Abstract
The scope of the impact of the Coronavirus disease 19 (COVID‐19) pandemic on living donor kidney transplantation (LDKT) practices across the world is not well‐defined. We received survey responses from 204 transplant centers internationally from May to June 2020 regarding the impact of the COVID‐19 pandemic on LDKT practices. Respondents represented 16 countries on five continents. Overall, 75% of responding centers reported that LDKT surgery was on hold (from 67% of North American centers to 91% of European centers). The majority (59%) of centers reported that new donor evaluations were stopped (from 46% of North American centers to 86% of European centers), with additional 23% of centers reporting important decrease in evaluations. Only 10% of centers reported slight variations on their evaluations. For the centers that continued donor evaluations, 40% performed in‐person visits, 68% by video, and 42% by telephone. Center concerns for donor (82%) and recipient (76%) safety were the leading barriers to LDKT during the pandemic, followed by patients concerns (48%), and government restrictions (46%). European centers reported more barriers related to staff limitations while North and Latin American centers were more concerned with testing capacity and insufficient resources including protective equipment. As LDKT resumes, 96% of the programs intend to screen donor and recipient pairs for coronavirus infection, most of them with polymerase chain reaction testing of nasopharyngeal swab samples. The COVID‐19 pandemic has had broad impact on all aspects of LDKT practice. Ongoing research and consensus‐building are needed to guide safe reopening of LDKT programs.
Keywords: COVID‐19, evaluation, kidney transplantation, living kidney donation, pandemic, practices, telehealth
Abbreviations
- AKI
acute kidney injury
- COVID‐19
Coronavirus Disease 2019
- ICU
intensive care units
- KPD
kidney paired donation
- LDKT
living donor kidney transplantation
- MERS‐CoV
Middle East Respiratory Syndrome Coronavirus
- PCR
polymerase chain reaction
- PPE
personal protective equipment
- SARS‐CoV
Severe Acute Respiratory Syndrome Coronavirus
- US
United States
1. INTRODUCTION
The emergence of Coronavirus disease 2019 (COVID‐19) has challenged healthcare systems across the world. 1 , 2 , 3 Organ transplant recipients on immunosuppressive therapy are considered high‐risk because of increased risk of mortality following infection with similar Coronaviruses, such as the Severe Acute Respiratory Syndrome Coronavirus (SARS‐CoV) or the Middle East Respiratory Syndrome Coronavirus (MERS‐CoV). 4 , 5 Initial reports have suggested a fatality rate of up to 10% in transplant recipients with COVID‐19, with many survivors developing progressive respiratory failure and acute respiratory distress syndrome. 6 , 7 In efforts to deal with this public health emergency, many hospitals have deferred elective procedures to better manage intensive care units (ICU) bed utilization and to properly allocate scarce resources, such as mechanical ventilators and personal protective equipment (PPE). Such practices have dramatically impacted transplant activity by limiting deceased donor recoveries, living donor surgeries, and organ transplant procedures. Although some hospitals have continued performing selected life‐saving procedures (such as cancer surgery, emergencies, heart and liver transplantation), early reports demonstrated that transplant volumes were negatively impacted by the pandemic. 8 , 9 , 10 , 11 , 12 In particular, the number of kidney transplants decreased worldwide. 8 , 10 , 11 , 12 While some deceased donor kidney transplant centers continued through the pandemic with restrictions, living donor kidney transplantation (LDKT) programs were more significantly curtailed. 10 , 11 , 12
To safeguard healthy donors and protect vulnerable immunosuppressed recipients, many LDKT centers altered their practices, including adjusting patient evaluation strategies, implementing new consent information, delaying or cancelling surgical procedures, and adjusting immediate and long‐term post‐operative care to prevent donor and recipient infection. 10 , 11 , 12 , 13 Multicenter, cross‐national data regarding the specific and detailed impact of COVID‐19 on LDKT care is scarce but vital to understand the global impact on transplant access. Understanding global patterns may provide context for assessing efforts to safely resume transplant care including re‐starting LDKT surgeries. 10 , 11 , 12 , 13 Herein, we report on a global survey to assess the impact of the COVID‐19 pandemic on the comprehensive elements of LDKT evaluation, surgery, follow‐up, and education practices.
2. MATERIAL AND METHODS
2.1. Survey design
This study was approved as Human Subject Exempt by the Saint Louis University Institutional Review Board. The survey instrument was developed by the study investigators. The final survey instrument comprised of 33 questions addressing the impact of COVID‐19 pandemic on LDKT local volume, donor and recipient evaluations, testing, consent, and post‐transplant care (Table S1). Several questions were specifically designed to understand the COVID‐19 impact on the kidney paired donation (KPD) programs. The survey also queried information on the participant role (nephrologist, transplant surgeon, clinical coordinator, social worker, administrator, or other) at the LDKT center. The survey was approved by the American Society of Transplantation (AST) Education Committee.
2.2. Survey administration
The target population was healthcare providers at kidney transplant centers active in 2020. Investigators contacted centers and transplant societies in their countries via email and list servs, and also invited participants to share the survey with other centers. United States results were collected first. 14 Additional participants were recruited from LDKT programs in Canada, Latin America, Europe, the Middle East, and Asia. Potential participants were derived from the working group's professional connections and emailed the survey through the Qualtrics Survey Software, with a focus on living donor program directors. Opportunity for self‐elected participation through a Qualtrics link was also posted to professional society list servs including that of the AST Outstanding Questions in Transplantation (which includes international membership), the Turkish Society of Nephrology and Canadian Blood Services Living Donation Advisory Committee.
The first page of the survey included a consent for participation. The decision to proceed with the questions of the survey indicates agreement with survey terms. Up to two reminders, a week apart, were provided for non‐responders. Qualtrics Survey Software (SAP Business Solutions, Walldorf, Germany) was utilized for mailing and data collection. Data were analyzed from distribution between 05/9/2020 and 06/20/2020.
2.3. Statistical analysis
Each program was represented only once in the analysis. For programs with multiple respondents, we selected a single participant to represent the program using a hierarchical algorithm. First, we prioritized responses with the most complete information (ie, least unanswered items). Next, we prioritized surveys submitted by nephrologists or transplant surgeons, over those from coordinators, social workers, administrators, or others. Lastly, we prioritized the earliest submitted questionnaire.
Responses to each survey question were described with percentages and frequencies. To obtain rates, we divided the number of program responses by the total number of programs who responded to the question, such that percentages reflect proportions of respondents, as per previous methods. 15 , 16 , 17 For questions where participants were asked to “select all that apply,” the denominator for calculating percentages was the number of participants responding to that question. Centers were then categorized by their geographical areas according to their location, as Latin America, Europe, Asia/Middle East, or North America.
Although the sample size of programs was too small for statistical significance (P > .05), stratification by region of the transplant center is presented to assess trends in the relationship of local disease prevalence with LDKT practices. All analyses were performed using R for windows version 1.2.5042 (RStudio Inc, Boston, MA).
3. RESULTS
3.1. Participants, countries, and regions
This report describes responses from 204 unique LDKT programs, from 16 countries, in five continents. Response rates to the voluntarily survey have varied. In those countries where information was requested from all LDKTs centers, rates of program representation varied from 19% in Canada, 35% in Turkey, 39% in Brazil, and 61% in the United States. Another 18 LDKT centers added responses to the survey from 14 additional countries, based on access through list servs. Most of the transplant centers that participated in the surveyed were tertiary medical centers, serving as major LDKT centers in their regions and/or countries, with annual LDKT volumes ranging from 1 to 221 in 2019. Participants were most often transplant nephrologists (63%), followed by transplant surgeons (25%) and coordinators (5%) (Table 1).
Table 1.
Characteristics of respondents of the international survey on LDKT
| Role in Transplant Program | Overall | Latin America | Europe | Asia/Middle East | North America |
|---|---|---|---|---|---|
| Surgeon | 25% (50/204) | 2% (1/40) | 3% (1/35) | 50% (3/6) | 37% (45/123) |
| Nephrologist | 63% (129/204) | 85% (34/40) | 91% (32/35) | 50% (3/6) | 49% (60/123) |
| Clinical Coordinator | 5% (10/204) | 10% (4/40) | 0% (0/35) | 0% (0/6) | 5% (6/123) |
| Social Worker | 1% (3/204) | 0% (0/40) | 0% (0/35) | 0% (0/6) | 2% (3/123) |
| Administrator | 2% (4/204) | 0% (0/40) | 0% (0/35) | 0% (0/6) | 3% (4/123) |
| Other | 4% (8/204) | 2% (1/40) | 6% (2/35) | 0% (0/6) | 4% (5/123) |
3.2. Living donor evaluation
Evaluation of donor candidates was significantly reduced during the COVID‐19 pandemic, with 59% of programs pausing living donor evaluation (Table 2). Centers that continued living donor evaluations generally used video‐based assessment (68%) or telephone‐based contact (42%). However, 40% of centers still used in‐clinic assessment (up to 83% among the centers located outside of North America).
Table 2.
Activity of LDKT centers during the COVID‐19 pandemic
| Overall | Latin America | Europe | Asia/Middle East | North America | |
|---|---|---|---|---|---|
| Have you continued living donor candidate evaluations during the COVID‐19 pandemic? | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) |
| Yes | 41% (84/203) | 25% (10/40) | 14% (5/35) | 50% (3/6) | 54% (66/122) |
| No | 59% (119/203) | 75% (30/40) | 86% (30/35) | 50% (3/6) | 46% (56/122) |
| If you have continued living donor candidate evaluations, what modalities do you use for patient interactions? Select all that apply. | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) |
| Assessment in clinic | 40% (40/101) | 83% (10/12) | 75% (3/4) | 60% (3/5) | 30% (24/80) |
| Telehealth: telephone‐based | 42% (42/101) | 33% (4/12) | 50% (2/4) | 40% (2/5) | 42% (34/80) |
| Telehealth: video‐based | 68% (69/101) | 17% (2/12) | 25% (1/4) | 0% (0/5) | 82% (66/80) |
| Did your center use telehealth for donor evaluation prior to the COVID‐19 pandemic? | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) |
| Yes | 13% (27/201) | 18% (7/40) | 12% (4/33) | 17% (1/6) | 12% (15/122) |
| No | 87% (174/201) | 82% (33/40) | 88% (29/33) | 83% (5/6) | 88% (107/122) |
| What elements of the living donor evaluation does your center use telehealth to perform? Select all that apply. | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) |
| Medical evaluation | 74% (89/121) | 80% (12/15) | 58% (7/12) | 25% (1/4) | 77% (69/90) |
| Surgical evaluation | 32% (39/121) | 27% (4/15) | 0% (0/12) | 50% (2/4) | 37% (33/90) |
| Social work evaluation | 63% (76/121) | 20% (3/15) | 17% (2/12) | 0% (0/4) | 79% (71/90) |
| ILDA evaluation | 54% (65/121) | 0% (0/15) | 0% (0/12) | 0% (0/4) | 72% (65/90) |
| Dietician evaluation | 50% (60/121) | 7% (1/15) | 8% (1/12) | 0% (0/4) | 64% (58/90) |
| Coordinator education | 64% (78/121) | 13% (2/15) | 50% (6/12) | 25% (1/4) | 77% (69/90) |
| Other | 16% (19/121) | 20% (3/15) | 25% (3/12) | 50% (2/4) | 12% (11/90) |
Denominators for percentages reflect respondents by item, within each region.
The majority of responding programs (87%) were not using telehealth prior to the pandemic. During the pandemic, LDKT programs reported using this technology predominantly for medical (74%), social work (63%), and independent living donor advocate (54%) evaluation. Conversely, surgical evaluation was still mainly done in‐person (at 68% of the responding centers overall). Importantly, 86% of responding centers require at least one in‐person evaluation for physical examination of the donor. Based on successful use during the pandemic, 87% of the centers intend to continue telehealth for their donor assessments after the pandemic, including 47% at higher than pre‐pandemic levels.
As a result of COVID‐19’s impact, all programs reported some reduction in donor evaluation volume, with more than 74% of programs reporting at least a 75% reduction in their average volume of donor evaluations. Among Asian/Middle Eastern respondents only 33% of centers stopped all evaluations compared with 80% of European centers (Figure 1A).
Figure 1.
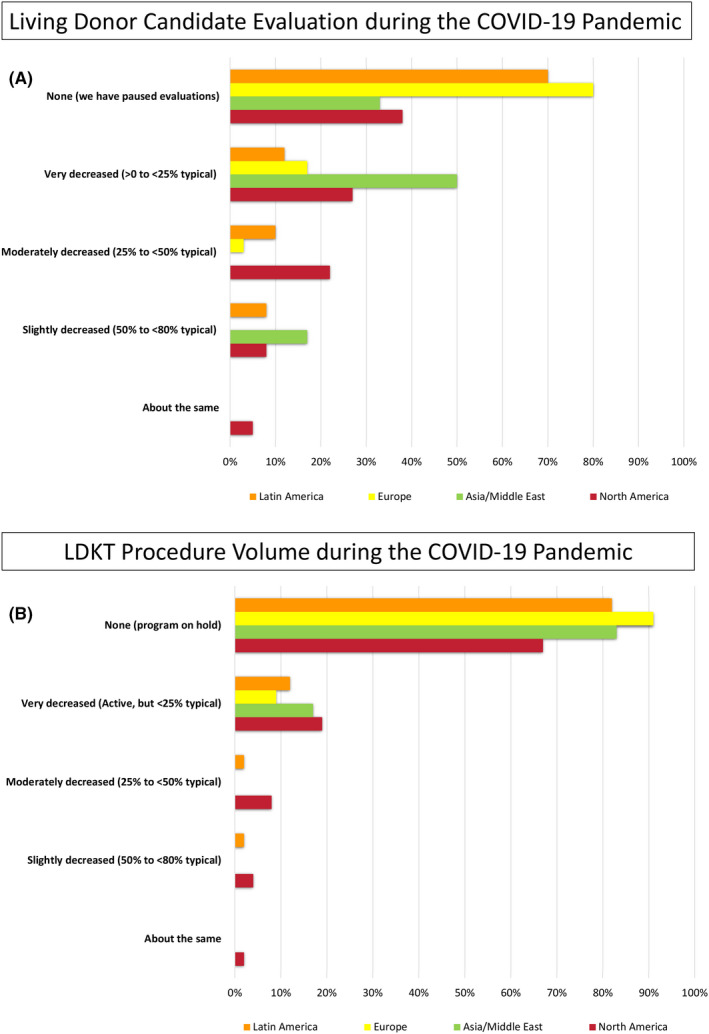
Volume changes in (A) living donor candidate evaluation and (B) LDKT procedure activity during the pandemic
3.3. Living donor testing
Responding programs identified several key barriers to proceeding with donor evaluation and testing during the COVID‐19 pandemic. Restrictions caused by local stay‐at‐home orders (71%) were the most common issue reported, followed by donor concern/refusal (59%), limited access to evaluation testing (52%), and reduced patient inquires (38%). Patterns appeared similar across different geographic regions (Figure 2).
Figure 2.
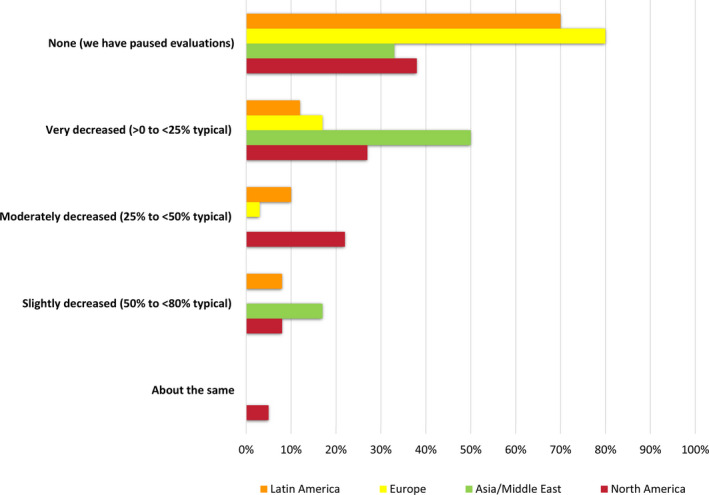
Barriers to living donor candidate evaluation during the pandemic
Most centers stopped their predonation laboratory, cardiac, and radiological testing procedures as a result of the COVID‐19 pandemic during the survey period (Table 3). Of those continuing testing, few could incorporate other local testing sites in community labs or utilize home‐based phlebotomy. Testing appeared to decline independently of continent.
Table 3.
LDKT testing during the COVID‐19 pandemic
| Overall | Latin America | Europe | Asia/Middle East | North America | |
|---|---|---|---|---|---|
| Have you continued living donor candidate lab testing during the pandemic? | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) |
| Yes | 43% (87/203) | 32% (13/40) | 17% (6/35) | 33% (2/6) | 54% (66/122) |
| No | 57% (116/203) | 68% (27/40) | 83% (29/35) | 67% (4/6) | 46% (56/122) |
| If you continued living donor candidate lab testing during the pandemic, where are labs performed? Select all that apply. | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) |
| Transplant hospital | 66% (59/89) | 67% (10/15) | 83% (5/6) | 100% (2/2) | 64% (42/66) |
| Community lab | 63% (56/89) | 40% (6/15) | 17% (1/6) | 0% (0/2) | 74% (49/66) |
| Home‐based phlebotomy service | 13% (12/89) | 7% (1/15) | 0% (0/6) | 0% (0/2) | 17% (11/66) |
| Have you continued other forms of living donor candidate testing during the pandemic (eg radiology, cardiac testing)? | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) |
| Yes | 32% (64/200) | 33% (13/39) | 9% (3/33) | 33% (2/6) | 38% (46/122) |
| No | 68% (136/200) | 67% (26/39) | 91% (30/33) | 67% (4/6) | 62% (76/122) |
Denominators for percentages reflect respondents by item, within each region.
3.4. LDKT surgery and pre‐operative screening
LDKT surgery has been negatively impacted by the pandemic. Almost all transplant centers reported a reduction in surgical activity. Volume was decreased by at least 75% of pre‐pandemic levels at 91% of responding programs, with 75% of programs halting LDKT completely (from 67% of North American centers to 91% of European centers) (Figure 1B). Among the barriers cited to proceeding with LDKT, center concerns for donor (82%) and recipient (76%) safety were the leading barriers to LDKT during the pandemic, followed by patient (donor and/or recipient) concerns (48%), other hospital (46%) and government restrictions (46%).
European, Asian, and Middle Eastern countries reported more barriers related to staff and resource diversion (Figure 3). North and Latin American centers were more concerned with testing capacity and insufficient resources. The majority of programs that reported interruptions also reported plans to re‐start LDKT within the next month (Table 4). Overall, 56% of programs elected to pause KPD activity. In Latin American, European and Asia/Middle Eastern countries, LDKT centers have either completely halted or have never participated in KPD exchanges.
Figure 3.
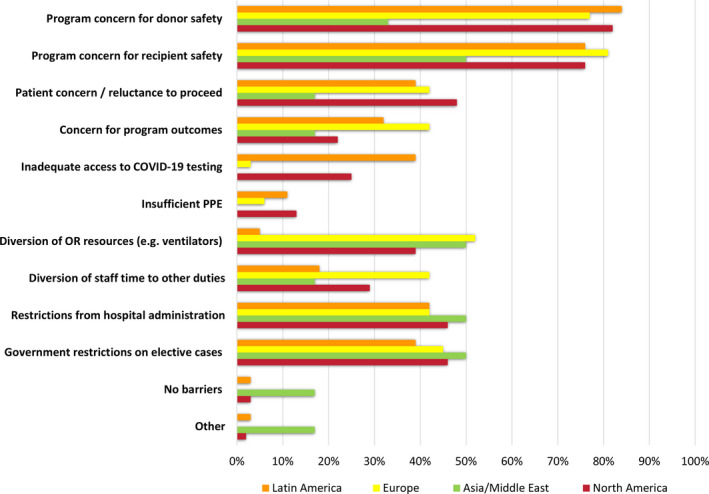
Barriers to living donor transplant encountered during the pandemic
Table 4.
LDKT surgery practices and pre‐operative screening during the COVID‐19 pandemic
| Overall | Latin America | Europe | Asia/Middle East | North America | |
|---|---|---|---|---|---|
| Would you approve a living donor candidate for surgery based on telehealth evaluation only, without physical exam? | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) |
| Yes | 14% (28/202) | 10% (4/39) | 11% (4/35) | 0% (0/6) | 16% (20/122) |
| No | 86% (174/202) | 90% (35/39) | 89% (31/35) | 100% (6/6) | 84% (102/122) |
| When are you planning to resume normal living donor transplantation procedures? | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) |
| Never interrupted | 4% (8/204) | 2% (1/40) | 0% (0/35) | 17% (1/6) | 5% (6/123) |
| Within the next 2 weeks | 23% (47/204) | 8% (3/40) | 20% (7/35) | 0% (0/6) | 30% (37/123) |
| Within the month | 23% (47/204) | 2% (1/40) | 34% (12/35) | 17% (1/6) | 27% (33/123) |
| When the incidence of local COVID‐19 cases has shown steady decline over 14 days | 19% (38/204) | 48% (19/40) | 29% (10/35) | 17% (1/6) | 7% (8/123) |
| When recommended by professional guidelines | 13% (27/204) | 30% (12/40) | 9% (3/35) | 50% (3/6) | 7% (9/123) |
| Program had paused, but now resumed | 18% (37/204) | 10% (4/40) | 9% (3/35) | 0% (0/6) | 24% (30/123) |
| When you resume living donation, when will you perform COVID‐19 testing in asymptomatic patients in relation to surgery? | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) |
| Within 24 hours | 30% (61/201) | 33% (13/39) | 34% (12/35) | 50% (3/6) | 27% (33/121) |
| Within > 24 to 48 hours | 40% (81/201) | 33% (13/39) | 29% (10/35) | 17% (1/6) | 47% (57/121) |
| Within > 48 to 72 hours | 26% (52/201) | 21% (8/39) | 34% (12/35) | 17% (1/6) | 26% (31/121) |
| Will not test asymptomatic patients | 3% (7/201) | 13% (5/39) | 3% (1/35) | 17% (1/6) | 0% (0/121) |
| What testing modality do you use for presurgical COVID‐19 testing for living donors? Select all that apply. | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) |
| PCR, nasopharyngeal swab | 98% (190/194) | 94% (31/33) | 97% (33/34) | 100% (6/6) | 99% (120/121) |
| PCR, other specimen | 2% (3/194) | 0% (0/33) | 3% (1/34) | 17% (1/6) | 1% (1/121) |
| Serum IgG Antibody | 21% (40/194) | 24% (8/33) | 29% (10/34) | 0% (0/6) | 18% (22/121) |
| Serum IgM Antibody | 15% (30/194) | 15% (5/33) | 24% (8/34) | 17% (1/6) | 13% (16/121) |
| Serum Antigen | 2% (3/194) | 0% (0/33) | 3% (1/34) | 0% (0/6) | 2% (2/121) |
| Where do you send presurgical COVID‐19 testing for donors and recipients? Select all that apply. | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) |
| Hospital lab | 83% (160/193) | 47% (16/34) | 85% (29/34) | 83% (5/6) | 92% (110/119) |
| Community lab | 12% (24/193) | 21% (7/34) | 6% (2/34) | 0% (0/6) | 13% (15/119) |
| Public health reference lab | 19% (36/193) | 53% (18/34) | 29% (10/34) | 17% (1/6) | 6% (7/119) |
| How has the COVID‐19 pandemic impacted kidney paired donation (KPD) at your center? | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) |
| Continue all KPD | 10% (20/200) | 3% (1/39) | 0% (0/34) | 0% (0/6) | 16% (19/121) |
| Continue only internal KPD | 4% (7/200) | 0% (0/39) | 0% (0/34) | 0% (0/6) | 6% (7/121) |
| Halt all KPD | 56% (113/200) | 5% (2/39) | 59% (20/34) | 33% (2/6) | 74% (89/121) |
| Center does not perform KPD | 30% (60/200) | 92% (36/39) | 41% (14/34) | 67% (4/6) | 5% (6/121) |
Denominators for percentages reflect respondents by item, within each region.
3.5. Impact of COVID‐19 on disease transmission practices
To ensure safe practice, 97% of responding programs plan to implement predonation testing for COVID‐19, of which 98% plan to test using polymerase chain reaction (PCR) nasopharyngeal swabs (Table 4). In addition, 21% of programs planning testing intend to use of serum IgG antibody testing, varying from no intention in Asia/Middle East to 29% intention in Europe. COVID‐19 testing is performed at the hospital laboratory in 83% of programs, while few reported using community‐based partners or a public health laboratory facility. Timing of testing varies by center, with 30% requiring testing within 24 hours, 40% within 48 hours and 26% within 72 hours of donation surgery. Of interest, 3% of centers do not intend to test asymptomatic patients. Program practices regarding requirement for self‐quarantining before donation is variable. Durations of recommended predonation self‐quarantine also vary. No quarantine is requested in 21% of responding programs, 54% require 7 to 14 days, while the remainder requires a variety of shorter lengths (Table 5). Centers in Europe and North America tended to request shorter quarantines than those in South and Latin America, and Asia/Middle East.
Table 5.
LDKT practices and disease transmission during the COVID‐19 pandemic
| Overall | Latin America | Europe | Asia/Middle East | North America | |
|---|---|---|---|---|---|
| How long do you ask local donors to self‐quarantine prior to donation surgery? | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) |
| No general quarantine request | 21% (41/194) | 6% (2/32) | 18% (6/33) | 0% (0/6) | 27% (33/123) |
| > 0 to 2 days | 3% (5/194) | 0% (0/32) | 0% (0/33) | 0% (0/6) | 4% (5/123) |
| 2 to 7 days | 14% (28/194) | 3% (1/32) | 12% (4/33) | 0% (0/6) | 19% (23/123) |
| 7 to 14 days | 54% (104/194) | 91% (29/32) | 64% (21/33) | 83% (5/6) | 40% (49/123) |
| Other | 8% (16/194) | 0% (0/32) | 6% (2/33) | 17% (1/6) | 11% (13/123) |
| If a donor has to travel to your center for surgery (ie, residence is not local), how long will you require them to quarantine prior to surgery (in addition to negative COVID‐19 testing)? | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) |
| No quarantine with negative COVID‐19 test | 24% (46/189) | 25% (8/32) | 15% (5/34) | 0% (0/5) | 28% (33/118) |
| > 0 to 2 days | 4% (8/189) | 9% (3/32) | 3% (1/34) | 0% (0/5) | 3% (4/118) |
| 2 to 7 days | 13% (25/189) | 6% (2/32) | 12% (4/34) | 20% (1/5) | 15% (18/118) |
| 7 to 14 days | 44% (84/189) | 47% (15/32) | 65% (22/34) | 60% (3/5) | 37% (44/118) |
| Refuse donor | 3% (6/189) | 6% (2/32) | 0% (0/34) | 20% (1/5) | 3% (3/118) |
| Prefer remote donation surgery | 3% (5/189) | 3% (1/32) | 0% (0/34) | 0% (0/5) | 3% (4/118) |
| Other | 8% (15/189) | 3% (1/32) | 6% (2/34) | 0% (0/5) | 10% (12/118) |
| Would your center accept a donor who has recovered from COVID‐19 infection and is PCR negative but Antibody positive? | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) |
| Yes | 66% (126/192) | 68% (26/38) | 66% (23/35) | 33% (2/6) | 66% (75/113) |
| No | 34% (66/192) | 32% (12/38) | 34% (12/35) | 67% (4/6) | 34% (38/113) |
| What measures does your center use to reduce risk of donor contracting COVID‐19 during surgical hospitalization? Select all that apply. | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) |
| Separate COVID‐19 and non‐COVID‐19 wards | 92% (180/195) | 94% (32/34) | 100% (34/34) | 83% (5/6) | 90% (109/121) |
| PPE use for patients and staff | 82% (159/195) | 74% (25/34) | 76% (26/34) | 83% (5/6) | 85% (103/121) |
| Staff screening | 51% (99/195) | 38% (13/34) | 44% (15/34) | 17% (1/6) | 58% (70/121) |
| Other | 7% (14/195) | 3% (1/34) | 3% (1/34) | 17% (1/6) | 9% (11/121) |
| How do you counsel living donors about COVID‐19 related risks? Select all that apply. | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) |
| The risk of contracting COVID‐19 is not impacted by donation | 43% (78/180) | 39% (12/31) | 47% (14/30) | 33% (2/6) | 44% (50/113) |
| The risk of complications is not impacted by donation | 34% (61/180) | 23% (7/31) | 50% (15/30) | 50% (3/6) | 32% (36/113) |
| COVID‐19 has been associated with acute kidney injury | 57% (102/180) | 71% (22/31) | 43% (13/30) | 33% (2/6) | 58% (65/113) |
| Other counseling | 19% (34/180) | 6% (2/31) | 0% (0/30) | 17% (1/6) | 27% (31/113) |
Denominators for percentages reflect respondents by item, within each region.
For patients who travel to the living donor recovery center, 76% of responding programs require additional quarantine, usually from 7 to 14 days (Table 5). A single responding program outside North America would opt for remote donation, using organ travel, compared to patient travel for a distant donor. Six centers would refuse a donor who required travel for donation. To further protect patients during hospitalization, 92% of responding programs have separate COVID‐19 wards and 82% require PPE and testing (51%) for all staff.
3.6. Living donor counseling
Forty‐three percent of LDKT centers have counseled donors that the risk of contracting COVID‐19 is not impacted by donation, 34% counsel that the risk of complications is not impacted by donation, and 57% educate donors that COVID‐19 has been associated with acute kidney injury (AKI) (Table 5). Agreement of responses on counseling appears similar across transplant center location.
3.7. Living donor follow‐up
Appropriate follow‐up of donor and recipient pairs has been challenged during the pandemic (Table 6). More than a third of responding LDKT centers completely stopped clinical and laboratory follow‐up during the pandemic. Forty‐eight percent of the centers reported continuing follow‐up without change in clinical evaluation and lab testing, while 15% have continued clinical follow‐up while deferring laboratory testing. Among those continuing follow‐ups, 33% reported conducting in‐person visits (33%) (including 67% at Latin American centers), while 57% of used video‐based telehealth assessment (including 73% at North American centers), and 65% used telephone contacts (including 82% at European centers). Among programs continuing laboratory follow‐up testing, most used hospital and community labs and only 14% used home‐based phlebotomy. The most common barriers to living donor follow‐up reported were hospital restrictions on elective visits (55%), followed by patients’ unwillingness to come for laboratory testing and on‐site visits (Figure 4).
Table 6.
LDKT follow‐up during the COVID‐19 pandemic
| Overall |
Latin America |
Europe |
Asia/Middle East |
North America | |
|---|---|---|---|---|---|
| Have you continued living donor follow‐up during the pandemic? (N = 203) | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) |
| Yes: clinical and labs | 48% (97/203) | 42% (17/40) | 37% (13/35) | 67% (4/6) | 52% (63/122) |
| Yes: clinical only, but labs deferred | 15% (31/203) | 5% (2/40) | 11% (4/35) | 33% (2/6) | 19% (23/122) |
| No (we have paused follow‐up) | 37% (75/203) | 52% (21/40) | 51% (18/35) | 0% (0/6) | 30% (36/122) |
| If you have continued clinical living donor follow‐up what modalities do you use for patient interactions? Select all that apply. (N = 136) | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) |
| Assessment in clinic | 33% (45/136) | 67% (14/21) | 53% (9/17) | 50% (3/6) | 21% (19/92) |
| Telehealth: telephone‐based | 65% (89/136) | 52% (11/21) | 82% (14/17) | 50% (3/6) | 66% (61/92) |
| Telehealth: video‐based | 57% (77/136) | 33% (7/21) | 18% (3/17) | 0% (0/6) | 73% (67/92) |
| If you continued living donor follow‐up lab testing during the pandemic, where are labs performed? Select all that apply. (N = 116) | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) |
| Transplant hospital | 69% (80/116) | 80% (16/20) | 88% (14/16) | 75% (3/4) | 62% (47/76) |
| Community lab | 69% (80/116) | 65% (13/20) | 31% (5/16) | 25% (1/4) | 80% (61/76) |
| Home‐based phlebotomy service | 14% (16/116) | 5% (1/20) | 6% (1/16) | 0% (0/4) | 18% (14/76) |
| Has your center used telehealth for living donor follow‐up prior to the COVID‐19 pandemic? (N = 200) | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) |
| Yes | 16% (33/200) | 20% (8/40) | 12% (4/33) | 17% (1/6) | 17% (20/121) |
| No | 84% (167/200) | 80% (32/40) | 88% (29/33) | 83% (5/6) | 83% (101/121) |
| Do you plan to use telehealth for living donor care after the COVID‐19 pandemic? (N = 200) | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) |
| Yes, at higher than pre‐pandemic utilization | 47% (94/200) | 25% (10/40) | 26% (9/34) | 33% (2/6) | 61% (73/120) |
| Yes, selectively | 40% (80/200) | 42% (17/40) | 65% (22/34) | 33% (2/6) | 32% (39/120) |
| No | 13% (26/200) | 32% (13/40) | 9% (3/34) | 33% (2/6) | 7% (8/120) |
Denominators for percentages reflect respondents by item, within each region.
Figure 4.
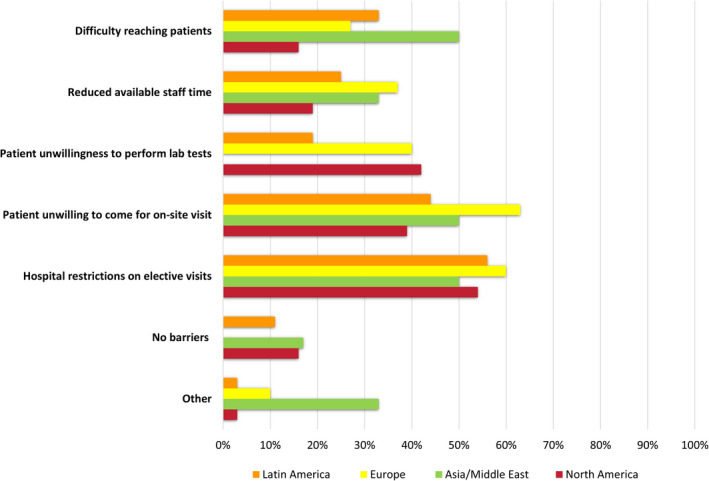
Barriers to living donor follow‐up during the pandemic
4. DISCUSSION
The COVID‐19 pandemic has significantly changed healthcare delivery across the world, 1 , 2 , 3 including profound impacts on organ transplantation. 8 , 9 , 10 , 11 To safeguard donors and to protect recipients, most LDKT centers adjusted their practices. 10 , 11 , 12 In this international survey of LDKT center practices, we assessed the impact of the COVID‐19 pandemic on LDKT evaluation, surgery, follow‐up, and education practices, and identified several major findings. First, the pandemic has decreased not only LDKT surgeries, but also donor evaluation, testing, and follow‐up across the world. Second, telehealth was unfrequently used by LDKT centers prior to the pandemic, and its use has grown significantly during the COVID‐19 crisis, although at different rates based on local resources and preferences. Third, multiple barriers underlie the change in the decreased LDKT activity, including center‐related concerns with donor/recipient safety, patient's reluctance to proceed, staff and resource limitations, and hospital and government restrictions. Finally, LDKT centers are in the process of resuming operations based on guidelines from national health authorities, transplantation societies, and local infection rates. Significant variation was documented in time and strategies to resume donation procedures, test and quarantine patients, and conduct necessary follow‐up.
Telehealth was not widely used in transplantation prior to the COVID‐19 pandemic. 18 , 19 , 20 , 21 Although its utilization by many specialties has expanded in response to restrictions on in‐person patient care, the use of remote evaluations for LDKT has not been prospectively evaluated. 10 , 11 , 22 , 23 , 24 Contrary to our expectation, the utilization of telehealth in LDKT has not grown uniformly across the globe. Many centers in Latin America and Asia still elect in‐person visits for donor candidate evaluation. European and North American centers seem to have adopted telehealth more rapidly. Most programs remain unwilling to use telehealth solely for evaluation, and conduct the surgical evaluation in‐person prior to donation. Insurance coverage may play a role in the utilization of telehealth tools and the increases observed during the pandemic may be related to payment reforms (at least in the US context). 25 Transplant centers can utilize telehealth tools for medical and surgical evaluation, social work assessment, psychological and dietary interviews, and informed consent. In addition, telehealth appears to have been widely adopted for post‐operative follow‐up. These data support the conclusion that remote evaluation and management via telehealth will benefit living donors, limiting infection, reducing burden, and hopefully increasing willingness to donate. Future studies should examine the impact of telehealth‐based communication on donor's perceived quality of care and completeness of follow‐up.
Centers reported a number of barriers to performing LDKT procedures. Some barriers were logistical and practical including governmental regulations and the acquisition of PPE and mechanical ventilators. Still, unavailability of the equipment was not at the top of the list of barriers to LKDT and most centers seem to have adapted their wards to COVID‐19 free‐zones and follow recommendations for appropriate use of resources in line with government and hospital guidance. 26 In fact, fear of additional complications in otherwise healthy donors and potential risk of infections in immunosuppressed recipients led to significant reduction in LDKT access despite the known overall health benefits of LDKT for recipients.
Transplant centers have been challenged during the pandemic to conduct appropriate patient follow‐up. It is paramount that donors and recipients are assured that their safety is important to every transplant program, especially during the early postdonation period when assessment of renal function is critical. 27 Outside of North American and Europe, programs that continued follow‐up relied largely on in‐person visits. While technological and cultural constraints may limit the rapid adoption of telehealth, insurers and policy makers should support telehealth options and remote lab draws to help ensure that donors are appropriately and safely followed after donation.
Respondents differed in how and when to test patients for COVID‐19 prior to LDKT, although nearly all endorsed the need for some form of testing. We were surprised that 21% of the surveyed LDKT centers were planning to test donors only by IgG serology. This has not been the recommendation of most of medical societies and the outcomes of this practice must be followed closely by the transplant community.
The indication and duration of predonation quarantine practices and the willingness to accept patients who have recovered from COVID‐19 were also not uniform among the surveyed centers. Some transplant professional societies have suggested 2‐week quarantine for patients who recovered from COVID‐19 infection and a negative PCR test for all patients, with testing as close as possible to the surgery date. 13 , 28 , 29 , 30 Another area that warrants analysis is the optimal management for donors who travel via aircraft to their donation center. The necessity of quarantine, government travel restrictions, and potentially financial hardship from work leave can influence decisions to donate and/or add liability to the donation process. Use of distant centers to perform surgeries and shipment of the kidney can be a viable alternative to donor travel. Although avoided by most centers, there is undoubtedly room for collaboration and expansion of regional networks, as feasibility and good outcomes have been demonstrated. 31 In terms of collaboration, the limited number of LDKT centers that participated in KPD exchanges during the pandemic is also notable. These chains have been extensively used in North America for almost two decades and there is certainly an opportunity for centers in Latin America, Europe, and Asia/Middle East to more broadly adopt remote donation. 32 , 33
Another intriguing finding of our survey relates to limitations in donor counseling. Most centers educate donors about associations of COVID‐19 and AKI, but fewer expand on the risks of donor complications. We are not aware of reports of a living donor who has acquired COVID‐19 and suffered AKI or required dialysis. However, it is possible that reduced renal function because of nephrectomy could increase the risk of severe AKI in those donors who acquire COVID‐19, which warrants close follow‐up during the pandemic. 34
Our study has the limitations inherent to the survey method, such as risks of recall biases and sampling bias. The findings represent practices as they are reported, and we cannot verify how accurately the reports represent actual practice at each LDKT program. The findings also represent local practices during the months of the survey and practices are likely to evolve over time, as the rate of infection varies over the course of the pandemic. Unfortunately, given the rapidly changing nature of the pandemic, we were not able to correlate center specific responses with current local infection rates. Additionally, practices reported at participating centers might differ from those at non‐responding centers and in other parts of the world, such as Africa, China, India, and Australia. Finally, the small number of centers limits statistical comparisons across regions and countries.
In conclusion, the COVID‐19 pandemic has had a universal impact on LDKT practice, including evaluation and counseling, testing, surgery, and post‐operative follow‐up care. Most surveyed centers stopped evaluating/testing donors and performing surgeries during the pandemic. Almost half of these centers stopped following donors despite telehealth options that allow the safe communication with patients. To safely resume LDKT, concerns related to donor and recipient safety, post‐transplant outcomes, patient reluctance, staff and resource limitations, and hospital and government restrictions must be overcome. Practices regarding resumption of surgeries, how and when to test and quarantine patients are not uniform, and best practices should be further investigated. Ongoing research is needed to support and optimize LDKT practice so that life‐saving surgery can be safely continued in the absence of an effective treatment or vaccine for COVID‐19.
AUTHOR CONTRIBUIONS
PRS, GFF, YK, DA: Study design, data interpretation, and writing of the paper. LSV: Study design, regulatory approvals, data analysis, and presentation. TSF, LRM, NNL, RAM: data interpretation and writing of the paper. MAS, KLL: Study design, regulatory approvals, data interpretation, and writing of the paper.
CONFLICT OF INTEREST
The authors of this manuscript have no conflicts of interest to disclose as described by Transplantation.
Funding information
KLL is supported by the Mid‐America Transplant/Jane A. Beckman Endowed Chair in Transplantation.
Supporting information
Table S1
ACKNOWLEDGEMENTS
The authors thank survey respondents, including members of the Turkish Society of Nephrology, Canadian Blood Services Living Donation Advisory Committee, and the American Society of Transplantation (AST) Outstanding Questions in Transplantation listservs. We also thank the AST Education Committee for review of the survey instrument. KLL is supported by the Mid‐America Transplant/Jane A. Beckman Endowed Chair in Transplantation.
Salvalaggio PR, Ferreira GF, Caliskan Y, et al. An International survey on living kidney donation and transplant practices during the COVID‐19 pandemic. Transpl Infect Dis.2021;23:e13526. 10.1111/tid.13526
Paolo R. Salvalaggio and Gustavo F. Ferreira are co first authors.
David A. Axelrod and Krista L. Lentine are co senior authors.
REFERENCES
- 1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;7(323):1239–1242. [DOI] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kumar D, Tellier R, Draker R, Levy G, Humar A. Severe Acute Respiratory Syndrome (SARS) in a liver transplant recipient and guidelines for donor SARS screening. Am J Transplant. 2003;3(8):977‐981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. AlGhamdi M, Mushtaq F, Awn N, Shalhoub S. MERS CoV infection in two renal transplant recipients: case report. Am J Transplant. 2015;15(4):1101‐1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu L, Gong N, Liu B, et al. Coronavirus disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Eur Urol. 2020;77(6):748‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short‐term outcome of 20 kidney transplant patients admitted for SARS‐CoV2 pneumonia. Kidney Int. 2020;97(6):1083‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Loupy A, Aubert O, Reese PP, Bastien O, Bayer F, Jacquelinet C. Organ procurement and transplantation during the COVID‐19 pandemic. Lancet. 2020;395(10237):e95‐e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lembach H, Hann A, McKay SC, et al. Resuming liver transplantation amid the COVID‐19 pandemic. Lancet Gastroenterol Hepatol. 2020;5(8):725‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boyarsky BJ, Po‐Yu Chiang T, Werbel WA, et al. Early impact of COVID‐19 on transplant center practices and policies in the United States. Am J Transplant. 2020;20(7):1809‐1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ahn C, Amer H, Anglicheau D, et al. Global transplantation COVID report march 2020. Transplantation. 2020;104(10):1974‐1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumar D, Manuel O, Natori Y, et al. COVID‐19: a global transplant perspective on successfully navigating a pandemic. Am J Transplant. 2020;20(7):1773‐1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ritschl PV, Nevermann N, Wiering L, et al. Solid organ transplantation programs facing lack of empiric evidence in the COVID‐19 pandemic: a by‐proxy society recommendation consensus approach. Am J Transplant. 2020;20(7):1826‐1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lentine KL, Vest LD, Schnitzler MA, et al. Survey of US living kidney donation and transplantation practices in the COVID‐19 era. Kidney Int Rep. 2020;5(11):1894–1905 10.1016/j.ekir.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mandelbrot DA, Pavlakis M, Danovitch GM, et al. The medical evaluation of living kidney donors: a survey of US transplant centers. Am J Transplant. 2007;7(10):2333‐2343. [DOI] [PubMed] [Google Scholar]
- 16. Mandelbrot DA, Pavlakis M, Karp SJ, Johnson SR, Hanto DW, Rodrigue JR. Practices and barriers in long‐term living kidney donor follow‐up: a survey of U.S. transplant centers. Transplantation. 2009;88(7):855‐860. [DOI] [PubMed] [Google Scholar]
- 17. Mandelbrot DA, Fleishman A, Rodrigue JR, Norman SP, Samaniego M. Practices in the evaluation of potential kidney transplant recipients who are elderly: a survey of U.S. transplant centers. Clin Transplant. 2017;31(10). [DOI] [PubMed] [Google Scholar]
- 18. Lee TC, Kaiser TE, Alloway R, Woodle ES, Edwards MJ, Shah SA. Telemedicine based remote home monitoring after liver transplantation: results of a randomized prospective trial. Ann Surg. 2019;270(3):564‐572. [DOI] [PubMed] [Google Scholar]
- 19. Pape L, de Zwaan M, Tegtbur U, et al. The KTx360 degrees ‐study: a multicenter, multisectoral, multimodal, telemedicine‐based follow‐up care model to improve care and reduce health‐care costs after kidney transplantation in children and adults. BMC Health Serv Res. 2017;17(1):587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murgia F, Corona B, Bianciardi F, Romano P, Tagliente I, Bella S. The application of telemedicine in the follow‐up of lung transplantation in a patient with cystic fibrosis. Clin Ter. 2014;165(5):e382‐e383. [DOI] [PubMed] [Google Scholar]
- 21. Mammas CS, Geropoulos S, Kavantzas N, et al. Telemedicine systems in organ transplantation: a feasibility and reliability study of the integrated teleradiological and tele‐pathological evaluation of the cardiac graft. Stud Health Technol Inform. 2014;202:303‐306. [PubMed] [Google Scholar]
- 22. Smith WR, Atala AJ, Terlecki RP, Kelly EE, Matthews CA. Implementation guide for rapid integration of an outpatient telemedicine program during the COVID‐19 Pandemic. J Am Coll Surg. 2020;231(2):216‐222.e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid‐19. N Engl J Med. 2020;382(18):1679‐1681. [DOI] [PubMed] [Google Scholar]
- 24. Serper M, Cubell AW, Deleener ME, et al. Telemedicine in liver disease and beyond: Can the COVID‐19 crisis lead to action? Hepatology. 2020;72(2):723‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Centers for Medicare & Medicaid Services (CMS) . President Trump Expands Telehealth Benefits for Medicare Beneficiaries During COVID‐19 Outbreak. March 17, 2020. Available at: https://www.cms.gov/newsroom/press‐releases/president‐trump‐expands‐telehealth‐benefits‐medicare‐beneficiaries‐during‐covid‐19‐outbreak. Accessed: 9/23/2020.
- 26. Wall AE, Pruett T, Stock P, Testa G. Coronavirus disease 2019: Utilizing an ethical framework for rationing absolutely scarce health‐care resources in transplant allocation decisions. Am J Transplant. 2020;20(9):2332–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Massie AB, Holscher CM, Henderson ML, et al. Association of early postdonation renal function with subsequent risk of end‐stage renal disease in living kidney donors. JAMA Surg. 2020;155(3):e195472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. American Society of Transplantation (AST) . 2019‐nCoV (Coronavirus): Recommendations and Guidance for Organ Donor Testing. https://www.myast.org/covid‐19‐information. Published 2020. Updated 10/5/2020. Accessed 10/5/2020, 2020.
- 29. American Society of Transplant Surgeons (ASTS) . Re‐engaging Organ Transplantation in the COVID‐19 Era. June 5, 2020. Available at: https://asts.org/advocacy/covid‐19‐resources/asts‐covid‐19‐strike‐force/re‐engaging‐organ‐transplantation‐in‐the‐covid‐19‐era#.X8wpwdhKj‐g. Accessed: 9/23/2020.
- 30. The Transplantation Society (TTS) . Guidance on Coronavirus Disease 2019 (COVID‐19) for Transplant Clinicians. Updated June 8, 2020. Avaiable at: https://tts.org/23‐tid/tid‐news/657‐tid‐update‐and‐guidance‐on‐2019‐novel‐coronavirus‐2019‐ncov‐for‐transplant‐id‐clinicians. Accessed: 9/23/2020. https://tts.org/tid‐about/tidpresidents‐message/23‐tid/tid‐news/657‐tid‐update‐and‐guidance‐on‐2019‐novel‐coronavirus‐2019‐ncov‐for‐transplant‐id‐clinicians. Accessed.
- 31. Treat EG, Miller ET, Kwan L, et al. Outcomes of shipped live donor kidney transplants compared with traditional living donor kidney transplants. Transpl Int. 2014;27(11):1175‐1182. [DOI] [PubMed] [Google Scholar]
- 32. Flechner SM, Thomas AG, Ronin M, et al. The first 9 years of kidney paired donation through the National Kidney Registry: Characteristics of donors and recipients compared with National Live Donor Transplant Registries. Am J Transplant. 2018;18(11):2730‐2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Segev DL, Gentry SE, Warren DS, Reeb B, Montgomery RA. Kidney paired donation and optimizing the use of live donor organs. JAMA. 2005;293(15):1883‐1890. [DOI] [PubMed] [Google Scholar]
- 34. Naicker S, Yang CW, Hwang SJ, Liu BC, Chen JH, Jha V. The Novel Coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97(5):824‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


