Increasing severity of ketoacidosis in newly diagnosed type 1 diabetes (T1D) has been reported during the coronavirus 2019 disease (COVID‐19) pandemic in Germany 1 and cases of delayed presentation of children with T1D have also been reported in Italy 2 and the United States. 3 There are significant differences in healthcare delivery systems and socioeconomic status (SES) between the United States and Europe, prompting us to evaluate if delayed presentation was observed in a large paediatric diabetes centre in the United States. In Pennsylvania, the first two cases of COVID‐19 were reported on March 6th 2020 and schools were closed on March 16th. All non‐essential businesses were closed by March 19 and from April onwards, all children admitted to the Children's Hospital of Philadelphia had severe acute respiratory syndrome coronavirus 2 (SARS CoV‐2) testing, 4 including those with newly diagnosed T1D.
We performed a retrospective chart review including children (<18 years) presenting to The Children's Hospital of Philadelphia with a new diagnosis of autoantibody (GAD, IAA, ICA512 or ZnT8) positive diabetes between March 16th and July 31st of 2020, and, as a representative comparison, during the same timeframe in 2017, 2018 and 2019. The study was approved by the Institutional Review Board at The Children's Hospital of Philadelphia. Diabetic ketoacidosis was defined as a venous pH <7.3 and/or bicarbonate level <15 mEq/L, and severe diabetic ketoacidosis was defined as a venous pH <7.1 and/or bicarbonate level <5 mEq/L. 5 Chi‐squared tests were used to compare categorical, and Mann–Whitney U tests to compare non‐normally distributed continuous variables.
There were 73 cases of newly diagnosed T1D between March 16th and July 31st 2020, lower than the average of 92.7 seen over the same timeframe in the preceding 3 years. Although a larger proportion of patients in 2020 presented in ketoacidosis (45.2% vs. 37.9%) and severe ketoacidosis (15% vs. 11.6%), neither were statistically significant (p = 0.3 and 0.4, respectively). When compared with previous years, a trend towards increased rates of ketoacidosis was seen in the first 6 weeks of the pandemic from March 16th to April 30th (63% in 2020 vs. 39% in previous years [56% 2019, 33% 2018, 43% 2017], p = 0.1), Figure 1. In the children with government insurance (low SES), more presented in severe ketoacidosis in 2020 although this was not statistically significant (3/11 [27.3%] vs. 4/57 [7%], p = 0.08), perhaps due to limited numbers in our study. The total rates of ketoacidosis were similar to prior years in this group (5/11 [45.5%] in 2020 vs. 26/57 [45.6%] in 2017–2019; p = 0.9).
FIGURE 1.
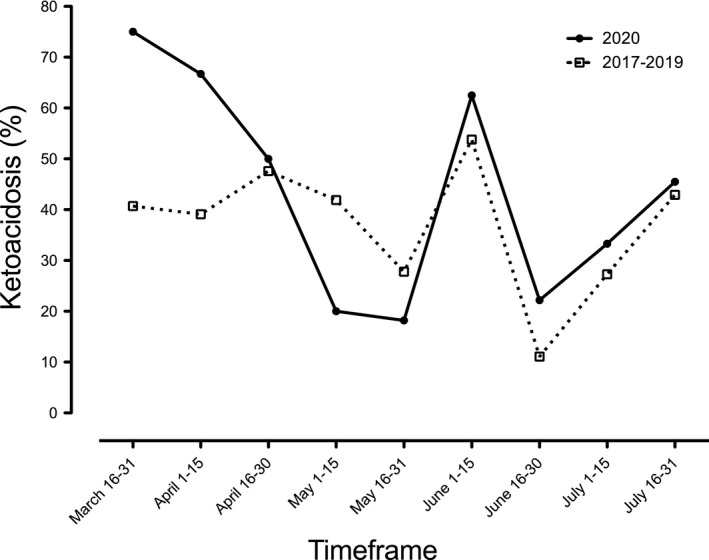
The incidence of ketoacidosis in children presenting with newly diagnosed type 1 diabetes between March 16th and July 31st 2020 compared with the same timeframe in 2017–2019. Note a peak in ketoacidosis seen in the first 6 weeks of the pandemic
Of those diagnosed with T1D in 2020, 68 were tested for SARS‐CoV2 and 2 were positive. Neither of these two patients had known COVID exposures. This included an otherwise well 3‐year‐old female who had polydipsia and polyuria over the preceding weeks. In the week prior to presentation with newly diagnosed T1D, she was also noted to have a diffuse rash on all four extremities and peeling lips. She presented in moderate ketoacidosis (pH 7.1, bicarbonate 10 mEq/L) and responded well to ketoacidosis management. The second SARS‐CoV‐2 positive patient was a 9‐year‐old male who reported a 1‐week history of polydipsia and polyuria (pH 7.25, bicarbonate 10.3 mEq/L). He was noted to have a low‐grade fever to 38.1°C during his admission, with no other symptoms.
During the COVID‐19 pandemic, our large diabetes centre has seen a trend towards increasing ketoacidosis during the first 6 weeks of the pandemic. As Figure 1 demonstrates, the pattern subsequently resembled that of prior years after the initial 6‐week period. The majority of the increase in ketoacidosis was seen in children with commercial insurance, whereas the increase in severe ketoacidosis was seen in those with government insurance. This possibly reflects an overall trend towards delayed presentation, but this study is limited by small patient numbers. We hypothesize that this corresponds to an initial reticence to seek medical attention (as was reported in the SARS epidemic of 2003 6 ) that dissipated as the pandemic continued. Furthermore, reduced interaction of paediatric patients with the healthcare system due to reduced rates of transmissible infections or trauma 2 may also reduce rates of incidental detection of hyperglycaemia in children with early T1D. In this setting, patients, parents and healthcare providers must be even more cognizant of the early symptoms of T1D 3 to ensure the timely diagnosis of this condition prior to the onset of ketoacidosis. As we instituted testing for SARS‐CoV‐2 in almost all patients admitted to hospital, only a small proportion (2/68) presenting in the first 4 months of the pandemic bore any potential of being acutely COVID‐19 related.
CONFLICT OF INTEREST
CPH and SMW have no financial relationships relevant to this article to disclose.
AUTHOR CONTRIBUTION
CPH and SMW designed this study and prepared the manuscript. CPH performed data analysis.
REFERENCES
- 1. Kamrath C, Mönkemöller K, Biester T, et al. Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID‐19 pandemic in Germany. JAMA. 2020;324(8):801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lazzerini M, Barbi E, Apicella A, Marchetti F, Cardinale F, Trobia G. Delayed access or provision of care in Italy resulting from fear of COVID‐19. Lancet Child Adolesc Health. 2020;4:e10‐e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cherubini V, Gohil A, Addala A, et al. Unintended consequences of COVID‐19: remember general pediatrics. J Pediatr. 2020;223:197‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Otto WR, Geoghegan S, Posch LC, et al. The epidemiology of SARS‐CoV‐2 in a pediatric healthcare network in the United States. J Pediatric Infect Dis Soc. 2020;9:523‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolfsdorf JI, Glaser N, Agus M, et al. ISPAD Clinical Practice Consensus Guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19(Suppl 27):155‐177. [DOI] [PubMed] [Google Scholar]
- 6. Chang HJ, Huang N, Lee CH, Hsu YJ, Hsieh CJ, Chou YJ. The impact of the SARS epidemic on the utilization of medical services: SARS and the fear of SARS. Am J Public Health. 2004;94:562‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]


