Abstract
COVID‐19 generates a complex systemic inflammatory response that can lead to death due to wide macrophage activation, endothelial damage, and coagulation in critically ill patients. SARS‐CoV‐2‐induced lung injury due to inflammatory mediated thrombosis could be similar to the livedoid vasculopathy in the skin, supporting a translational comparison of these clinical settings. In this article, we discuss anticoagulation, suppression of inflammatory response, and hyperbaric oxygen therapy in the context of severe COVID‐19 and livedoid vasculopathy.
Keywords: COVID‐19, heparin, hyperbaric oxygenation, livedoid, thrombosis, vasculopathy
1. INTRODUCTION
The COVID‐19 pandemic has resulted in more than 800 000 deaths (as of August 17, 2020) worldwide. This global scenario of sickness and death has had the most severe effect on public health of any crisis in the last 100 years. No effective drug or vaccine is yet available. Nevertheless, in medicine, we must learn from the experiences of the past and through comparison with other clinical settings. Besides the foremost lung involvement due to direct infection by SARS‐CoV‐2, critically ill patients often develop a complex state of immunologically mediated thrombosis and endothelial damage that can lead to systemic compromise and death.
Zhang et al 1 described the first three Chinese patients with coagulopathy and antiphospholipid antibodies (anticardiolipin IgA and anti‐β2‐glycoprotein I IgA and IgG), who developed multiple cerebral infarctions during their COVID‐19 course. Iba et al 2 published an excellent review of the thrombotic complications and coagulopathy that often occur in COVID‐19 patients. These authors named this hypercoagulable state “COVID‐19‐associated coagulopathy” whose primary causes involve macrophage activation and damage to the endothelial cells, which follow the activation of the complement system, von Willebrand factor and factor VIII serum elevations, the presence of antiphospholipid antibodies, an increase in pro‐inflammatory cytokines as IL‐1β and IL‐6, an increase in the serum levels of fibrinogen, D‐dimers, activated partial thromboplastin time (aPTT), and a prolongation of the prothrombin time (PT).
Among critically ill patients, the result of this thromboinflammatory response has frequently been found to present as microthrombosis and venous thrombosis in the lungs, brain, kidney, skin, liver, and gastrointestinal system, leading to multiple organ dysfunction. 2 In the early stages of COVID‐19, inflammation and coagulation phenomena are limited to the lungs; however, as the disease evolves to its hyperinflammatory phase, these phenomena progress toward sepsis‐induced coagulopathy (SIC) or disseminated intravascular coagulation. 2
According to our experience of inpatient and outpatient dermatological practice, especially with the treatment of livedoid vasculopathy (LV), we observed some similarities between the early stages of COVID‐19 and the pathological findings in the thrombo‐occlusive ulcers in skin lesions of LV patients, as well as some therapeutic alternatives common to these diseases.
The pathogenesis of LV is yet to be understood, with the main mechanism being hypercoagulability and inflammation playing a secondary role 3 , as opposed to the sequence observed in COVID‐19 during the early phases within the lungs. Both diseases may benefit from corticosteroids and could be treated by the combined therapy of hyperbaric oxygen therapy (HBOT) and anticoagulation.
The histopathology of LV is characterized by intraluminal thrombosis, proliferation of the endothelium, and segmental hyalinization of dermal vessels, often only in the lower limbs. 3 Nevertheless, lymphocyte infiltrate can be present and perpetuate thrombotic phenomena, leading to skin necrosis (Figure 1).
FIGURE 1.
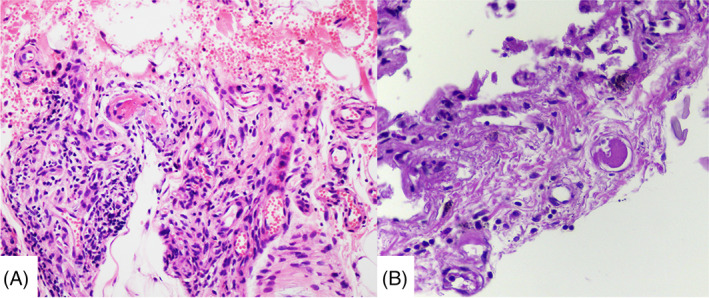
Livedoid vasculopathy in the skin: dermal necrosis with hemorrhagic, lymphocyte infiltrate, and endothelial damage with fibrin microthrombi (HE 400×)
Similarly, pulmonary endothelial damage with microthrombi (Figure 1A) and hemorrhagic lung infarction by septal necrosis (Figure 2B) were observed. In addition, the inflammatory process is accompanied by fibrinoid plugs (Figure 2C) and lymphocytic interstitial infiltration (Figure 2D).
FIGURE 2.
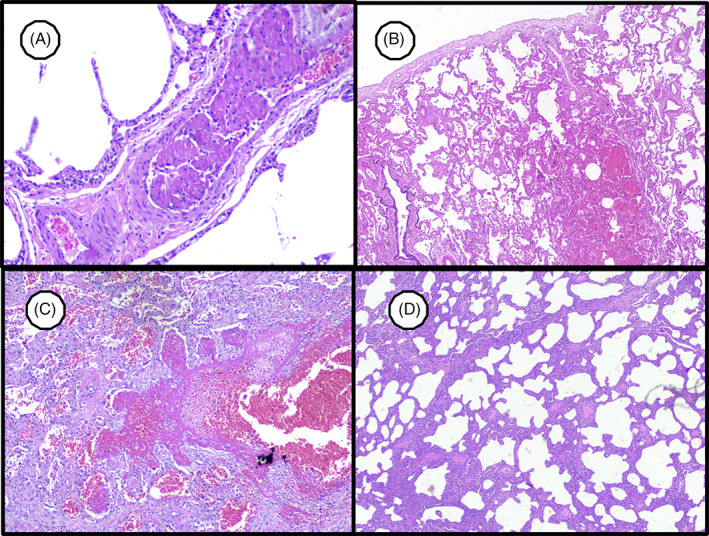
SARS‐CoV‐2‐induced lung injury. A, Lung venous microthrombi (400×). B, Hemorrhagic lung infarction (200×). C, Acute fibrinoid organizing pneumonia (200×). D, Cellular interstitial pneumonia with lymphocytes (100×)
In this article, we present a rationale for the efficacy of anticoagulation and hyperbaric oxygen therapy in both disorders.
2. HYPERBARIC OXYGEN THERAPY
In 2003, Yang et al 4 described two patients with an intractable LV whose ulcers were successfully treated with HBOT. After this, other authors published case reports with similarly satisfactory results in LV patients. 5 , 6 , 7 , 8 , 9 , 10 , 11
In February 2020, Zhong et al 12 described the first case report of successful HBOT in a severely ill COVID‐19 patient who was failing standard respiratory support (not intubated) and whose disease course was reversed with eight HBOT sessions at 200 kPa over a total treatment time of 95 minutes.
Subsequently, Chen et al 13 reported five severe or moderate acute respiratory distress syndrome (ARDS) patients with COVID‐19 pneumonia who had been treated under HBOT. These five patients were 24 to 69 (mean 47.6) years old and received three to eight (mean 4.6) sessions of HBOT in addition to routine therapies. 13 These patients' daily average oxygen saturation levels (SpO2) were restored to above 95% after one to eight HBOT treatments. Following HBOT, patients' PaO2 and SaO2 levels had significantly increased (P < .05) and their lactate levels had declined. The patients' peripheral blood lymphocytes were obviously elevated after HBOT treatments (P < .05) and their fibrinogen and D‐dimer serum levels had decreased. Chest computerized tomography (CT) scans obtained during or after HBOT showed significantly improved imaging status of lung lesions in each patient. 13
The HBOT methods applied by Chen et al 13 were described as a chamber compression of 2.0 ATA (absolute atmospheric pressure) for patient 1 and 1.6 ATA for the other four patients, in 15 minutes. The bottom time was 90 minutes in the first treatment and 60 minutes in the second. Decompression to atmospheric pressure took place over 20 minutes. 13
Regarding the safety of disease control and the prevention of airborne contagion, a hyperbaric chamber and oxygen inhalation system are perfect gas management systems for disease control due to their properties of closed, one‐way gas flow, all‐fresh‐air, and relatively independent gas lines for medical staff and patients, which suggest that the risk of infection for medical staff in the chamber is not higher than in the ward.
Rigorous measures of disease control and prevention were applied for HBOT. 13 For the treatment of COVID‐19 patients, the measures for disease control outside the chamber were the same as in the infection wards, such as implementation of separate paths for medical staff and patients and the distinction of infectious areas. 13 Disinfection measures in the chamber were further strengthened to similar levels as in infectious ward areas. 13 In regards to the treatment procedure, patients respired with built‐in breathing apparatus immediately upon entering the chamber. 13 The chamber maintained continuous ventilation with a high volume of fresh air. 13
Guo et al 14 reported the same success in two other male COVID‐19 patients, each of whom met at least one of the following criteria: shortness of breath; a respiratory rate (RR) of ≥30 breaths per minute; finger pulse oxygen saturation (SpO2) of ≤93% at rest; and an oxygen index with a P/F ratio of PaO2/FiO2 ≤ 300 mm Hg. Patients were treated with HBOT of 1.5 atm with an oxygen concentration of more than 95% for 60 minutes per treatment once a day for 1 week. No patient became critically ill; they demonstrated a decreasing trend of SO2 and their P/F ratios were immediately reversed and increased daily. In addition, their lymphocyte counts and ratios reflecting immune function gradually recovered, their D‐dimer corresponding to peripheral circulation disorders and serum cholinesterase (reflecting liver function) improved. Subsequent chest CTs showed that patients' pulmonary inflammation had clearly subsided.
A single‐center prospective study was also conducted on HBOT as an adjunct to standard therapy for a pilot cohort of 40 COVID‐19‐positive patients with respiratory distress at New York University Winthrop Hospital (registered at clinicaltrial.gov under the identifier NCT04332081). 15
The initial result of this study was that following infection by SARS‐CoV‐2, morbidity and mortality from this condition are due to the incidence of ARDS, which is defined as a condition of extremely low arterial oxygen concentration or hypoxia with a ratio of partial pressure of arterial oxygen and a fraction of inspired oxygen (PaO2/FiO2 ratio) of ≤300, as well as bilateral opacities not fully explained by effusions, lobar/lung collapse, or nodules identified by chest radiography or CT. 16 ARDS as seen in severe COVID‐19 is characterized by difficulty in breathing and low blood oxygen levels. 17 , 18 As a result, some patients may experience complications with secondary bacterial and fungal infections. ARDS may lead directly to respiratory failure, which is the cause of death in 70% of fatal COVID‐19 cases. 17 The development of ARDS is likely the product of inflammation mounted by the patient's response to the virus and secondary bacterial infections. 18
Harch PG 19 analyzed the outcomes of the first two Chinese with COVID‐19 for whom HBOT was applied 12 , 13 and reinforced the application of HBOT in this clinical scenario based on sound physiology and Henry's law. Named after the English physician William Henry, this law defines the relationship between the partial pressure of gases overlying a solution and the gases' ability to dissolve in that solution. 20
Henry's law states that when a gaseous mixture (eg, the atmosphere) is in contact with a solution, the amount of any gas in that mixture that dissolves in the solution is directly proportionate to the partial pressure of that gas. 20 The partial pressure of a gas is the amount of pressure that the gas contributes to the total pressure of that gas mixture. 20 According to Henry's law, if the pressure of a gas over liquid increases, the amount of gas dissolved in the liquid will increase proportionally. As the gas pressure decreases, the amount of gas dissolved in the solution drops. 20 In COVID‐19 lung injury, the alveolar‐capillary barrier is damaged by an inflammatory exudate with edema and a lymphocytic infiltrate, leading to reduced gas exchange in the distal airway spaces. 19 Additionally, there is associated microvascular thrombosis in the perialveolar blood vessels (Figure 2).
Dr. Richard Levitan, an emergency physician at Bellevue Hospital in New York City, made some striking patient observations regarding blood O2 levels that he shared in a New York Times opinion piece (April 20, 2020). 18 He noted that the initial stage of COVID‐19 is only now being understood as “silent hypoxia,” alluding to its “insidious, hard‐to‐detect nature.” 18 In Levitan's observations, SpO2 fell from the normal range of 94‐100% to as low as 50%, but patients did not experience any dyspnea until the depleted levels reached critical values. This was most likely due to the fact that CO2 continued to be released. By the time CO2 started to accumulate, a feeling of breathlessness developed and many COVID‐19 patients declined quickly into respiratory failure. 18
According to Henry's law, the HBOT acts by (a) dissolving oxygen in the inflamed alveolar‐capillary barrier, (b) increasing the diffusion rate of oxygen, (c) the diffusion distance of oxygen, (d) increasing the dissolution of oxygen in blood plasma, (e) achieving more oxygen saturation of hemoglobin in the red blood cells, and (f) achieving the best delivery of oxygen to the microcirculation and tissue. 19 The next result is a reversal of the downward spiral of COVID‐19 patients. 19 Elevated systemic levels of oxygen secondary to HBOT has been traditionally misunderstood in terms of respiratory metabolite effects with a transient hyperoxemia that dissipates once the patient leaves the chamber. 19
A similar clinical setting was observed during the Spanish flu pandemic of 1918, when doctor Orval Cunningham of Kansas City, USA, applied HBOT (pressure and oxygen) with a near‐identical pressure of 1.6 ATA (1 ATA = 101.32 kPa) to a moribund patient with agonal breathing. He exhibited the same dramatic reversal of his condition that was observed by the Chinese physicians in Wuhan's patients with COVID‐19. 19 , 21
HBOT is based on intermittent inhalation of 100% oxygen in a pressurized chamber under 1.5 to 3.0 ATA. All HBOT properties meet the demand of aerobic metabolism in hypoperfused regions of the body, 22 such as the alveoli suffering from edema, low lung surfactant production, tissue inflammation, and areas of perialveolar microthrombosed capillaries found in patients with COVID‐19. Besides offering better oxygen perfusion, HBOT also has anti‐inflammatory properties.
The main side effects of HBOT are limited to the pulmonary and neurological (eg, visual impairment, tinnitus, nausea, facial spasms, dizziness, and disorientation) systems. 22 Pulmonary toxicity usually manifests with tracheobronchial irritation. Oxygen toxicity has also been previously reported. 22 However, the adverse effects of HBOT seem of relatively small concern in COVID‐19 cases considering the rather limited number of patients in whom such therapy could be considered and applied. 22
Several of HBOT's effects in reducing patients' inflammatory state have been described, 22 such as (a) modulation of oxidative stress, including lipid peroxidation, and increase in antioxidant enzymes, such as activity of iNOS in leukocytes and eNOS in platelets; (b) modulation of cytokine levels, reduction of TNF‐α, and lung neutrophil sequestration; and (c) increase in synthesis of HIF‐1α (hypoxia‐inducible factor‐1 alpha) via an oxidative stress response, mediated in part by thioredoxin, leading to increased recruitment of stem cells.
Hyperbaric exposition and decompression induce activation of fibrinolysis, even in the absence of detectable gas bubbles. 23 Fibrinolytic activity increases mainly due to decreases in concentration and activity of plasminogen activator inhibitor‐1 (PAI‐1). 23 Other HBOT effects under coagulation/fibrinolytic pathways include (a) increasing red blood cell (RBC) deformation (erythrocyte rheology), allowing RBCs to perfuse areas that they otherwise could not due to capillary and small arteriolar damage from purpura fulminans or duet to impaired RBC deformability, contributing to sludging and oxygen offload inability. 24 ; (b) attenuating reperfusion injury and platelet aggregation 24 ; (c) capillary and fibroblast proliferation 24 ; (d) collagen production 24 ; and (e) prevention of alteration in the coagulation cascade and arterial blood gas in an experimental zymosan‐induced model of multiple organ failure syndrome. 25
Endothelial cells represent one‐third of the total lung cells. 24 Baseline endothelial damage may be chronically caused by increased adiponectin in diabetic and obese patients; this effect is related to activation of inflammasome NLRP3 and autocrine production of IL‐1β. 24 Additional damage to pulmonary endothelial dysfunctional cells is acutely provoked by infections and, in turn, causes excess thrombin generation and reduced fibrinolysis. 26 Additionally, hypoxia may lead to increased expression and hypercoagulability of HIF‐1α. 26 Therefore, a high rate of thrombotic episodes is reported in patients with COVID‐19, while increased vascular permeability seems to be strongly related to increased thrombosis (inflammatory mediated). 26 In lymphopenia with organ failure, increased vascular permeability has been strongly correlated with severe lymphopenia. 26 Figure 3 summarizes the possible mechanisms by which HBOT may act in both LV and COVID‐19 patients to repair inflammation, coagulation, and tissue damage.
FIGURE 3.
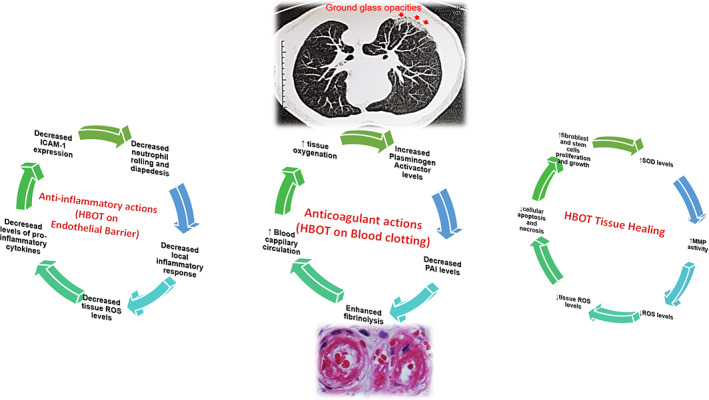
Possible mechanisms of hyperbaric oxygen therapy in COVID‐19 patients. Some actions of hyperbaric oxygen therapy, such as acting as an anti‐inflammatory agent, enhancing the fibrinolytic system, and promoting tissue damage repair. At the top of the figure, a CT image of the chest of a male patient is suggestive of radiological COVID‐19 findings, such as ground‐glass opacity in the posterior lung segment. At the bottom of the figure, a characteristic vaso‐occlusive phenomenon can be observed in a patient with LV. HBOT, hyperbaric oxygen therapy; ↑, enhance; ↓, decrease; MMP, metalloproteinase; PAI, plasminogen inhibitor; ROS, reactive oxygen species; SOD, superoxide‐dismutase
3. ANTICOAGULATION
LV is histologically characterized by hyaline vascular alterations in the sub‐intima, leakage of erythrocytes, and fibrinous substance without evidence of leukocytoclastic vasculitis (Figure 1). Lymphocyte infiltrate and thrombosis of the dermal vessels and tissue ischemia may also occur. 7
We have personally witnessed the excellent results of anticoagulation therapy in patients suffering from LV. 27 , 28 Warfarin (targeted INR 2.0), heparin, and rivaroxaban have been used with positive results in pain resolution and wound repair. 27 , 28
In Italy, a randomized controlled trial is ongoing to compare efficacy and safety of high doses of low‐molecular‐weight heparin (LMWH) (ie, enoxaparin 70 IU/kg twice daily) compared to a standard prophylactic dose (ie, enoxaparin 4000 IU once daily) in hospitalized patients with COVID‐19 who do not require invasive mechanical ventilation. 29
Activation of the coagulation system has been linked to the onset of ARDS in patients during the hyperinflammatory phase of COVID‐19. 30 Since macrophage activation and endothelial damage lead to a greater expression of tissue factor and other coagulative factors, median plasma concentrations of tissue factor and plasminogen activator inhibitor‐1 are significantly higher up to day 7 in patients with ARDS compared to those without ARDS. 30 Coagulopathy follows the hyperinflammation and arises from thrombin generation mediated by localized tissue factor, neutrophil extracellular traps, and depression of fibrinolysis mediated by plasminogen activator in the lungs associated with an increase in PAI‐1. 30 , 31
High levels of IL‐6 and IL‐8 have been associated with SARS‐CoV‐1 and SARS‐CoV‐2. 30 In patients with an exacerbated inflammatory state during COVID‐19, these cytokines may aggravate the hypercoagulable state for the following reasons: (a) IL‐1β, IL‐6, and IL‐8 can cause abnormal clot lysis, as demonstrated through analysis of thromboelastography; (b) IL‐8 can cause hypercoagulation (ie, clots form faster) with an increase in cross‐linking of fibrin fibers; (c) inflammation stimulates coagulation through increased intravascular tissue factor (TF) expression and downregulation of the fibrinolytic pathway; and (d) IL‐6 can increase expression of fibrinogen, factor VIII, and von Willebrand factor (vWF), as well as activation of endothelial cells, increased platelet production, and reduced levels hemostasis inhibitors, such as antithrombin and protein S. These findings demonstrate how cytokines and inflammation activate coagulation and point toward the presence of a possible loop mechanism that could be amplified in a setting like that of COVID‐19. 30 , 31
Wichmann et al 32 conducted a prospective cohort study in 12 consecutive COVID‐19‐positive deaths in Hamburg, Germany, including findings from complete autopsies, postmortem computed tomography, and histopathologic and virologic analysis. The autopsies revealed deep venous thrombosis in seven of 12 patients (58%) in whom venous thromboembolism was not suspected before death; pulmonary embolism was the direct cause of death in four patients. Postmortem computed tomography revealed reticular infiltration of the lungs with severe bilateral, dense consolidation, whereas histomorphologically diffuse alveolar damage was observed in eight patients. In all patients, SARS‐CoV‐2 RNA was detected in the lung at high concentrations, viremia was found in 6 of 10 patients, and 5e of 12 patients demonstrated high viral RNA titers in the liver, kidney, or heart. 32
Our group performed 49 autopsies in COVID‐19 patients, all of which presented severe pulmonary damage, including 25% with thromboembolism and at least 75% with microinfarction, highlighting that inflammation and endothelial damage per se act as local stimuli for coagulation.
Histopathology of the lungs revealed diffuse alveolar damage consistent with early ARDS in eight cases. Predominant findings were hyaline membranes, activated pneumocytes, microvascular thrombi, capillary congestion, and protein‐enriched interstitial edema. 31 Microthrombi were found within small lung arteries and occasionally within the prostate but not in other organs. 32
COVID‐19 may predispose patients to venous thromboembolism in several ways. 32 The coagulation system may be activated by many different viruses, including HIV, dengue virus, and Ebola virus. 31 In particular, coronavirus infections may be a trigger for venous thromboembolism, and several pathogenetic mechanisms are involved, including endothelial dysfunction characterized by increased levels of vWF; systemic inflammation by toll‐like receptor activation; and a procoagulatory state by TF pathway activation. 32 Direct activation of the coagulation cascade by a cytokine storm is also conceivable. 32 In this context, some professional societies have already made recommendations for antithrombotic therapy for patients with COVID‐19. 33
Heparin has two different mechanisms of inhibition on the NF‐κB signaling pathway: one focuses on inhibiting translocation of the transcription factor into the nucleus 33 and the other one has been explained as the ability of heparin to interfere non‐specifically with the binding of NF‐κB to DNA in the nucleus. 33 Hence, leukocyte adhesion and activation, as well as pro‐inflammatory cytokine production, are downregulated as a result of the inhibitory effect of heparin on the NF‐κB signaling. 34
Administering heparin to patients will not only activate their antithrombins but may also affect the functional state of a variety of other proteins. 35 By competing with cell‐surface heparan sulfate (HS) for protein binding, heparin will displace proteins from their HS‐mediated anchoring and thus disrupt associated function. 35
The effects of potential value in COVID‐19 treatment include the prevention of viral adhesion as well as promotion of antiinflammatory activity based on inhibition of neutrophil chemotaxis and leukocyte migration. 35 The recurrent involvement of proteins bound to cell‐surface HS is striking. 35 Binding of a viral protein to cell‐surface HS is often the first step in a cascade of interactions that are required for viral entry and initiation of the infection. 35 Heparin interacts with the receptor‐binding domain of the SARS‐CoV‐2 Spike S1 protein and heparin use may have the potential to prevent viral adhesion. 35 A retrospective clinical study found that use of LMWH in the treatment of COVID‐19 patients resulted in significantly lower plasma levels of IL‐6, a key player in the “cytokine storm” associated with the severe outcomes of this viral disease. 35 , 36
This review aim to contribute with additional information that may be applied in the futures randomized‐controlled trails (RCT) on COVID‐19 treatment, based on our experience with similar pathological findings of microthrombosis in cutaneous blood vessels found in patients with livedoid vasculopathy. Unfortunately, a systematic review including RCT for COVID‐19 treatment with anticoagulation (warfarin, heparin or new direct oral anticoagulants) and/or HBOT were not showed published results disposable until September 23, 2020, as demonstrated in Table 1. 15 , 29 , 37 , 38 , 39 , 40 , 41 , 42 , 43
TABLE 1.
Summary of distinct studies and observations, including retrospective, prospective or randomized‐controlled trials published or in perspective of enrollment using anticoagulation and/or oxygen hyperbaric therapy
| Authors | Country | Study design | Heparin | Warfarin | DOACs | Hyperbaric oxygen therapy (HBOT) |
|---|---|---|---|---|---|---|
| Tang et al 37 | China | Retrospective |
99 inpatients between 499 with severe COVID‐19 received heparin (mainly with low molecular weight heparin) for 7 days or longer. D‐dimer, prothrombin time, and age were positively, and platelet count was negatively, correlated with 28‐day mortality in multivariate analysis. No difference in 28‐day mortality was found between heparin users and non‐users (30.3% vs 29.7%, P = .910). But the 28‐day mortality of heparin users was lower than non‐users in patients with SIC (sepsis‐induced coagulopathy) score ≥ 4 (40.0% vs 64.2%, P = .029), or D‐dimer >6‐fold of upper limit of normal (32.8% vs 52.4%, P = .017). |
|||
| Ayerbe et al 38 | Spain | Retrospective | 2075 patients in 17 Spanish hospitals between first March and the 20th of April 2020. Several treatments were applied (heparin, hydroxychloroquine, azithromycin, steroids, tocilizumab, a combination of lopinavir with ritonavir, and oseltamivir). Heparin was associated with lower mortality when the model was adjusted for age and gender, with OR (95% CI) 0.55 (0.37‐0.82) P = .003. This association remain significant when saturation of oxygen < 90%, and temperature > 37°C were added to de model with OR 0.54 (0.36‐0.82) P = .003, and also when all the other drugs were included as covariates OR 0.42 (0.26‐0.66) P < .001. | |||
| Marietta et al 29 | Italy |
Prospective. Protocol version 1.2 of 11/05/2020. Recruitment start (expected): 08/06/2020 Recruitment finish (expected): 30 April 2021 Trial registration EudraCT 2020‐001972‐13, registered on 17 April, 2020 |
Inpatients will be recruited from 7 Italian Academic and non‐Academic Internal Medicine Units, 2 Infectious Disease Units and 1 Respiratory Disease Unit. Control Group (Low‐Dose LMWH): patients in this group will be administered Enoxaparin (Inhixa®) at standard prophylactic dose (ie, 4000 UI subcutaneously once day). Intervention Group (High‐Dose LMWH): patients in this group will be administered Enoxaparin (Inhixa) at dose of 70 IU/kg every 12 hours. | |||
| Wilkinson et al 39 | United Kingdom |
Prospective. EudraCT 2020‐001736‐95, registered 28 April 2020. |
ACCORD is a seamless, Phase 2, adaptive, randomized controlled platform study, designed to rapidly test candidate agents in the treatment of COVID‐19. Current candidate experimental arms include bemcentinib, MEDI3506, acalabrutinib, zilucoplan and nebulized heparin with others to be added over time. | |||
| Busani et al 40 | Italy |
Prospective. Inpatients will be recruited from 8 Italian Academic and non‐Academic Intensive Care Units |
Recruitment of 210 participants will be completed in approximately 10 months. Three groups of patients: (1) LMWH (low molecular weight heparin) group: patients in this group will be administered enoxaparin at standard prophylactic dosage. (2) LMWH + steroid group: patients in this group will receive enoxaparin at standard prophylactic dosage and methylprednisolone. (3) UFH (unfractionated heparin) + steroid group: patients in this group will receive UFH at therapeutic dosages and methylprednisolone. | |||
| Kharma et al 41 | Qatar |
Prospective. Single centre parallel group, superiority, randomized (1:1 allocation ratio) controlled trial. ANTI‐CO Trial” in ClinicalTrials.org with the registration number: NCT04445935. Registered on June 24, 2020. |
The authors will enroll a total of 100 patients (50 in each group). The intervention group will receive the anticoagulant bivalirudin intravenously with a target aPTT of 45‐70 seconds for three days while the control group will stay on the standard treatment with low‐molecular‐weight heparins /unfractionated heparin subcutaneously | |||
| Barco et al 42 | Switzerland | Prospective. OVID study. RCT, open‐label study, no blinding procedures will be used. | 1000 patients: 500 patients randomized to the intervention group will receive subcutaneous enoxaparin at the recommended dose of 4000 IU anti‐Xa activity (40 mg/0.4 mL) once daily for 14 days. Other 500 patients randomized to the comparator group will receive no anticoagulation. | |||
| None study | ||||||
| Testa et al 43 | Italy | Observational. Patients hospitalized between February 22 and March 15. | Of the 1039 patients hospitalized between February 22 and March 15, 2020 with COVID‐19 pneumonia and candidates for antiviral therapy (lopinavir, ritonavir, or darunavir), 32 were on treatment with a DOAC (dabigatran apixaban, rivaroxaban and edoxaban). DOAC was stopped in 20 and continued in the remaining 12. On average, C‐trough levels were 6.14 times higher during hospitalization than in the pre‐hospitalization period. DOAC patients treated with antiviral drugs show an alarming increase in DOAC plasma levels. | |||
| New York University Winthrop Hospital. 15 | USA | Prospective. Open Label Single‐Center Study of Emergency Hyperbaric Oxygen for Respiratory Distress in patients with COVID‐19. NYU Winthrop Hospital. ClinicalTrials.gov identifier NCT04332081 | Standard treatment plus HBOT for 40 COVID19‐positive patients with respiratory distress. The patient will receive 90 minutes of hyperbaric oxygen at 2.0 ATA with or without airbreaks per the hyperbaric physician. |
4. CONCLUSION
We reviewed the mechanisms involved in inflammation and coagulation in patients with COVID‐19, as well as their similarities with the microthrombosis observed in LV. Our experience in treating patients with LV with excellent responses to HBOT and LMWH regimens acts as reasoning for their translation from dermatology to treatment of severely ill COVID‐19 patients. A rational randomized controlled prospective study assessing treatment of COVID‐19 with and without HBOT and LMWH is necessary as a proof of concept in the efficacy and safety of this approach for moderate to severe COVID‐19 until an effective antiviral drug becomes available.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHORS CONTRIBUTION
Paulo Ricardo Criado: article conception, article writing, Figure 1 and 3 conception, final article organization and submission. Helio Amante Miot: article writing, Figure 1 conception, final article organization. Thais Prota Hussein Pincelli: article writing, final article organization, English review. Alexandre Todorovic Fabro: article writing, Figure 1 and 2 conception, final article organization.
Criado PR, Miot HA, Pincelli TPH, Fabro AT. From dermatological conditions to COVID‐19: Reasoning for anticoagulation, suppression of inflammation, and hyperbaric oxygen therapy. Dermatologic Therapy. 2021;34:e14565. 10.1111/dth.14565
“What is death, but a letting go of breath? […].What is life but a drawing in of breath?” – Adrian Rice, “Breath.”
Contributor Information
Paulo Ricardo Criado, Email: prcriado@uol.com.br.
Hélio Amante Miot, Email: heliomiot@fmb.unesp.br.
Alexandre Todorovic Fabro, Email: fabro@fmrp.usp.br.
DATA AVAILABILITY STATEMENT
Data Availability Statement: Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and Antiphospholipid antibodies in patients with Covid‐19. N Engl J Med. 2020;382(17):e38. 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iba T, Levy JH, Connors JM, Warkentin TE, Thachil J, Levi M. The unique characteristics of COVID‐19 coagulopathy. Version 2. Crit Care. 2020;24(1):360. 10.1186/s13054-020-03077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vasudevan B, Neema S, Verma R. Livedoid vasculopathy: a review of pathogenesis and principles of management. Indian J Dermatol Venereol Leprol. 2016;82(5):478‐488. 10.4103/0378-6323.183635. [DOI] [PubMed] [Google Scholar]
- 4. Yang CH, Ho HC, Chan YS, Liou LB, Hong HS, Yang LC. Intractable livedoid vasculopathy successfully treated with hyperbaric oxygen. Br J Dermatol. 2003;149(3):647‐652. 10.1046/j.1365-2133.2003.05546.x. [DOI] [PubMed] [Google Scholar]
- 5. Juan WH, Chan YS, Lee JC, Yang LC, Hong HS, Yang CH. Livedoid vasculopathy: long‐term follow‐up results following hyperbaric oxygen therapy. Br J Dermatol. 2006;154(2):251‐255. 10.1111/j.1365-2133.2005.06843.x. [DOI] [PubMed] [Google Scholar]
- 6. Antunes J, Filipe P, André M, Fraga A, Miltenyi G, Marques GM. Livedoid vasculopathy associated with plasminogen activator inhibitor‐1 promoter homozygosity (4G/4G) and prothrombin G20210A heterozygosity: response to t‐PA therapy. Acta Derm Venereol. 2010;90(1):91‐92. 10.2340/00015555-0760. [DOI] [PubMed] [Google Scholar]
- 7. Bollmann PW, Shimada AK, Michalany NS, Manhani AR, Giglio AD. Livedoid vasculopathy: fast involution after anticoagulant and hyperbaric oxygen therapy. Einstein (Sao Paulo). 2011;9(2):212‐215. 10.1590/S1679-45082011RC1676. [DOI] [PubMed] [Google Scholar]
- 8. Bhutani S, Verma R, Verghese G. Livedoid vasculopathy managed with hyperbaric oxygen therapy. Med J Armed Forces India. 2012;68(4):389‐391. 10.1016/j.mjafi.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Banham ND. Livedoid vasculopathy successfully treated with hyperbaric oxygen. Diving Hyperb Med. 2013;43(1):35‐36. [PubMed] [Google Scholar]
- 10. Verma V. Livedoid vasculopathy managed with hyperbaric oxygen therapy. Med J Armed Forces India. 2013;69(2):202‐203. 10.1016/j.mjafi.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ray R, Sharma A, Vasudevan B, Sridhar J, Deo R, Mohanty CS. Livedoid vasculopathy with Hyperhomocysteinemia responding to hyperbaric oxygen therapy. Indian J Dermatol. 2015;60(5):524. 10.4103/0019-5154.159657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhong XL, Tao XL, Tang YC, et al. Effect of hyperbaric oxygen therapy to treat hypoxia in severe novel coronavirus pneumonia patients: first case report. Chin J Nauti and Hyperb Med. 2020;27. 10.3760/cma.j.issn.1009-6906.2020.0001. [DOI] [Google Scholar]
- 13. Chen R, Zhong X, Tang Y, Liang Y, Li B, Tao X, Liao C. The outcomes of hyperbaric oxygen therapy to severe and critically ill patients with COVID‐19 pneumonia. https://oxycamaras.com.br/wp-content/uploads/2020/04/Outcome-of-HBOT-to-COVID19.pdf.pdf.pdf.pdf.pdf
- 14. Guo D, Pan S, Wang M, Guo Y. Hyperbaric oxygen therapy may be effective to improve hypoxemia in patients with severe COVID‐2019 pneumonia: two case reports. Undersea Hyperb Med 2020. Second‐Quarter;47(2):181–187. [PubMed] [Google Scholar]
- 15. HBO2 for COVID‐19: clinical trials at clinicaltrials.Gov. Undersea Hyperb Med. 2020;47(2):299‐307. [PubMed] [Google Scholar]
- 16. ARDS Definition Task Force , Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526‐2533. 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 17. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Maio A, Hightower LE. COVID‐19, acute respiratory distress syndrome (ARDS), and hyperbaric oxygen therapy (HBOT): what is the link? Cell Stress Chaperones. 2020;18:1‐4. 10.1007/s12192-020-01121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harch PG. Hyperbaric oxygen treatment of novel coronavirus (COVID‐19) respiratory failure. Med Gas Res. 2020;10(2):61‐62. 10.4103/2045-9912.282177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Avishay DM, Tenny KM. Henry's law. [Updated 2019 Jul 16]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK544301/ [Google Scholar]
- 21. Sellers LM. The fallibility of the forrestian principle. “Semper primus pervenio maxima cum VI”. Laryngoscope. 1964;74:613‐633. [DOI] [PubMed] [Google Scholar]
- 22. Paganini M, Bosco G, Perozzo FAG, et al. The role of hyperbaric oxygen treatment for COVID‐19: a review. Adv Exp Med Biol. 2020. 10.1007/5584_2020_568. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 23. Radziwon P, Olszański R, Tomaszewski R, et al. Decreased levels of PAI‐1 and alpha 2‐antiplasmin contribute to enhanced fibrinolytic activity in divers. Thromb Res. 2007;121(2):235‐240. [DOI] [PubMed] [Google Scholar]
- 24. Cooper JS, Allinson P, Keim L, et al. Hyperbaric oxygen: a useful adjunct for purpura fulminans: case report and review of the literature. Undersea Hyperb Med. 2014;41(1):51‐57. [PubMed] [Google Scholar]
- 25. Imperatore F, Cuzzocrea S, De Lucia D, et al. Hyperbaric oxygen therapy prevents coagulation disorders in an experimental model of multiple organ failure syndrome. Intensive Care Med. 2006;32(11):1881‐1888. [DOI] [PubMed] [Google Scholar]
- 26. Marchetti M. COVID‐19‐driven endothelial damage: complement, HIF‐1, and ABL2 are potential pathways of damage and targets for cure. Ann Hematol. 2020;99(8):1701‐1707. 10.1007/s00277-020-04138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Di Giacomo TB, Hussein TP, Souza DG, Criado PR. Frequency of thrombophilia determinant factors in patients with livedoid vasculopathy and treatment with anticoagulant drugs—a prospective study. J Eur Acad Dermatol Venereol. 2010;24(11):1340‐1346. [DOI] [PubMed] [Google Scholar]
- 28. Franco Marques G, Criado PR, Alves Batista Morita TC, Cajas García MS. The management of livedoid vasculopathy focused on direct oral anticoagulants (DOACs): four case reports successfully treated with rivaroxaban. Int J Dermatol. 2018;57(6):732‐741. [DOI] [PubMed] [Google Scholar]
- 29. Marietta M, Vandelli P, Mighali P, et al. Randomised controlled trial comparing efficacy and safety of high versus low low‐molecular weight heparin dosages in hospitalized patients with severe COVID‐19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID‐19 HD): a structured summary of a study protocol. Trials. 2020;21(1):574. 10.1186/s13063-020-04475-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Magro G. COVID‐19: review on latest available drugs and therapies against SARS‐CoV‐2. Coagulation and inflammation cross‐talking. Virus Res. 2020;286:198070. 10.1016/j.virusres.2020.198070 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Merad M, Martin JC. Pathological inflammation in patients with COVID‐19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID‐19. Ann Intern Med. 2020;173(4):268‐277. 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020;75(23):2950‐2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alkhoury H, Hautmann A, Fuhrmann B, et al. Studies on the mechanisms of anti‐inflammatory activity of heparin‐ and Hyaluronan‐containing multilayer coatings‐targeting NF‐κB Signalling pathway. Int J Mol Sci. 2020;21(10):3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lindahl U, Li JP. Heparin ‐ an old drug with multiple potential targets in Covid‐19 therapy. J Thromb Haemost. 2020. 10.1111/jth.14898. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shi C, Wang C, Wang H, et al. The potential of low molecular weight heparin to mitigate cytokine storm in severe COVID‐19 patients: a retrospective clinical study. medRxiv. 2020. 10.1111/cts.12880. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094‐1099. 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ayerbe L, Risco C, Ayis S. The association between treatment with heparin and survival in patients with Covid‐19. J Thromb Thrombolysis. 2020;50(2):298‐301. 10.1007/s11239-020-02162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wilkinson T, Dixon R, Page C, et al. ACCORD: a multicentre, seamless, phase 2 adaptive randomisation platform study to assess the efficacy and safety of multiple candidate agents for the treatment of COVID‐19 in hospitalised patients: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):691. 10.1186/s13063-020-04584-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Busani S, Tosi M, Mighali P, et al. Multi‐Centre, three arm, randomized controlled trial on the use of methylprednisolone and unfractionated heparin in critically ill ventilated patients with pneumonia from SARS‐CoV‐2 infection: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020. Aug 17;21(1):724. 10.1186/s13063-020-04645-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kharma N, Roehrig S, Shible AA, et al. Anticoagulation in critically ill patients on mechanical ventilation suffering from COVID‐19 disease, the ANTI‐CO trial: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):769. 10.1186/s13063-020-04689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barco S, Bingisser R, Colucci G, et al. Enoxaparin for primary thromboprophylaxis in ambulatory patients with coronavirus disease‐2019 (the OVID study): a structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21(1):770. 10.1186/s13063-020-04678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Testa S, Prandoni P, Paoletti O, et al. Direct oral anticoagulant plasma levels' striking increase in severe COVID‐19 respiratory syndrome patients treated with antiviral agents: the Cremona experience. J Thromb Haemost. 2020;18(6):1320‐1323. 10.1111/jth.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data Availability Statement: Data sharing is not applicable to this article as no new data were created or analyzed in this study.


