Abstract
The goal of this study was to assess the clinical effectiveness and safety profile of the COVID‐19 treatment protocol (containing both hydroxychloroquine (HCQ) and azithromycin) in an Iraqi specialised hospital.
Methods
This prospective study used a pre‐ and post‐intervention design without a comparison group. The intervention was routine Ministry of Health (MOH) approved the management of COVID‐19 for all patients. The study was conducted in a public healthcare setting in Baghdad, Iraq from March 1st to May 25, 2020. The study outcome measures included the changes in clinical and biochemical parameters during the hospitalisation period. Paired t‐test and Chi‐square test were used to compare the measures of vital signs, lab tests and symptoms before and after treatment.
Results
The study included 161 patients who were admitted with positive RT‐PCR and clinical symptoms of COVID‐19. In terms of severity, 53 (32.9%) patients had amild condition, 47 (29.2%) had moderate condition, 35 (21.7%) had severe condition and 26 (16.1%) had critical condition. Most patients (84.5%) recovered and were discharged without symptoms after testing negative with RT‐PCR, while 11 (6.8%) patients died during the study period. The signs and symptoms of COVID‐19 were reduced significantly in response to a therapy regimen containing HCQ and azithromycin. The most common reported side effects were stomach pain, hypoglycemia, dizziness, itching, skin rash, QT prolongation, arrhythmia, and conjunctivitis.
Conclusions
This natural trial showed that the COVID‐19 regimen containing both HCQ and azithromycin can be helpful to promote the recovery of most patients and reduced their signs and symptoms significantly. It also shows some manageable side effects mostly those related to heart rhythm. In the absence of FDA‐approved medications to treat COVID‐19, the repurposing of HCQ and azithromycin to control the disease signs and symptoms can be useful.
Keywords: azithromycin, COVID‐19, effectiveness, hydroxychloroquine, natural trial, safety, treatment
What is already known about this topic?
There is a controversy about using hydroxychloroquine and azithromycin to treat COVID‐19 because of uncertainly about their effectiveness and safety.
What does this article add?
The experience with Iraqi protocol to treat COVID‐19 can help to answer some questions about the effectiveness and safety of this therapy combination which can offer some support to the international community in facing such a vicious pandemic.
This natural trial showed that the COVID‐19 regimen containing both HCQ and azithromycin can be helpful to promote the recovery of most patients and reduced their signs and symptoms significantly.
The trial was not registered because it is a natural trial (quasi‐experiment) which was conducted according to the Iraqi Ministry of Health protocol by a regular healthcare team who is planning to share its experience with the international scientific community.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is a respiratory tract infection caused by a newly emergent coronavirus that was first recognised in Wuhan, China, in December 2019. 1 Genetic sequencing of the virus suggests that it is a beta‐corona virus closely linked to the SARS virus. 2 While most people with COVID‐19 develop the only mild or uncomplicated illness, approximately 14% develop a severe disease that requires hospitalisation and oxygen support, and 5% require admission to an intensive care unit. 3 In severe cases, COVID‐19 can be complicated by acute respiratory distress syndrome (ARDS), sepsis and septic shock, multiorgan failure, including acute kidney injury and cardiac injury. 3 Older age and co‐existing diseases have been reported as risk factors for death. 4 On March 12, the WHO officially declared the COVID‐19 outbreak a pandemic. 5 As of June 24, 2020, COVID‐19 was confirmed in 188 countries with a total confirmed cases of 9,295,365 and more than 478,289 deaths. 6 Iraq had 36,702 confirmed cases, 16,814 recovered cases and 1,330 deaths associated with COVID‐19 pandemic as of June 24, 2020. 6
The pandemic of COVID‐19 is caused by the novel severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) and presents a challenge in regard to identifying effective drugs for prevention and treatment. Up to the time of writing this manuscript, there were no medications approved by the US Food and Drug Administration (FDA) to treat COVID‐19. 7 However, the FDA recently created a new emergency programme, the Coronavirus Treatment Acceleration Programme (CTAP), aimed at speeding up research for the development of COVID‐19 treatments. 8 , 9 As of May 11, 2020, more than 144 clinical trials had been launched to test COVID‐19 treatments, including some drug repurposing or repositioning. 8 , 9 Researchers are also testing medications typically used to treat other conditions to see if they are also effective in treating COVID‐19. For example, hydroxychloroquine (HCQ) and chloroquine are two medications that have been used for many decades to treat malaria and autoimmune conditions such as rheumatoid arthritis and lupus, 10 and azithromycin is an antibiotic commonly used to treat bacterial infections such as bronchitis and pneumonia. They have been shown to have some in vitro activity against viruses such as influenza A and Zika. 11
We are ethically committed to share the experience of Iraqi healthcare providers with COVID‐19 treatment with the scientific community to help answer some questions about the effectiveness and safety of HCQ and azithromycin containing regimen. The Iraqi Scientific Committee at the Ministry of Health (MOH) adopted a treatment protocol on March 1st, 2020 several days after the detection of the first COVID‐19 case in Iraq on February 24, 2020 ( Table 1). 12 The goal of this study was to assess the clinical effectiveness and safety profile of the current COVID‐19 treatment protocol (containing both HCQ and azithromycin) in an Iraqi specialised hospital.
TABLE 1.
Iraqi COVID‐19 treatment protocol (13)
| Therapy regimen | Case Severity |
|---|---|
|
Hydroxychloroquine PO (400mg BID first day then 200 mg BID for 5 days), Azithromycin PO (500mg on the first day, then 250 mg daily for 5 days) |
Covid‐19 patients without pneumonia |
|
Hydroxychloroquine PO (400mg BID first day then 200 mg BID for 14 days), Azithromycin PO (500mg on the first day, then 250 mg daily for 14 days), Tamiflu 75 mg PO BID for 5 days |
Covid‐19 patients with pneumonia in the ward |
|
Hydroxychloroquine PO (400mg BID first day then 200 mg BID for 14 days), Azithromycin PO (500mg on the first day, then 250 mg daily for 14 days), Tamiflu 75 mg PO BID for 5 days, KALETRA (Lopinavir 200 mg /ritonavir 50 mg) BID for 5 days and antibiotic(s) accordingly |
Covid‐19 patients with pneumonia in the ICU |
“PO” means the medication is taken by mouth. ICU = intensive care unit.
BID, twice a day.
2. METHODS
2.1. Study designs, duration and setting
This prospective study used a pre‐ and post‐intervention design. The intervention was the routine MOH approved the management of COVID‐19 for all patients. In other words, this was a natural clinical trial (quasi‐experiment) with no comparison group. The study was conducted in a public healthcare centre, Al‐Shifaa Centre for COVID‐19 pandemic treatment, Medical City, Baghdad, Iraq. The study included all adult patients with a confirmed COVID‐19 infection that were admitted to this hospital over 85 days (from March 1st to May 25, 2020). They were defined as confirmed cases of coronavirus COVID‐19 after the result of a nasopharyngeal sample using Reverse transcription‐polymerase chain reaction (RT‐PCR) comes positive.
Exclusion criteria included known allergy to hydroxychloroquine (HCQ) or chloroquine, any contraindications to HCQ or chloroquine and azithromycin including retinopathy and prolonged QT, patients with severely reduced in left ventricular (LV) function, severely reduced in renal function and G6PD deficiency in addition to pregnant and lactating women.
2.2. Follow‐up protocol and measures
The research team (clinical pharmacist and three specialist physicians) was part of the medical team in the centre. They prospectively collected patient medical information from patients, patient records and attending physicians. The information included demographics, disease and treatment characteristics and clinical outcome. Patient's clinical conditions and routine biochemical tests were assessed at baseline and then every day to evaluate the effect of therapy protocol.
2.3. Study outcomes
The study outcome measures were assessed at baseline (at admission) and daily after hospitalisation (post‐intervention) until the discharge of the recovered patient or death. The study outcome measures included the changes in clinical and biochemical parameters during hospitalisation period such as the disappearance of clinical symptoms, virologic clearance and occurrence of side effects. The routine assessment of vital signs was conducted three times daily. Virologic clearance was measured using real‐time PCR which was retested at day 6 post hospitalisation. Complete recovery was defined as two negative RT‐PCR tests (which were usually performed on days 6th and 7th of hospitalisation). Fourteen days after hospital discharge, recovered patients were retested in outpatient clinics using RT‐PCR.
Other outcome measures included the time to clinical recovery (TTCR) and final clinical prognosis (recovery vs death). The TTCR is defined as the time to the disappearance of clinical symptoms (eg, cough and shortness of breath, SOB) and a normal body temperature and respiratory rate for more than 72 hours.
2.4. Study patients
Study participants with confirmed cases of COVID‐19 were transferred to the study specialised centre after having a positive RT‐PCR test at one of the Medical City Hospitals. After admission, COVID‐19 patients were classified according to clinical evaluation to mild cases (no pneumonia on a CT scan), moderate cases (pneumonia on a CT scan), severe cases (respiratory rate ≥ 30 breaths /min, oxygen saturation ≤ 93% or patients with pneumonia on a CT scan) and critical cases (respiratory failure/need mechanical ventilation). All patients were treated according to the MOH treatment protocol which relies on patient severity status (Table 1).
To assess radiological changes, a chest CT scan was conducted before starting the treatment (day 0) and after 5 days of treatment (day 6). Pulmonary status was classified into three levels: Exacerbated, unchanged, and improved. Medication safety was also assessed by monitoring adverse drug reactions (ADRs), vital signs and lab tests. Routine lab tests during the treatment period included liver function tests, renal function tests and fasting blood sugar. Liver function tests included alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and total bilirubin. Renal function was assessed using serum urea and creatinine (s.cr). The WHO‐UPPSALA causality assessment criteria were used to determine the causality of adverse drug reactions (ADRs) of hydroxychloroquine and azithromycin. 13
All data were recorded in the patient medical record. Consent was obtained from the patients before giving the therapy regimen. The researchers de‐identified patient names and addresses to keep patient confidentiality. The study was approved by the Higher Ethical Committee at Medical City Hospitals.
2.5. Statistical analysis
Statistical Package for the Social Sciences 22 (SPSS, Chicago, IL, USA) was used to analyse the data. For descriptive analysis, categorical variables were presented as frequencies and percentages, while continuous variables were described using mean and standard deviation. A paired t‐test was used to compare the means of vital signs and lab tests before and after treatment. The Chi‐square test was used to compare categorical variables (presence of fever and cough) before and after treatment. A P‐value < .05 was considered to be statistically significant.
3. RESULTS
The study included 161 patients [52 (32.3%) women, 109 (67.7%) men] who were admitted with positive RT‐PCR and clinical symptoms of COVID‐19 (Table 2). The participants’ mean age was 44.3 (±17.0) years and average bodyweight was 81.3 (±9.6). The disease was prevalent amongst the middle age group (41‐50 years). One‐fifth of patients (19.3%) acquired the infection when they were visiting Iran. Approximately two‐thirds (63.4%) of patients were infected by contact with a family member, while 17 (10.6%) patients obtained COVID‐19 from an unknown person. Some patients were healthcare providers/staff (6.8%) who were infected because of their work in hospitals. In terms of severity, 53 (32.9%) patients had mild condition, 47 (29.2%) had moderate condition, 35 (21.7%) had severe condition, and 26 (16.1%) had critical condition. Approximately, one‐quarter (23.6%) of the patients were admitted to the respiratory care unit (RCU) (Table 2). All the 11 deceased patients were admitted in critical condition and had co‐existing diseases.
TABLE 2.
Demographic and disease characteristics for patients
| Characteristics | N (%) |
|---|---|
| Age (y) | |
| 18‐30 | 35 (21.73) |
| 31‐40 | 30 (18.63) |
| 41‐50 | 41 (25.46) |
| 51‐60 | 31 (19.25) |
| 61‐80 | 24 (14.90) |
| Total | 161 (100) |
| Gender | |
| Female | 52 (32.3) |
| Male | 109 (67.7) |
| Exposure and transmission | |
| Travellers returning from affected areas | 32 (19.9) |
| Exposure direct contact with patient (most of them the same family) | 102 (63.35) |
| Contact with unknown person | 17 (10.55) |
| Healthcare provider/ staff | 11(6.83) |
| Co‐existing diseases | |
| Hypertension | 38 (23.60) |
| Diabetes with A1c > 7.6% | 37 (22.98) |
| Ischemic heart diseases | 8 (4.96) |
| Chronic kidney diseases | 4 (2.48) |
| Asthma | 8 (4.96) |
| History of transplant or other;; Immunosuppression | 2 (1.24) |
| Hepatitis B, C | 2 (1.24) |
| Systemic lupus erythematosus (SLE) | 1 (0.62) |
| Pulmonary hypertension | 1 (0.62) |
| Lymphocytopenia at admission | 30 (18.63) |
| Condition severity | |
| Mild | 53 (32.91) |
| Moderate | 47 (29.19) |
| Sever | 35 (21.73) |
| Critical | 26 (16.14) |
| Needed respiratory care unit (RCU) | 38 (23.60) |
| Needed oxygen therapy | 61 (37.88) |
| Characteristic | Mean (ST Dev) |
| Age (y) | 44.60 ± 16.44 |
| Body weight (kg) | 81.79 ± 9.64 |
| Duration of starting symptoms before admission (days) | 5.24 ± 2.57 |
The most common symptoms of COVID‐19 patients at admission were cough (64.6%), headache (56.5%), shortness of breath (SOB) (47.2%), sore throat (41.0%), diarrhoea (36.6%), exertional dyspnea (32.9%), abdominal pain (31.1%), tiredness (31.1%), loss of appetite (29.8%), vomiting (23.6%), chest tightness (15.5%) (Table 3).
TABLE 3.
COVID‐19 patient symptoms at admission
| Symptom | Frequency (N) | Percent (%) |
|---|---|---|
| Cough | 104 | 64.6 |
| Headache | 91 | 56.5 |
| Shortness of breath (SOB) | 76 | 47.2 |
| Sore throat | 66 | 41.0 |
| Diarrhea | 59 | 36.6 |
| Exertional dyspnea | 53 | 32.9 |
| Abdominal pain | 50 | 31.1 |
| Tiredness | 50 | 31.1 |
| Loss of appetite | 48 | 29.8 |
| Vomiting | 38 | 23.6 |
| Chest tightness | 25 | 15.5 |
| Sneezing | 7 | 4.3 |
| Runny nose | 2 | 1.2 |
| Loss of taste/smell | 1 | 0.6 |
| Total | 161 | 100.0 |
Most patients (84.5%) recovered and were discharged without symptoms after testing negative RT‐PCR. However, 14 (8.7%) patients were discharged with mild symptoms (mild cough and/or exertional dyspnea). All recovered patients were required to be retested with RT‐PCR 14 days after their hospital discharge.
Moreover, 11 (6.8%) patients died during the study period. Eight of them died before completing the five‐day treatment course (six died within the first 24 hours and two died after 24 hours), while three died after one week of treatment in the intensive care unit (ICU) (Table 4). The deceased patients were admitted to the hospital in critical condition.
TABLE 4.
The clinical outcomes of COVID‐19 patients
| Patient category | N (%) |
|---|---|
| Total number of admitted patients | 161 (100) |
| Recovered patients | 136 (84.47) |
| Discharged (recovered) patients with mild symptoms | |
| Negative RT‐PCR with mild cough or exertional dyspnea | 14 (8.69%) |
| Death | |
| Within 24 hours | 6 (3.72%) |
| After 24 hours | 2 (1.24%) |
| During the treatment | 3 (1.86%) |
| Total death | 11 (6.83) |
| Hospitalisation days for recovered patients | |
| Mean ± SD | 12.88 ± 5.53 |
Abbreviation: SOB, shortness of breath.
The average period of patient hospitalisation was 12.9 (±5.5) days. However, the duration of treatment was up to 14 days (for patients with pneumonia). The standard COVID‐19 regimen which included hydroxychloroquine (HCQ) and azithromycin had a significant positive effect (P‐value < .05) on patient body temperature, cough, SOB, respiratory rate (RR), pulse rate (PR), and fasting blood sugar (Table 5). In other words, those signs and symptoms of COVID‐19 patients were reduced significantly after using a therapy regimen containing HCQ and azithromycin. However, there were non‐significant effects of treatment (P‐value ≥ .05) on systolic blood pressure (SBP), diastolic blood pressure (DBP), AST, ALT, ALP, total bilirubin, serum urea, and serum creatinine (Figures 1 and 2).
TABLE 5.
The effect of treatment on clinical condition and biochemical parameters of patients with COVID‐19
| Parameters | Before treatment | After treatment | P‐value |
|---|---|---|---|
| Symptoms | N (%) | N (%) | |
| Cough | 104 (64.60%) | 14 (8.70%) | .0000* |
| SOB | 76 (47.21%) | 11 (6.83%) | .0000* |
| Fever | 38.25 ± 0.64 | 36.53 ± 0.39 | .0000* |
| Vital signs | Mean ± SD | Mean ± SD | |
| RR | 23.43 ± 5.55 | 19.66 ± 2.32 | .0000* |
| SBP | 129.27 ± 16.68 | 126.57 ± 14.88 | .1260 |
| DBP | 83.77 ± 10.64 | 84.76 ± 10.46 | .4015 |
| PR | 96.36 ± 8.31 | 78.21 ± 3.05 | .0000* |
| Lab tests | |||
| FBS mmol/L | 9.61 ± 5.65 | 6.95 ± 1.72 | .0000* |
| WBCs *103 | 11.20 ± 3.33 | 6.67 ± 1.97 | .0000* |
| Hg g\dl | 12.74 ± 1.75 | 12.70 ± 1.54 | .7969 |
| S.Cr | 1.00 ± 0.94 | 0.95 ± 0.69 | .6451 |
| Urea | 26.04 ± 34.77 | 25.61 ± 33.85 | .9112 |
| AST | 25.32 ± 13.16 | 27.20 ± 9.34 | .1387 |
| ALT | 33.63 ± 28.36 | 34.80 ± 20.15 | .6925 |
| ALP | 106.47 ± 71.16 | 107.27 ± 47.38 | .9054 |
| Bilirubin | 0.73 ± 0.70 | 0.75 ± 0.57 | .7391 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DBP, diastolic blood pressure; FBS, fasting blood sugar; PR, pulse rate; RR, respiratory rate; S.Cr, serum creatinine; SBP, systolic blood pressure; SOB, shortness of breath; WBCs, white blood cells.
Significant P‐value ≤ 0.05 according to paired T‐test or Chi‐square.
FIGURE 1.
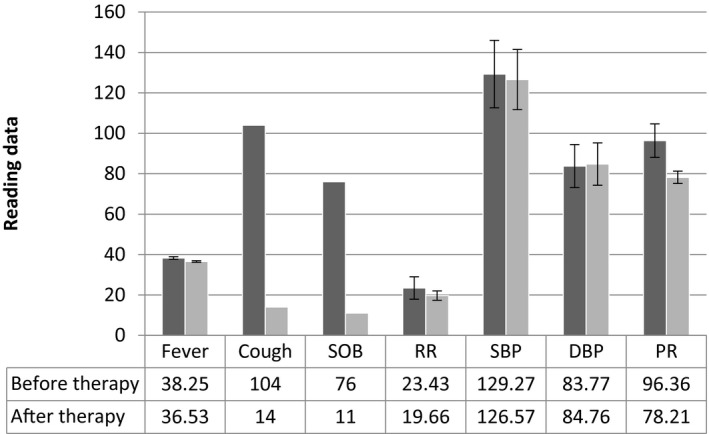
The effect of treatment on patient clinical condition (signs and symptoms). Before therapy was measured at baseline (at admission). Vital sign measurements after therapy equal the average of the last three‐day measurements. Frequency of patients with symptoms (cough/SOB) after therapy was measured on the last day before discharge. SOB, shortness of breath, RR, respiratory rate, SBP, systolic blood pressure, DBP, diastolic blood pressure, PR, pulse rate
FIGURE 2.
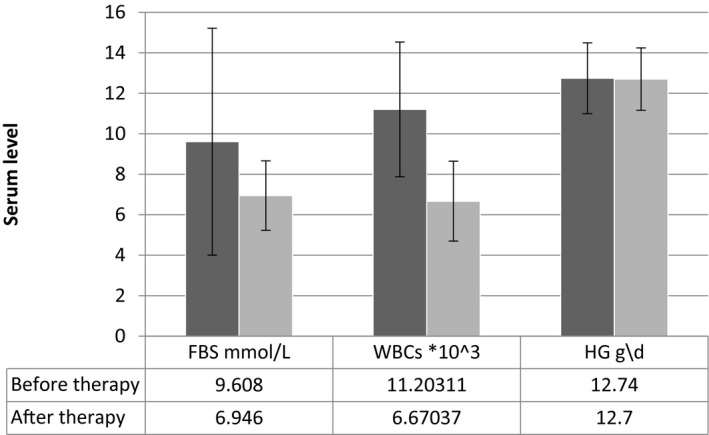
The effect of treatment on FBS, WBCs, and Hg levels. FBS, fasting blood sugar; WBCs, white blood cells; Hg, hemoglobin
In terms of adverse drug reactions (ADRs), the most commonly reported ones were stomach pain/cramps, hypoglycemia, headaches, mood changes, dizziness, itching, diarrhoea, muscle weakness, nausea, skin rash, vomiting, QT prolongation, arrhythmia, and conjunctivitis (Figure 3). Most ADRs were possible to be caused by HCQ and/or azithromycin. It means they also can be caused by the disease (COVID‐19). Moreover, three ADRs including arrhythmia, QT prolongation and conjunctivitis were probably caused by the two medications (Table 6). In other words, they unlikely to be attributed to COVID‐19 disease. The electrocardiograph (ECG) of six (3.7%) patients recorded QTc prolongation, but not more than 450ms. Additionally, arrhythmia was reported in three (1.9%) other patients (Table 6). The medical team stopped the HCQ and azithromycin treatment for three patients who experienced arrhythmia. Then electrolyte levels (calcium and potassium) were measured and corrected to treat the arrhythmia. However, this treatment stoppage occurred after completing the first five days of the therapy course.
FIGURE 3.
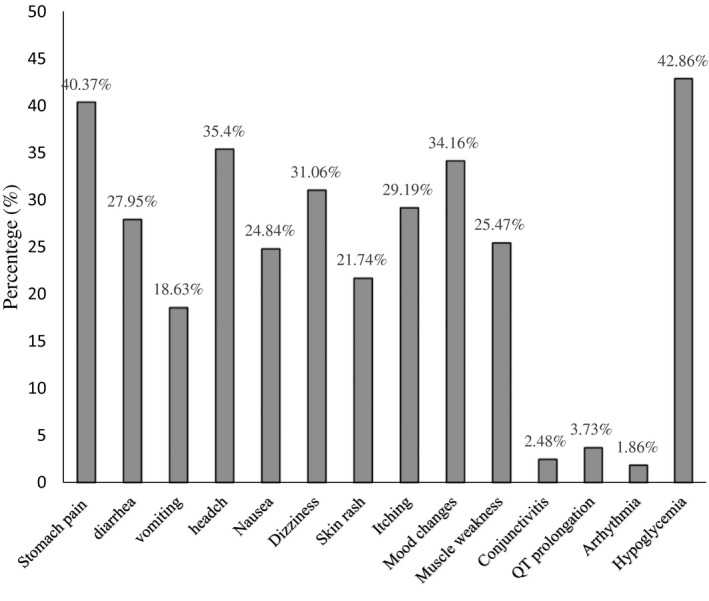
The reported adverse reactions of treatment on patients with COVID‐19
TABLE 6.
The reported adverse reactions of HCQ and azithromycin containing treatment on patients with COVID‐19
| Side effects | N (%) | Causality term |
|---|---|---|
| Stomach pain or cramps | 65 (40.37%) | possible |
| Hypoglycemia | 60 (42.86%) | possible |
| Headaches | 57 (35.40%) | possible |
| Mood changes | 55 (34.16) | unlikely |
| Dizziness | 50 (31.06% | possible |
| Itching | 47 (29.19%) | possible |
| Diarrhoea | 45 (27.95%) | possible |
| Muscle weakness | 41 (25.47%) | possible |
| Nausea | 40 (24.84%) | possible |
| Skin rash | 35 (21.74%) | possible |
| Vomiting | 30 (18.63%) | possible |
| QT prolongation | 6 (3.73%) | probable |
| Conjunctivitis | 4 (2.48%) | probable |
| Arrhythmia | 3 (1.86%) | probable |
Probable = Unlikely to be attributed to disease or other drugs. Possible = Could also be explained by disease or other drugs. Unlikely = Disease or other drugs provide plausible explanations 13
4. DISCUSSION
The pandemic of coronavirus disease 2019 (COVID‐19) caused by the SARS‐CoV‐2 presents a challenge in terms of identifying effective drugs for prevention and treatment. 14 HCQ has a long history as a safe and inexpensive drug for malaria and autoimmune diseases despite some eye and cardiac side effects. 15
During the study period (from March 1 to May 25, 2020) and before the exponential increase in COVID‐19 cases in Iraq (>1000 cases/day) which started on June 5, 2020, admission to one of the MOH quarantine facilities was required by law for confirmed cases. Thus, all patients who tested positive were treated in government COVID‐19 specialised facilities. The study centre was one of the 22 specialised COVID‐19 centres across the country. The number of participants in this study represents 3.5% of the total Iraqi COVID‐19 cases during the study period. The therapy regimen was the same across all 22 specialised COVID‐19 in Iraq as it was implemented by the MOH. According to the MOH therapy protocol, HCQ and azithromycin should be given for all COVID‐19 cases and other anti‐viral agents/antibiotics can be added for severe and critical cases. 12 During the study period, the use of convalescent plasma to treat COVID‐19 had not been started in the study setting.
In this study, the treatment regimen (containing both HCQ and azithromycin) appeared to help promote recovery in 150 COVID‐19 patients (83.2%) in the study centre. Although 16% of patients were admitted in critical condition and 26% were admitted to the RCU, the mortality rate was only 6.8%. It is worth mentioning that most deceased patients were admitted in critical condition and died before receiving a full course of treatment. The same MOH therapy regimen facilitate recovery in 2,811 cases out of 4,632 COVID‐19 patients in Iraq during the study period, and only 163 deaths (3.52%) were reported. 6 This high recovery rate was probably because of the effectiveness of the treatment protocol and close disease monitoring by healthcare providers. They work hard despite several challenges facing the Iraqi healthcare system including a shortage in the number of quarantine facilities, a shortage in personal protective equipment (PPE) and limited RT‐PCR testing. 16
The average hospitalisation period was 12.9 (±5.5 days) given there was a small number of admissions per day during the study period and no high demand for hospital beds. This duration may reflect the fact that about two‐thirds of admitted patients developed pneumonia and needed time to recover. Moreover, all those who tested negative with RT‐PCR on days 6 and 7 after admission were discharged on day 8. The results of our study were comparable to a French clinical trial to evaluate the role of HCQ and Azithromycin on respiratory viral loads in patients with confirmed COVID‐19. 17 The study found a significant reduction of the viral carriage in the HCQ/azithromycin group compared with the control group at day 6 post‐inclusion. The study reported 100% viral clearance in nasopharyngeal swabs with the combination of HCQ and azithromycin compared with 57.1% in HCQ alone group and 12.5% in patients who did not receive HCQ (P < .001). 17 Chloroquine and HCQ appear to block viral entry into cells by inhibiting the glycosylation of host receptors, proteolytic processing, and endosomal acidification. 18 , 19 These agents also have immunomodulatory effects through the attenuation of cytokine production and inhibition of autophagy and lysosomal activities in host cells. 19 , 20 HCQ has in‐vitro activity against SARS‐CoV‐2 with half‐maximal effective concentration (EC50) of 6.14 μM which is lower than that of chloroquine (EC50 = 23.90 μM) after 24 hours of growth. 18
Moreover, some manageable side effects of HCQ were reported during the treatment period. These side effects may disappear during treatment as the body adjusts to the medicine. 21 The reported side effects included stomach pain/cramps, hypoglycemia, headaches, mood changes, dizziness, itching, diarrhoea, muscle weakness, nausea, skin rash, and vomiting. Those are common HCQ side effects and appear to be dose‐dependent and most often occur with loading doses of 800 mg or higher. 22 In this study, we used an HCQ maintenance dose of 400 mg daily for a short‐term course which is relatively safe since the recommended daily dose was not exceeded. The most severe and life‐threatening complications from the use of hydroxychloroquine include QT interval prolongation and the resultant risk of ventricular arrhythmias. 23 In our study, the electrocardiogram (ECG) monitoring showed that six patients developed QT interval prolongation, but not more than 450ms which did not require medical intervention. In contrast, three patients developed arrhythmia and needed medical intervention which consisted of stopping the treatment and normalising the blood electrolytes (potassium and calcium). The incidence of QT interval prolongation with using chloroquine and hydroxychloroquine is highly dependent on baseline ECG findings, with risk exacerbated using concomitant QT‐interval prolonging medications. 24 The combination of azithromycin with HCQ frequently prolongs the QT interval in a clinically significant manner, increasing over time and requiring additional monitoring. 25
Chinese Centre for Disease Control and Prevention includes chloroquine in the treatment guideline for COVID‐19 patients which gave a high recovery rate (94.5%). 26 A recent study relying on a multinational registry analysis which was published on May 22, 2020, found no evidence of hydroxychloroquine effectiveness to treat COVID‐19. 27 However, this study was retracted after some expressed concerns about its findings. On March 28, 2020, the US FDA issued Emergency Use Authorisations (EUA) that allowed the use of hydroxychloroquine to treat COVID‐19. On June 15, 2020, the FDA revoked this EUA because of the absence of scientific evidence. 28 This controversy about using hydroxychloroquine to treat COVID‐19 is because of the absence of findings based on well‐designed randomised controlled trials (RCTs). Although there is a debate about the effectiveness and safety of using hydroxychloroquine, the new Iraqi guideline (on June 1, 2020) still includes HCQ to treat mild and moderate cases of COVID‐19 since it has been showing promising results and because of the absence of superior alternatives, in addition to its affordability and availability.
This study had some limitations. It was conducted in a single‐centre without a comparison group. Single‐centre findings may not be generalisable. However, a universal therapy regimen has been implemented by the health top authority across all country healthcare settings. Lack of a comparison group can limit the determination of causality between the therapy and the cure or good prognosis. Additionally, some disease‐induced symptoms overlap with ADRs during treatment period.
5. CONCLUSIONS
This natural trial showed that the COVID‐19 regimen containing both HCQ and azithromycin is helpful in treating the majority of patients and reduced their signs and symptoms significantly. It also causes some manageable side effects mostly those related to heart rhythm. In the absence of FDA‐approved medications to treat COVID‐19, the repurposing of HCQ and azithromycin to control the disease signs and symptoms is potentially useful. The study showed that using this therapy combination (HCQ and azithromycin) is promising and can fill the gap until more effective treatments are found. Future randomised controlled trial can give more definite answers about the effectiveness and safety of such therapy protocol.
CONFLICT OF INTEREST
No conflict of interest to declare and the study received no fund.
AUTHOR CONTRIBUTIONS
Hassan M Abbas, BS Pharm, MS, PhD, Participated in study conceptualisation/designing, writing manuscript and approval of the article. Ali Azeez Al‐Jumaili, BS Pharm, MS, MPH, PhD, Participated in study designing, analyses, drafting and revising the manuscript, in addition, to prepare the manuscript for submission. Kawthar F Nassir, BS Pharm, FIBMS, Participated in study designing, collecting data, writing the manuscript and approval of the article. Muhammed Waheeb Al‐Obaidy, MD, FCCP, FRCPE, Participated in study designing, collecting data and approval of the article Adnan Mohammed Al Jubouri, MD, FCCP, FRCPE, Participated in study designing, collecting data and approval of the article. Basim Dhawi Dakhil, MD, FIBMS, Participated in study designing, writing manuscript and approval of the article. Mohammed Mahir Abdulelah, MD, FIBMS, Participated in study designing, collecting data and approval of the article. Qutaiba Ahmed Al Khames, BS Pharm, MS, PhD, Participated in data analysis, writing manuscript and approval of the article. All the co‐authors approved the last version of the manuscript.
ACKNOWLEDGEMENTS
The authors would like to thank Dr Jawad Rasheed, Dr Ali AL‐Garrawy and all the physicians and healthcare provider in Al‐Shifaa Centre for Corona pandemic at Medical City, Baghdad who helped the research team conduct the study. If they had not bravely risked their lives to treat the COVID‐19 cases, there would not be such a high recovery rate amongst patients.
Abbas HM, Azeez Al‐Jumaili A, Nassir KF, et al. Assessment of COVID‐19 Treatment containing both Hydroxychloroquine and Azithromycin: A natural clinical trial. Int J Clin Pract.2021;75:e13856. 10.1111/ijcp.13856
REFERENCES
- 1. Guo Y‐R, Cao Q‐D, Hong Z‐S, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak‐ A n update on the status. Mil Med Res. 2020;7(1):1‐10. 10.1186/s40779-020-00240-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. MacKenzie JS, Smith DW. COVID‐19: A novel zoonotic disease caused by a coronavirus from China: What we know and what we don’t. Microbiol Aust. 2020;41(1):45‐50. 10.1071/MA20013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tamblyn S. Clinical management of patients with moderate to severe COVID‐19 ‐ Interim Guidance. Public Heal Agency Canada. 2020;1‐20. [Google Scholar]
- 4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cucinotta D, Vanelli M. WHO declares COVID‐19 a pandemic. Acta Biomed. 2020;91(1):157‐160. 10.23750/abm.v91i1.9397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. And C for SS, Engineering (CSSE) JHU (JHU). COVID‐19 Dashboard. https://coronavirus.jhu.edu/map.html. Published 2020. Accessed June 24, 2020.
- 7. Ullah MA, Araf Y, Sarkar B, Moin AT, Reshad RAI. Pathogenesis, Diagnosis and Possible Therapeutic Options for COVID‐19. 2020; 10.20944/PREPRINTS202004.0372.V1 [DOI]
- 8. FDA . Coronavirus Treatment Acceleration Program (CTAP). https://www.FDA.gov/drugs/coronavirus‐covid‐19‐drugs/coronavirus‐treatment‐acceleration‐program‐ctap. 2020. Accessed June 3, 2020.
- 9. D’Acquarica I, Agranat I. Chiral switches of chloroquine and hydroxychloroquine: potential drugs to treat COVID‐19. Drug Discov Today. 2020;. 10.1016/j.drudis.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gbinigie K, Frie K. Should chloroquine and hydroxychloroquine be used to treat COVID‐19? A rapid review. BJGP Open. 2020;4(2):bjgpopen20X101069. 10.3399/bjgpopen20X101069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Damle B, Vourvahis M, Wang E, Leaney J, Corrigan B. Clinical pharmacology perspectives on the antiviral activity of azithromycin and use in COVID‐19. Clin Pharmacol Ther. 2020;1‐11. 10.1002/cpt.1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Allawi JS, Abbas HM, Rasheed JI, et al. The first 40‐days experience and clinical outcomes in the management of coronavirus covid‐19 crisis. Single center preliminary study. J Fac Med Baghdad. 2019;61(3,4). [Google Scholar]
- 13. Center WHO‐TUM . WHO Causality assessment. Good Pharmacovigil Pract Guid. 2009;(3):39. [Google Scholar]
- 14. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and coronavirus disease‐2019 (COVID‐19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. 10.1016/j.ijantimicag.2020.105924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haładyj E, Sikora M, Felis‐Giemza A, Olesinska M. Antimalarials ‐ are they effective and safe in rheumatic diseases? Reumatologia. 2018;56(3):164‐173. 10.5114/reum.2018.76904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mikhael EM, Al‐Jumaili AA. Can developing countries face novel coronavirus outbreak alone? The Iraqi situation. Public Heal Pract. 2020;1:100004. 10.1016/j.puhip.2020.100004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gautret P, Lagier J‐C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949. 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Al‐Bari MAA. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol Res Perspect. 2017;5(1):1‐13. 10.1002/prp2.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): A Review. JAMA‐J Am Med Assoc. 2020;323(18): 10.1001/jama.2020.6019 [DOI] [PubMed] [Google Scholar]
- 20. Meyerowitz EA, Vannier AGL, Friesen MGN, et al. Rethinking the role of hydroxychloroquine in the treatment of COVID‐19. FASEB J. 2020;34(5):6027‐6037. 10.1096/fj.202000919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ikeda Y, Rokutanda MO. The side effect and the retention rate of hydroxychloroquine in Japan. St Luke’s Int Hosp Tokyo, Japan. 2018;5(Suppl 1):A1‐A129. [Google Scholar]
- 22. Tétu P, Hamelin A, Lebrun‐Vignes B, et al. Prevalence of hydroxychloroquine‐induced side‐effects in dermatology patients: A retrospective survey of 102 patients. Ann Dermatol Venereol. 2018;145(6–7):395‐404. 10.1016/j.annder.2018.03.168 [DOI] [PubMed] [Google Scholar]
- 23. O’Laughlin JP, Mehta PH, Wong BC. Life threatening severe QTc prolongation in patient with systemic lupus erythematosus due to hydroxychloroquine. Case Reports Cardiol. 2016;2016:1‐4. 10.1155/2016/4626279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gérard A, Romani S, Fresse A, et al. “Off‐label” use of hydroxychloroquine, azithromycin, lopinavir‐ritonavir and chloroquine in COVID‐19: A survey of cardiac adverse drug reactions by the French Network of Pharmacovigilance Centers. Therapies. 2020; 10.1016/j.therap.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramireddy A, Chugh H, Reinier K, et al. Experience with Hydroxychloroquine and Azithromycin in the COVID‐19 Pandemic: Implications for QT Interval Monitoring. medRxiv. 2020;21(1):1–9. 10.1016/j.solener.2019.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chinese Centre for Disease Control and Prevention. COVID‐19 Prevention and Control. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mehra MR, Desai SS, Ruschitzka F, Patel AN. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID‐19: a multinational registry analysis. Lancet. 2020; 10.1016/S0140-6736(20)31180-6 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28. Coronavirus ( COVID‐19 ) Update : FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine Inquiries. 2020;1‐3.


