Abstract
COVID‐19 pandemic leads to health challenges globally, and its diverse aspects need to be uncovered. Multi‐organ injuries have been reported by describing potential SARS‐CoV‐2 entrance routes: ACE2 and TMPRSS2. Since these cell surface receptors’ expression has been disclosed within the male reproductive system, its susceptibility to being infected by SARS‐CoV‐2 has been summarised through this literature review. Expression of ACE2 and TMPRSS2 at RNA or protein level has been reported across various investigations indicates that the male genitalia potentially is vulnerable to SARS‐CoV‐2 infection. Presence of SARS‐CoV‐2 within semen samples and following direct viral damage, secondary inflammatory response causing orchitis or testicular discomfort and finally the amount of viral load leading testicular damage and immune response activation are among probable underlying mechanisms. Therefore, genital examination and laboratory tests should be considered to address the male reproductive tract complications and fertility issues.
Keywords: ACE2, COVID‐19, male genital tract, spermatogenesis, TMPRSS2
1. INTRODUCTION
As novel severe acute respiratory syndrome coronavirus (SARS‐CoV‐2) is spreading worldwide since the first cases reported in Wuhan, China, in December 2019, several biological scientists’ attention has been focused on its pathogenesis (Adhikari et al., 2020; Park et al., 2020; WHO, 2020, July, 9). Respiratory symptoms have been reported significantly among confirmed cases, initiating by nasopharyngeal entrance ending up in Acute Respiratory Distress Syndrome (ARDS) in severe cases by provoking cytokine storm (Huang et al., 2020; Jiang et al., 2020). However, other target organs have become a significant concern, such as cardiovascular system damage and kidney injury (Li, Yang, et al., 2020; Zhou, Zhang et al., 2020). Moreover, digestive system disturbances, coagulopathies, even CNS dysfunction and dermatologic manifestations have raised the diagnosis and treatment complexity (Baig et al., 2020; Li, Yang, et al., 2020; Wong et al., 2020). However, less attention has been drawn to reproductive organs while being susceptible to SARS‐CoV‐2 as discussed below.
According to viruses’ characteristics and replication processes within the host cell, attachment and penetration mechanisms are of great importance across viral infections; thus, SARS‐CoV‐2 RNA‐virus pathogenesis could be described (Guo, Cao, et al., 2020).
Prior research demonstrated the capability of SARS‐CoV‐2 entry via angiotensin‐converting enzyme 2 (ACE2) receptor among ACE2‐expressing cells in the human body (Wang, Zhang, et al., 2020; Zhou, Yang, et al., 2020), which binds to virus proteins and activates surface transmembrane protease serine 2 (TMPRSS2) to facilitate the virus‐receptor fusion (Letko et al., 2020; Oberfeld et al., 2020).
ACE2 expression in various human tissues such as pulmonary, cardiovascular, renal, gastrointestinal tract and urogenital systems makes them potentially vulnerable to the virus (Guo, Yu, et al., 2020; Sommerstein et al., 2020; Wang, Zhang, et al., 2020). Several scientific works has been done around various symptoms and different organ failures, except the reproductive system, which is one of the least noticed organs containing the ACE2 receptor.
Furthermore, TMPRSS2, as an essential part of virus entrance and activation, has significantly expressed in the prostate (Ko et al., 2015). TMPRSS2, as a transmembrane protease, is expressed in high levels in the intestine, also in the prostate, colon, salivary gland and stomach at lower levels. Moreover, the proteolytic role of TMPRSS2 in other viruses such as human coronaviruses, human metapneumovirus, human parainfluenza and influenza A virus have described previously (Huret et al., 2003).
Since reproductive health is of an ever‐growing issue among populations, and its adverse sequels could lead to severe complications, varying from physical disturbances to psychological difficulties, this review study tried to pose a significant challenge to short‐ and long‐term genital complications of SARS‐CoV‐2 and to illustrate the picture of SARS‐CoV‐2 male reproductive involvement due to potential target of direct damage by virus‐receptor binding activity (Li, Yin, et al., 2020; Qing & Gallagher, 2020).
This literature review developed an attempt to male fertility problems associated with COVID‐19 infection, suggesting more attention to the history of COVID‐19 infection among infertility cases in practice, whether the patients were infected previously and have presented with the complications, or newly diagnosed ones with COVID‐19. Therefore, after the pandemic, including COVID‐19 infection to the infertility causes in men might be necessary.
2. MATERIAL AND METHODS
For this literature review, three datasets consisting: PubMed/MEDLINE, Science Direct and Google Scholar were searched for terms “COVID‐19”, “Male genital Tract”, “Spermatogenesis” and “Orchitis” in first place. Secondly “ACE‐2” and “TMPRSS2” were searched. We focused on journals with high quality according to their validity and reliability scores in terms of their impact factor.
Since the subject has recently been introduced, we considered it as a whole and then discussed in detail.
3. VIRAL INFECTIONS AND MALE GENITAL TRACT
The importance of viral infections and involvement of the male genitalia has highlighted in several studies, leading to detection of over 30 viruses shedding into semen to date (Salam & Horby, 2017; Tortorec et al., 2020). In the context of more familiar ones: mumps virus (MuV), human immunodeficiency virus (HIV), hepatitis viruses and zika virus (ZIKV) are among those which spreading through viremia and breaking the blood–testis barrier, led to various complications such as orchitis, epididymitis and sperm count or quality alterations (Dejucq & Jégou, 2001; Liu et al., 2018; Salam & Horby, 2017).
The blood–testis barrier, formed by the Sertoli cell junctional complex for seminiferous separation into basal components, is vital to regulation of nutrients, molecules and immune compartments for seminiferous tubules (Jonathan et al., 2016). According to the literature, blood–testis barrier damages cause immune deficit and consequent defective spermatogenesis and sterility (Jiang et al., 2014) (Figure 1a).
FIGURE 1.
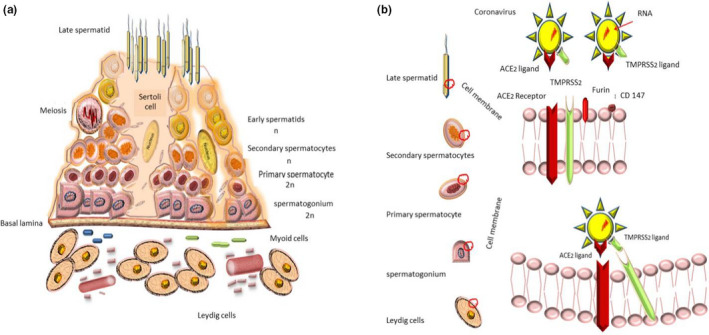
(a) The spermatogenesis process is initiated by spermatogonia's mitosis, and meiosis led to spermatid formation. Blood‐testis‐barrier organising by Sertoli cells makes specific virus blockade environment. (b) It is speculated that co‐expression is necessary for SARS‐CoV‐2 entrance. Controversial results display various levels of ACE2 and TMPRSS2 expression among various stages of reproductive cells of male genitalia. Recently, Furin and CD147 have also been suggested as another potential host cell receptors for SARS‐CoV‐2
Recent discoveries have raised the possibility of the genital system's invasion by SARS‐CoV‐2 aside from other tissues (Qing & Gallagher, 2020).
4. SARS‐COV‐2 INVASION MECHANISM
Although evidence regarding preceding studies suggested a similar mechanism to SARS‐CoV (Hoffmann, Kleine‐Weber, Krüger, et al., 2020; Mason, 2020; Nadeem et al., 2020). recent investigations revealed the most probable process for SARS‐CoV‐2 invasion: virus‐host cell fusion mediating by surface spike (S) glycoprotein of virus binding ACE2 cellular receptor (Hoffmann, Kleine‐Weber, Schroeder, et al., 2020). The process triggers by cellular membrane serine protease TMPRSS2, which cleaves S protein into S1 and S2 leading virus endocytosis, translation and replication (Nadeem et al., 2020; Oberfeld et al., 2020; Qing & Gallagher, 2020; South et al., 2020). ACE2 internalisation causes a decline in ACE2 levels at the cell surface, resulting in less angiotensin 2 (Ang2) degradation to angiotensin 1–7 (Ang1‐7), which points out deleterious lung injury, inflammation and fibrosis (South et al., 2020). Therefore, the ACE2 receptor acts as the entrance gate in one hand, and the cell‐protective barrier on the other hand (Zhang et al., 2020). Nevertheless, cluster of differentiation 147 (CD147) as an alternative receptor for SARS‐CoV‐2 spike protein and furin as the cleaving protease have proposed in most recent investigations due to appearing in protein sequence of the SARS‐CoV‐2 (Wang, Chen, et al., 2020) (Figures 1b,2,3 and 4). Additionally, Stanley et al. considered receptor basigin (BSG) and its cysteine protease cathespin (CSTL) as another potential receptor‐mediated virus entry as a result of high co‐expression in early and late primary spermatocytes by scRNAseq data (Stanley et al., 2020).
FIGURE 2.
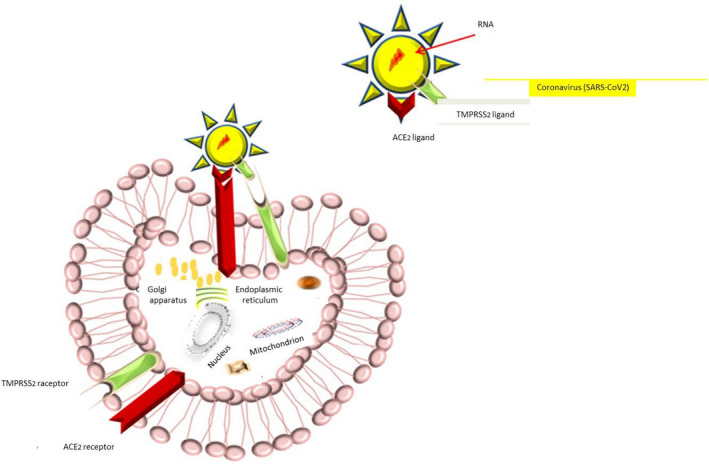
After the virus and target cell fusion, ACE2 is internalised and declined at the surface level of the target cell
FIGURE 3.
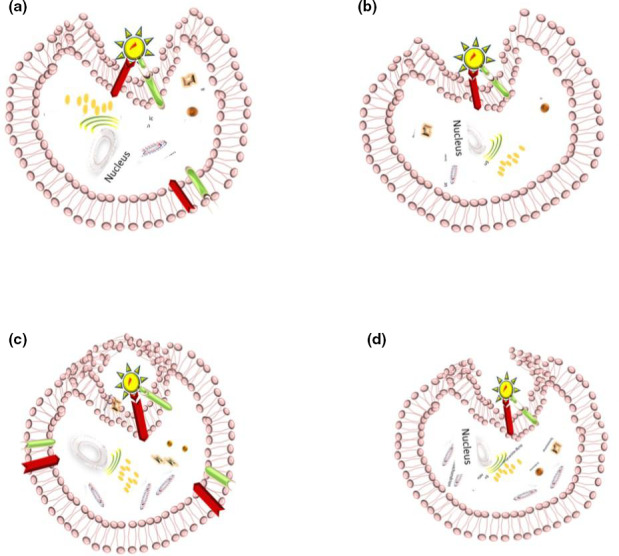
The virus binds to ACE2 receptor at the cell surface. The cell invasion is facilitated by TMPRSS2, which leads to SARS‐CoV‐2 endocytosis
FIGURE 4.
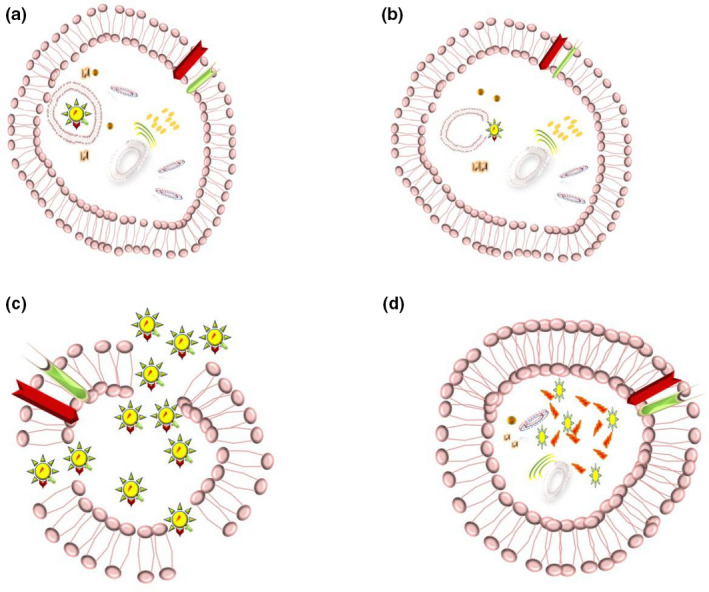
After the entrance and the process of translation to RNA and protein synthesis, the virus causes direct cell damage utilising host cell structures
The presence of both ACE2 and TMPRSS2 within target cells has hypothesised in this study as necessary components of the host cell to be affected by SARS‐CoV‐2. Additionally, evidence regarding the presence of both ACE2 and TMPRSS2 among male genital tissue, suggests its virus vulnerability (Wang & Xu, 2020) (Figure 1b).
5. ACE2 PRINCIPLES
The integral membrane glycoprotein ACE2 was discovered as ACE homolog in 2000, distributing hypertension, heart function and diabetes through its ability in Ang2 to Ang1‐7 conversion. After SARS emergence in 2003, it has been implicated as the virus gateway by its high affinity to viral spike glycoprotein (Tikellis & Thomas, 2012; Turner et al., 2004).
Since pneumonia has elucidated as the most critical manifestation of the disease, most literature has focused on ACE2 containing cells among the pulmonary system, admitting 83% expressing of ACE2 receptors in alveolar epithelial cells type2. However, extrapulmonary tissues have recognised as possible reservoirs for the virus (Zhang et al., 2020). Recent studies have provided some basis for ACE2 high levels of expression in the male reproductive system as well, and subsequent infertility issues (Pan et al., 2013; Wang & Xu, 2020).
6. ACE2 AND THE MALE REPRODUCTIVE SYSTEM
In 2004, the expression of ACE2 was reported in adult Leydig and Sertoli cells of the human testis, although the exact mechanism in male reproductive function was not defined (Douglas et al., 2004). Further studies exhibited the role of ACE2 Ang1‐7 Mas receptor axis in spermatogenesis regulation of rats: Ang1‐7 receptor blockage led to a decrease in testis and seminal vesicles weight, seminiferous epithelium, daily sperm production, the overall rate of spermatogenesis and increase in apoptotic cells in the seminiferous epithelium (Leal et al., 2009; Leung & Sernia, 2003). Recently, more attention has been focused on tracing ACE2 expression in human tissues, which indicated high enrichment within Leydig and Sertoli cells of the testis, and seminal vesicles (Fan et al., 2020). Likewise, Wang et al. collected the ACE2 level of expression's data across various testicular cell types: Sertoli and Leydig cells in first place and Spermatogonia in second place. In contrast, somewhat expression was reported in epithelial and somatic cells, different stages of spermatids and spermatocytes (Wang & Xu, 2020).
Moreover, the highest level of ACE2 mRNA expression in testis alongside small intestine, kidney, heart and adipose tissue among 31 standard human tissue samples has been reported in recent literature (Li, Li, et al., 2020). Meanwhile, ACE2 receptor expression in the prostate has disclosed by collecting bulk RNA‐seq profile of two public databases; also testis and seminal vesicle ACE2 expression at both RNA and protein level among top 10 high expressed organs (Dai et al., 2020; Tikellis & Thomas, 2012; Xu et al., 2020). Recently, another observation in India has confirmed the presence of ACE2 receptor expression in testicles among 68 median aged men positive for the virus, even at protein levels (Shastri et al., 2020).
However, the expression of ACE2 on the cell surface seems not to be sufficient for virus entrance and activation, and TMPRSS2 also has a notable role in this regard (Chen et al., 2010; Qing & Gallagher, 2020).
7. TMPRSS2 PRINCIPLES
The transmembrane serine protease TMPRSS2 regulating by androgen has declared to have a significant role in epithelial sodium homeostasis, angiogenesis and tubulogenesis via its proteolytic cascades, besides serves as a cell receptor by signalling conduction (Chen et al., 2010; Ko et al., 2015). Previous studies across primary and metastatic prostate cancer displayed predominant expression in prostate, also low levels in colon, stomach, epididymis, breast and even testicular Leydig cells and kidney (Lucas et al., 2008).
8. TMPRSS2 AND THE MALE REPRODUCTIVE SYSTEM
TMPRSS2, as one of the approved SARS‐CoV‐2 invasion compartments, has recorded highly expressed in prostate luminal cells and released in seminal fluid as part of prostatosomes. The accumulating body of evidence has been suggested prostatosome as a regulating factor and results in normal sperm function and reproductive health (Chen et al., 2010; Hoffmann, Kleine‐Weber, Schroeder, et al., 2020). Additionally, TMPRSS2 expression among all cell clusters of testis has been reported by RNA‐seq profiling, especially in spermatogonia and spermatids (Wang & Xu, 2020). However, RNA and protein expression scores vary in male tissues. Despite the inconsistent level of TMPRSS2 expression in male genitalia, which has been reported by different datasets, somehow expression has been recorded within the prostate, seminal vesicles and testes (Uhlén et al., 2015).
Further studies on TMPRSS2 and its contributions to male genital tissues are necessary for future investigations.
9. ACE2 AND TMPRSS2 CO‐EXPRESSION FOR COVID‐19
Recent research efforts have displayed ACE2 and TMPRSS2 co‐expressing in hillock and club cells of the prostate (Song, Seddighzadeh, et al., 2020). Besides, Wang and colleagues have recorded TMPRSS2 expressed in spermatogonia and spermatids, while ACE2 enriched in Leydig and Sertoli cells and spermatogonia (Wang & Xu, 2020). This finding sheds light on male reproductive tract vulnerability invasion by SARS‐CoV‐2 and provides some basis for further investigations over male fertility problems (Figures 3, 5).
FIGURE 5.
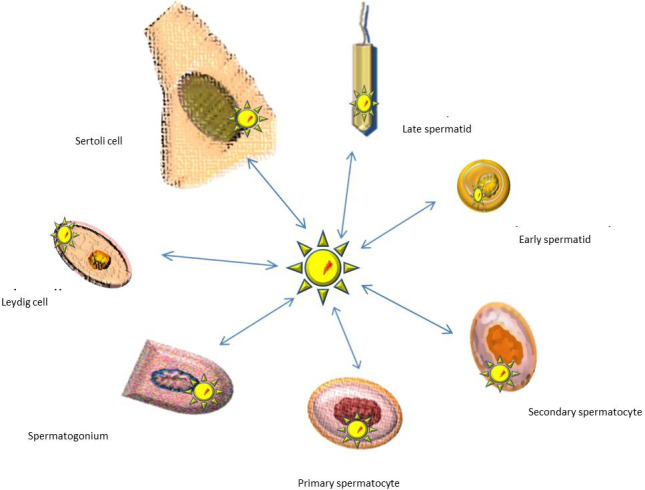
Various cell lines being affected by the virus have an impact on sperm dysfunction and consequent infertility
In contrast to the mentioned hypothesis, Pan and colleagues in the first cohort study analysed 34 male adult patients’ semen samples for SARS‐CoV‐2 detection during the recovery period. The results showed no virus; however, 19% of scrotal discomfort was reported; also, ACE2 and TMPRSS2 sparsely were enriched in testicular cells by RNA profiling experiments (Pan et al., 2020). In consistent with these findings, 34 men (18 recovered, 14 control and 2 through acute phase of infection) were investigated in a University Hospital in Duesseldorf, Germany, to find SARS‐CoV‐2 RNA among semen samples. Although no RNA was detected in semen samples of participants, sperm quality impairment in counts, motility or concentration was recorded in patients who faced the moderate course of disease (Holtmann et al., 2020).
Previously orchitis was reported in autopsy specimens of six patients who died of SARS in 2006 (Xu et al., 2006); however, no more research was applied to find the specific pathology.
10. HYPOTHESISED UNDERLYING MECHANISMS
We speculated that SARS‐CoV‐2 could be able to infect the male genital tract according to the abovementioned process. Afterwards, we discussed underlying mechanisms:
1) Direct damage has proposed at first place via virus‐receptor binding; however, this damage is also dependent on its replication and stabilisation capabilities inside the target cells (Salam & Horby, 2017). Xu and colleagues reported no positive staining testis by SARS‐CoV infected patients with orchitis in 2006 (Xu et al., 2006). Recently, Song et al. collected 12 semen samples of SARS‐CoV‐2 infected patients during the recovery period to diagnose COVID‐19 by polymerase chain reaction (PCR) test, resulting in no positive sample (Song, Wang, et al., 2020). Interestingly, one recent cohort study in China has reported positive semen samples of COVID‐19 patients: four positive for SARS‐CoV‐2 samples during the acute phase of the disease, and two positive ones during the recovery period among total 38 semen samples of infected patients (Li, Jin, et al., 2020).
2) Secondary inflammatory response by inflammatory cytokines has suggested as another underlying mechanism; like most infectious diseases, fever has accused of testicular damage due to the destruction of germ cells in constant high temperature, and leucocyte infiltration by destroying Leydig cells and following a decline in testosterone (T) level (Xu et al., 2006). Similarly, in one recent study in China, SARS‐CoV‐2 infected males were compared to healthy men in reproductive age to assess the changes in sex hormone levels. Consequently, the possibility of hypogonadism and Leydig cell destruction were hypothesised due to decline in T: LH ratio. It is worth noting that the potential effect of various therapies, corticosteroids as an example, on hypothalamic–pituitary–gonadal axis must need to be taken into consideration (Ma et al., 2020).
Additionally, a couple of investigations have suggested the role of sex steroid abnormalities in the severity of symptoms and poor prognosis among COVID‐19 patients (Giagulli et al., 2020; Stopsack et al., 2020).
3) Additionally, viral load has recommended as an influential factor in following complications like testicular damage, on one hand (Hikmet et al., 2020), and immune response activation, adaptive and innate, to fight against the virus on the other hand (Li, Li, et al., 2020).
Further studies are required to address this issue by more patients’ evaluation and examination, more semen samples collecting during different phases of the disease, additional hormonal function analysis and more studies in both RNA and protein expression levels.
Conclusively, we suggest physicians gain more attention to male genital examinations besides pulmonary, cardiovascular and other involved organs. These examinations should be done in the course of the acute and recovery phase to recognise any genital discomfort complaints, orchitis, epididymitis or infertility problems.
Furthermore, exploring laboratory findings such as sex hormone levels and semen analysis is suggested to be considered to diagnose sperm dysfunctions and subsequent fertility problems during short‐ and long‐term periods in patients and the ones who recovered COVID‐19.
Taken together, patients’ evaluation in terms of fertility has recommended to urologists and other physicians as a result of the potential male reproductive tract's involvement during the infection.
ACKNOWLEDGMENTS
We thank the University of Tabriz for funding support.
Sheikhzadeh Hesari F, Hosseinzadeh SS, Asl Monadi Sardroud MA. Review of COVID‐19 and male genital tract. Andrologia.2021;53:e13914. 10.1111/and.13914
REFERENCES
- Adhikari, S. P. , Meng, S. , Wu, Y.‐J. , Mao, Y.‐P. , Ye, R.‐X. , Wang, Q.‐Z. , Sun, C. , Sylvia, S. , Rozelle, S. , Raat, H. , & Zhou, H. (2020). Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID‐19) during the early outbreak period: A scoping review. Infectious Diseases of Poverty, 9(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig, A. M. , Khaleeq, A. , Ali, U. , & Syeda, H. (2020). Evidence of the COVID‐19 virus targeting the CNS: tissue distribution, host‐virus interaction, and proposed neurotropic mechanisms. ACS Chemical Neuroscience, 11(7), 995–998. 10.1021/acschemneuro.0c00122 [DOI] [PubMed] [Google Scholar]
- Chen, Y.‐W. , Lee, M.‐S. , Lucht, A. , Chou, F.‐P. , Huang, W. , Havighurst, T. C. , Kim, K. , Wang, J. K. , Antalis, T. M. , Johnson, M. D. , & Lin, C. Y. (2010). TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells. The American Journal of Pathology, 176(6), 2986–2996. https://ajp.amjpathol.org/article/S0002‐9440(10)60818‐8/pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, Y.‐J. , Hu, F. , Li, H. , Huang, H.‐Y. , Wang, D.‐W. , & Liang, Y. (2020). A profiling analysis on the receptor ACE2 expression reveals the potential risk of different type of cancers vulnerable to SARS‐CoV‐2 infection. Annals of Translational Medicine, 8(7), 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejucq, N. , & Jégou, B. (2001). Viruses in the mammalian male genital tract and their effects on the reproductive system. Microbiology and Molecular Biology Reviews, 65(2), 208–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas, G. C. , O’Bryan, M. K. , Hedger, M. P. , Lee, D. K. , Yarski, M. A. , Smith, A. I. , & Lew, R. A. (2004). The novel angiotensin‐converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology, 145(10), 4703–4711. [DOI] [PubMed] [Google Scholar]
- Fan, C. , Li, K. , Ding, Y. , Lu, W. L. , & Wang, J. (2020). ACE2 expression in kidney and testis may cause kidney and testis damage after 2019‐nCoV infection. MedRxiv. https://www.medrxiv.org/content/10.1101/2020.02.12.20022418v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giagulli, V. A. , Guastamacchia, E. , Magrone, T. , Jirillo, E. , Lisco, G. , De Pergola, G. , & Triggiani, V. (2020). Worse progression of COVID‐19 in men: Is Testosterone a key factor? Andrology. 1–12. 10.1111/andr.12836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, L. , Yu, K. , Li, D. , Yang, H. , Liu, L. , Fan, J. , Sun, N. , & Yang, X. (2020). Potential pathogenesis of multiple organ injury in COVID‐19. Preprints. [Google Scholar]
- Guo, Y. R. , Cao, Q. D. , Hong, Z. S. , Tan, Y. Y. , Chen, S. D. , Jin, H. J. , Tan, K. S. , Wang, D. Y. , & Yan, Y. (2020). The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak ‐ an update on the status. Mil Med Res, 7(1), 11. 10.1186/s40779-020-00240-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikmet, F. , Mear, L. , Uhlen, M. , & Lindskog, C. (2020). The protein expression profile of ACE2 in human tissues. bioRxiv. 16(7), e9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , Krüger, N. , Mueller, M. A. , Drosten, C. , & Pöhlmann, S. (2020). The novel coronavirus 2019 (2019‐nCoV) uses the SARS‐coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv. 10.1101/2020.01.31.929042 [DOI] [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , Schroeder, S. , Krüger, N. , Herrler, T. , Erichsen, S. , Schiergens, T. S. , Herrler, G. , Wu, N.‐H. , Nitsche, A. , Müller, M. A. , Drosten, C. , & Pöhlmann, S. (2020). SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmann, N. , Edimiris, P. , Andree, M. , Doehmen, C. , Baston‐Buest, D. , Adams, O. , Kruessel, J.‐S. , & Bielfeld, A. P. (2020). Assessment of SARS‐CoV‐2 in human semen—a cohort study. Fertility and Sterility, 114(2), 233–238. 10.1016/j.fertnstert.2020.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. I. , Zhang, L. I. , Fan, G. , Xu, J. , Gu, X. , Cheng, Z. , Yu, T. , Xia, J. , Wei, Y. , Wu, W. , Xie, X. , Yin, W. , Li, H. , Liu, M. , … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. The Lancet, 395(10223), 497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huret, J. L. , Dessen, P. , & Bernheim, A. (2003). An internet database on genetics in oncology. Oncogene, 22(13), 1907. 10.1038/sj.onc.1206225 [DOI] [Google Scholar]
- Jiang, F. , Deng, L. , Zhang, L. , Cai, Y. , Cheung, C. W. , & Xia, Z. (2020). Review of the clinical characteristics of coronavirus disease 2019 (COVID‐19). Journal of General Internal Medicine, 35(5), 1545–1549. 10.1007/s11606-020-05762-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, X.‐H. , Bukhari, I. , Zheng, W. , Yin, S. , Wang, Z. , Cooke, H. , & Shi, Q.‐H. (2014). Blood‐testis barrier and spermatogenesis: Lessons from genetically‐modified mice. Asian Journal of Andrology, 16(4), 572–580. 10.4103/1008-682X.125401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonathan, G. , Julia, H. , & Ralph, B. (2016). Blood–testis barrier and Sertoli cell function: Lessons from SCCx43KO mice. Reproduction, 151(2), R15–R27. 10.1530/REP-15-0366 [DOI] [PubMed] [Google Scholar]
- Ko, C.‐J. , Huang, C.‐C. , Lin, H.‐Y. , Juan, C.‐P. , Lan, S.‐W. , Shyu, H.‐Y. , Wu, S.‐R. , Hsiao, P.‐W. , Huang, H.‐P. , Shun, C.‐T. , & Lee, M.‐S. (2015). Androgen‐induced TMPRSS2 activates matriptase and promotes extracellular matrix degradation, prostate cancer cell invasion, tumor growth, and metastasis. Cancer Research, 75(14), 2949–2960. 10.1158/0008-5472.CAN-14-3297 [DOI] [PubMed] [Google Scholar]
- Leal, M. C. , Pinheiro, S. V. B. , Ferreira, A. J. , Santos, R. A. S. , Bordoni, L. S. , Alenina, N. , Bader, M. , & França, L. R. (2009). The role of angiotensin‐(1–7) receptor Mas in spermatogenesis in mice and rats. Journal of Anatomy, 214(5), 736–743. 10.1111/j.1469-7580.2009.01058.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letko, M. , Marzi, A. , & Munster, V. (2020). Functional assessment of cell entry and receptor usage for SARS‐CoV‐2 and other lineage B betacoronaviruses. Nat Microbiol, 5(4), 562–569. 10.1038/s41564-020-0688-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, P. , & Sernia, C. (2003). The renin‐angiotensin system and male reproduction: New functions for old hormones. Journal of Molecular Endocrinology, 30(3), 263–270. 10.1677/jme.0.0300263 [DOI] [PubMed] [Google Scholar]
- Li, B. O. , Yang, J. , Zhao, F. , Zhi, L. , Wang, X. , Liu, L. , Bi, Z. , & Zhao, Y. (2020). Prevalence and impact of cardiovascular metabolic diseases on COVID‐19 in China. Clinical Research in Cardiology, 109(5), 531–538. 10.1007/s00392-020-01626-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, D. , Jin, M. , Bao, P. , Zhao, W. , & Zhang, S. (2020). Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Network Open, 3(5), e208292. 10.1001/jamanetworkopen.2020.8292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M.‐Y. , Li, L. , Zhang, Y. , & Wang, X.‐S. (2020). Expression of the SARS‐CoV‐2 cell receptor gene ACE2 in a wide variety of human tissues. Infectious Diseases of Poverty, 9, 1–7. 10.1186/s40249-020-00662-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R. , Yin, T. , Fang, F. , Li, Q. , Chen, J. , Wang, Y. , Hao, Y. , Wu, G. , Duan, P. , Wang, Y. , Cheng, D. , Zhou, Q. I. , Zafar, M. I. , Xiong, C. , Li, H. , Yang, J. , & Qiao, J. (2020). Potential risks of SARS‐Cov‐2 infection on reproductive health. Reproductive BioMedicine Online. 10.1016/j.rbmo.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W. , Han, R. , Wu, H. , & Han, D. (2018). Viral threat to male fertility. Andrologia, 50(11), e13140. 10.1111/and.13140 [DOI] [PubMed] [Google Scholar]
- Lucas, J. , True, L. , Hawley, S. , Matsumura, M. , Morrissey, C. , Vessella, R. , & Nelson, P. (2008). The androgen‐regulated type II serine protease TMPRSS2 is differentially expressed and mislocalized in prostate adenocarcinoma. The Journal of Pathology, 215(2), 118–125. 10.1002/path.2330 [DOI] [PubMed] [Google Scholar]
- Ma, L. , Xie, W. , Li, D. , Shi, L. , Mao, Y. , Xiong, Y. , & Zhang, M. (2020). Effect of SARS‐CoV‐2 infection upon male gonadal function: A single center‐based study. MedRxiv. 10.1101/2020.03.21.20037267 [DOI] [Google Scholar]
- Mason, R. J. (2020). Pathogenesis of COVID‐19 from a cell biology perspective. European Respiratory Journal, 55(4), 2000607. 10.1183/13993003.00607-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem, M. S. , Zamzami, M. A. , Choudhry, H. , Murtaza, B. N. , Kazmi, I. , Ahmad, H. , & Shakoori, A. R. (2020). Origin, potential therapeutic targets and treatment for coronavirus disease (COVID‐19). Pathogens, 9(4), 307. 10.3390/pathogens9040307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberfeld, B. , Achanta, A. , Carpenter, K. , Chen, P. , Gilette, N. M. , Langat, P. , Said, J. T. , Schiff, A. E. , Zhou, A. S. , Barczak, A. K. , & Pillai, S. (2020). SnapShot: COVID‐19. Cell, 181(4), 954–954.e951. 10.1016/j.cell.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, F. , Xiao, X. , Guo, J. , Song, Y. , Li, H. , Patel, D. P. , Spivak, A. M. , Alukal, J. P. , Zhang, X. , Xiong, C. , Li, P. S. , & Hotaling, J. M. (2020). No evidence of SARS‐CoV‐2 in semen of males recovering from COVID‐19. Fertility and Sterility, 113, 1135–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, P.‐P. , Zhan, Q.‐T. , Le, F. , Zheng, Y.‐M. , & Jin, F. (2013). Angiotensin‐converting enzymes play a dominant role in fertility. International Journal of Molecular Sciences, 14(10), 21071–21086. 10.3390/ijms141021071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, M. , Thwaites, R. S. , & Openshaw, P. J. (2020). COVID‐19: Lessons from SARS and MERS. European Journal of Immunology, 50(3), 308. 10.1002/eji.202070035 [DOI] [Google Scholar]
- Qing, E. , & Gallagher, T. (2020). SARS coronavirus redux. Trends in Immunology, 41(4), 271–273. 10.1016/j.it.2020.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salam, A. P. , & Horby, P. W. (2017). The breadth of viruses in human semen. Emerging Infectious Diseases, 23(11), 1922–1924. 10.3201/eid2311.171049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shastri, A. , Wheat, J. , Agrawal, S. , Chaterjee, N. , Pradhan, K. , Goldfinger, M. , & Shastri, J. (2020). Delayed clearance of SARS‐CoV2 in male compared to female patients: High ACE2 expression in testes suggests possible existence of gender‐specific viral reservoirs. MedRxiv. 10.1101/2020.04.16.20060566 [DOI] [Google Scholar]
- Sommerstein, R. , Kochen, M. M. , Messerli, F. H. , & Gräni, C. (2020). Coronavirus disease 2019 (COVID‐19): Do angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers have a biphasic effect? Journal of the American Heart Association, 9(7), e016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, C. , Wang, Y. , Li, W. , Hu, B. , Chen, G. , Xia, P. , & Yao, B. (2020). Detection of 2019 novel coronavirus in semen and testicular biopsy specimen of COVID‐19 patients. MedRxiv. 10.1101/2020.03.31.20042333 [DOI] [Google Scholar]
- Song, H. , Seddighzadeh, B. , Cooperberg, M. R. , & Huang, F. W. (2020). Expression of ACE2, the SARS‐CoV‐2 receptor, and TMPRSS2 in prostate epithelial cells. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South, A. M. , Diz, D. I. , & Chappell, M. C. (2020). COVID‐19, ACE2, and the cardiovascular consequences. American Journal of Physiology‐Heart and Circulatory Physiology, 318(5), H1084–H1090. 10.1152/ajpheart.00217.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley, K. E. , Thomas, E. , Leaver, M. , & Wells, D. (2020). Coronavirus disease‐19 and fertility: Viral host entry protein expression in male and female reproductive tissues. Fertility and Sterility, 114(1), 33–43. 10.1016/j.fertnstert.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopsack, K. H. , Mucci, L. A. , Antonarakis, E. S. , Nelson, P. S. , & Kantoff, P. W. (2020). TMPRSS2 and COVID‐19: Serendipity or Opportunity for Intervention? Cancer Discovery, 10(6), 779–782. Retrieved from https://cancerdiscovery.aacrjournals.org/content/candisc/10/6/779.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikellis, C. , & Thomas, M. (2012). Angiotensin‐Converting Enzyme 2 (ACE2) Is a Key Modulator of the Renin Angiotensin System in Health and Disease. International Journal of Peptides, 2012, 1–8. 10.1155/2012/256294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorec, A. L. , Matusali, G. , Mahé, D. , Aubry, F. , Mazaud‐Guittot, S. , Houzet, L. , & Dejucq‐Rainsford, N. (2020). From ancient to emerging infections: The Odyssey of viruses in the male genital tract. Physiological Reviews, 100(3), 1349–1414. 10.1152/physrev.00021.2019 [DOI] [PubMed] [Google Scholar]
- Turner, A. J. , Hiscox, J. A. , & Hooper, N. M. (2004). ACE2: From vasopeptidase to SARS virus receptor. Trends in Pharmacological Sciences, 25(6), 291–294. 10.1016/j.tips.2004.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen, M. , Fagerberg, L. , Hallstrom, B. M. , Lindskog, C. , Oksvold, P. , Mardinoglu, A. , Sivertsson, A. , Kampf, C. , Sjostedt, E. , Asplund, A. , Olsson, I. , Edlund, K. , Lundberg, E. , Navani, S. , Szigyarto, C.‐A.‐K. , Odeberg, J. , Djureinovic, D. , Takanen, J. O. , … Ponten, F. (2015). Tissue‐based map of the human proteome. Science, 347(6220), 1260419. 10.1126/science.1260419 [DOI] [PubMed] [Google Scholar]
- Wang, K. , Chen, W. , Zhou, Y.‐S. , Lian, J.‐Q. , Zhang, Z. , Du, P. , Chen, Z.‐N. (2020). SARS‐CoV‐2 invades host cells via a novel route: CD147‐spike protein. bioRxiv, 2020.2003.2014.988345. 10.1101/2020.03.14.988345 [DOI] [Google Scholar]
- Wang, Q. , Zhang, Y. , Wu, L. , Niu, S. , Song, C. , Zhang, Z. , Lu, G. , Qiao, C. , Hu, Y. , Yuen, K. Y. , Wang, Q. , Zhou, H. , Yan, J. , & Qi, J. (2020). Structural and functional basis of SARS‐CoV‐2 entry by using human ACE2. Cell, 181, 894‐904.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. , & Xu, X. (2020). scRNA‐seq profiling of human testes reveals the presence of the ACE2 Receptor, A Target for SARS‐CoV‐2 infection in spermatogonia, leydig and sertoli cells. Cells, 9(4), 920. 10.3390/cells9040920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . (2020). Coronavirus disease (COVID‐19) Situation Report‐171. Retrieved from https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200709-covid-19-sitrep-171.pdf?sfvrsn=9aba7ec7_2 [Google Scholar]
- Wong, S. , Lui, R. , & Sung, J. (2020). Covid‐19 and the digestive system. Journal of Gastroenterology and Hepatology, 35, 10.1111/jgh.15047 [DOI] [PubMed] [Google Scholar]
- Xu, H. , Zhong, L. , Deng, J. , Peng, J. , Dan, H. , Zeng, X. , Li, T. , & Chen, Q. (2020). High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. International Journal of Oral Science, 12(1), 1–5. 10.1038/s41368-020-0074-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. , Qi, L. , Chi, X. , Yang, J. , Wei, X. , Gong, E. , & Gu, J. (2006). Orchitis: A complication of severe acute respiratory syndrome (SARS). Biology of Reproduction, 74(2), 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Penninger, J. M. , Li, Y. , Zhong, N. , & Slutsky, A. S. (2020). Angiotensin‐converting enzyme 2 (ACE2) as a SARS‐CoV‐2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Medicine, 46(4), 586–590. 10.1007/s00134-020-05985-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, M. , Zhang, X. , & Qu, J. (2020). Coronavirus disease 2019 (COVID‐19): A clinical update. Frontiers of Medicine, 14(2), 126–135. 10.1007/s11684-020-0767-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, P. , Yang, X.‐L. , Wang, X.‐G. , Hu, B. , Zhang, L. , Zhang, W. , Si, H.‐R. , Zhu, Y. , Li, B. , Huang, C.‐L. , Chen, H.‐D. , Chen, J. , Luo, Y. , Guo, H. , Jiang, R.‐D. , Liu, M.‐Q. , Chen, Y. , Shen, X.‐R. , Wang, X. I. , Shi, Z.‐L. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579(7798), 270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]


