Summary
This study aimed to evaluate changes in sleep during the COVID‐19 outbreak, and used data‐driven approaches to identify distinct profiles of changes in sleep‐related behaviours. Demographic, behavioural and psychological factors associated with sleep changes were also investigated. An online population survey assessing sleep and mental health was distributed between 3 April and 24 June 2020. Retrospective questions were used to estimate temporal changes from before to during the outbreak. In 5,525 Canadian respondents (67.1% females, 16–95 years old: Mean ± SD = 55.6 ± 16.3 years), wake‐up times were significantly delayed relative to pre‐outbreak estimates (p < .001, = 0.04). Occurrences of clinically meaningful sleep difficulties significantly increased from 36.0% before the outbreak to 50.5% during the outbreak (all p < .001, g ≥ 0.27). Three subgroups with distinct profiles of changes in sleep behaviours were identified: “Reduced Time in Bed”, “Delayed Sleep” and “Extended Time in Bed”. The “Reduced Time in Bed” and “Delayed Sleep” subgroups had more adverse sleep outcomes and psychological changes during the outbreak. The emergence of new sleep difficulties was independently associated with female sex, chronic illnesses, being employed, family responsibilities, earlier wake‐up times, higher stress levels, as well as heavier alcohol use and television exposure. The heterogeneity of sleep changes in response to the pandemic highlights the need for tailored interventions to address sleep problems.
Keywords: chronotype, COVID‐19, mental health, pandemic, sleep
1. INTRODUCTION
Since first appearing in Wuhan, China in December 2019, the coronavirus (COVID‐19) pandemic has caused widespread increases in stress (Salari et al., 2020), a phenomenon likely to influence sleep (Åkerstedt, 2006; Van Reeth et al., 2000). Furthermore, efforts to mitigate the spread of this virus have led to drastic changes in daily life. These factors are likely to affect sleep patterns, a phenomenon that may have serious downstream impacts on physical and mental health. Since the pandemic is a complex multifaceted situation, there is a need to investigate potentially heterogeneous patterns of changes in sleep and how they may relate to the psychological response to the pandemic.
Early COVID‐19 studies from Asia and Europe reported sleep disturbances in up to a third of their samples (Lin et al., 2020; Qiu et al., 2020; Voitsidis et al., 2020). Increases in sleep complaints and hypnotic use compared with population‐based data collected before the pandemic have also been observed (Beck et al., 2020). However, the COVID‐19 pandemic may not affect everyone in the same manner. For example, 15.6% of older adults reported sleeping less than usual following the pandemic, while 27.1% reported sleeping more (Emerson, 2020), suggesting high inter‐individual variability. Some aspects of confinement could improve sleep (Bryson, 2020). Working or attending school from home may result in more flexible schedules, which could possibly relieve part of the social jet lag and sleep deprivation that previously affected some individuals. This may be particularly true for those with a predisposition to later sleep schedules, such as adolescents/younger adults and people with evening chronotypes (Altena et al., 2020). Inter‐individual variability in sleep changes may also be influenced by the degree to which people are engaging in maladaptive coping strategies during the pandemic, including increased consumption of alcohol, cigarettes and hypnotics, as well as more frequent screen time (Beck et al., 2020; Stanton et al., 2020; Sun et al., 2020).
Furthermore, certain aspects of sleep might be affected differentially by this pandemic. For instance, sleep quantity could increase due to more flexible schedules, but sleep quality might deteriorate due to the psychological distress associated with this global crisis (Robillard et al., 2020). Accordingly, early findings from China showed that insomnia symptoms increased despite prolonged time in bed and total sleep time (Li et al., 2020), a finding that aligns with the fact that increased sleep windows can lead to sleep fragmentation and poorer sleep quality (Grandner & Kripke, 2004). There is thus a need to assess potential interactions between different sleep features. Notably, changes in controllable sleep‐related behaviours, such as the time at which one chooses to go to bed, wakes up, and the overall time spent in bed, may lead to changes in sleep quantity and quality. Little is known about the different profiles of sleep changes that may emerge during the pandemic, and their relationship with demographic, behavioural and psychological factors.
The present study aimed to: (a) assess perceived changes in subjective sleep patterns during the COVID‐19 outbreak relative to retrospective pre‐outbreak estimates; (b) identify distinct profiles of changes in sleep‐related behaviours taking place during the outbreak and determine how they relate to sleep outcomes; (c) identify factors associated with sleep changes during the outbreak; and (d) determine if individuals with different profiles of sleep changes have different psychological responses to the outbreak.
2. METHODS
2.1. Study design
An online longitudinal population survey including validated questionnaires and custom‐built questions pertaining to the pandemic was distributed between 3 April and 24 June 2020 via websites, social media, and multiple organizations and hospitals across Canada (See Supporting Information or ClinicalTrials.gov: NCT04369690). The survey was available in both English and French, and was developed and conducted following guidelines from the Checklist for Reporting Results of Internet E‐Surveys (CHERRIES; Eysenbach, 2004). Retrospective questions were used to estimate perceived temporal changes across two time referents: from “before the outbreak” (i.e. in the last month before the outbreak) to “during the outbreak” (i.e. in the 7 days prior to filling out the survey). Electronic informed consent was obtained from each participant. This study was approved by the Clinical Trials Ontario‐Qualified Research Ethics Board (Protocol #2131).
2.2. Participants
The following exclusion criteria were used for the current report: younger than 16 years old; shift worker; travelled to a different time zone in the last 30 days; located outside of Canada; and missing data for the main study outcomes.
2.3. Measures
2.3.1. Sleep
Respondents completed the Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989) to characterize sleep behaviour profiles (bed and wake‐up times, and the time spent in bed), sleep‐onset latency, total sleep time, sleep efficiency and global subjective sleep quality. The Reduced Morningness−Eveningness Questionnaire (rMEQ; Adan & Almirall, 1991) was used to estimate chronotypes. Difficulties pertaining to sleep initiation, sleep maintenance and early morning awakenings were rated on the first three items of the Quick Inventory of Depressive Symptomatology (QIDS‐SR16; Rush et al., 2003; Soehner et al., 2014). Clinically meaningful sleep difficulties were identified from these QIDS‐SR16 items based on the following thresholds: a score of at least two on the first item (i.e. reflecting sleep initiation difficulties: “I take at least 30 min to fall asleep, more than half the time”), a score of 3 on the second and third items (i.e. reflecting sleep maintenance difficulties and early morning awakenings, respectively: “I awaken more than once a night and stay awake for 20 min or more, more than half the time” and “I awaken at least 1 hour before I need to, and can't go back to sleep”).
To characterize the discrepancy between behavioural sleep schedules and circadian preferences, a circadian preference misalignment index was calculated as the time difference between the sleep midpoint based on the actual bed and wake‐up times reported on the PSQI, and the sleep midpoint based on preferred bed and wake‐up times reported on the rMEQ. Positive values on this index indicate that the actual sleep schedule is later than the preferred sleep schedule, whereas negative values indicate that the actual sleep schedule is earlier than the preferred sleep schedule.
2.3.2. Mental health
In addition to the QIDS‐SR16 which was also used to assess depression symptoms, respondents assessed stress levels on the 10‐item version of the Perceived Stress Scale (PSS10; Cohen & Williamson, 1988), and anxiety symptoms on the Generalized Anxiety Disorder 7 (GAD‐7; Spitzer et al., 2006; see Supporting Information). For analyses pertaining to mental health, QIDS‐SR16 scores were recalculated, while discarding the first three items (i.e. the sleep items) to avoid circularity.
2.3.3. Minimal clinically important difference between the two time referents
For the PSQI and all mental health metrics, occurrences of minimal clinically important differences between the pre‐outbreak and outbreak time referents were calculated based on previously established thresholds: difference of at least three points on the PSQI (Hughes et al., 2009); 28.0% change on the PSS (Eskildsen et al., 2015); difference of at least four points on the GAD‐7 (Toussaint et al., 2020); and a 28.5% change on the QIDS‐SR16 (Masson & Tejani, 2013).
2.4. Statistical analyses
To address the first study aim, analyses of covariance with one repeated measure (pre‐outbreak versus outbreak) were used to assess changes in subjective sleep parameters, while controlling for age, sex and the time elapsed since the pandemic declaration by the World Health Organization (WHO). Occurrences of minimal clinically important differences on the PSQI were reported for the overall sample, and McNemar's tests were used to compare the proportions of individuals who had new clinically meaningful sleep difficulties during the outbreak relative to pre‐outbreak estimates.
To address the second aim, K‐means cluster analysis was used to identify distinct profiles of changes in sleep behaviours taking place during the pandemic (i.e. change scores calculated as outbreak minus pre‐outbreak values), based on three sleep parameters derived from the PSQI: bedtime, time in bed and wake‐up time. The NbClust package in R was used to determine the optimal number of clusters (Charrad et al., 2014). To validate the resulting clusters, mixed ANCOVAs with one repeated measure (two time referents: pre‐outbreak versus outbreak) and one independent factor (cluster subgroups) controlling for the time elapsed since the pandemic declaration were run on the variables on which the clustering was based. To determine how the resulting behavioural change subgroups identified from cluster analyses were affected by changes in sleep outcomes during the outbreak, these subgroups were compared using mixed ANCOVAs with one repeated measure (two time referents) and one independent factor (behavioural change subgroups) while controlling for the time elapsed since the pandemic declaration on the following variables: sleep‐onset latency, total sleep time, sleep efficiency, total PSQI score, and the circadian preference misalignment index. The proportion of individuals with new clinically meaningful sleep difficulties and increased medication use were compared across these subgroups with Chi‐squared analyses.
To address the third aim, a multivariate logistic regression assessed how the emergence of new clinically meaningful sleep difficulties relates to: the time elapsed since the start of the pandemic, demographic factors (i.e. age, sex and current chronic illnesses), initial sleep/circadian profile before the outbreak (self‐reported diagnoses of sleep disorders, initial level of sleep disturbances on the PSQI, chronotype), changes in bedtime and wake‐up time since the outbreak relative to pre‐outbreak estimates, current stress levels (PSS), and social and behavioural factors known to influence sleep [i.e. occupational status, family responsibilities (having children or being the primary care giver of a person with a disability or chronic illness), living with others versus alone, alcohol consumption, and the time spent doing physical activity or watching television]. Mann–Whitney U test and Chi‐squared tests were used to compare behavioural change subgroups in terms of demographic factors, initial sleep profile before the outbreak, chronotypes, and social and behavioural factors known to influence sleep.
To address the fourth aim, Chi‐squared tests were used to compare the proportion of minimal clinically important worsening on the PSS, GAD‐7 and QIDS‐SR16 between the behavioural change subgroups.
For Chi‐squared analyses, Cramer's V was used as an effect size, with 0.10, 0.30 and 0.50 as the thresholds for small, medium and large effect sizes, respectively (Kim, 2017). For all other analyses, partial eta‐squared () was used to determine effect sizes with the following thresholds: > 0.02 (small), > 0.13 (medium) and > 0.26 (large; Cohen, 1988). For all analyses, given the relatively large sample size and the risk of artificial p‐value deflation (Lin et al., 2013), only results with both a p‐value < 0.050 and an effect size above the following threshold (i.e. ≥ 0.02, V ≥ 0.01, B ≥ 0.01 or Cogen's g ≥ 0.05) were interpreted as significant.
3. RESULTS
3.1. Global changes in subjective sleep parameters, sleep difficulties and sleeping medication use
The study population included 5,525 respondents between 16 and 95 years of age (mean ± SD: 55.6 ± 16.3 years old) with 67.1% (3,705) females and a median time elapsed since the pandemic declaration of 62 days (IQR: 12). Further sample characteristics are presented in Table 1.
TABLE 1.
Characteristics of the study population at the time of the survey completion
| n | %Missing | Mean ± SD or % (frequency) | |
|---|---|---|---|
| General demographics | |||
| Time elapsed since the outbreak (days) | 5,525 | 0.0 | 56.9 ± 13.4 |
| Age (years) | 5,525 | 0.0 | 54.6 ± 16.3 |
| Biological sex (Females) | 5,522 | 0.1 | 67.1 (3,705) |
| Ethnicity (Caucasian) | 5,312 | 3.9 | 88.3 (4,692) |
| Education | 5,525 | 0.0 | |
| University | 63.9 (3,531) | ||
| College | 22.0 (1,218) | ||
| No college | 14.0 (776) | ||
| Family responsibilities | |||
| Has underage children | 5,524 | < 0.1 | 64.2 (3,546) |
| Primary caregiver | 5,309 | 3.9 | 7.7 (408) |
| Socioeconomic, occupational and living situation | |||
| Total family income | 5,160 | 6.6 | |
| < $30K | 7.0 (360) | ||
| $30k–$100K | 42.6 (2,199) | ||
| > $100K | 50.4 (2,601) | ||
| Employed | 5,524 | < 0.1 | 51.1 (2,825) |
| Working from homea | 2,631 | 2.5 | 69.4 (1,827) |
| Living with others | 5,302 | 4.0 | 76.1 (4,207) |
| Health | |||
| Alcohol consumption (≥ 7 drinks per week) | 5,525 | 0.0 | 28.4 (1,567) |
| Chronic illnessb | 5,278 | 4.5 | 68.8 (3,631) |
| Mental disorder diagnosis | 5,511 | 0.3 | 29.2 (1,610) |
| Sleep | |||
| Any sleep disorder diagnosis | 5,525 | 0.0 | 21.6 (1,192) |
| Insomnia | 5,525 | 0.0 | 6.1 (338) |
| Sleep‐related breathing disorder | 5,525 | 0.0 | 13.4 (740) |
| Restless leg syndrome | 5,525 | 0.0 | 4.8 (265) |
| Nightmare disorder | 5,525 | 0.0 | 0.7 (39) |
| Circadian disorder | 5,525 | 0.0 | 0.7 (36) |
| Hypersomnia | 5,525 | 0.0 | 0.7 (39) |
| Chronotype | 4,723 | 14.5 | |
| Morning type | 41.5 (1,958) | ||
| Neither type | 46.1 (2,179) | ||
| Evening type | 12.4 (586) | ||
| Medication use | |||
| Sleeping aids (prescribed or over the counter) | 5,396 | 2.3 | 27.3 (1,474) |
| Antidepressant | 4,879 | 11.7 | 22.1 (1,078) |
| Anxiolytics and/or benzodiazepines | 4,879 | 11.7 | 9.2 (447) |
Characteristics of the survey responders regarding general demographics, socioeconomic, occupational (athe question of whether one was working from home was asked only to those who had stated that they were actively working), living situation, health (be.g. hypertension, diabetes, arthritis), sleep and medication use at the time of the survey completion.
SD, standard deviation.
Across the study population, there was considerable inter‐individual variability in subjective sleep parameters before and during the outbreak (Table 2). On average, after controlling for age, sex and the time elapsed since the start of the outbreak, there was a significant 28‐min delay in wake‐up times relative to pre‐outbreak estimates (F 1,5,274 = 209.4, p < .001, = 0.04). No other difference met the adjusted significance thresholds. Of the entire sample, 5.8% (n = 292) underwent a minimal clinically important improvement on the PSQI, and 17.5% (n = 874) underwent a minimal clinically important worsening.
TABLE 2.
Estimated changes in sleep parameters during the outbreak
| n | %Missing | Pre‐outbreak | Outbreak | F | p |
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||||
| Sleep‐onset latency (min) | 5,272 | 4.6 | 24.3 | 23.7 | 30.1 | 28.7 | 52.3 | < .001 | 0.01 | |
| Bedtime (clock time) | 5,286 | 4.3 | 21:05 | 5:14 | 20:05 | 6:48 | 29.4 | < .001 | 0.01 | |
| Wake‐up time (clock time) | 5,278 | 4.5 | 7:02 | 1:31 | 7:30 | 1:45 | 209.5 | < .001 | 0.04 | |
| Time in bed (hr) | 5,265 | 4.7 | 8.4 | 1.3 | 8.5 | 1.4 | 28 | < .001 | 0.01 | |
| Total sleep time (hr) | 5,453 | 1.3 | 7.3 | 1.2 | 7.2 | 1.5 | 13.3 | < .001 | < 0.01 | |
| Sleep efficiency (%) | 5,206 | 5.8 | 88.1 | 14.0 | 85.7 | 16.0 | 2.3 | .127 | < 0.01 | |
| PSQI total score (0–21) | 4,996 | 9.6 | 6.11 | 3.41 | 6.71 | 3.96 | 10.7 | .001 | < 0.01 | |
Repeated‐measures ANCOVAs comparing sleep parameters from pre‐outbreak to outbreak estimates while controlling for age, sex and the time elapsed since the pandemic declaration by the World Health Organization.
, partial eta‐squared; PSQI, Pittsburg Sleep Quality Index; SD, standard deviation.
During the outbreak, there was a significant increase in the emergence of clinically meaningful sleep difficulties pertaining to sleep initiation, sleep maintenance and early morning awakenings (all p < .001, Cohen's g ≥ 0.27; Figure 1). The proportion of individuals endorsing any type of sleep difficulties increased from 36.0% (n = 1,988) before the outbreak to 50.5% (n = 2,750) during the outbreak.
FIGURE 1.
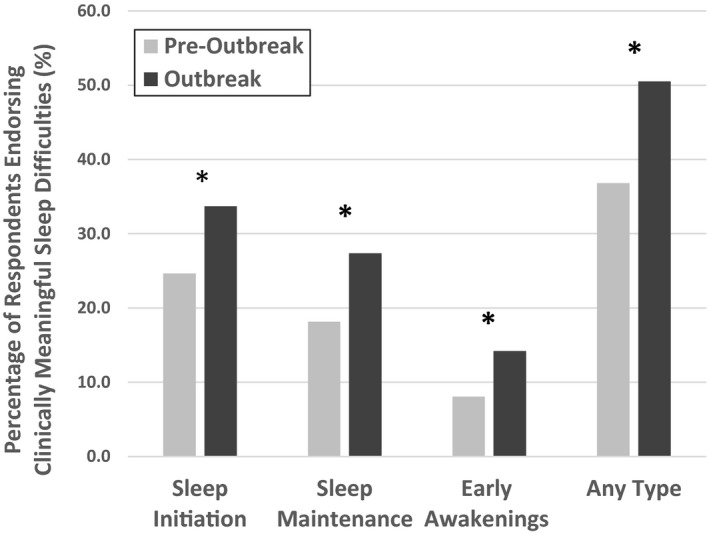
Changes in clinically meaningful sleep difficulties. Percentages of respondents endorsing clinically meaningful difficulties with sleep initiation, sleep maintenance, early awakening, or any type of sleep difficulties from pre‐outbreak to outbreak estimates. *p < .001, Cohen'sg ≥ 0.27 from McNemar's tests
Across the entire sample, 8.0% (n = 433) of respondents reported an increase in the frequency of sleeping medication use (prescribed or over the counter) during the outbreak compared to before the outbreak.
3.2. Distinct profiles of changes in sleep behaviours
3.2.1. Cluster validation
When searching for subgroups with consistent changes in sleep behaviours, a 3‐cluster solution was found. After controlling for the time elapsed since the start of the outbreak, significant time by clusters interactions were found for all variables on which the cluster analysis was based: bedtime (F 2,5,256 = 2,209.2, p < .001, = 0.46), wake‐up time (F 2,5,256 = 2,609.7, p < .001, = 0.48), and time in bed (F 2,5,256 = 1,699.2, p < .001, = 0.39; Figure 2). One of the clusters was characterized by no significant change in bedtime and significantly later wake‐up times (p < .001, = 0.02) during the outbreak compared to pre‐outbreak estimates, leading to a lengthening of time in bed (p = .001, = 0.03; “Extended Time in Bed” subgroup; n = 3,515). Another cluster had later bedtimes (p <.001, = 0.03) and earlier wake‐up times (p = .001, = 0.02), leading to a shorter time in bed (p <.001, = 0.08; “Reduced Time in Bed” subgroup; n = 686). The last cluster had later bedtimes (p <.001, = 0.15) and later wake‐up times (p <.001, = 0.21), with a small lengthening of time in bed (p <.001, = 0.02; “Delayed Sleep” subgroup; n = 1,059).
FIGURE 2.
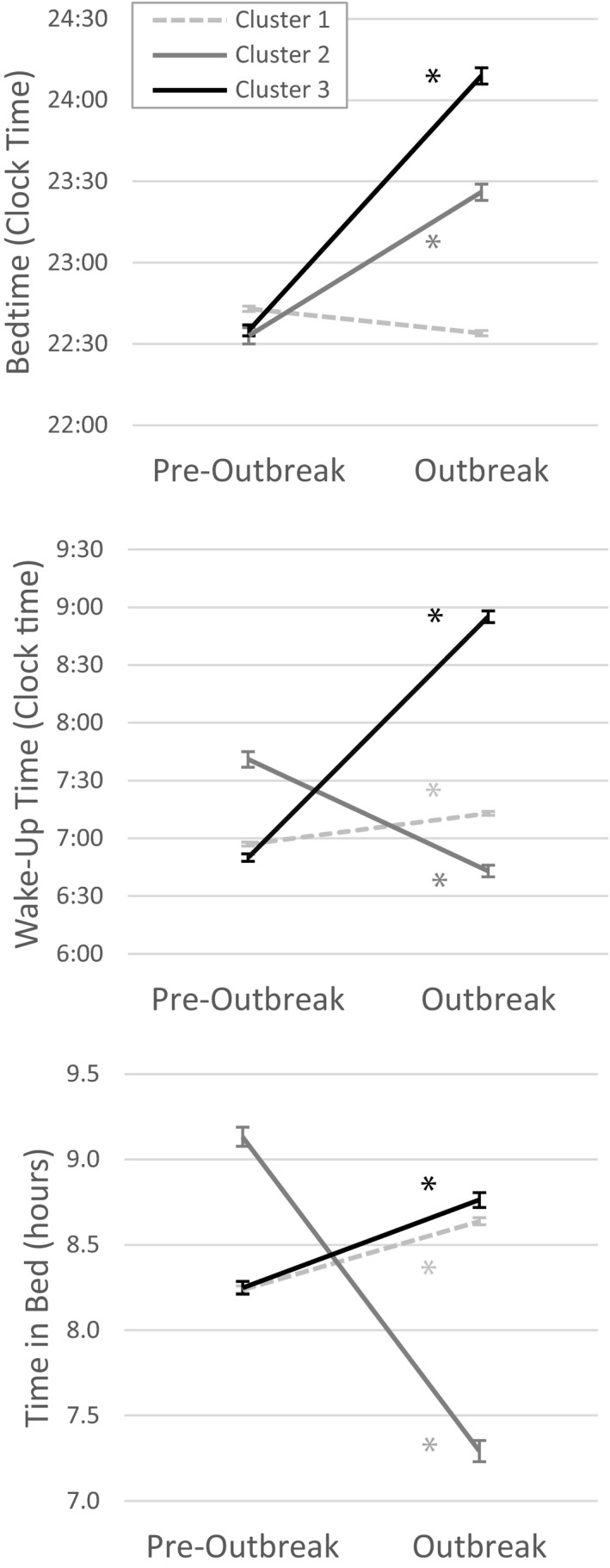
Cluster validation to identify subgroups with distinct profiles of changes in sleep behaviours. Validation of the cluster solution: pre‐outbreak to outbreak changes in the sleep behaviour variables included in the cluster analysis (bedtime [lower panel], wake‐up time [middle panel] and time in bed [lower panel]) across the three cluster groups. Error bars indicate the standard error of the mean. Cluster 1: “Extended TiB (Time in Bed)”; Cluster 2: “Reduced TiB”; Cluster 3: “Delayed Sleep” (*p ≤ .001, ≥ 0.02)
3.2.2. Changes in sleep outcomes across the behavioural change subgroups during the pandemic
Significant time by behavioural change subgroup interactions were found for sleep‐onset latency (F 2,5,228 = 109.3, p < .001, = 0.04), total sleep time (F 2,5,197 = 297.5, p < .001, = 0.10), sleep efficiency (F 2,5,197 = 167.8, p < .001, = 0.06), total PSQI scores (F 2,2,598 = 21.2, p < .001, = 0.02) and the circadian preference misalignment index (F 2,5,005 = 2,848.6, p < .001, = 0.53; Figure 3). From pre‐outbreak estimates to current states during the pandemic, the “Extended Time in Bed” subgroup had no significant change in any of these sleep outcomes (p ≥ .001, < 0.01). The “Reduced Time in Bed” subgroup had a significant shortening of total sleep time (p = .001, = 0.02) and no significant difference in any other sleep outcomes (p ≥ .010, ≤ 0.01). The “Delayed Sleep” subgroup had a significant lengthening in sleep‐onset latency, and no significant difference in total sleep time, sleep efficiency or PSQI total scores (p > .021, < 0.01). The “Delayed Sleep” subgroup also had a large increase in the circadian preference misalignment index (p < .001, = 0.24). Specifically, before the outbreak, their actual sleep schedules were earlier than their preferred schedules but, during the outbreak, their actual sleep schedules shifted later than their preferred schedules.
FIGURE 3.
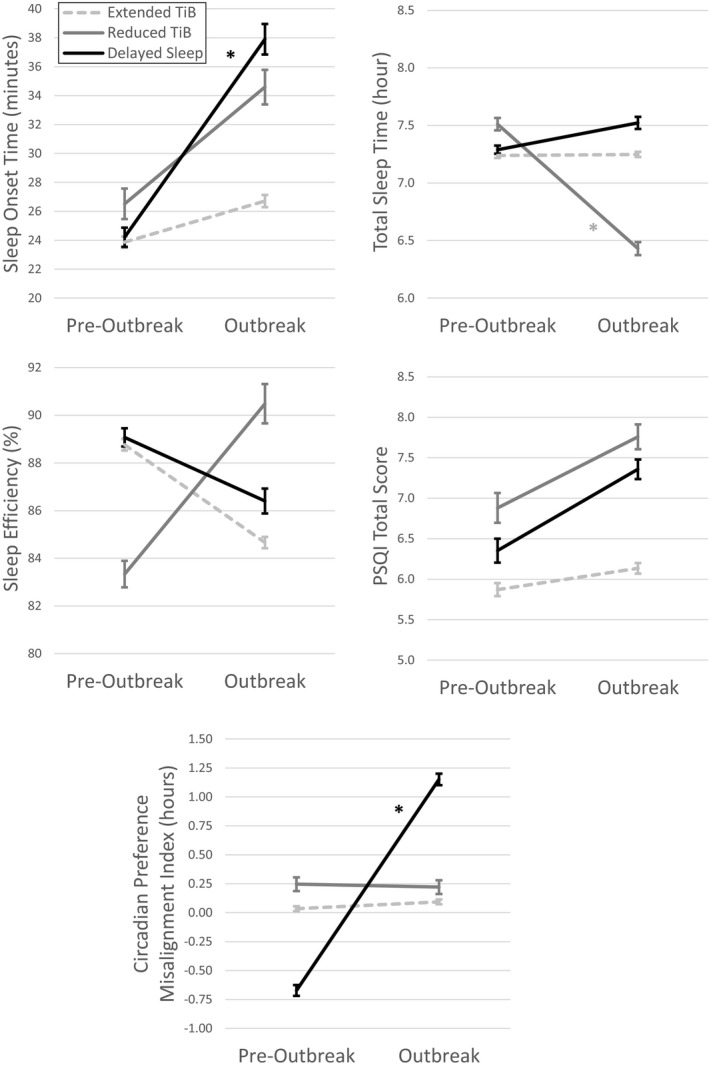
Pre‐outbreak to outbreak changes in sleep‐onset time, total sleep time, sleep efficiency, PSQI total score and the circadian preference misalignment index in each subgroup with distinct sleep behaviour profiles. Error bars indicate the standard error of the mean. TiB, time in bed; PSQI, Pittsburg Sleep Quality Index (*p < .05 and > 0.02)
The proportion of individuals with new clinically meaningful sleep difficulties was found to differ significantly between behavioural change subgroups. These differences were observed for sleep initiations difficulties [Chi‐squared (2) = 159.3, p < .001, V = 0.12], sleep maintenance difficulties [Chi‐squared (2) = 82.2, p < .001, V = 0.09], and early morning awakenings [Chi‐squared (2) = 226.4, p < .001, V = 0.15; Figure 4]. Specifically, the highest occurrences of symptoms suggestive of sleep initiation difficulties were found in the “Delayed Sleep” subgroup. The highest occurrences of worsening in sleep maintenance and morning awakenings were reported in the “Reduced Time in Bed” subgroup.
FIGURE 4.
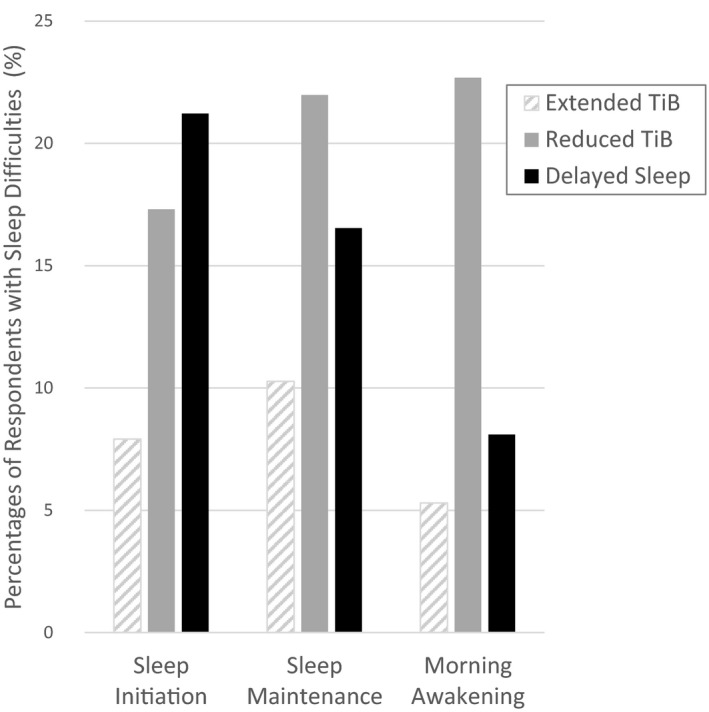
Proportions of respondents reporting new clinically meaningful difficulties with sleep initiation, sleep maintenance and morning awakening during the outbreak relative to pre‐outbreak estimates in subgroups with distinct profiles of sleep behaviours. TiB: time in bed. Chi‐squared (2) > 82.2, p < .001, V > 0.09
Within the “Reduced Time in Bed” and “Delayed Sleep” subgroups, 10.6% of respondents reported an increase in sleeping medication use, compared with 6.6% in the “Extended Time in Bed” subgroup. However, this difference did not reach the adjusted significance thresholds (Chi‐squared [2] = 24.71, p < .001, V = 0.05).
3.3. Factors associated with sleep changes
A multivariate logistic regression model estimating the emergence of new clinically meaningful sleep difficulties during the outbreak explained 19.5% (Nagelkerke R 2) of the variance. The following factors were found to be independently associated with the emergence of clinically meaningful sleep difficulties after controlling for covariates (Table 3): being female, being employed, having family responsibilities, having a chronic illness, lower level of sleep disturbances before the outbreak, waking up early, higher stress levels, consuming more than six alcoholic drinks per week, and spending more than 30 min watching television per week.
TABLE 3.
Factors associated with the emergence of clinically meaningful sleep difficulties identified using the multivariate logistic regression model
| B | SE | Exp(B) | 95% CI | p | ||
|---|---|---|---|---|---|---|
| LL | UL | |||||
| Time elapsed since the outbreak (per 7 days) | −0.006 | 0.003 | 0.994 | 0.988 | 1.001 | .079 |
| Demographic factors | ||||||
| Age (per 10 years) | −0.002 | 0.004 | 0.998 | 0.990 | 1.006 | .602 |
| Male sex (versus female) | −0.525 | 0.107 | 0.592 | 0.480 | 0.729 | < .001 |
| Current chronic illnesses (versus none) | 0.224 | 0.103 | 1.251 | 1.022 | 1.532 | .030 |
| Sleep/circadian profile before the outbreak | ||||||
| Diagnosis of a sleep disorder (versus none) | 0.000 | 0.114 | 1.000 | 0.800 | 1.251 | .997 |
| Pre‐outbreak PSQI (per unit on a 0‐21 scale ) | −0.159 | 0.017 | 0.853 | 0.826 | 0.882 | < .001 |
| rMEQ (scale from 4 to 26) | −0.020 | 0.015 | 0.980 | 0.952 | 1.009 | .167 |
| Changes in controllable sleep behaviours | ||||||
| Bedtime (per hour) | 0.020 | 0.037 | 1.020 | 0.948 | 1.098 | .589 |
| Wake‐up time (per hour) | −0.076 | 0.034 | 0.927 | 0.867 | 0.992 | .028 |
| Current stress levels | ||||||
| PSS (per unit on a 0–40 scale) | 0.108 | 0.006 | 1.115 | 1.101 | 1.128 | < .001 |
| Social and behavioural factors | ||||||
| Employed (versus unemployed) | 0.205 | 0.103 | 1.228 | 1.003 | 1.503 | .047 |
| Family responsibilitiesa(versus none) | 0.368 | 0.110 | 1.445 | 1.165 | 1.793 | .001 |
| Living with others (versus alone) | 0.126 | 0.117 | 1.134 | 0.902 | 1.426 | .282 |
| Drinking ≥ 7 drinks per week (versus < 7 drinks per week) | 0.208 | 0.097 | 1.231 | 1.019 | 1.488 | .031 |
| Spent 30 min or less per week (versus more than 30 min): | ||||||
| Exercising | 0.045 | 0.089 | 1.046 | 0.878 | 1.246 | .614 |
| Watching television | −0.339 | 0.150 | 0.713 | 0.531 | 0.956 | .024 |
Coefficient parameters from the logistic regression. B: unstandardized coefficients (calculated per unit for continuous variables, except for the time elapsed since the start of the outbreak, which was calculated for each 7 days, as well as age which was calculated per 10 units). Units (for continuous variables) and reference groups (for categorical variables) are presented in parentheses in the first column.
SE, standard error of B; Exp(B), exponentiation of the B coefficient, i.e. odds ratio; CI, confidence interval of Exp(B); LL, lower limit; UL, upper limit; PSQI, Pittsburg Sleep Quality Index; rMEQ, Reduced Morningness−Eveningness Questionnaire; PSS, Cohen's Perceived Stress Scale.
Family responsibilities: having an underage child or being the primary caregiver of someone with a disability or a chronic illness.
Demographic and behavioural factors in each behavioural change subgroup are presented in the Supporting Information section (Table S1). Compared with the “Extended Time in Bed” subgroup, the “Reduced Time in Bed” and “Delayed Sleep” subgroups had a higher proportion of females, people with mental disorders and people using psychotropic medication. Compared with the two other subgroups, the “Delayed Sleep” subgroup was younger, had higher rates of employment, a higher proportion of respondents working from home, a lower proportion of individuals with family responsibilities, and a slightly shorter time elapsed since the pandemic declaration. There was a progressive decrease in the proportions of morning types and a progressive increase in the proportions of evening types from the “Extended Time in Bed” subgroup to the “Reduced Time in Bed” and the “Delayed Sleep” subgroups (Chi‐squared (4) = 232.0, p < .001, V = 0.11; Figure S1).
3.4. Psychological changes and profiles of sleep changes
Significant differences in the proportion of individuals who had a clinically important worsening in stress (PSS; Chi‐squared = 84.8, p < .001, V = 0.13), anxiety (GAD‐7; Chi‐squared = 186.0, p < .001, V = 0.19) and depression (QIDS‐SR16; Chi‐squared = 247.9, p < .001, V = 0.22) from pre‐outbreak to during the outbreak were found across the behavioural change subgroups (Figure 5). Specifically, compared with the “Extended Time in Bed” subgroup, both the “Reduced Time in Bed” and “Delayed Sleep” subgroups had higher proportions of clinically important worsening in stress (PSS; p < .001, V > 0.11), anxiety (GAD‐7; p < .001, V > 0.16) and depression (QIDS‐SR16; p < .001, V > 0.15). No other difference in minimally meaningful psychological worsening between subgroups reached the significance thresholds.
FIGURE 5.
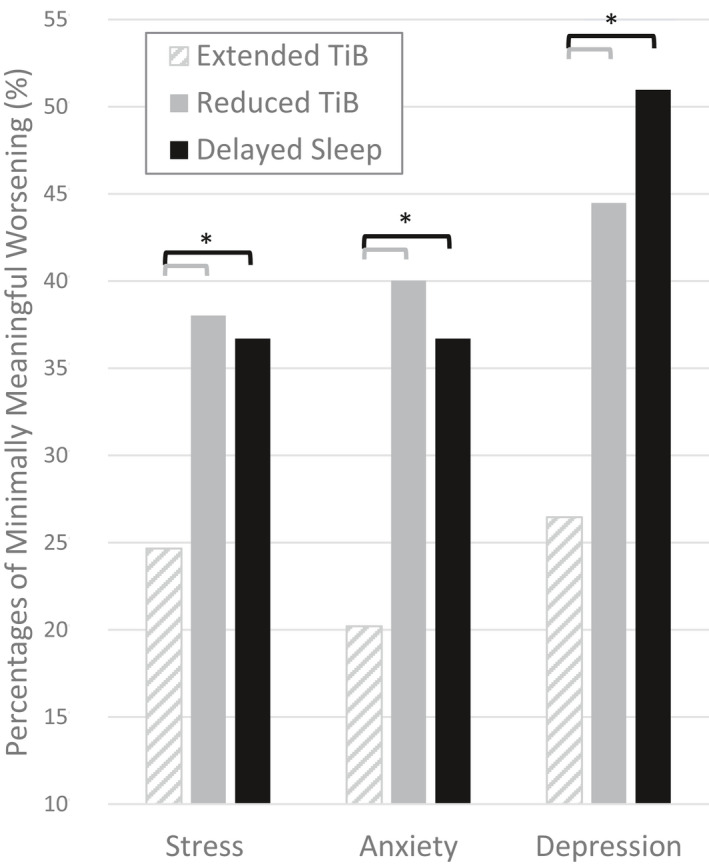
Proportions of respondents with minimally meaningful worsening of symptoms of stress, anxiety and depression from pre‐outbreak to during the outbreak in each subgroup with distinct sleep behaviour profiles. TiB: time in bed; Stress: Perceived Stress Scale; Anxiety: Generalized Anxiety Disorder 7; Depression: Quick Inventory of Depressive Symptomatology recalculated without the items pertaining to sleep (*p < .001, V > 0.11)
4. DISCUSSION
In a population sample of over 5,000 Canadians, we observed variable changes in subjective sleep and sleeping medication use during the COVID‐19 pandemic relative to pre‐outbreak estimates, and identified several factors independently associated with these changes. Using a data‐driven approach, we identified three distinct profiles of changes in sleep‐related behaviours, and observed that these profiles are linked to different changes in sleep quantity and quality, as well as to different psychological responses to the pandemic. To our knowledge, this is the first report of distinct multidimensional profiles of changes in sleep‐related behaviours in response to a global external stressor affecting multiple aspects of daily life.
Our finding that over half of the study population had clinically meaningful sleep difficulties during the pandemic and that several respondents reported increased use of sleeping medications are consistent with other reports (Li et al., 2020; Lin et al., 2020; Voitsidis et al., 2020). This represents a 15% increase in the proportion of individuals with clinically meaningful sleep difficulties relative to pre‐outbreak estimates. Importantly, our results highlight considerable variability in the profiles of sleep changes taking place during the pandemic, confirming previous comments anticipating that the pandemic may have heterogeneous effects on sleep (Altena et al., 2020; Becker & Gregory, 2020). In the present study, while some individuals reported increased sleep difficulties and worsening in global sleep quality compared with pre‐outbreak estimates, about 6% reported clinically meaningful improvements in global sleep quality. This may notably be influenced by some aspects of confinements such as working from home, which enables a later wake‐up time for some people (Hurley, 2020) and variability in the stress response to the pandemic (Robillard et al., 2020). Cluster analysis identified three distinct subgroups based on changes in controllable sleep behaviours emerging during the outbreak: the “Extended Time in Bed”, the “Reduced Time in Bed” and the “Delayed Sleep” subgroups. The younger age of the “Delayed Sleep” subgroup may align with a recent US survey differentiating sleep habits during the pandemic among generations, with generation Z (18–22 years old) and millennials (23–38 years old) going to bed later than any other generation during confinement (Sleep Standards, 2020). Individuals from the “Delayed Sleep” subgroup were also more likely to be working from home and less likely to have family responsibilities, suggesting that they may have had more flexibility to change their sleep schedule.
Importantly, the different profiles of changes in behavioural sleep parameters taking place during the pandemic may contribute to the heterogeneity of changes in sleep quantity and quality. Individuals who actively shortened their sleep window by going to bed later and waking up earlier had shorter sleep durations, and more sleep maintenance and early morning awakenings difficulties. Those who delayed their sleep−wake cycle had a complete reversal of the misalignment between their circadian preference and their actual sleep schedules (with their actual sleep schedules occurring later than their preferred sleep schedules), and this was accompanied by prolonged sleep‐onset latency and higher occurrences of sleep initiation difficulties. While confinement may have enabled better alignment of sleep schedules with circadian preference for some individuals, others may have undergone an excessive delay in sleep schedules .
These behavioural change subgroups also differed in terms of chronotypes, with a progressive shift towards eveningness from the “Extended Time in Bed” cluster to the “Reduced Time in Bed” and “Delayed Sleep” clusters. This may indicate that when some of the socio‐occupational constraints of daily life lift off in the context of a global stressor, evening types may revert to bedtime schedules that are better aligned with their circadian preference, but that the actual time at which they manage to fall asleep may be further delayed. While some of these individuals are able to sleep in later (i.e. “Delayed Sleep” cluster), others seem unable to extend their wake‐up time, thereby leading to sleep curtailing (i.e. “Reduced Time in Bed”).
These distinct profiles of changes in sleep behaviours were associated with different psychological responses to the pandemic. The “Reduced Time in Bed” and “Delayed Sleep” subgroups had higher proportions of people with clinically meaningful worsening in stress, anxiety and depressive symptoms. Considering the bi‐directional relationship between sleep and mental health (Kaneita et al., 2009), more adverse psychological reactions to the outbreak may actively worsen sleep, and poorer sleep may in turn worsen stress, anxiety and depression. If left unattended, these sleep disturbances may thus actively worsen mental health.
Similar to other studies, we confirmed that females were more vulnerable to behavioural changes leading to reduced sleep duration and prolonged sleep latency, and to clinically meaningful sleep difficulties during the COVID‐19 outbreak (Losada‐Baltar et al., 2020; Qiu et al., 2020). This may relate to the fact that females are more prone to both stress‐related disorders (Li & Graham, 2017) and insomnia (Hohagen et al., 1993). In addition, we observed that some maladaptive coping strategies, such as elevated alcohol consumption and spending more time watching television, were independently associated with worsening in clinically meaningful sleep difficulties. The pandemic may impose higher stress on individuals who need to adjust to work‐related changes imposed by confinement measures, those with family responsibilities, those needing to maintain early wake‐up times, and those struggling with chronic medical conditions. This may explain in part why, in addition to the direct relationship between current stress levels and sleep problems, these factors were associated with new sleep difficulties. Of note, behavioural changes leading to reduced sleep duration and prolonged sleep latency were also more prevalent in individuals with mental disorders and in those taking psychotropic medications, many of which are known to alter sleep (Riemann & Nissen, 2011). In line with these findings, there have been previous indications that individuals with mental disorders may be especially prone to new sleep difficulties due to the COVID‐19 outbreak (Li et al., 2020). Altogether, these factors represent vulnerability indicators that could help identify people with the most pressing needs for sleep interventions during and following the pandemic.
This study has several limitations. Firstly, it was based on subjective sleep measures. Data collection spanned over nearly 3 months starting in early April (although statistical models were adjusted for time differences). In mid‐March 2020, several Canadian provinces declared the state of emergency, and federal restrictions were imposed on crossing Canadian borders as infected cases were on the rise. By mid‐April, Canada reached a peak of 2,000 COVID‐19 cases emerging each day, and around the end of June, numbers dropped to about 300 cases per day (Government of Canada, 2020). Many confinement measures persisted over that period in Canada, but other aspects of the pandemic also likely to influence sleep may have changed during that period. There were slight differences between the behavioural change subgroups for the time elapsed since the pandemic declaration, which may suggest that changes in sleep behaviours may evolve dynamically across this period of turmoil, a phenomenon that should be assessed by longitudinal studies. Another limitation pertains to the fact that retrospective reports to estimate sleep prior to the outbreak may introduce recall bias, but recent evidence supports the use of retrospective sleep assessments when prospective assessment is not feasible (Dietch et al., 2019). The sample comprised a high proportion of white, middle‐aged adults females, with high levels of education, employment and income, as well as a high proportion of morning types. As some of these features may be linked to better resources to cope with the pandemic, thereby lowering the degree of psychological and sleep difficulties relative to other parts of the population, this limits the generalizability of these results. It is also important to note that negative outcomes reported herein may have arisen not only in response to the pandemic, but also in relation to several other concurrent social stressors (e.g. police violence, international human rights breeches, and political turmoil) that occurred during the period of data collection. Furthermore, other factors that could influence sleep, such as neurological conditions, were not accounted for in the analyses.
Overall, the current findings highlight the need to rapidly build and deploy adapted interventions to address distinct profiles of sleep problems that may have emerged during the pandemic. These may range from sleep health education to cognitive‐behavioural therapy for insomnia in those with prominent sleep difficulties, or chronobiotic therapies in those with delayed bedtimes (e.g. morning bright light exposure and evening exogenous melatonin intake). It is also foreseeable that there may be an increase in individuals who may require professional guidance to taper off from sleeping medications started or increased during the pandemic. While some of these sleep problems may be transient, it should be a high priority to ensure they do not evolve into chronic sleep disorders.
The sleep profiles identified in this study, as well as their demographic and behavioural correlates, may help identify those at risk of specific sleep difficulties and develop tailored interventions accounting for inter‐individual differences. Considerable efforts have been made by the scientific and clinical sleep community during the COVID‐19 pandemic to stress the importance of sleep health to better face the challenges posed by the pandemic and provide information about good sleep habits (Li et al., 2020; www.sleeponitcanada.ca/covid‐19‐2/). The fact that more than half of survey respondents endorsed symptoms suggestive of clinically meaningful sleep difficulties during the outbreak reinforces the need to continue increasing awareness in the general public, and to increase access to large‐scale accessible evidence‐based sleep interventions (Altena et al., 2020; Morin & Carrier, 2020).
Conflict of interest
No conflict of interest declared.
Author contributions
All co‐authors were involved in the following: interpretation of data, revising the manuscript critically for the accuracy and important intellectual content, and final approval of the version to be published. All co‐authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. RR, MP, ES, MS, RG, TK, JE and LQ were additionally involved in study conception and design. RR, TK, JE and LQ were involved in the participants' recruitment as site primary investigators. RR conducted the analyses. RR, TK, EL, KD and AM wrote the initial manuscript draft.
Supporting information
Suppinfo
ACKNOWLEDGEMENTS
The authors wish to thank all the participants who gave their time to fill out this extensive survey during this difficult period. The authors also extend their gratitude to the individuals who kindly provided their comments on the survey content and format during the development stage, to Rachel Théoret, Samantha Kenny, Rebecca Burdayron and Christopher Kalogeropoulos for their help with preparing some documents for ethics application, to the ethics boards who rapidly and diligently provided insights on this project to enable a timely launch, to the organizations who helped circulate the survey in their networks, including the Canadian sleep promotion campaign Sleep On It Canada! (sleeponitcanada.ca) and Ottawa Public Health, and to NIVA inc, for their advice on distribution strategies. The authors thank the Clinical Investigation Unit at the Ottawa Hospital Research Institute, the University of Ottawa Heart Institute, the Royal Ottawa Mental Health Centre, and the Centre for Addiction and Mental Health for assistance with recruiting participants.
Robillard R, Dion K, Pennestri M‐H, et al. Profiles of sleep changes during the COVID‐19 pandemic: Demographic, behavioural and psychological factors. J Sleep Res.2021;30:e13231. 10.1111/jsr.13231
DATA AVAILABILITY STATEMENT
Proposals to access data from this study can be submitted to the corresponding author and may be made available upon data sharing agreement.
REFERENCES
- Adan, A. , & Almirall, H. (1991). Horne & Östberg morningness‐eveningness questionnaire: A reduced scale. Personality and Individual Differences, 12(3), 241–253. 10.1016/0191-8869(91)90110-W [DOI] [Google Scholar]
- Åkerstedt, T. (2006). Psychosocial stress and impaired sleep. Scandinavian Journal of Work, Environment and Health, 32(6), 493–501. 10.5271/sjweh.1054 [DOI] [PubMed] [Google Scholar]
- Altena, E. , Baglioni, C. , Espie, C. A. , Ellis, J. , Gavriloff, D. , Holzinger, B. , Schlarb, A. , Frase, L. , Jernelöv, S. , & Riemann, D. (2020). Dealing with sleep problems during home confinement due to the COVID‐19 outbreak: Practical recommendations from a task force of the European CBT‐I Academy. Journal of Sleep Research, 29(4), e13052, 10.1111/jsr.13052 [DOI] [PubMed] [Google Scholar]
- Beck, F. , Léger, D. , Fressard, L. , Peretti‐Watel, P. , & Verger, P. (2020). Covid‐19 health crisis and lockdown associated with high level of sleep complaints and hypnotic uptake at the population level. Journal of Sleep Research, e13119, 10.1111/jsr.13119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, S. P. , & Gregory, A. M. (2020). Editorial Perspective: Perils and promise for child and adolescent sleep and associated psychopathology during the COVID‐19 pandemic. Journal of Child Psychology and Psychiatry and Allied Disciplines, 61(7), 757–759. 10.1111/jcpp.13278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson, W. J. (2020). Circadian rhythm sleep‐wake disorders and the COVID‐19 pandemic. Journal of Clinical Sleep Medicine, 16(8), 1423. 10.5664/jcsm.8540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse, D. J. , Reynolds, C. F. , Monk, T. H. , Berman, S. R. , & Kupfer, D. J. (1989). The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Charrad, M. , Ghazzali, N. , Boiteau, V. , & Niknafs, A. (2014). NbClust: An R Package for Determining the best number of clusters in a data set. Journal of Statistical Software, 10.18637/jss.v061.i06 61, 1–36. [DOI] [Google Scholar]
- Cohen, J. (1988). Statistical power analysis for the behavioural sciences (2nd ed.). Lawrence Earlbaum Associates. [Google Scholar]
- Cohen, S. , & Williamson, G. (1988). Perceived stress in a probability sample of the United States. In Spacapam S., & Oskamp S. (Eds.), The social psychology of health. Sage Publications. [Google Scholar]
- Dietch, J. R. , Sethi, K. , Slavish, D. C. , & Taylor, D. J. (2019). Validity of two retrospective questionnaire versions of the Consensus Sleep Diary: The whole week and split week Self‐Assessment of Sleep Surveys. Sleep Medicine, 63, 127–136. 10.1016/j.sleep.2019.05.015 [DOI] [PubMed] [Google Scholar]
- Emerson, K. G. (2020). Coping with being cooped up: Social distancing during COVID‐19 among 60+ in the United States. Revista Panamericana De Salud Publica/Pan American Journal of Public Health, 44, e81. 10.26633/RPSP.2020.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskildsen, A. , Dalgaard, V. L. , Nielsen, K. J. , Andersen, J. H. , Zachariae, R. , Olsen, L. R. , & Christiansen, D. H. (2015). Cross‐cultural adaptation and validation of the danish consensus version of the 10‐item perceived stress scale. Scandinavian Journal of Work, Environment and Health, 41(5), 486–490. 10.5271/sjweh.3510 [DOI] [PubMed] [Google Scholar]
- Eysenbach, G. (2004). Improving the quality of web surveys: The Checklist for Reporting Results of Internet E‐Surveys (CHERRIES). Journal of Medical Internet Research, 6(3), e34. 10.2196/jmir.6.3.e34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Government of Canada . (2020). Epidemiological summary of COVID‐19 cases in Canada ‐ Canada.ca. Retrieved October 9, 2020, from https://health‐infobase.canada.ca/covid‐19/epidemiological‐summary‐covid‐19‐cases.html?stat=num&measure=total#a2
- Grandner, M. A. , & Kripke, D. F. (2004). Self‐reported sleep complaints with long and short sleep: A nationally representative sample. Psychosomatic Medicine, 66(2), 239. 10.1097/01.PSY.0000107881.53228.4D [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohagen, F. , Rink, K. , Käppler, C. , Schramm, E. , Riemann, D. , Weyerer, S. , & Berger, M. (1993). Prevalence and treatment of insomnia in general practice ‐ A longitudinal study. European Archives of Psychiatry and Clinical Neuroscience, 242(6), 329–336. 10.1007/BF02190245 [DOI] [PubMed] [Google Scholar]
- Hughes, C. M. , McCullough, C. A. , Bradbury, I. , Boyde, C. , Hume, D. , Yuan, J. , Quinn, F. , & McDonough, S. M. (2009). Acupuncture and reflexology for insomnia: A feasibility study. Acupuncture in Medicine, 27(4), 163–168. 10.1136/aim.2009.000760 [DOI] [PubMed] [Google Scholar]
- Hurley, D. (2020). Sleep neurologists call it ’COVID‐Somnia’—Increased sleep disturbances linked to the pandemic. Neurology Today, 20(13), 1–26. 10.1097/01.NT.0000694012.58759.9c [DOI] [Google Scholar]
- Kaneita, Y. , Yokoyama, E. , Harano, S. , Tamaki, T. , Suzuki, H. , Munezawa, T. , Nakajima, H. , Asai, T. , & Ohida, T. (2009). Associations between sleep disturbance and mental health status: A longitudinal study of Japanese junior high school students. Sleep Medicine, 10(7), 780–786. 10.1016/j.sleep.2008.06.014 [DOI] [PubMed] [Google Scholar]
- Kim, H.‐Y. (2017). Statistical notes for clinical researchers: Chi‐squared test and Fisher’s exact test. Restorative Dentistry & Endodontics, 42(2), 152–155. 10.5395/rde.2017.42.2.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. H. , & Graham, B. M. (2017). Why are women so vulnerable to anxiety, trauma‐related and stress‐related disorders? The potential role of sex hormones. The Lancet Psychiatry, 4(1), 73–82. 10.1016/S2215-0366(16)30358-3 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Qin, Q. , Sun, Q. , Sanford, L. D. , Vgontzas, A. N. , & Tang, X. (2020). Insomnia and psychological reactions during the COVID‐19 outbreak in China. Journal of Clinical Sleep Medicine, 16(8), 1417–1418. 10.5664/jcsm.8524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, L.‐Y. , Wang, J. , Ou‐yang, X.‐Y. , Miao, Q. , Chen, R. , Liang, F.‐X. , Zhang, Y.‐P. , Tang, Q. , & Wang, T. (2020). The immediate impact of the 2019 novel coronavirus (COVID‐19) outbreak on subjective sleep status. Sleep Medicine, 10.1016/j.sleep.2020.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, M. , Lucas, H. C. , & Shmueli, G. (2013). Too big to fail: Large samples and the p‐value problem. Information Systems Research, 24(4), 906–917. 10.1287/isre.2013.0480 [DOI] [Google Scholar]
- Losada‐Baltar, A. , Jiménez‐Gonzalo, L. , Gallego‐Alberto, L. , Pedroso‐Chaparro, M. D. S. , Fernandes‐Pires, J. , & Márquez‐González, M. (2020). “We’re staying at home”. Association of self‐perceptions of aging, personal and family resources and loneliness with psychological distress during the lock‐down period of COVID‐19. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 10.1093/geronb/gbaa048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson, S. C. , & Tejani, A. M. (2013). Minimum clinically important differences identified for commonly used depression rating scales. Journal of Clinical Epidemiology, 66(7), 805. 10.1016/j.jclinepi.2013.01.010 [DOI] [PubMed] [Google Scholar]
- Morin, C. M. , & Carrier, J. (2020). The acute effects of the COVID‐19 pandemic on insomnia and psychological symptoms. Sleep Medicine, 10.1016/j.sleep.2020.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, J. , Shen, B. , Zhao, M. , Wang, Z. , Xie, B. , & Xu, Y. (2020). A nationwide survey of psychological distress among Chinese people in the COVID‐19 epidemic: Implications and policy recommendations. General . Psychiatry, 33(2), 10.1136/gpsych-2020-100213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann, D. , & Nissen, C. (2011). Sleep and psychotropic drugs. Morin C. & Espie C. (Eds.), In The Oxford Handbook of Sleep and Sleep Disorders (pp. 190–222). Oxford: Oxford University Press. [Google Scholar]
- Robillard, R. , Saad, M. , Edwards, J. D. , Solomonova, E. , Pennestri, M.‐H. , Daros, A. , & Kendzerska, T. (2020). Social, Financial and Psychological Stress during an Emerging Pandemic: Observations from a Population Web‐Based Survey in the acute phase of the COVID‐19 pandemic. MedRxiv, 10.1101/2020.06.29.20142638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush, A. J. , Trivedi, M. H. , Ibrahim, H. M. , Carmody, T. J. , Arnow, B. , Klein, D. N. , Markowitz, J. C. , Ninan, P. T. , Kornstein, S. , Manber, R. , Thase, M. E. , Kocsis, J. H. , & Keller, M. B. (2003). The 16‐item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS‐C), and self‐report (QIDS‐SR): A psychometric evaluation in patients with chronic major depression. Biological Psychiatry, 54(5), 573–583. 10.1016/S0006-3223(02)01866-8 [DOI] [PubMed] [Google Scholar]
- Salari, N. , Hosseinian‐Far, A. , Jalali, R. , Vaisi‐Raygani, A. , Rasoulpoor, S. , Mohammadi, M. , Rasoulpoor, S. , & Khaledi‐Paveh, B. (2020). Prevalence of stress, anxiety, depression among the general population during the COVID‐19 pandemic: A systematic review and meta‐analysis. Globalization and Health, 16(1), 1–11. 10.1186/s12992-020-00589-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleep Standards (2020). Sleep habits post lockdown in the U.S. ‐ New study. Retrieved August 27, 2020, from https://sleepstandards.com/sleep‐habits‐post‐quarantine/#Demographics
- Soehner, A. M. , Kaplan, K. A. , & Harvey, A. G. (2014). Prevalence and clinical correlates of co‐occurring insomnia and hypersomnia symptoms in depression. Journal of Affective Disorders, 167, 93–97. 10.1016/j.jad.2014.05.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer, R. L. , Kroenke, K. , Williams, J. B. W. , & Löwe, B. (2006). A brief measure for assessing generalized anxiety disorder: The GAD‐7. Archives of Internal Medicine, 166(10), 1092–1097. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- Stanton, R. , To, Q. G. , Khalesi, S. , Williams, S. L. , Alley, S. J. , Thwaite, T. L. , Fenning, A. S. , & Vandelanotte, C. (2020). Depression, anxiety and stress during COVID‐19: Associations with changes in physical activity, sleep, tobacco and alcohol use in Australian adults. International Journal of Environmental Research and Public Health, 17(11), 4065. 10.3390/ijerph17114065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y. , Li, Y. , Bao, Y. , Meng, S. , Sun, Y. , Schumann, G. , Kosten, T. , Strang, J. , Lu, L. , & Shi, J. (2020). Brief report: Increased addictive internet and substance use behavior during the COVID‐19 pandemic in China. American Journal on Addictions, 29(4), 268–270. 10.1111/ajad.13066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint, A. , Hüsing, P. , Gumz, A. , Wingenfeld, K. , Härter, M. , Schramm, E. , & Löwe, B. (2020). Sensitivity to change and minimal clinically important difference of the 7‐item Generalized Anxiety Disorder Questionnaire (GAD‐7). Journal of Affective Disorders, 265, 395–401. 10.1016/j.jad.2020.01.032 [DOI] [PubMed] [Google Scholar]
- Van Reeth, O. , Weibel, L. , Spiegel, K. , Leproult, R. , Dugovic, C. , & Maccari, S. (2000). Interactions between stress and sleep: From basic research to clinical situations. Sleep Medicine Reviews, 4(2), 201–220. 10.1053/smrv.1999.0097 [DOI] [Google Scholar]
- Voitsidis, P. , Gliatas, I. , Bairachtari, V. , Papadopoulou, K. , Papageorgiou, G. , Parlapani, E. , Syngelakis, M. , Holeva, V. , & Diakogiannis, I. (2020). Insomnia during the COVID‐19 pandemic in a Greek population. Psychiatry Research, 113076, 10.1016/j.psychres.2020.113076 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppinfo
Data Availability Statement
Proposals to access data from this study can be submitted to the corresponding author and may be made available upon data sharing agreement.


