Abstract
Telogen effluvium (TE) is one of the most common form of hair loss in women. Many triggers have been identified, as stress, drugs, trauma, endocrine disease, nutritional deficiencies, and febrile states. We report three cases of TE occurred after severe Sars‐Cov‐2 infection and provide our clinical management, according to Sars‐Cov‐2 hygiene measures. Only one case report has been found in the literature associating anagen effluvium during severe Sars‐Cov‐2 infection. Other studies reported the exacerbation of a preexisting TE, correlated to the stress of lockdown. In our cases, patients never had a TE diagnosis before and did not report previous evident hair loss. TE can be associated with post severe Sars‐Cov‐2 infection. From our revision of the literature, this is the first case‐series describing TE in post severe Sars‐Cov‐2 patients. Further studies are needed to evaluate the relationship between TE and Sars‐Cov‐2 infection.
Keywords: alopecia, hair disorders, infection‐bacterial/fungal/viral, therapy‐systemic
1. INTRODUCTION
Telogen effluvium (TE) is one of the most common form of hair loss in women and it usually involves less than 50% of hair with diffuse, nonscarring club hair shedding. 1 In the majority of cases, its pathomechanism is characterized by abnormal shift in follicular cycling with diffuse synchronization of hair follicles in the telogen phase, driving to a global increased number of hair follicles predisposed to fall. Sometimes hair fall is dramatic with evident thinning. 2 Important psychological implications are generally associated, in women above all, often showing an anxious habitus, exacerbated by worsening of the hair condition. 3 Acute TE is usually self‐limiting in 6 month, while chronic TE exceeds 6 months. 4 Triggering factors for TE can be various, such as stress, drugs, trauma, endocrine disease, nutritional deficiencies, and febrile states. 2 We report three cases of TE that occurred after severe Sars‐Cov‐2 infection. All the patients were women, worried about their significant hair loss, and showing great concern since they never had TE diagnosis before. From our revision of the literature, only one case‐report 5 shows the association between Sars‐cov‐2 infection and anagen effluvium in a patient with severe respiratory disease.
2. CASE 1
A 62‐year‐old woman was hospitalized in the Sars‐Cov‐2 unit of United Hospital of Ancona for 1 month owing to the acute onset of distress respiratory syndrome characterized by severe dyspnea, fever (39°C), and oxygen saturation (Sp02) of 89%,. Nasopharyngeal swab for Sars‐Cov‐2 RNA research was positive and computed tomography (CT) scan showed bilateral interstitial pneumonia. The patient had previous history of autoimmune thrombocytopenia for 2 years and osteoporosis. She started continuous positive airway pressure (CPAP) therapy for 2 weeks and then switched to a gradual reduction of oxygen administration from 5 L/min to room air. Enoxaparin 6000 IU once a day, ceftriaxone 1 g three times a day, tocilizumab 400 mg only one time, and lopinavir/ritonavir 400/100 mg twice a day were administered, with progressive improvement of general conditions and recovery from viral infection (two Sars‐cov‐2 swab negative 24 hours apart).
The patient came to our dermatological clinic 3 months after hospital discharge for generalized hair loss, started 75 days after the febrile peak. On physical examination, a widespread reduction in hair density was evident (Figure 1A). The reported stress level was 7 on a visual analog scale ranging from 1 to 10. Cell blood count and leukocyte formula, kidney and liver function tests, serum levels of ferritin, vitamin B12, folic acid, magnesium (Mg), iron (Fe), zinc (Zn), copper (Cu), and thyroid stimulating hormone (TSH) were within normal ranges, whereas an autoimmune thrombocytopenia (120 000/mL) was detected, compatible with the patient's chronic disease. She had not any skin manifestations related to Sars‐Cov‐2 infection during the acute phase. The pull test was strongly positive, with club shedding hair, and the modified wash test 6 (MWT) was compatible with TE. Trichoscopy revealed reduction in hair density, and some empty follicles, with integrity of the stem and absence of miniaturized hair (Figure 1B). Diagnosis of acute TE was confirmed, and we reassured the patient her hair would grow back again. Oral supplementation with sulfur amino acid/vit B6 and daily topical application of peptide mimicking hair growth factor lotion were prescribed.
FIGURE 1.
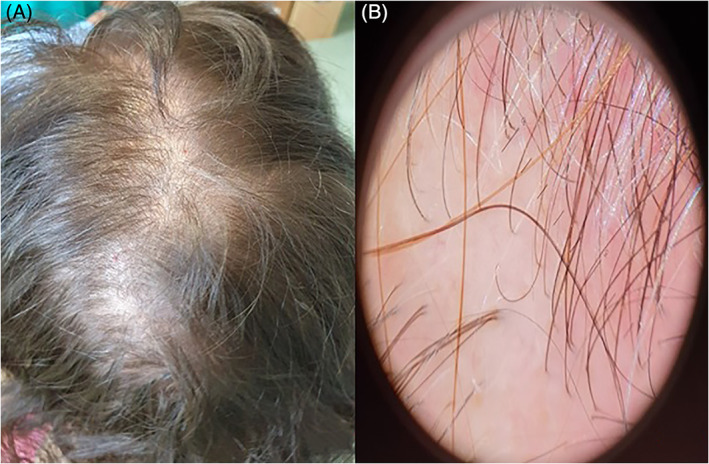
A, widespread reduction in hair density. B, Trichoscopy revealed a reduction in hair density and some empty follicles, with the integrity of the stem and the absence of miniaturized hair
3. CASE 2
A 74‐year‐old woman was hospitalized in the Sars‐Cov‐2 unit of United Hospital of Ancona for 1 month. She complained of the acute onset of moderate dyspnea, SpO2 92%, and feverish peak of 39.2°C. Patient resulted positive for Sars‐Cov‐2 RNA research on nasopharyngeal swab. Chest radiography showed extensive right basal parenchymal thickening. The patient had several metabolic comorbidities including diabetes mellitus, systemic hypertension, and dyslipidemia in treatment with gliclazide 60 mg once a day, acarbose 100 mg twice a day, ezetimibe/simvastatin 10/10 mg once a day, sitagliptin 50 mg once a day.
She was treated with enoxaparin 6000 IU once a day, ceftriaxone 1 g three times a day, oxygen therapy (15 L/min, FiO2 50%), and lopinavir/ritonavir 400/100 mg twice a day, withdrawed 24 hours after first administration for diarrhea. One week after her admission, respiratory conditions rapidly worsened, with SpO2 decreased up to 85%. Blood count and leucocyte formula were within normal range, while C‐reactive protein (7 ng/mL) and erythrocyte sedimentation rate (70 mm/h) were increased. CT scan showed bilateral interstitial pneumonia and CPAP was administered for 1 week, followed by progressive reduction of oxygen therapy.
The patient came to our attention 3 months after hospital discharge, complaining hair loss started 60 days after febrile peak. On physical examination, we found hair thinning and reduced central hair density (Figure 2A). The reported stress level on visual analog scale was 8/10. Serum levels of ferritin, TSH, Fe, Mg, Zn, Cu, B12, and folates were within normal ranges. The pull test and MWT were positive for club shedding hair, suggesting acute TE. Trichoscopy showed a mild reduction in hair density without any other pathological findings (Figure 2B). The patient was treated with sulfur amino acid/vit B6 oral supplementation and peptide mimicking hair growth factor lotion topical daily application.
FIGURE 2.
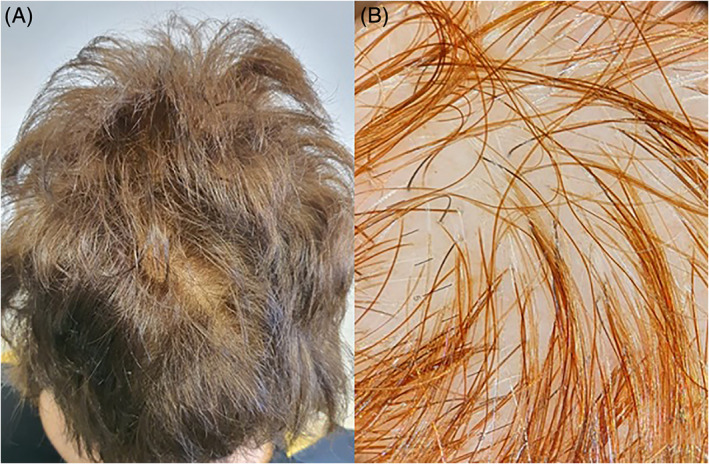
A, Hair thinning and reduced central hair density. B, Trichoscopy showed a mild reduction in hair density without any other pathological findings
4. CASE 3
A 58‐year‐old female patient was hospitalized in the Sars‐Cov‐2 unit of United Hospital of Ancona for 21 days since the sudden onset of bilateral interstitial pneumonia. She had positive Sars‐Cov‐2 nasopharyngeal swab, feverish peak 39°C, SpO2 93%, and diarrheal symptoms, associated with previous weight loss of 4 kg in last 2 weeks. The treatment consisted of oxygen therapy, 15 L/min FiO2 50%, then progressively reduced to room air, enoxaparin 6000 IU once a day, lopinavir/ritonavir 400/100 mg twice a day. One day after hospitalization, the patient reported the appearance of diffuse itching and flushing, treated with chlorphenamine maleate 10 mg once a day and lopinavir/ritonavir withdrawal.
At the time of dermatological examination, 3 months later, she referred that hair loss started 36 days after febrile peak. On physical examination, we found diffuse reduced hair density without regression to the temporal regions (Figure 3A). The reported stress level on visual analog scale was 6/10. Fe, ferritin, TSH, Mg, Zn, Cu, B12, and folates were within normal ranges. The pull test and MWT were strongly positive for TE, with club shedding hair. Trichoscopy showed a mild reduction in hair density with reduced thickness in some among the analyzed hair (Figure 3B). These clinical features were indicative of acute TE arising on a previous form of initial androgenetic alopecia (AGA). We prescribed a galenic lotion with minoxidil 5%, peptide mimicking hair growth factor lotion, and oral supplementation with sulfur amino acid/vit B6.
FIGURE 3.
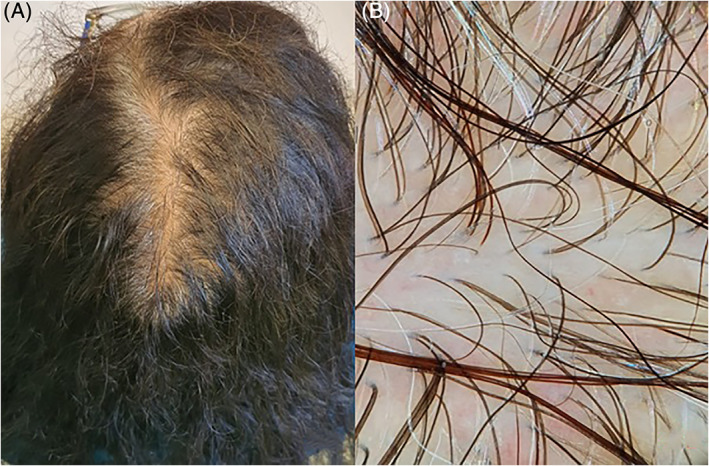
A, Diffuse reduced hair density without regression to the temporal regions. B, Trichoscopy showed a mild reduction in hair density with some with some hair of reduced thickness
5. DISCUSSION
5.1. Clinical implications
TE effluvium often occurs 2 to 3 months following trigger events, including severe infective episodes. 2 In literature association between acute TE and Dengue, human immunodeficiency virus infection, influenza, typhoid fever, scarlet fever, pneumonia, pertussis, tuberculosis, and malaria have already been reported. 2 , 7 , 8
In our cases, time interval between the onset of hair shedding and Sars‐Cov‐2 infection (chronologically identified in the first feverish peak) was respectively 75, 60, and 36 days for the three described cases (Table 1). This timing suggests that in cases 1 and 2 Sars‐Cov‐2 infection could have acted as trigger event for TE. In case 3 hospitalization was delayed due to the limited intestinal manifestation of the infection, which began 2 weeks earlier the appearance of fever and respiratory symptoms.
TABLE 1.
The clinical characteristics of three patients with Sars‐cov‐2 associated Telogen Effluvium (TE)
| Age (years) | Onset of hair shedding (days from febrile peak) | Febrile peak (°C) | Medical history | |
|---|---|---|---|---|
| Case 1 | 62 | 75 | 39 |
|
| Case 2 | 74 | 60 | 39.2 |
|
| Case 3 | 58 | 36 | 39 |
|
| Clinical findings | Medications during hospitalization | |||
| Case 1 |
|
|
||
| Case 2 |
|
|
||
| Case 3 |
|
|
||
| Treatment | Diagnosis | |||
| Case 1 |
|
Acute TE | ||
| Case 2 |
|
Acute TE | ||
| Case 3 |
|
Acute TE on initial AGA a | ||
Androgenetic alopecia (AGA).
In 1919 9 the epidemic influenza caused acute TE 2 weeks to 3 months after the feverish peak and Savil 10 reported that most of the patients showed hair fall has begun 2 to 6 weeks after the onset of fever. Following these considerations, in absence of other triggers (eg, stressful or major life event, marked weight loss and extreme dieting), acute TE in patient 3 could be strongly associated to Sars‐Cov‐2 infection. On the other hand, case 3 is less likely related to the stress resulting from hospitalization in semi‐intensive unit, because the time interval occurred between hospital access and onset of TE (36 days) was outside the typical range of 2 to 3 months. Differently, emotional stress could have had a part in TE development in both cases 1 and 2, whose hospitalization date preceded the TE onset of about 2 months and half, and 2 months.
In particular, patients who have experienced a long hospitalization for severe Sars‐Cov‐2 infection showed a consistent increase in pro‐inflammatory cytokines, (Interleukin 1b, Interleukin 6, tumor necrosis factor α and type 1, and 2 interferon) 11 which can explain infection‐related skin manifestations, such as urticaria, livedoid vasculopathy, chicken‐pox like rash, and Covid toes. 12 , 13 Cytokine‐storm can promote TE development by damaging hair‐producing matrix cells, 14 and particularly it has already been demonstrated that high levels of interferons associated with acute TE. 15
However, an involvement of enoxaparin in the development of TE cannot be ruled out. Watras et al 16 highlighted the role of anticoagulants, including enoxaparin, 17 in inducing TE. They described that TE started 3 weeks after drug administration and resolved in 1 month after its suspension. The median dose used in the study (100 IU/mL pro Kg twice a day) was higher than those in our cases (about 100 IU/mL pro Kg once a day), thus it seems less plausible is heparin the cause of TE rather than the Sars‐Cov‐2 infection.
Drug‐induced acute TE was excluded for all chronic home therapies. On the other hand, only two case‐reports 18 , 19 showed the association of ritonavir/lopinavir and acute TE in HIV‐positive patients. In our cases 2 and 3 the administration of ritonavir/lopinavir was suspended after 1 day respectively due to the appearance of diarrhea and itching/flushing. In case 1 the patient received ritonavir/lopinavir for 1 month, while the cases in the literature reported at least 2 months of continuous therapy before the onset of hair loss. We believe these drugs cannot be responsible for acute TE given the short duration of treatment. We also excluded ceftriaxone and chlorphenamine maleate as a cause of acute TE, due to the absence of cases in the literature, and tocilizumab, which seems to be responsible for regrowth phenomena in AGA. 20
Furthermore, we found that chronic autoimmune illness, as chronic thrombocytopenia, may be associated with chronic TE, 21 but this was ruled out as in our cases all patients complained of sudden new hair loss, compatible with acute TE. It is important to notice that Sars‐Cov‐2 infection can be related with hematological findings, including autoimmune thrombocytopenia. 22 In case 1 the patient had a history of chronic autoimmune thrombocytopenia for 2 years. The platelet count remained unchanged during and after hospitalization, maintaining the previous values (120 000 platelets/mL). For this reason, we assume that the Sars‐Cov‐2 infection did not cause the autoimmune thrombocytopenia.
Finally, the therapeutic approach to acute TE involves the identification and elimination of the trigger, 21 presumably identifiable in our cases with severe Sars‐Cov‐2 infection. The drugs held responsible for the acute TE should also be changed or discontinued. In our cases, although a drug‐induced acute TE is not very probable, heparin and newly introduced therapies have been suspended upon hospital discharge. In most cases, these measures lead to a resolution of the acute TE with follow‐up alone, informing the patient that the hair fall can continue for up to 6 months and then recover progressively. 21
A food supplement may improve the quality of the hair and provide an adequate nutritional setting for hair regrowth, using sulfur amino acids and vitamin B complex. Topical peptides mimicking hair growth factor is also described as beneficial in TE. 23
Topical minoxidil 5% can be considered in chronic TE but has only been proposed to patient 3 as suffering from a concomitant AGA, not previously diagnosed. In the other patients, we preferred not to administer minoxidil as it may result in an increased initial hair fall, due to a shortening of the telogen phase and an early entry into the anagen phase of the resting follicles. 24 Patients were already psychologically involved and the risk of an initial hair worsening could have further destabilized them.
5.2. Management implications
The Sars‐Cov‐2 pandemic has had a significant impact on the daily routine of people around the world. In Italy, the government imposed 8 weeks‐long complete lockdown period starting from March 9th (phase 1 period). Clinical activities were reorganized allowing hospital admission only to urgent patients. The new Italian Decree of first June 2020 established the end of the partial lockdown period (phase 2 period) and the resumption of elective clinical activities. However, a whole series of constraints to reduce the risk of infection and new spread of the virus has been introduced. 25 , 26 Our dermatological clinic, an Italian second level Dermatology Center, has developed organizational strategies aimed at guaranteeing the avoidance of crowding (eg, placing seats in waiting room at a minimum distance of 1 m from each other, setting the accesses to the outpatient clinic by spacing the appointments between patients every 30 minutes).
Hygienic measures, as hand washing with hydroalcoholic solution, surgical mask for patients and healthcare workers, and body temperature measurement with a thermoscanner system have been maintained until now. 27 , 28
These measures allowed us to visit even elective patients who needed a first‐person evaluation, especially for the psychological implications of their pathology, such as hair loss. In this regards several studies 29 , 30 highlighted an exacerbation of chronic TE in patients forced to stay at home.
All patients reported increased stress level (average value 8.2/10) 30 and much more apprehension about hair loss during the quarantine. During the phase 1 period, remote clinical assistance system has been proposed with teledermatology, to avoid patients coming into the hospital. However, some clinical and diagnostic limitations, as the impossibility to perform pull test and trichoscopy, makes teledermatology unable to replace completely a first outpatient visit. For this reason, we decided to personally manage new cases of hair loss, following the previous Sars‐Cov‐2 hygiene measures.
6. CONCLUSIONS
Clinicians should be aware that Sars‐Cov‐2 infection can be associated with delayed TE. To the best of our knowledge, this is the first case‐series describing TE in post severe Sars‐Cov‐2 patients. Only one case report has been found in the literature describing association between anagen effluvium and severe Sars‐Cov‐2 infection. 5 Other studies 3 , 4 reported the exacerbation of a preexisting TE, correlated to the stress of lockdown. In our cases patients never had a TE diagnosis before and did not report previous evident hair loss. Further studies are warranted to reach the precise etio‐pathogenesis of the condition based on histopathological and/or immunohistochemical workup.
CONFLICTS OF INTEREST
The authors declare no conflicts of interests.
AUTHOR CONTRIBUTION
All authors have contributed to the writing of the manuscript.
Rizzetto G, Diotallevi F, Campanati A, et al. Telogen effluvium related to post severe Sars‐Cov‐2 infection: Clinical aspects and our management experience. Dermatologic Therapy. 2021;34:e14547. 10.1111/dth.14547
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study
REFERENCES
- 1. Trueb RM. Diffuse hair loss. In: Blume‐Peytavi U, Tosti A, Whiting DA, Trueb R, eds. Hair Growth and Disorders. 1st ed. Berlin: Springer; 2008:259‐272. [Google Scholar]
- 2. Malkud S. Telogen effluvium: a review. J Clin Diagn Res. 2015;9(9):WE01‐WE03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dinh QQ, Sinclair R. Female pattern hair loss: current treatment concepts. Clin Interv Aging. 2007;2:189. [PMC free article] [PubMed] [Google Scholar]
- 4. Torres F, Tosti A. Female pattern alopecia and telogen effluvium: figuring out diffuse alopecia. Semin Cutan Med Surg. 2015;34(2):67‐71. [DOI] [PubMed] [Google Scholar]
- 5. Shanshal M. COVID‐19 related anagen effluvium [published online ahead of print, 2020 Jul 16]. J Dermatolog Treat. 2020;1‐2. 10.1080/09546634.2020.1792400. [DOI] [PubMed] [Google Scholar]
- 6. Guarrera M, Cardo PP, Rebora A. Assessing the reliability of the modified wash test. G Ital Dermatol Venereol. 2011;146(4):289‐294. [PubMed] [Google Scholar]
- 7. Chu C‐B, Yang C‐C. Dengue‐associated telogen effluvium: a report of 14 patients. Dermatologica Sinica. 2017;35(3):124‐126. [Google Scholar]
- 8. Bernstein GM, Crollick JS, Hassett JM Jr. Postfebrile telogen effluvium in critically ill patients. Crit Care Med. 1988;16:98‐99. [DOI] [PubMed] [Google Scholar]
- 9. Hazen HH. Postinfluenzal alopecia. JAMA. 1919;72:1452. [Google Scholar]
- 10. Savil A. The Hair and Scalp – A Clinical Study. 4th ed. London: E. Arnold & Co.; 1952:85. [Google Scholar]
- 11. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the cytokine storm' in COVID‐19. J Infection. 2020;80(6):607‐613. 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garg S, Garg M, Prabhakar N, Malhotra P, Agarwal R. Unraveling the mystery of Covid‐19 cytokine storm: from skin to organ systems. Dermatol Ther. 2020;e13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diotallevi F, Campanati A, Bianchelli T, et al. Skin involvement in SARS‐CoV‐2 infection: case series. J Med Virol. 2020;92:2332‐2334. 10.1002/jmv.26012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Headington JT. Telogen effluvium. New concepts and review. Arch Dermatol. 1993;129(3):356‐363. [DOI] [PubMed] [Google Scholar]
- 15. Olsen EA, Rosen ST, Vollmer RT, et al. Interferon alfa‐2a in the treatment of cutaneous T cell lymphoma. J Am Acad Dermatol. 1989;20(3):395‐407. [DOI] [PubMed] [Google Scholar]
- 16. Watras MM, Patel JP, Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? A review of the literature. Drugs Real World Outcomes. 2016;3(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang YY, Po HL. Enoxaparin‐induced alopecia in patients with cerebral venous thrombosis. J Clin Pharm Thera. 2006;31(5):513‐517. [DOI] [PubMed] [Google Scholar]
- 18. Borrás‐Blasco J, Belda A, Rosique‐Robles D, Casterá E, Abad J, Amorós‐Quiles I. Hair loss induced by lopinavir‐ritonavir. Pharmacotherapy. 2007;27(8):1215‐1218. [DOI] [PubMed] [Google Scholar]
- 19. Chrysos G, Mikros S, Kokkoris S, Pastelli A, Kontochristopoulos G. Alopecia induced by lopinavir plus ritonavir therapy in an HIV patient. J Drugs Dermatol. 2007;6(7):742‐743. [PubMed] [Google Scholar]
- 20. Vidon C, Bossert M, Lohse‐Walliser A, Godfrin‐Valnet M, Balblanc JC, Wendling D. Hair‐cycle changes in two patients taking tocilizumab. Joint Bone Spine. 2014;81(1):100‐101. [DOI] [PubMed] [Google Scholar]
- 21. Mysore V, Parthasaradhi A, Kharkar RD, et al. Expert consensus on the management of Telogen effluvium in India. Int J Trichology. 2019;11(3):107‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bomhof G, Mutsaers PGNJ, Leebeek FWG, et al. COVID‐19‐associated immune thrombocytopenia. Br J Haematol. 2020;190(2):e61‐e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo EL, Katta R. Diet and hair loss: effects of nutrient deficiency and supplement use. Dermatol Pract Concept. 2017;7:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186‐194. [DOI] [PubMed] [Google Scholar]
- 25. Radi G, Diotallevi F, Campanati A, Offidani A. Global coronavirus pandemic (2019‐nCOV): implication for an Italian medium size dermatological clinic of a II level hospital. J Eur Acad Dermatol Venereol. 2020;34:e213‐e214. [DOI] [PubMed] [Google Scholar]
- 26. Campanati A, Brisigotti V, Diotallevi F, et al. Active implications for dermatologists in “SARS‐CoV‐2 ERA”: personal experience and review of literature. J Eur Acad Dermatol Venereol. 2020;34:1626‐1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Diotallevi F, Radi G, Campanati A, et al. Time to restart: protocol of resumption of activities of a dermatological clinic of a level ii hospital in the COVID‐19 era. Int J Dermatol. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Radi G, Simonetti O, Diotallevi F, et al. How can I take care of you? The dermatologist meets patients' needs during the COVID‐19 pandemic. Dermatol Ther. 2020;e13740 [published online ahead of print, 2020 Jun 1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Turkmen D, Altunisik N, Sener S, Colak C. Evaluation of the effects of COVID‐19 pandemic on hair diseases through a web‐based questionnaire. Dermatol Ther. 2020;e13923 [published online ahead of print, 2020 Jun 28]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rivetti N, Barruscotti S. Management of telogen effluvium during the COVID‐19 emergency: psychological implications. Dermatol Ther. 2020;e13648 [published online ahead of print, may 22, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study


