of disease states, with altered baseline Nrf2a levels rendering pancreatic β-cells vulnerable to subsequent redox active xenobiotic exposures. Concordant with our results, in healthy human pancreatic β-cells Nrf2 is largely absent; Nrf2 activation is indicated as a coping mechanism for stressed β-cells, accompanied by reduced insulin secreting capacity [57,58]. Thus, while activation of Nrf2 in β-cells can promote cell survival, these cells are not as effective in regulating blood glucose levels.
The transcriptomic profiles of β-cells are well-conserved in zebrafish and humans [59]. Within humans, Nrf2 expression is known to be very low in pancreatic β-cells. Gclc, the catalytic subunit of the enzyme that catalyzes GSH synthesis is also expressed at very low levels. Moreover, Gstπ, a gene transcriptionally regulated by Nrf2 is expressed at 2.5 fold lower levels in β-cells than in exocrine pancreatic cells [60].
A wide array of xenobiotics and pharmaceuticals impact developmental GSH profiles, and, consequently Nrf2. Nrf2 is highly pleiotropic, with many functions in biological processes like inflammation, lipid and xenobiotic metabolism and DNA repair, in addition to the central role it plays in cancer [27,28,61,62]. While Nrf2 is widely studied in the context of its protective activities, there is also a dark side including upregulation in cancer cells, where it can confer cytoprotection from chemotherapeutics, and in mutagenesis where genes upregulated by Nrf2 can lead to increased bioactivation of pro-carcinogens [[62], [63], [64]]. This underscores the need to better characterize the functions of Nrf2 during development. This study, to the best of our knowledge, is the first to interrogate Nrf2a expression patterns in the endocrine pancreas in situ during embryogenesis. During the course of this study, we discovered that treatment with redox modulators induced sustained changes in Nrf2a protein levels.
To maintain data fidelity, all images were acquired under the same microscope settings. An unfortunate consequence of this was the inability to quantitatively analyze the images due to certain treatment conditions (e.g. 776 μM tBOOH) saturating the fluorescence detector. We acknowledge that this is a limitation of the current study. However, the study is informative about endogenous and modulated redox patterns in the developing pancreas. Furthermore, it can be used to inform future studies where changes in Nrf2a protein and protein glutathionylation levels can be quantified.
Disruptions in developmental Nrf2a signaling that manifest as subtle changes, as demonstrated in this study, are easily overlooked until disease occurs. These subtle changes in pancreatic morphology could predispose an individual to develop metabolic disease later in life. Indeed, in multiple epidemiological studies in humans, toxicant exposure has been closely associated with increased rates of metabolic disorders and diabetes [65]. Moreover, we find critical windows of susceptibility to redox disruptions for the endocrine pancreas. This could have implications for identifying sensitive windows of pancreas development in humans.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are grateful to Dr. Jason M Hansen from Brigham Young University for advice on the BioGEE experiments, Dr. Laura N Vandenberg from University of Massachusetts, Amherst for advice on the histology experiments and Dr. James J Chambers from the University of Massachusetts Amherst IALS LMF Nikon Center of Excellence for assistance with confocal microscopy. We thank Dr. Mark Hahn at the Woods Hole Oceanographic Institution for a critical read of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101788.
7. Funding.
This work was supported by National Institutes of Health R01ES025748 (to ART-L).
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig. S1.
α-cell cluster area of zebrafish embryos presented in Fig. 1 α-cell cluster area in Tg(gcga:eGFP) zebrafish at 96 hpf following a transient exposure to the stated redox modulating chemical beginning at A) 24 hpf B) 48 hpf, or C) 72 hpf then raised in clean conditions and imaged at 96 hpf (n = 30–37 fish). Different letters indicate significant differences (p < 0.05) as determined by a ONE-WAY ANOVA followed by a Fisher's LSD Post-Hoc Test.
Fig. S2.
Pancreatic Nrf2a expression in response to tBOOH exposure. Grayscale split channel images from composites presented in Fig. 3. Genotypes, channels and treatments specified in image. All scale bars represent 20 μm. Solid lines denote exocrine pancreas, dashed lines represent endocrine pancreas.
Fig. S3.
Pancreatic protein gluathionylation patterns in response to tBOOH exposure. Grayscale split channel images from composites presented in Fig. 3. Genotypes, channels and treatments specified in image. All scale bars represent 20 μm. Solid lines denote exocrine pancreas, dashed lines represent endocrine pancreas.
Fig. S4.
Pancreatic Nrf2a patterns in response to tBHQ and SFN exposure. Grayscale split channel images from composites presented in Fig. 4, Fig. 5. White arrow points to an ectopic β-cell outside the primary islet. Genotypes, channels and treatments specified in image. All scale bars represent 20 μm. Solid lines denote exocrine pancreas, dashed lines represent endocrine pancreas.
Fig. S5.
Pancreatic protein glutathionylation in response to tBHQ and SFN exposure. Grayscale split channel images from composites presented in Fig. 4, Fig. 5. Genotypes, channels and treatments specified in image. All scale bars represent 20 μm. Solid lines denote exocrine pancreas, dashed lines represent endocrine pancreas.
References
- 1.Association A.D. Economic costs of diabetes in the U.S. In 2017. Diabetes Care. 2018;41(5):917–928. doi: 10.2337/dci18-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan F.C., Brissova M. Pancreas development in humans. Curr. Opin. Endocrinol. Diabetes Obes. 2014;21(2):77–82. doi: 10.1097/MED.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobs H.M., Sant K.E., Basnet A., Williams L.M., Moss J.B., Timme-Laragy A.R. Embryonic exposure to Mono(2-ethylhexyl) phthalate (MEHP) disrupts pancreatic organogenesis in zebrafish (Danio rerio) Chemosphere. 2018;195:498–507. doi: 10.1016/j.chemosphere.2017.12.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sant K.E., Jacobs H.M., Borofski K.A., Moss J.B., Timme-Laragy A.R. Embryonic exposures to perfluorooctanesulfonic acid (PFOS) disrupt pancreatic organogenesis in the zebrafish, Danio rerio. Environ. Pollut. 2017;220(Pt B):807–817. doi: 10.1016/j.envpol.2016.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sant K.E., Jacobs H.M., Xu J., Borofski K.A., Moss L.G., Moss J.B., Timme-Laragy A.R. Assessment of toxicological perturbations and variants of pancreatic islet development in the zebrafish model. Toxics. 2016;4(3) doi: 10.3390/toxics4030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rousseau M.E., Sant K.E., Borden L.R., Franks D.G., Hahn M.E., Timme-Laragy A.R. Regulation of Ahr signaling by Nrf2 during development: effects of Nrf2a deficiency on PCB126 embryotoxicity in zebrafish (Danio rerio) Aquat. Toxicol. 2015;167:157–171. doi: 10.1016/j.aquatox.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pi J., Bai Y., Zhang Q., Wong V., Floering L.M., Daniel K., Reece J.M., Deeney J.T., Andersen M.E., Corkey B.E., Collins S. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;56(7):1783–1791. doi: 10.2337/db06-1601. [DOI] [PubMed] [Google Scholar]
- 8.Pi J., Zhang Q., Fu J., Woods C.G., Hou Y., Corkey B.E., Collins S., Andersen M.E. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol. Appl. Pharmacol. 2010;244(1):77–83. doi: 10.1016/j.taap.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roma L.P., Jonas J.C. Nutrient metabolism, subcellular redox state, and oxidative stress in pancreatic islets and beta-cells. J. Mol. Biol. 2020;432(5):1461–1493. doi: 10.1016/j.jmb.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Miki A., Ricordi C., Sakuma Y., Yamamoto T., Misawa R., Mita A., Molano R.D., Vaziri N.D., Pileggi A., Ichii H. Divergent antioxidant capacity of human islet cell subsets: a potential cause of beta-cell vulnerability in diabetes and islet transplantation. PloS One. 2018;13(5) doi: 10.1371/journal.pone.0196570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.J.N. Ham, D.A. Stoffers, Role of pdx-1 in B-cell growth, in: R.N. Kulkarni (Ed.), Islet Cell Growth Factors, Landis Bioscience2011, pp. 39-56.
- 12.Timme-Laragy A.R., Hahn M.E., Hansen J.M., Rastogi A., Roy M.A. Redox stress and signaling during vertebrate embryonic development: regulation and responses. Semin. Cell Dev. Biol. 2018;80:17–28. doi: 10.1016/j.semcdb.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu S.C. Regulation of glutathione synthesis. Mol. Aspect. Med. 2009;30(1–2):42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meister A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1988;263(33):17205–17208. [PubMed] [Google Scholar]
- 15.Salas-Vidal E., Lomeli H., Castro-Obregon S., Cuervo R., Escalante-Alcalde D., Covarrubias L. Reactive oxygen species participate in the control of mouse embryonic cell death. Exp. Cell Res. 1998;238(1):136–147. doi: 10.1006/excr.1997.3828. [DOI] [PubMed] [Google Scholar]
- 16.Thompson L.P., Al-Hasan Y. Impact of oxidative stress in fetal programming. Journal of Pregnancy. 2012;2012:8. doi: 10.1155/2012/582748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rastogi A., Clark C.W., Conlin S.M., Brown S.E., Timme-Laragy A.R. Mapping glutathione utilization in the developing zebrafish (Danio rerio) embryo. Redox Biol. 2019;26:101235. doi: 10.1016/j.redox.2019.101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimmel C.B., Ballard W.W., Kimmel S.R., Ullmann B., Schilling T.F. Stages of embryonic development of the zebrafish. Dev. Dynam. 1995;203(3):253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 19.Timme-Laragy A.R., Goldstone J.V., Imhoff B.R., Stegeman J.J., Hahn M.E., Hansen J.M. Glutathione redox dynamics and expression of glutathione-related genes in the developing embryo. Free Radic. Biol. Med. 2013;65:89–101. doi: 10.1016/j.freeradbiomed.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiso N., Moro E., Argenton F. Zebrafish pancreas development. Mol. Cell. Endocrinol. 2009;312(1–2):24–30. doi: 10.1016/j.mce.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Brigelius-Flohe R., Flohe L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxidants Redox Signal. 2011;15(8):2335–2381. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dominko K., Dikic D. Glutathionylation: a regulatory role of glutathione in physiological processes. Arh. Hig. Rada. Toksikol. 2018;69(1):1–24. doi: 10.2478/aiht-2018-69-2966. [DOI] [PubMed] [Google Scholar]
- 23.Cooper A.J., Pinto J.T., Callery P.S. Reversible and irreversible protein glutathionylation: biological and clinical aspects. Expet Opin. Drug Metabol. Toxicol. 2011;7(7):891–910. doi: 10.1517/17425255.2011.577738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen T., Nioi P., Pickett C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009;284(20):13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen J.M., Harris C. Redox control of teratogenesis. Reprod. Toxicol. 2013;35:165–179. doi: 10.1016/j.reprotox.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Sant K.E., Hansen J.M., Williams L.M., Tran N.L., Goldstone J.V., Stegeman J.J., Hahn M.E., Timme-Laragy A. The role of Nrf1 and Nrf2 in the regulation of glutathione and redox dynamics in the developing zebrafish embryo. Redox Biol. 2017;13:207–218. doi: 10.1016/j.redox.2017.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hahn M.E., Timme-Laragy A.R., Karchner S.I., Stegeman J.J. Nrf2 and Nrf2-related proteins in development and developmental toxicity: insights from studies in zebrafish (Danio rerio) Free Radic. Biol. Med. 2015;88(Pt B):275–289. doi: 10.1016/j.freeradbiomed.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams L.M., Timme-Laragy A.R., Goldstone J.V., McArthur A.G., Stegeman J.J., Smolowitz R.M., Hahn M.E. Developmental expression of the Nfe2-related factor (Nrf) transcription factor family. PloS One. 2013;8(10) doi: 10.1371/journal.pone.0079574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen G.M., d'Arcy Doherty M. Free radical mediated cell toxicity by redox cycling chemicals. Br. J. Canc. Suppl. 1987;8:46–52. [PMC free article] [PubMed] [Google Scholar]
- 30.Hoarau E., Chandra V., Rustin P., Scharfmann R., Duvillie B. Pro-oxidant/antioxidant balance controls pancreatic beta-cell differentiation through the ERK1/2 pathway. Cell Death Dis. 2014;5:e1487. doi: 10.1038/cddis.2014.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerber P.A., Rutter G.A. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxidants Redox Signal. 2017;26(10):501–518. doi: 10.1089/ars.2016.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uruno A., Furusawa Y., Yagishita Y., Fukutomi T., Muramatsu H., Negishi T., Sugawara A., Kensler T.W., Yamamoto M. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol. Cell Biol. 2013;33(15):2996–3010. doi: 10.1128/MCB.00225-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Timme-Laragy A.R., Karchner S.I., Franks D.G., Jenny M.J., Harbeitner R.C., Goldstone J.V., McArthur A.G., Hahn M.E. Nrf2b: a novel zebrafish paralog of the oxidant-responsive transcription factor NF-E2-related factor 2 (NRF2) J. Biol. Chem. 2012;287:4609–4627. doi: 10.1074/jbc.M111.260125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.diIorio P.J., Moss J.B., Sbrogna J.L., Karlstrom R.O., Moss L.G. Sonic hedgehog is required early in pancreatic islet development. Dev. Biol. 2002;244(1):75–84. doi: 10.1006/dbio.2002.0573. [DOI] [PubMed] [Google Scholar]
- 35.Pauls S., Zecchin E., Tiso N., Bortolussi M., Argenton F. Function and regulation of zebrafish nkx2.2a during development of pancreatic islet and ducts. Dev. Biol. 2007;304(2):875–890. doi: 10.1016/j.ydbio.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 36.Mukaigasa K., Nguyen L.T., Li L., Nakajima H., Yamamoto M., Kobayashi M. Genetic evidence of an evolutionarily conserved role for Nrf2 in the protection against oxidative stress. Mol. Cell Biol. 2012;32(21):4455–4461. doi: 10.1128/MCB.00481-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi M., Itoh K., Suzuki T., Osanai H., Nishikawa K., Katoh Y., Takagi Y., Yamamoto M. Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system. Gene Cell. 2002;7(8):807–820. doi: 10.1046/j.1365-2443.2002.00561.x. [DOI] [PubMed] [Google Scholar]
- 38.Hahn M.E., McArthur A.G., Karchner S.I., Franks D.G., Jenny M.J., Timme-Laragy A.R., Stegeman J.J., Woodin B.R., Cipriano M.J., Linney E. The transcriptional response to oxidative stress during vertebrate development: effects of tert-butylhydroquinone and 2,3,7,8-tetrachlorodibenzo-p-dioxin. PloS One. 2014;9(11):e113158. doi: 10.1371/journal.pone.0113158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sant K.E., Jacobs H.M., Moss L.G., Moss J.B., Timme-Laragy A.R. Assessment of toxicological perturbations and variants of pancreatic islet development in the zebrafish model. Toxics. 2016;4(3):20. doi: 10.3390/toxics4030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.OECD, Histopathology Guidance Document for the Medaka Extended One-Generation Reproduction Test (MEOGRT), OECD2014.
- 41.OECD . FET) Test; 2013. Test No. 236: Fish Embryo Acute Toxicity. [Google Scholar]
- 42.Zhitkovich A., N-Acetylcysteine Antioxidant, aldehyde scavenger, and more. Chem. Res. Toxicol. 2019;32(7):1318–1319. doi: 10.1021/acs.chemrestox.9b00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kubo E., Chhunchha B., Singh P., Sasaki H., Singh D.P. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci. Rep. 2017;7(1):14130. doi: 10.1038/s41598-017-14520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sullivan D.M., Wehr N.B., Fergusson M.M., Levine R.L., Finkel T. Identification of oxidant-sensitive proteins: TNF-alpha induces protein glutathiolation. Biochemistry. 2000;39(36):11121–11128. doi: 10.1021/bi0007674. [DOI] [PubMed] [Google Scholar]
- 45.Zhou R., Lin J., Wu D. Sulforaphane induces Nrf2 and protects against CYP2E1-dependent binge alcohol-induced liver steatosis. Biochim. Biophys. Acta. 2014;1840(1):209–218. doi: 10.1016/j.bbagen.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zagorski J.W., Turley A.E., Dover H.E., VanDenBerg K.R., Compton J.R., Rockwell C.E. The Nrf2 activator, tBHQ, differentially affects early events following stimulation of Jurkat cells. Toxicol. Sci. 2013;136(1):63–71. doi: 10.1093/toxsci/kft172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alia M., Ramos S., Mateos R., Bravo L., Goya L. Response of the antioxidant defense system to tert-butyl hydroperoxide and hydrogen peroxide in a human hepatoma cell line (HepG2) J. Biochem. Mol. Toxicol. 2005;19(2):119–128. doi: 10.1002/jbt.20061. [DOI] [PubMed] [Google Scholar]
- 48.Koulajian K., Ivovic A., Ye K., Desai T., Shah A., Fantus I.G., Ran Q., Giacca A. Overexpression of glutathione peroxidase 4 prevents beta-cell dysfunction induced by prolonged elevation of lipids in vivo. Am. J. Physiol. Endocrinol. Metabol. 2013;305(2):E254–E262. doi: 10.1152/ajpendo.00481.2012. [DOI] [PubMed] [Google Scholar]
- 49.Harmon J.S., Bogdani M., Parazzoli S.D., Mak S.S., Oseid E.A., Berghmans M., Leboeuf R.C., Robertson R.P. beta-Cell-specific overexpression of glutathione peroxidase preserves intranuclear MafA and reverses diabetes in db/db mice. Endocrinology. 2009;150(11):4855–4862. doi: 10.1210/en.2009-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rundlof A.K., Carlsten M., Arner E.S. The core promoter of human thioredoxin reductase 1: cloning, transcriptional activity, and Oct-1, Sp1, and Sp3 binding reveal a housekeeping-type promoter for the AU-rich element-regulated gene. J. Biol. Chem. 2001;276(32):30542–30551. doi: 10.1074/jbc.M101452200. [DOI] [PubMed] [Google Scholar]
- 51.Mayer M.P., Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005;62(6):670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mailloux R.J., Fu A., Robson-Doucette C., Allister E.M., Wheeler M.B., Screaton R., Harper M.E. Glutathionylation state of uncoupling protein-2 and the control of glucose-stimulated insulin secretion. J. Biol. Chem. 2012;287(47):39673–39685. doi: 10.1074/jbc.M112.393538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carvalho A.N., Marques C., Guedes R.C., Castro-Caldas M., Rodrigues E., van Horssen J., Gama M.J., S-Glutathionylation of Keap1 A new role for glutathione S-transferase pi in neuronal protection. FEBS Lett. 2016;590(10):1455–1466. doi: 10.1002/1873-3468.12177. [DOI] [PubMed] [Google Scholar]
- 54.Adler V., Yin Z., Fuchs S.Y., Benezra M., Rosario L., Tew K.D., Pincus M.R., Sardana M., Henderson C.J., Wolf C.R., Davis R.J., Ronai Z. Regulation of JNK signaling by GSTp. EMBO J. 1999;18(5):1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fujimoto K., Polonsky K.S. Pdx1 and other factors that regulate pancreatic beta-cell survival. Diabetes Obes. Metabol. 2009;11(Suppl 4):30–37. doi: 10.1111/j.1463-1326.2009.01121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawamori D., Kajimoto Y., Kaneto H., Umayahara Y., Fujitani Y., Miyatsuka T., Watada H., Leibiger I.B., Yamasaki Y., Hori M. Oxidative stress induces nucleo-cytoplasmic translocation of pancreatic transcription factor PDX-1 through activation of c-Jun NH(2)-terminal kinase. Diabetes. 2003;52(12):2896–2904. doi: 10.2337/diabetes.52.12.2896. [DOI] [PubMed] [Google Scholar]
- 57.Sharma R.B., O'Donnell A.C., Stamateris R.E., Ha B., McCloskey K.M., Reynolds P.R., Arvan P., Alonso L.C. Insulin demand regulates beta cell number via the unfolded protein response. J. Clin. Invest. 2015;125(10):3831–3846. doi: 10.1172/JCI79264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szabat M., Page M.M., Panzhinskiy E., Skovso S., Mojibian M., Fernandez-Tajes J., Bruin J.E., Bround M.J., Lee J.T., Xu E.E., Taghizadeh F., O'Dwyer S., van de Bunt M., Moon K.M., Sinha S., Han J., Fan Y., Lynn F.C., Trucco M., Borchers C.H., Foster L.J., Nislow C., Kieffer T.J., Johnson J.D. Reduced insulin production relieves endoplasmic reticulum stress and induces beta cell proliferation. Cell Metabol. 2016;23(1):179–193. doi: 10.1016/j.cmet.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 59.Tarifeno-Saldivia E., Lavergne A., Bernard A., Padamata K., Bergemann D., Voz M.L., Manfroid I., Peers B. Transcriptome analysis of pancreatic cells across distant species highlights novel important regulator genes. BMC Biol. 2017;15(1):21. doi: 10.1186/s12915-017-0362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Segerstolpe A., Palasantza A., Eliasson P., Andersson E.M., Andreasson A.C., Sun X., Picelli S., Sabirsh A., Clausen M., Bjursell M.K., Smith D.M., Kasper M., Ammala C., Sandberg R. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metabol. 2016;24(4):593–607. doi: 10.1016/j.cmet.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hayes J.D., Dinkova-Kostova A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014;39(4):199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 62.Rojo de la Vega M., Chapman E., Zhang D.D. NRF2 and the hallmarks of cancer. Canc. Cell. 2018;34(1):21–43. doi: 10.1016/j.ccell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sporn M.B., Liby K.T. NRF2 and cancer: the good, the bad and the importance of context. Nat. Rev. Canc. 2012;12(8):564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murray J.R., de la Vega L., Hayes J.D., Duan L., Penning T.M. Induction of the antioxidant response by the transcription factor NRF2 increases bioactivation of the mutagenic air pollutant 3-nitrobenzanthrone in human lung cells. Chem. Res. Toxicol. 2019;32(12):2538–2551. doi: 10.1021/acs.chemrestox.9b00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Edwards J., Ackerman C. A review of diabetes mellitus and exposure to the environmental toxicant cadmium with an emphasis on likely mechanisms of action. Curr. Diabetes Rev. 2016;12(3):252–258. doi: 10.2174/1573399811666150812142922. [DOI] [PMC free article] [PubMed] [Google Scholar]