Dear Editor,
Generalized pustular psoriasis is a rare manifestation of psoriasis that can be triggered by a variety of factors including viral infections, drugs, and the rapid tapering of systemic corticosteroids. 1
Herein, we report the case of a 60‐year‐old male patient who presented to the hospital on 3 July 2020, with vomiting, diarrhea, myalgia, and cough. The past medical history included hypertension, osteopenia, and subacute thyroiditis. The nasal swab polymerase chain reaction test for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was positive. Oxygen saturation was 98% without an oxygen mask and the patient was discharged with naproxen and hydroxychloroquine. On 18 July 2020, he returned to the hospital with respiratory distress; the computed tomography (CT) scan of the chest revealed bilateral ground‐glass opacities (Figure 1). Meropenem, linezolid, vitamin D3, heparin, and intravenous pulse methylprednisolone were added to the treatment protocol. On 27 July, he was discharged on prednisolone 30 mg/day. Two days later, the patient developed fever (39°C) and widespread erythematous patches and pustules (Figure 1). He also complained of bilateral edema of the lower extremities, but color Doppler sonography revealed no signs of deep venous thrombosis. Further questioning indicated that the patient had a history of psoriasis during childhood. A skin biopsy was taken with differential diagnoses of pustular psoriasis and acute generalized exanthematous pustulosis (AGEP); the histopathologic findings were compatible with the former (Figure 1). Laboratory evaluations revealed a white blood cell count of 7.2 × 103/L (neutrophil and lymphocyte differentiation of 82% and 8%, respectively), a creatinine level of 1.2 mg/dL, a blood urea nitrogen level of 21 mg/dL, mild hypocalcemia (7.4 mg/dL), and negative stool, urine, and blood cultures. Oral acitretin 25 mg/day was initiated and hydroxychloroquine was discontinued. Oral prednisolone was gradually tapered and, fortunately, there was a gradual improvement in the pustular lesions (Figure 2).
FIGURE 1.
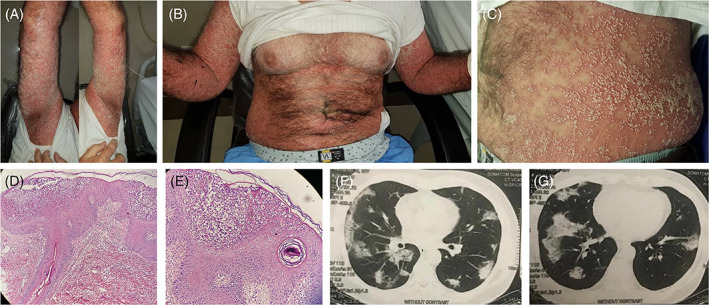
Widespread pustules on a background of erythrodermic skin in the patient before treatment (A‐C). Psoriasiform acanthosis; absence of the granular layer with large intraepidermal pustule formation; some tortuous vessels and perivascular lymphocytic and neutrophilic infiltration are seen in the superficial dermis; there is no evidence of viral cytopathic effects (D, H&E ×200; E, H&E ×400). Bilateral ground‐glass opacities and consolidations compatible with COVID‐19 (F, G)
FIGURE 2.
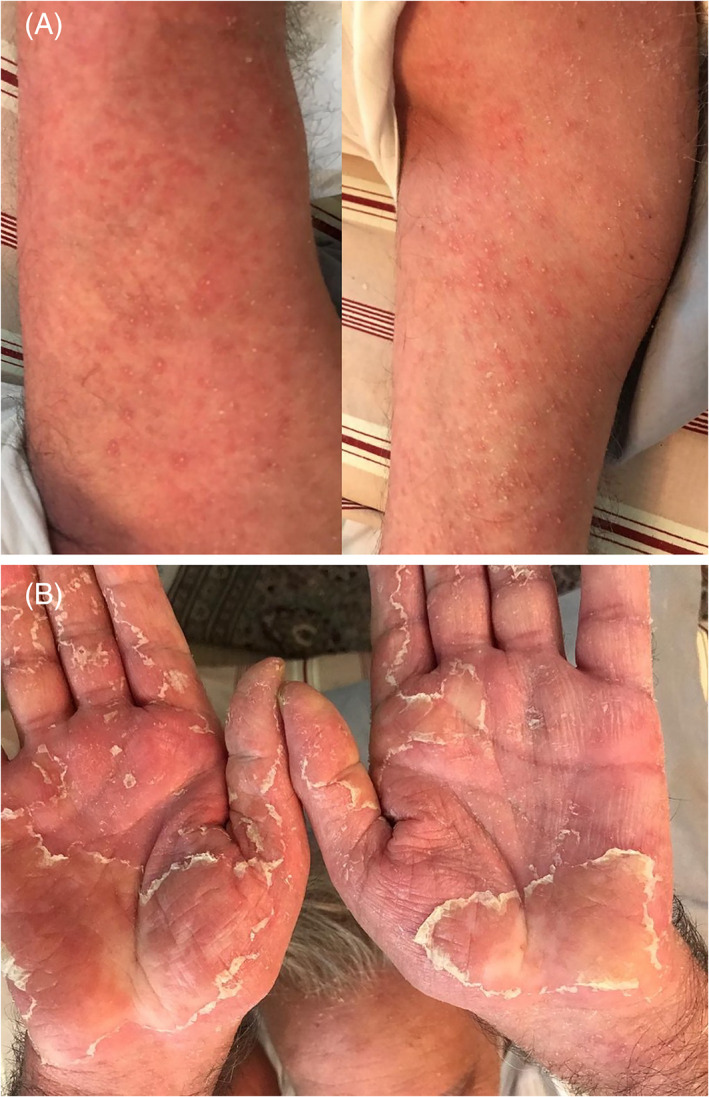
Marked improvement in the pustular lesions and erythema 2 weeks after treatment with acitretin (A, B)
Psoriasis is a chronic inflammatory disease that can be aggravated by viral infections (especially rhinoviruses and coronaviruses), in addition to pregnancy, hypocalcemia, and a variety of medications. 1 , 2 , 3 During this pandemic, psoriasis flares have been reported in coronavirus disease 2019 (COVID‐19) patients with a history of psoriasis. 4 Both viral infections and medications used for treatment may play roles in these flares. A possible mechanism for psoriatic flares following viral infections is the induction of a hyperinflammatory state, with SARS‐CoV‐2 possibly being involved. 2 , 4 Moreover, hydroxychloroquine, one of the most important drugs used widely as a potential treatment for COVID‐19, can induce psoriasis or lead to recurrence or exacerbation of psoriatic lesions. 5 It is proposed that hydroxychloroquine can weaken the outer surface of the skin and lead to abnormal keratinocyte proliferation while interfering with cholesterol metabolism, which is essential for the epidermal barrier integrity; it can hence give rise to psoriasis flares. 5 Although reducing the dose of the systemic corticosteroid in our patient was not rapid, its role should not be overlooked.
Important differential diagnoses for pustular psoriasis include AGEP and drug reactions due to the consumption of a new drug like hydroxychloroquine. However, the clinical course and features of these entities are usually similar to a certain limit. 6 Previous history of psoriasis, a long interval between starting the medication and skin eruption and certain pathological features (eg, prominent acanthosis, suprapapillary epidermal thinning, and tortuous papillary dermal capillaries) support the diagnosis of pustular psoriasis. This is while in AGEP, there is no clear history of psoriasis and a shorter interval between drug consumption and skin eruption prevails. Furthermore, the histological findings of AGEP include prominent edema of the papillary dermis, necrotic keratinocytes, and prominent eosinophil infiltration. 6
In this case, the patient had a childhood history of psoriasis. In addition, the time interval between the commencement of hydroxychloroquine and the pustular reaction was fairly long (26 days) in comparison with what is expected in AGEP, where approximately 90% of cases occur 4 days after drug usage.
To our knowledge, this is the first reported case of generalized pustular psoriasis following COVID‐19 affliction. In this pandemic, the number of newly diagnosed patients with psoriasis or psoriatic patients experiencing a relapse of skin lesions as erythrodermic, pustular, or plaque‐type psoriasis is expected to rise 7 ; both infection‐induced hyperinflammation and medications play pivitol roles in this phenomenon. Paying attention to psoriasis is important in the history of COVID‐19 patients to be treated with hydroxychloroquine or systemic corticosteroids and it is recommended to discontinue the use of hydroxychloroquine in COVID‐19 patients who develop psoriasis or experience a recurrence of skin lesions. In such situations, nonimmunosuppressive drugs such as systemic retinoids should be used as far as possible for the treatment of psoriasis.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHORS CONTRIBUTION
M.S.D were involved in the diagnosis and management of the patient and have been responsible for the clinical part of the manuscript. M.A. reported the result of histopathological evaluation. R.D. and F.A. did literature review and drafted the manuscript. F.A. and M.S.D. were responsible for final editing of the manuscript. All authors have read and approved the final manuscript. F.A. and R.D. are corresponding authors.
Contributor Information
Reem Diab, Email: reemdiab1990@gmail.com.
Fahimeh Abdollahimajd, Email: fabdollahimajd@yahoo.com, Email: fabdollahimajd@sbmu.ac.ir.
DATA AVAILABILITY STATEMENT
All data are included in this published article.
REFERENCES
- 1. Mirza HA, Badri T, Kwan E. Generalized pustular psoriasis. [Updated 2020 Sep 15. StatPearls [Internet. Treasure Island, FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK493189/. [PubMed] [Google Scholar]
- 2. Abdelmaksoud A, Goldust M, Vestita M. Comment on “COVID‐19 and psoriasis: is it time to limit treatment with immunosuppressants? A call for action”. Dermatol Ther. 2020;33(4):e13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abdollahimajd F, Niknezhad N, Haghighatkhah HR, Namazi N, Niknejad N, Talebi A. Angiotensin‐converting enzyme and subclinical atherosclerosis in psoriasis: is there any association? A case‐control study. J Am Acad Dermatol. 2020;82(4):980‐981. [DOI] [PubMed] [Google Scholar]
- 4. Kutlu O, Metin A. A case of exacerbation of psoriasis after oseltamivir and hydroxychloroquine in a patient with COVID‐19: will cases of psoriasis increase after COVID‐19 pandemic? Dermatol Ther. 2020;33(4):e13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sachdeva M, Mufti A, Maliyar K, Lytvyn Y, Yeung J. Hydroxychloroquine effects on psoriasis: a systematic review and a cautionary note for COVID‐19 treatment. J Am Acad Dermatol. 2020;83(2):579‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Behrangi E, Hallaji Z, Ghiasi M, et al. Hydroxychloroquine‐induced unusual generalized pustular cutaneous reaction as a new clinical entity: a case series. Immunoregulation. 2020;3(1):67‐72. [Google Scholar]
- 7. Ghalamkarpour F, Pourani MR, Abdollahimajd F, Zargari O. A case of severe psoriatic erythroderma with COVID‐19. J Dermatol Treat. 2020;1‐3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in this published article.


