Abstract
Background
Recent trials with dexamethasone and hydrocortisone have demonstrated benefit in patients with coronavirus disease 2019 (COVID‐19). Data on methylprednisolone are limited.
Methods
Retrospective cohort of consecutive adults with severe COVID‐19 pneumonia on high‐flow oxygen (FiO2 ≥ 50%) admitted to an academic centre in New York, from 1 March to 15 April 2020. We used inverse probability of treatment weights to estimate the effect of methylprednisolone on clinical outcomes and intensive care resource utilization.
Results
Of 447 patients, 153 (34.2%) received methylprednisolone and 294 (65.8%) received no corticosteroids. At 28 days, 102 patients (22.8%) had died and 115 (25.7%) received mechanical ventilation. In weighted analyses, risk for death or mechanical ventilation was 37% lower with methylprednisolone (hazard ratio 0.63; 95% CI 0.47‐0.86; P = .003), driven by less frequent mechanical ventilation (subhazard ratio 0.56; 95% CI 0.40‐0.79; P = .001); mortality did not differ between groups. The methylprednisolone group had 2.8 more ventilator‐free days (95% CI 0.5‐5.1; P = .017) and 2.6 more intensive care‐free days (95% CI 0.2‐4.9; P = .033) during the first 28 days. Complication rates were not higher with methylprednisolone.
Conclusions
In nonintubated patients with severe COVID‐19 pneumonia, methylprednisolone was associated with reduced need for mechanical ventilation and less‐intensive care resource utilization without excess complications.
Keywords: COVID‐19, healthcare resource utilization, intensive care unit, mechanical ventilation, methylprednisolone, pneumonia
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is associated with a spectrum of respiratory impairment, ranging from mild upper respiratory infection to fulminant viral pneumonia, referred to as coronavirus disease 2019 (COVID‐19). Pathological features are consistent with adult respiratory distress syndrome (ARDS), resembling those observed in severe acute respiratory syndrome (SARS) and middle east respiratory syndrome (MERS). 1 Despite that patients with COVID‐19 frequently demonstrate a proinflammatory cytokine profile, 2 where corticosteroids and other immunosuppressive agents can be useful, 3 experience with SARS and MERS 4 , 5 and meta‐analyses from influenza pneumonia studies 6 , 7 have initially prevented widespread empirical use of corticosteroids in COVID‐19 pneumonia.
In a large randomized clinical trial (RCT) conducted in the United Kingdom, 8 low‐dose dexamethasone reduced mortality among ventilated patients with COVID‐19 pneumonia, and to a lesser extent among those requiring supplemental oxygen. Following these positive results, a number of prospective studies with hydrocortisone and dexamethasone in COVID‐19 stopped enrolling patients, reporting favourable interim results. 9 , 10 , 11 These positive results have been corroborated by a meta‐analysis of prospective studies with corticosteroids in COVID‐19. 12 However, data on methylprednisolone, an intermediate‐acting corticosteroid, 13 have been limited to date. 12 This agent has been used in most RCTs supporting corticosteroids in the management of ARDS in the intensive care unit (ICU), 14 and thus, many ICU physicians feel comfortable with this agent. Mechanistically, methylprednisolone achieves higher lung tissue‐to‐plasma levels compared to dexamethasone in animal models and thus may be more beneficial for lung injury. 15 Initial observational experience with methylprednisolone in COVID‐19 has been positive, with mortality benefit reported in an early study from China 16 and reduced healthcare resource utilization reported in a study from Detroit, MI. 17 Similarly, a recent observational study from China reported reduced progression to critical illness with methylprednisolone. 18 However, a randomized trial with this agent in patients with severe COVID‐19 pneumonia requiring supplemental oxygen or mechanical ventilation reported no difference in mortality. 19
In this retrospective cohort study, we evaluated the impact of methylprednisolone on outcomes, healthcare resource utilization, and complications in 447 nonintubated adults with severe COVID‐19 pneumonia on high‐flow oxygen therapy.
2. MATERIALS AND METHODS
2.1. Study population
We reviewed the medical records of 1019 adults (≥18 years old) admitted to Stony Brook University Hospital (Stony Brook, NY, USA) from 1 March to 15 April 2020 with COVID‐19 confirmed by polymerase chain reaction for SARS‐CoV‐2. Among these patients, we identified those with severe COVID‐19 pneumonia, defined as fever or suspected respiratory infection, plus ≥1 of the following: respiratory rate >30 breaths/min; severe respiratory distress; or oxygen saturation <93% on room air 20 who required high‐flow oxygen (non‐rebreather mask, Venturi mask with FiO2 ≥ 50% or high‐flow nasal cannula, bilevel or continuous positive airway pressure [BiPAP or CPAP]). We considered BiPAP or CPAP as modalities of advanced oxygen therapy rather than forms of mechanical ventilation. We excluded patients who (a) died or required mechanical ventilation within <24 hours from admission; (b) were admitted in critical condition due to nonrespiratory causes and subsequently tested positive for SARS‐CoV‐2; and (d) received other corticosteroids (Figure 1). Demographics, concomitant conditions and medications, smoking history, vital signs, laboratory data and in‐hospital therapies, were extracted from medical records. Race and ethnicity were based on standard census definitions. Smoking was self‐reported. Institutional guidelines during this period recommended corticosteroids only in moderate‐to‐severe ARDS in mechanically ventilated patients, followed by slow tapering every 3 days, over 2‐3 weeks. For nonintubated patients, the use of corticosteroids was considered on a case‐by‐case basis. Patients who received corticosteroids only after mechanical ventilation were not included in this analysis. We recorded the use of other COVID‐19–targeted therapies, including tocilizumab, hydroxychloroquine, azithromycin and remdesivir. Follow‐up data were collected until death, hospital discharge or readmission for COVID‐19–related causes. We used the 28‐day outcome framework based on the approach of the RECOVERY trial. 8 Patients not readmitted by 28 days were considered alive and out of the hospital for the purposes of healthcare resource utilization analysis. Patients still hospitalized by the date of database lock (4 July 2020) were censored as alive for mortality analysis. The study was approved by the Institutional Review Board of Stony Brook University.
Figure 1.
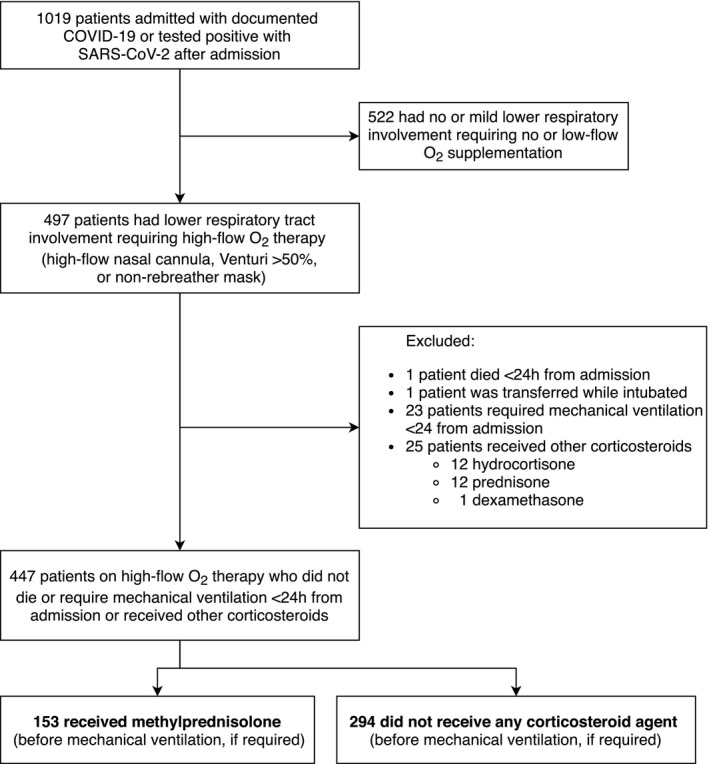
Flow chart of study population
2.2. Endpoints
The primary endpoint was death or mechanical ventilation at 28 days. Secondary endpoints were (a) 28‐day mortality; (b) 28‐day need for mechanical ventilation, accounting for competing mortality; (c) hospital‐, ICU‐ and ventilator‐free days during the first 28 days. We also evaluated the following complications: (a) bacteremia, (b) nosocomial pneumonia (hospital‐acquired and ventilator‐associated) and (c) gastrointestinal bleeding. Bacteremia was defined based on positive blood cultures; microbes isolated only once and belonging to commensal skin microbiota groups were considered contaminants. Nosocomial pneumonia was defined as positive sputum cultures ≥48 hours after admission; fungi isolated from sputum were considered colonization unless associated with fungemia.
2.3. Statistical analysis
Based on our initial institutional experience with COVID‐19, approximately 50% of patients had severe respiratory presentation requiring high‐flow O2 therapy. Among these patients, the rate of combined death or need for mechanical ventilation at 28 days was approximately 50%. We estimated that 426 patients would provide 80% power to demonstrate a one‐third reduction (HR = 0.66) of the primary endpoint with methylprednisolone (assuming a standard deviation of 0.5 for the distribution of the exposure of interest and a correlation r = 0.1 with other covariates as there was no systematic indication for methylprednisolone) at the 2‐sided α = 0.05, allowing for 5% attrition due to <24‐hour events. Therefore, we planned to review 1000 consecutive patients; we slightly exceeded that number because we were adding patients on a weekly basis.
The effect of methylprednisolone on the primary endpoint was estimated with flexible parametric survival (Royston‐Parmar) models to allow for time‐dependent effects. 21 The effect on mechanical ventilation was modelled using Fine and Gray competing‐risk models to account for competing mortality. 22 Methylprednisolone effects were weighted by the inverse probability of treatment based on the propensity score for methylprednisolone use, 23 , 24 a method that has been shown to produce minimal bias among propensity score methods. 23 , 25 The score for methylprednisolone use was calculated with a logistic regression model including age, sex, race, ethnicity, smoking, body mass index, hypertension, diabetes, coronary artery disease, atrial fibrillation, congestive heart failure, asthma, chronic lung disease, home use of angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker, immunocompromised status (>20 mg daily prednisone for ≥1 month; human immunodeficiency virus infection; post‐transplant immunosuppressive status; current malignancy, high‐dose chemotherapy, or stem cell transplant within the past year), symptoms duration, oxygen saturation, FiO2 needed at presentation, and admission values of creatinine, C‐reactive protein, lymphocyte count, D‐dimer, procalcitonin, liver function tests and N‐terminal pro‐B‐type natriuretic peptide. The effect of methylprednisolone on hospital‐, ICU‐ and ventilator‐free days during the first 28 days was estimated with generalized linear models, whereas the effect on complications was estimated with count data models; all models were weighted by the inverse probability of treatment. In all analyses, we additionally adjusted for the use of tocilizumab, hydroxychloroquine and azithromycin. We used multiple imputations (N = 15) with chained equations for missing covariate values and combined the estimates. 26 , 27 In sensitivity analyses, we (a) excluded those on chronic corticosteroids at home and (b) left‐truncated time at risk before administration of methylprednisolone. In exploratory analyses, we evaluated (a) the effect of methylprednisolone timing (from symptom onset) on the primary endpoint; and (b) any dose‐response effect of methylprednisolone. We used STATA 15.1 (StataCorp, College Station, TX) for all statistical analyses.
3. RESULTS
3.1. Baseline characteristics
A total of 447 adults with severe COVID‐19 pneumonia qualified for our study and constituted our analytic cohort. Of these, 153 (34.2%) received methylprednisolone while still on high‐flow oxygen (i.e. before mechanical ventilation) and 294 (65.6%) did not receive any corticosteroid. The baseline characteristics according to methylprednisolone use are presented in Table 1. Patients who received methylprednisolone were more likely to have history of asthma and had slightly longer duration of symptoms, lower oxygen saturation, higher respiratory rate, and higher N‐terminal pro‐B‐type natriuretic peptide and C‐reactive protein, but lower interleukin‐6 (in those available) levels.
Table 1.
Patient characteristics according to use of methylprednisolone (N = 447)
| Characteristics | Methylprednisolone (N = 153) | No Corticosteroids (N = 294) | P value a |
|---|---|---|---|
| Age, years | 62 (53‐72) | 61 (48‐74) | .37 |
| Female, N (%) | 49 (32.3) | 107 (36.4) | .40 |
| Race, N (%) | |||
| White | 122 (79.7) | 241 (82.0) | .82 |
| Black | 21 (13.7) | 35 (11.9) | |
| Asian | 10 (6.5) | 18 (6.1) | |
| Hispanic, N (%) | 45 (29.4) | 105 (35.7) | .20 |
| Body mass index, kg/m2 | 29.7 (26.3 ‐ 34.6) | 29.3 (26.1 ‐ 33.7) | .68 |
| Comorbidities, n (%) | |||
| Hypertension | 89 (58.2) | 165 (56.1) | .69 |
| Diabetes | 52 (34.0) | 95 (32.3) | .75 |
| Coronary artery disease | 24 (15.7) | 43 (14.6) | .78 |
| Atrial fibrillation | 17 (11.1) | 40 (13.6) | .55 |
| Chronic lung disease | 20 (13.1) | 28 (9.5) | .26 |
| Chronic kidney disease | 12 (7.8) | 34 (11.6) | .25 |
| Congestive heart failure | 17 (11.1) | 25 (8.5) | .40 |
| Asthma | 17 (11.1) | 14 (4.8) | .017 |
| Immunocompromised | 11 (7.2) | 22 (7.5) | .99 |
| Medication use, N (%) | |||
| ACE inhibitor | 25 (16.3) | 44 (15.0) | .78 |
| Angiotensin receptor blocker | 31 (20.3) | 42 (14.3) | .11 |
| Statins | 58 (37.9) | 116 (39.5) | .76 |
| Presentation characteristics | |||
| Duration of symptoms, days | 7 (4‐10) | 7 (3‐8) | .048 |
| O2 saturation, % | 90 (85‐93) | 91 (88‐93) | .006 |
| Temperature, °C | 38.1 (37.4‐38.9) | 38.2 (37.5‐39.0) | .45 |
| Systolic blood pressure, mmHg | 124 (110‐142) | 126 (112‐141) | .82 |
| Diastolic blood pressure, mmHg | 72.5 (65‐79) | 74 (66‐82) | .14 |
| Heart rate, beats/min | 99.5 (86‐110) | 99.5 (86.5‐112) | .50 |
| Respiratory rate, breaths/min | 24 (18‐30) | 20 (18‐26) | .026 |
| Laboratory findings | |||
| Corrected QT on ECG, ms | 436 (419‐455) | 439 (418‐462) | .41 |
| NT‐proBNP, pg/mL | 266 (86‐1145) | 161 (42‐883) | .046 |
| Troponin, ng/mL | 0.01 (0.01‐0.01) | 0.01 (0.01‐0.01) | .39 |
| Creatine phosphokinase, IU/L | 202 (78‐459) | 156 (74‐361) | .76 |
| ESR, mm/h | 52 (33‐78) | 57 (31‐80) | .99 |
| C‐reactive protein, mg/dL | 12.5 (7.0‐20.9) | 11.4 (5.9‐18.0) | .089 |
| D‐Dimer, ng/mL | 372 (258‐714) | 349 (237‐771) | .43 |
| Procalcitonin, ng/mL | 0.23 (0.14‐0.46) | 0.20 (0.12‐0.49) | .50 |
| Ferritin, ng/mL | 992 (509‐1610) | 893 (474‐1467) | .32 |
| Lactate dehydrogenase, IU/L | 418 (331‐549) | 396 (295‐524) | .11 |
| Interleukin‐6, pg/mL b | 55.2 (19.0‐99.2) | 67.9 (35.8‐115.0) | .045 |
| Lymphocyte count, K/uL | 0.81 (0.62‐1.05) | 0.83 (0.55‐1.15) | .65 |
| Creatinine, mg/dL | 0.94 (0.76‐1.26) | 0.97 (0.77‐1.31) | .42 |
| Alanine aminotransferase, IU/L | 34 (21‐61) | 34 (21‐54) | .35 |
| Aspartate aminotransferase, IU/L | 48 (33‐75) | 46 (31‐64) | .16 |
| International normalized ratio | 1.2 (1.1‐1.3) | 1.2 (1.1‐1.3) | .88 |
| Concomitant therapies, N (%) | |||
| Tocilizumab | 38 (24.8) | 70 (23.8) | .82 |
| Hydroxychloroquine | 67 (43.8) | 193 (65.6) | <.001 |
| Azithromycin | 54 (35.3) | 160 (54.4) | <.001 |
| Remdesivir | 3 (2.0) | 3 (1.0) | .42 |
Values are N (%) or median (25th, 75th percentile).
Abbreviations: ACE, angiotensin‐converting enzyme; ESR, erythrocyte sedimentation rate; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Mann–Whitney test for continuous variables and Fisher's exact test for categorical variables.
Available in 117/153 (76.5%) and 196/294 (66.7%) patients in the methylprednisolone (+) and (−) groups, respectively.
3.2. Use of methylprednisolone
Patients started methylprednisolone a median of 2 days after admission (25th‐75th percentile, 1‐4) and 10 days after symptom onset (7‐14). The median time from start of high‐flow oxygen to methylprednisolone administration was 1 day (0‐2). The median daily dose was 160 mg (120‐180). The median dose per unit of body weight was 1.78 mg/kg/d (1.33‐2.23). Median duration of methylprednisolone therapy was 5 days (25th‐75th percentile, 4‐6) for full‐dose and 10 days (5‐14) for the entire course, including tapering.
3.3. Methylprednisolone and clinical outcomes
A total of 217 (48.5%) patients met the 28‐day primary endpoint of death or mechanical ventilation. Specifically, 102 patients (22.8%) died (52 after mechanical ventilation) and 115 (25.7%) had received mechanical ventilation and survived at 28 days.
Among patients who received methylprednisolone, 71/153 (46.4%) met the primary endpoint at 28 days vs 146/294 (49.7%) among those who did not. Using inverse probability of treatment weights, the weighted 28‐day rate of the primary endpoint was 45.6% in the methylprednisolone group vs 52.5% in the control group, Figure 2. The hazard ratio (HR) over the 28‐day period was 0.63 (95% CI 0.47‐0.86; P = .003). The effect of methylprednisolone was time dependent. During the first week, the HR was 0.43 (95% CI 0.29‐0.63; P < .001) but the benefit attenuated significantly afterwards and was no longer evident after 5 days, Figure 3.
Figure 2.
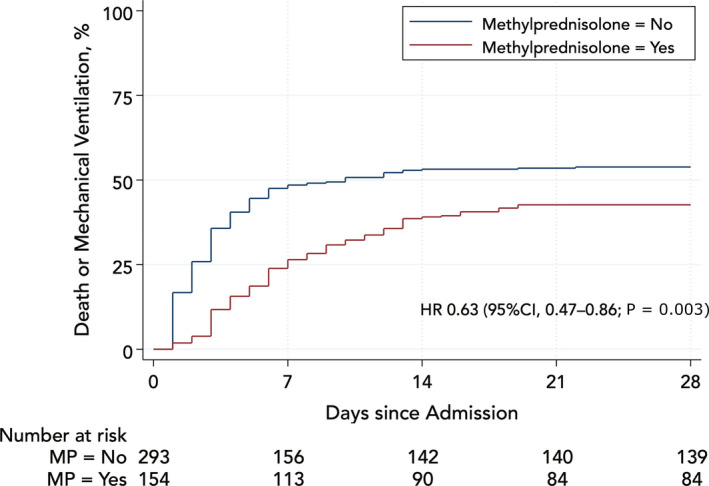
Rates of death or mechanical ventilation in methylprednisolone groups weighted by the inverse probability of treatment (propensity score). MP, methylprednisolone
Figure 3.
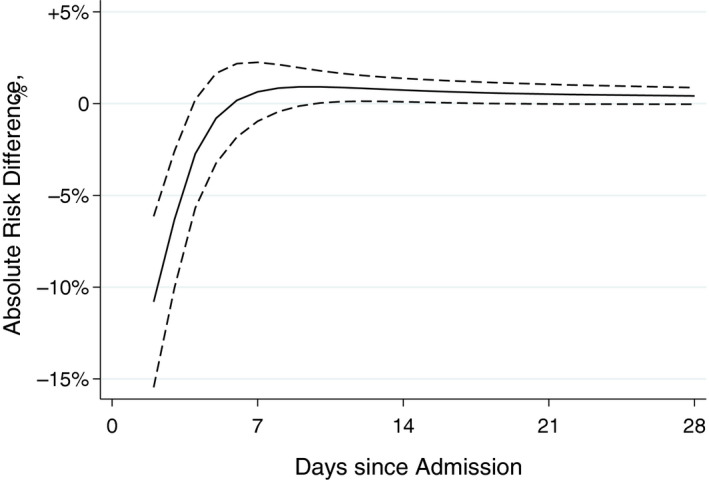
Absolute risk reduction for death or mechanical ventilation with use of methylprednisolone weighted by the inverse probability of treatment. Solid line denotes estimate; dashed lines denote 95% confidence interval
Among patients who received methylprednisolone, 50/153 (32.7%) required mechanical ventilation at 28 days vs 114/294 (38.8%) among those who did not. In weighted models, the cumulative incidence of mechanical ventilation at 28 days, accounting for competing mortality, was 25.6% in patients who received methylprednisolone vs 41.1% in the control group; the corresponding subhazard ratio was 0.56 (95% CI 0.40‐0.79; P = .001), Figure 4. The effect of methylprednisolone was time dependent. During the first week, the subhazard ratio was 0.43 (95% CI 0.30‐0.63; P < .001) and the benefit attenuated significantly afterwards.
Figure 4.
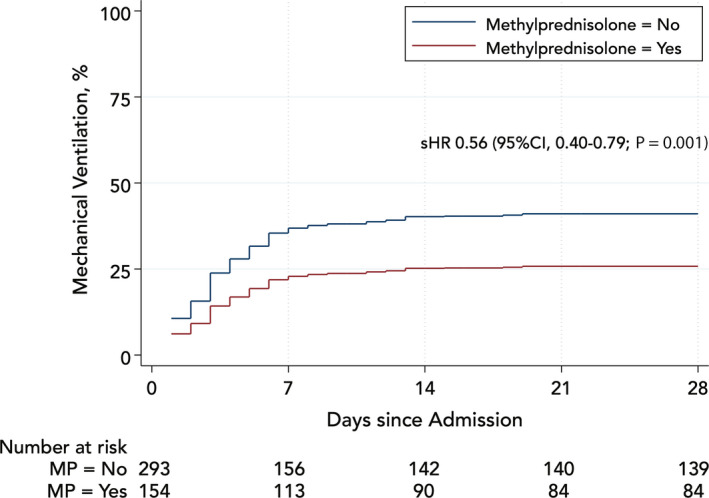
Cumulative incidence of mechanical ventilation, accounting for competing mortality, in methylprednisolone groups weighted by the inverse probability of treatment. MP, methylprednisolone
Mortality at 28 days was not different between patients who received methylprednisolone vs those who did not in crude (24.2% vs 22.1%; log‐rank test P = .64) and weighted (22.6% vs 23.3%, respectively; Cox test P = .87) analyses, Figure S1.
Among the 114 patients who required mechanical ventilation and had not received corticosteroids before intubation, 93/114 (81.6%) received corticosteroids. The 28‐day mortality was 29.8% among those who received post‐intubation corticosteroids vs 34.9% among those who did not. The corresponding hazard ratio in weighted models was 0.77 (95% CI 0.32‐1.86; P = .56). The results were similar when the 50 patients who received methylprednisolone before mechanical ventilation and continued afterwards were included (HR 0.72; 95% CI 0.31‐1.71; P = .46).
3.4. Methylprednisolone and healthcare resource utilization
Patients had a median of 10 hospital‐free days (0‐18) during the first 28 days; 24 ICU‐free days (6‐28); and 28 ventilator‐free days (7‐28). In weighted analyses, the methylprednisolone group had 2.8 more ventilator‐free days (95% CI 0.5‐5.1; P = .017) and 2.6 more ICU‐free days (95% CI 0.2‐4.9; P = .033) during the first 28 days (Table 2). Hospital‐free days did not differ.
Table 2.
Healthcare resource utilization at 28 d according to use of methylprednisolone (estimates weighted according to inverse probability of treatment with methylprednisolone)
| Methylprednisolone | Weighted Estimate | |||
|---|---|---|---|---|
| Yes (N = 153) | No (N = 294) | Δ (95% CI) | P | |
| Patients requiring ICU admission, % | 44.4 | 45.8 | −1.4 (−11.5, 8.7) | .79 |
| Patients requiring mechanical ventilation, % | 29.7 | 42.6 | −12.9 (−22.5, −0.32) | .009 |
| Ventilator‐free days, mean (SE) | 20.8 (0.9) | 18.0 (0.7) | 2.8 (0.5, 5.1) | .017 |
| Days outside the ICU, mean (SE) | 19.6 (0.9) | 17.0 (0.7) | 2.6 (0.2, 4.9) | .033 |
| Days outside the hospital, mean (SE) | 8.7 (0.8) | 9.5 (0.6) | −0.8 (−2.9, 1.3) | .45 |
All estimates are weighted by the inverse probability of treatment using the propensity score for methylprednisolone use.
Abbreviations: CI, confidence interval; ICU, intensive care unit; SE, standard error.
3.5. Complications
Bacteremia occurred in 33 (7.4%) of patients (5.0 events per 1000 patient‐days). Nosocomial pneumonia occurred in 48 (10.7%) of patients (7.3 per 1000 patient‐days). Gastrointestinal bleeding occurred in 18 patients (2.7 per 1000 patient‐days). In both crude and weighted analyses, the rates of bacteremia, nosocomial pneumonia and gastrointestinal bleeding were not higher among patients who received methylprednisolone vs those who did not (Table 3).
Table 3.
Complication rates at 28 d according to use of methylprednisolone (estimates weighted according to inverse probability of treatment with methylprednisolone)
| Methylprednisolone | Weighted Estimate | |||
|---|---|---|---|---|
| Yes (N = 153) | No (N = 294) | IRR (95%CI) | P | |
| Bacteremia, per 1000 patient‐days | 3.8 | 5.5 | 0.58 (0.29‐1.18) | .14 |
| Nosocomial pneumonia, per 1000 patient‐days | 4.3 | 9.0 | 0.43 (0.23‐0.82) | .010 |
| Gastrointestinal bleeding, per 1000 patient‐days | 2.2 | 3.6 | 0.54 (0.19‐1.52) | .24 |
Abbreviations: CI, confidence interval; IRR, incidence rate ratio.
3.6. Sensitivity analyses
The results did not materially change when we excluded 3 patients (2 in the methylprednisolone group) who were receiving chronic oral corticosteroids at home (weighted HR for the primary endpoint: 0.64; 95% CI 0.47‐0.87; P = .005). In an analysis with left‐truncated time for methylprednisolone recipients (to exclude time before methylprednisolone administration), the effect on the primary endpoint persisted (weighted HR 0.69; 95% CI 0.50‐0.95; P = .025).
3.7. Exploratory analyses
In an exploratory analysis, 82 patients received methylprednisolone on day 10 or later vs 71 before day 10. The benefit on the primary endpoint with corticosteroids was driven by administration on day 10 of symptoms or later. The weighted HR for the primary endpoint was 0.44 (95% CI: 0.29‐0.67; P < .001) with administration on day 10 or later (vs no corticosteroids); administration before day 10 of symptoms was not associated with benefit (weighted HR 0.84; 95% CI: 0.58‐1.21; P = .35).
Methylprednisolone initial daily dose was not associated with the primary endpoint; the weighted HR per 10 mg was 0.99 (95% CI: 0.95‐1.02; P = .47). When the dose/weight ratio was used, the weighted HR per 1 mg/kg/d was 0.92 (95% CI 0.66‐1.29; P = .63). However, the dispersion of methylprednisolone dose was relatively small, with a coefficient of variation of 41.6% for absolute and 39.7% for per body weight, providing thus limited power to detect a dose‐response effect.
4. DISCUSSION
In this single‐centre observational study, early administration of methylprednisolone (i.e. before mechanical ventilation) was associated with lower rates of the composite of death or mechanical ventilation at 28 days among patients with severe COVID‐19 pneumonia. This benefit was driven by reduced need for mechanical ventilation by 37%, with a front‐loaded effect that lasted for approximately 5 days. We did not observe an effect on mortality. Methylprednisolone was associated with more ventilator‐ and ICU‐free days, albeit hospital‐free days were similar between groups. Rates of superimposed infections or gastrointestinal bleeding were not higher with methylprednisolone. In an exploratory analysis, the optimal time for initiation of methylprednisolone appeared to be 10 days from symptoms onset or later.
Our results supplement those of the RECOVERY trial 8 and a number of inconclusive clinical trials that were stopped prematurely out of ethical concerns after the positive findings of RECOVERY. 9 , 10 , 11 In RECOVERY, 10 days of 6 mg dexamethasone daily reduced mortality in patients requiring supplementary oxygen; the benefit was even more prominent among patients requiring mechanical ventilation. 8 In the multinational REMAP‐CAP trial, a 7‐day fixed‐dose course of hydrocortisone or shock‐dependent hydrocortisone in severe COVID‐19, compared with no hydrocortisone, resulted in higher odds of improvement in organ support–free days within 21 days, but the trial was stopped early and no strategy met prespecified criteria for superiority. 9 In the French CAPE COVID trial, low‐dose hydrocortisone in severe respiratory COVID‐19 did improve death or ongoing respiratory support by 21 days but did not meet prespecified significance either. 10 In the Brazilian CoDEX trial, open‐label high‐dose dexamethasone (20 mg/d for 5 days, then 10 mg/d for 5 days) in moderate or severe ARDS from COVID‐19 led to 2.6 more ventilator‐free days through day 28, but mortality was not different. 11 No effect on mortality was reported also from the Metcovid trial in Brazil using 0.5 mg/kg methylprednisolone twice daily for 5 days in patients with COVID‐19 requiring supplemental oxygen or mechanical ventilation. 19 In concordance with CoDEX and Metcovid, methylprednisolone in our study was associated with 2.8 more ventilator‐free and 2.6 more ICU‐free days but no difference in mortality. Also, our cohort consisted of nonintubated patients on supplementary oxygen who were a decade younger compared to the corresponding RECOVERY subgroup. In all, our data support the use of methylprednisolone in these patients.
Corticosteroids have been an important therapeutic option when anti‐inflammatory and/or immunosuppressive effects are needed. COVID‐19 is associated with a cytokine profile characterized by activation of multiple pathways, besides interleukins. 3 , 28 Predictors of mortality in a study of confirmed COVID‐19 cases in Wuhan, China, included elevated ferritin and interleukin‐6, suggesting that virally driven hyperinflammation increases mortality. 3 Diffusing across cell membranes, corticosteroids bind to receptors and glucocorticoid response elements, which are associated with genes that either suppress or stimulate transcription. In contrast to other target‐specific immunomodulating therapies, corticosteroids act broadly, inhibiting multiple pathways in the inflammatory process. 13 Because corticosteroids act intracellularly, the effects persist even when detection in the plasma is absent. 13 In our study, patients who received methylprednisolone had lower levels of interleukin‐6 at baseline compared to those who did not. However, interleukin‐6 was missing in 30% of patients in our cohort, as this biomarker was not mandated by institutional protocol, and administration of methylprednisolone was not driven by interleukin‐6 levels in our institution. Therefore, although the theoretical assumption is that corticosteroids would be more beneficial in patients presenting with more intense inflammation, this hypothesis needs to be explicitly tested.
Corticosteroids carry their own side effects and even life‐threatening complications. Pathogenetically, studies have shown that viral replication in SARS‐CoV‐2 is high during the first week of the disease and wears off progressively, 29 followed by intense inflammatory process. 30 Studies with corticosteroids in SARS and MERS showed impaired virus clearance. 5 which should be taken into consideration as there is lack of SARS‐CoV‐2–specific data. Therefore, identifying the time of clinical transition from viral replication to inflammation is of critical importance and that could be the optimal point for initiation of treatment, as corticosteroids have strong and pleiotropic anti‐inflammatory effects. In our study, the benefit was observed after 10 days from symptom onset. The duration of treatment might be an additional key to balance effectiveness on inflammation while minimizing side effects. In our study, the benefit from methylprednisolone attenuated significantly over time and was no longer evident after 5 days. Of note, in the Metcovid trial, which demonstrated benefit with methylprednisolone, the duration of treatment was 5 days. Although our study was not powered to investigate the optimal duration of treatment, we did not observe any adverse signal of increased side effect rates within the limits of our study. 19
In our study, we observed significant decrease in the need for mechanical ventilation as well as days spent on mechanical ventilation and ICU with methylprednisolone. Areas hit hard by the COVID‐19 pandemic face considerable healthcare system constraints, especially related to the availability of ICU beds and ventilators, with potentially detrimental effect on mortality. 31 Therefore, early interventions during admission that can preserve those resources could be critical in areas of the world with limited resources or high incidence of COVID‐19. In this fight, methylprednisolone is an accessible therapeutic option, both in terms of availability and in terms of cost.
Our study has several limitations. First, there are inherent limitations in an observational study. We used a propensity score‐based method (inverse probability of treatment weighting) to minimize bias. However, it is likely that there is still residual confounding by indication and other unobserved confounders, which may have biased our results, especially considering that COVID‐19 is a new entity and we may have thus omitted factors that are associated with the treatment or the outcome. Second, there is a possibility that we have missed deaths after discharge, and this may have artificially inflated days out of hospital within the 28‐day period that was used for outcomes assessment in our analysis. However, these occurrences should be rare and confined to non‐COVID‐19–related deaths, as all COVID‐19–related readmissions took place in our centre. Third, we studied methylprednisolone in patients on high‐flow oxygen, and therefore, our results are not generalizable to milder forms of COVID‐19, although optimal benefit was noted when methylprednisolone was given after 10 days from symptom onset, which is approximately the time of clinical deterioration and need for high‐flow oxygen. Fourth, as evidence on effective COVID‐19 therapies was lacking when the pandemic hit our area in early March 2020, the use and timing of other therapies was variable. Finally, a larger sample size would have allowed us to assess the effect on mortality and complication rates more confidently.
5. CONCLUSIONS
In this observational study, early administration of methylprednisolone was associated with significantly reduced needs for mechanical ventilation and intensive care in patients with severe COVID‐19 pneumonia. Prospective controlled studies will be needed to demonstrate which corticosteroid agent provides the most benefit in this setting and determine the optimal timing and duration in this high‐risk population.
CONFLICT OF INTEREST
There are no potential conflicts of interest and/or financial disclosures associated with this work.
Supporting information
Fig S1
ACKNOWLEDGEMENTS
The authors wish to thank our Renaissance School of Medicine students for their help with data collection and cleaning: Jacquelyn Nakamura, Jenny Fung, Joshua Abata, Nikitha Karkala, Stella T. Tsui, Simrat Dhaliwal, Alexandra Coritsidis and Sahil Rawal.
Papamanoli A, Yoo J, Grewal P, et al. High‐dose methylprednisolone in nonintubated patients with severe COVID‐19 pneumonia. Eur J Clin Invest.2021;51:e13458. 10.1111/eci.13458
REFERENCES
- 1. Schaller T, Hirschbühl K, Burkhardt K, et al. Postmortem examination of patients with COVID‐19. JAMA. 2020;323(24):2518. 10.1001/jama.2020.8907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):1525‐1531. 10.1371/journal.pmed.0030343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arabi YM, Mandourah Y, Al‐Hameed F, et al. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757‐767. 10.1164/rccm.201706-1172OC [DOI] [PubMed] [Google Scholar]
- 6. Ni Y‐N, Chen G, Sun J, Liang B‐M, Liang Z‐A. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta‐analysis. Crit Care. 2019;23(1):99. 10.1186/s13054-019-2395-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou Y, Fu X, Liu X, et al. Use of corticosteroids in influenza‐associated acute respiratory distress syndrome and severe pneumonia: a systemic review and meta‐analysis. Sci Rep. 2020;10(1):3044. 10.1038/s41598-020-59732-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horby P, Lim WS; RECOVERY Collaborative Group , et al. Dexamethasone in hospitalized patients with Covid‐19 — Preliminary Report. N Engl J Med. 2020:NEJMoa2021436. 10.1056/NEJMoa2021436 [Epub ahead of print]. [DOI] [Google Scholar]
- 9. The Writing Committee for the REMAP‐CAP Investigators . Effect of hydrocortisone on mortality and organ support in patients with severe COVID‐19: the REMAP‐CAP COVID‐19 corticosteroid domain randomized clinical trial. JAMA. 2020;324(13):1317‐1329. 10.1001/jama.2020.17022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dequin P‐F, Heming N, Meziani F, et al. Effect of hydrocortisone on 21‐day mortality or respiratory support among critically Ill patients with COVID‐19: a randomized clinical trial. JAMA. 2020;324(13):1298. 10.1001/jama.2020.16761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator‐free in patients with moderate or severe acute respiratory distress syndrome and COVID‐19. JAMA. 2020;324(13):1‐10. 10.1001/jama.2020.17021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. WHO Rapid Evidence Appraisal for COVID‐19 Therapies (REACT) Working Group ; Sterne JAC, Murthy S, et al. Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID‐19: a meta‐analysis. JAMA. 2020;324(13):1‐12. 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Williams DM. Clinical pharmacology of corticosteroids. Respir Care. 2018;63(6):655‐670. 10.4187/respcare.06314 [DOI] [PubMed] [Google Scholar]
- 14. Annane D, Pastores SM, Rochwerg B, et al. Guidelines for the diagnosis and management of Critical Illness‐Related Corticosteroid Insufficiency (CIRCI) in critically ill patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Crit Care Med. 2017;45(12):2078‐2088. 10.1097/CCM.0000000000002737 [DOI] [PubMed] [Google Scholar]
- 15. Ayyar VS, Song D, DuBois DC, Almon RR, Jusko WJ. Modeling corticosteroid pharmacokinetics and pharmacodynamics, Part I: Determination and prediction of dexamethasone and methylprednisolone tissue binding in the rats. J Pharmacol Exp Ther. 2019;370(2):318‐326. 10.1124/jpet.119.257519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):1‐10. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fadel R, Morrison AR, Vahia A, et al. Early short course corticosteroids in hospitalized patients with COVID‐19. Clin Infect Dis. 2020;71(16):2114‐2120. 10.1093/cid/ciaa601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang R, Xiong Y, Ke H, Chen T, Gao S. The role of methylprednisolone on preventing disease progression for hospitalized patients with severe COVID‐19. Eur J Clin Invest. 2020;50(11):e13412. 10.1111/eci.13412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jeronimo CMP, Farias MEL, Val FFA, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with COVID‐19 (Metcovid): a randomised, double‐blind, phase IIb, placebo‐controlled trial. Clin Infect Dis. 2020. 10.1093/cid/ciaa1177 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization . Clinical guidance of severe SARI when Covid19 disease is suspected. Who. Published 2020. https://apps.who.int/iris/bitstream/handle/10665/178529/WHO_MERS_Clinical_15.1_eng.pdf?sequence=1&isAllowed=y. Accessed May 21, 2020.
- 21. Royston P, Parmar MKB. Flexible parametric proportional‐hazards and proportional‐odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21(15):2175‐2197. 10.1002/sim.1203 [DOI] [PubMed] [Google Scholar]
- 22. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496‐509. 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 23. Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013;32(16):2837‐2849. 10.1002/sim.5705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thomas L, Li F, Pencina M. Using propensity score methods to create target populations in observational clinical research. JAMA. 2020;323(5):466‐467. 10.1001/jama.2019.21558 [DOI] [PubMed] [Google Scholar]
- 25. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661‐3679. 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377‐399. 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 27. Reiter JP. Small‐sample degrees of freedom for multi‐component significance tests with multiple imputation for missing data. Biometrika. 2007;94(2):502‐508. 10.1093/biomet/asm028 [DOI] [Google Scholar]
- 28. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420‐422. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature. 2020;581(7809):465‐469. 10.1038/s41586-020-2196-x [DOI] [PubMed] [Google Scholar]
- 30. García LF. Immune Response, Inflammation, and the Clinical Spectrum of COVID‐19. Front Immunol. 2020;11:1441. 10.3389/fimmu.2020.01441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Quah P, Li A, Phua J, Phua J. Mortality rates of patients with COVID‐19 in the intensive care unit: a systematic review of the emerging literature. Crit Care. 2020;24(1):285. 10.1186/s13054-020-03006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1


