Acute lung injury resulting from SARS‐CoV‐2 infection has led to high number of prolonged invasive mechanical ventilation. Role and timing of tracheostomy for patients requiring critical care for coronavirus disease 2019 (COVID 19) remains unclear. So far, published data on early versus late tracheostomy are conflicting. Actual recommendations suggest that this procedure should be considered in patients with COVID 19 when mechanical ventilation is anticipated to be over 10‐15 days. 1
Tracheostomy is a highly aerosol generating procedure and exposes healthcare providers to viral contamination via air droplets. 2 Recommendations have been suggested for safe surgical tracheostomy during COVID‐19 pandemic by several societies. 3 , 4 , 5 , 6 In order to ensure maximal protection to staff performing the procedure, full personal protective equipment (PPE) is strongly recommended. 7 This includes N95 mask, goggles or face shiels, surgical gown and gloves. The use of such equipment may affect communication, visibility and other non‐technical skills. 8 However, it remains essential to prevent healthcare workers contamination. In addition to PPE, tracheostomy should be performed in a negative‐pressure room. Utilisation of techniques which minimise aerosolisation is recommended when performing tracheostomy.
We describe a tracheostomy technique for patients requiring prolonged mechanical ventilation. We aim to minimise aerosol contamination, using flow‐controlled ventilation (FCV) provided by a new ventilator, the Evone® (Ventinova medical BV), through a specifically designed cuffed endotracheal tube, the Tritube® (Ventinova Medical BV). 9
FCV system is designed to maintain constant flow during inspiration and expiration. The main specificity of this ventilator is that it provides active expiration. Tritube® is a 40‐cm‐long, narrow‐bore tube (outer diameter = 4.4 mm) with three independent lumens for, respectively, pressure measurement, ventilation and cuff inflation.
Patient, already intubated and sedated, is anesthetised and a deep neuromuscular block is ensured. Once ventilation and fresh gas flow are stopped at end expiration, the endotracheal tube (ETT) is cut at its proximal end. This allows easier insertion of the Tritube® through the standard ETT at the desired depth.
After checking both tubes’ length markings, the Tritube® is introduced into the ETT and is pushed down as caudally as possible into the trachea, in order to protrude the distal end of the ETT. Tritube® cuff is inflated. Ventilation with Evone® FCV system through Tritube® can then be started. (Figure 1).
FIGURE 1.
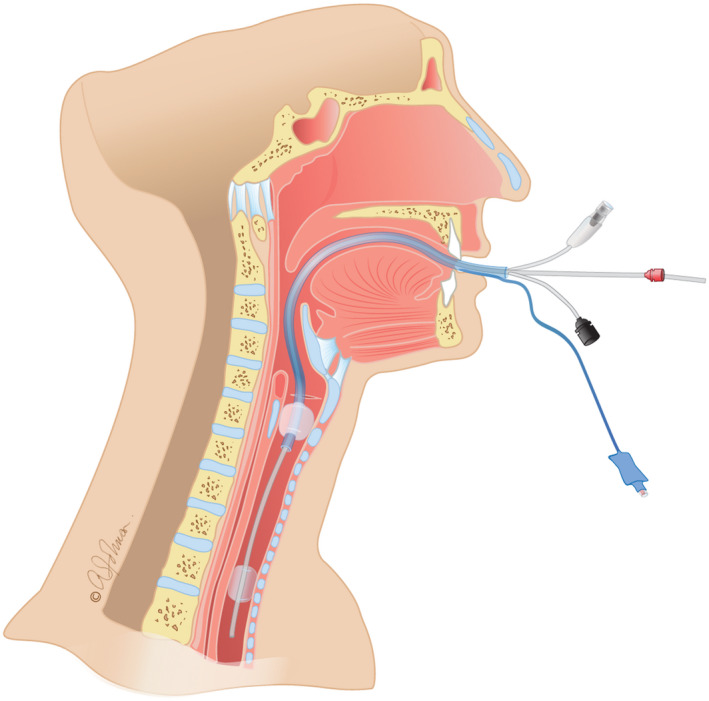
Introduction of the Tritube® through the endotracheal tube
ETT cuff can be deflated safely and lifted above the vocal cords to allow a good working space for the surgeon. (Figure 2).
FIGURE 2.
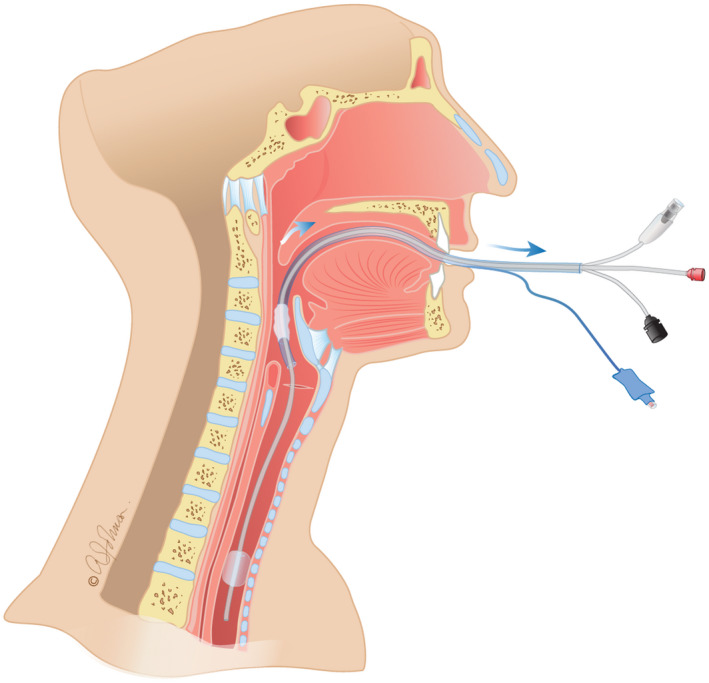
Endotracheal tube is lifted above the vocal cords
Trachea is opened by the surgeon without risk of aerosolisation, as Tritube® cuff is inflated and isolates the ventilated lower airways. The small diameter of the Tritube® allows the tracheal cannula to be inserted while the Tritube® is still in place. Cannula's cuff can be inflated and patient is ventilated through the cannula with conventional ventilator. (Figure 3).
FIGURE 3.
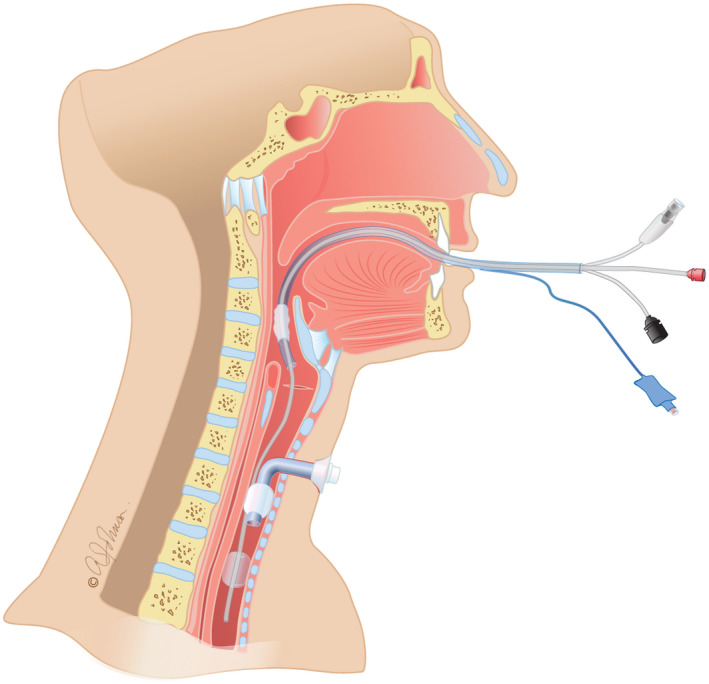
Insertion of the tracheostomy cannula
After deflating its cuff, Tritube® can be removed with the ETT around it. Tritube® passes easily beside the inflated tracheostomy cannula cuff. (Figure 4).
FIGURE 4.
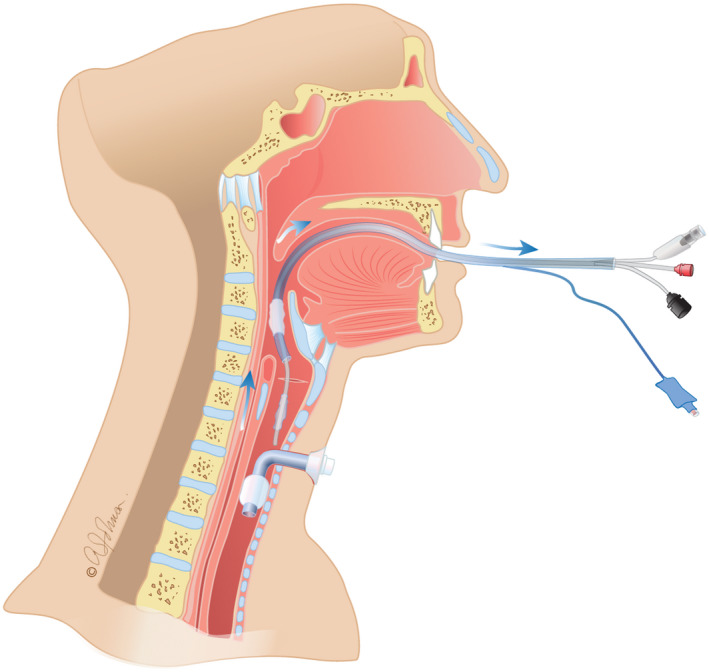
Tritube's® removal, together with endotracheal tube
Safety profile and effectiveness of FCV with Evone® system and Tritube® has been demonstrated by Meulemans and coll. No adverse effects had been described. 9 , 10
The technique we suggest has many advantages.
First, it decreases dramatically the aerosolisation of viral particles during the tracheostomy as closed system of ventilation is provided during the entire surgical procedure.
Second, apnoea time is decreased as patient is ventilated even during cannula insertion. This allows safe conduction of tracheostomy in very hypoxic patient.
Third, this technique could also be performed for percutaneous tracheostomy as it allows safe fiberoscopic visualisation during critical moments. Tritube® diameter allows easy fiberoscopy while Evone® insures ventilation with minimal aerosolisation.
The main goal of this letter was to present a safe tracheostomy technique protecting healthcare providers from aerosolisation during COVID‐19 pandemic. Further studies should be conducted regarding the use of FCV with Tritube® as a protective tool in ENT surgery for operating room teams.
DISCLOSURE
The authors declare no competing interests.
Funding information
Support was provided solely from institutional and departmental sources.
PRIOR PRESENTATIONS
Not applicable.
ACKNOWLEDGEMENTS
Illustrations realised by Wag Design and Communications.
REFERENCES
- 1. Lamb CR, Desai NR, Angel L, et al. Use of tracheostomy during the COVID‐19 pandemic: American College of Chest Physicians/American Association for Bronchology and Interventional Pulmonology/Association of Interventional Pulmonology Program Directors Expert Panel Report. Chest. 2020;158:1499‐1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pichi B, Mazzola F, Bonsembiante A, et al. CORONA‐steps for tracheotomy in COVID‐19 patients: a Staff‐safe method for airway management. Oral Oncol. 2020;105:104682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sommer DD, Engels PT, Weitzel EK, et al. Recommendations from the CSO‐HNS taskforce on performance of tracheotomy during the COVID‐19 pandemic. J Otolaryngol Head Neck Surg. 2020;49:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lima DS, Ribeiro Junior MF, Vieira‐Jr HM, Campos T, Saverio SD. Alternatives for establishing a surgical airway during the COVID‐19 pandemic. Alternativas para o estabelecimento de via aérea cirúrgica durante a pandemia de COVID‐19. Rev Col Bras Cir. 2020;47:e20202549. [DOI] [PubMed] [Google Scholar]
- 5. Mecham JC, Thomas OJ, Pirgousis P, Janus JR. Utility of tracheostomy in patients with COVID‐19 and other special considerations. Laryngoscope. 2020;130 1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takhar A, Walker A, Tricklebank S, et al. Recommendation of a practical guideline for safe tracheostomy during the COVID‐19 pandemic. Eur Arch Otorhinolaryngol. 2020;21:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization (WHO) . Rational use of personal protective equipment (PPE) for coronavirus disease 2019 (COVID‐19); 2020. http://apps.who.int/iris/handle/10665/331498
- 8. Yánez Benítez C, Güemes A, Aranda J, et al. Impact of personal protective equipment on surgical performance during the COVID‐19 pandemic. World J Surg. 2020;44:2842‐2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meulemans J, Jans A, Vermeulen K, Vandommele J, Delaere P, Vander PV. Evone® flow‐controlled ventilation during upper airway surgery: a clinical feasibility study and safety assessment. Front Surg. 2020;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmidt J, Günther F, Weber J, et al. Flow‐controlled ventilation during ear, nose and throat surgery: a prospective observational study. Eur J Anaesthesiol. 2019;36:327‐334. [DOI] [PubMed] [Google Scholar]


