Abstract
Background
The first case of COVID-19 infection in Saudi Arabia was reported in Qatif on March 2nd, 2020. Here, we describe the clinical characteristics of the initial COVID-19 patients in that area.
Methods
This is an observational study describing the clinical presentation, radiographic and laboratory data of COVID-19 cases.
Results
From March 1st, 2020 to April 5th, 2020 we identified a total of 82 adult COVID-19 patients. The median age of the patients was 50 years, with a range of 30 to 60 years and most of patients were female 54 (65.9%). Of all the patients, 29 (35.4%) were contacts and 43 (52.4%) were returning travelers, mainly from Iraq (65% of the total returning travelers). Comorbidities were present in 50% of patients, G6PD deficiency in 33%, hypertension in 27%, and diabetes mellitus in 26%. Chest radiographs were abnormal in 46% of symptomatic and 15.5% of asymptomatic patients (P value = 0.0035). Of all patients, 4 (4.87%) required intensive care admission. There was no significant difference in time to negative RT-PCR with mean days to negativity of 13.6 and 16.9 for asymptomatic and symptomatic group, respectively (P value = 0.42).
Conclusions
In the initial Epicenter of the COVID-19 in Saudi Arabia, the majority of the patients were asymptomatic and were returning travelers. Comorbidities were present in nearly half of the patients.
Keywords: COVID-19, SARS-CoV-2, Asymptomatic, Clinical presentation, Hospitalization
Introduction
The emergence in late December 2019 of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) had caused a global pandemic. The SARS-CoV-2 is genetically similar to SARS-CoV [1]. The initial cluster of cases were identified in Wuhan city, China and were related to a seafood market and later SARS-CoV-2 infections were secondary to community transmission from one person to another [1,2]. Subsequently, studies demonstrated human-to-human transmission of SARS-CoV-2 through either droplets or direct contact [3]. Initial cases occurred mainly among travelers from China and those who have had contact with such travelers. However, ongoing local transmission has driven the initial outbreaks in all countries around the globe including countries in Asia, Europe, North America and the Middle East [4,5]. The initial reported cases of COVID -19 in the Arabian Gulf region were related to travelers who came from Iran and Iraq to Bahrain and Kuwait [6,7]. The first case of the COVID-19 infection reported in Saudi Arabia was in Qatif, Eastern province in a person who came from Iran [6]. The Kingdom of Saudi Arabia had taken extensive preemptive strategies to curtail the spread of SARS-CoV-2 transmission [8,9]. There are limited published studies about COVID-19 in patients from the Gulf Cooperation Council (GCC) [[10], [11], [12]], and thus here we present the clinical presentation of the initial cases that were diagnosed in Saudi Arabia.
Methods
This is an observational descriptive study of adult patients (more than 14 years of age) with confirmed SARS-CoV-2 infection from March 1st, 2020 until April 5th, 2020 at Qatif Central Hospital. All patients who were diagnosed at that time were required by the Saudi Ministry of Health to be hospitalized including those who were asymptomatic [7]. The diagnosis of SARS-CoV-2 SARS-CoV-2 infection was based on a positive real-time reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay of SARS-CoV-2 based on nasal and pharyngeal swab specimens as described previously [13].
We used a pre-determined data extraction excel sheet to gather the different variables. The sociodemographic, epidemiological, clinical signs and symptoms, comorbidities, laboratory and radiological results were extracted from available medical records. We included time to clinical improvement (time from admission to discharge), need for intensive care unit (ICU) admission and invasive ventilation, presence of multiorgan failure, or mortality. The onset of the disease was defined as the day when symptoms appeared. Thrombocytopenia was defined as a platelet count of less than 150,000 cells per cubic millimeter according to American society of hematology. Lymphocytopenia was considered when lymphocyte count was ≤1500 per cubic millimeter [10,14]. Fever was defined as an axillary temperature of 37.5 °C or higher, and ARDS was defined according to the Berlin definition [15]. The study was approved by the institutional research ethic committee of Qatif central hospital (QCH), Eastern province, Saudi Arabia (QCH-SREC0195/2020).
Statistical analysis
Descriptive analysis of demographics, patients clinical and laboratory characteristics were expressed as frequencies and percentages for categorical data and mean and standard deviation (SD) or median and interquartile range (IQR) for continuous data. Comparison of asymptomatic and mildly symptomatic disease was done using chi square (χ2) test or Fisher exact test as appropriate for categorical outcomes. Student t-test was used for continuous variables. Univariate and multivariate logistic regression methods were used to adjust for the effect of age, gender, comorbid illness, and outcome. All analyses were performed using IBM Statistics SPSS version 21. P value of ≤0.05 was considered significant.
Results
During the study period between March 1, 2020 and April 5,2020, there was a total of 82 confirmed COVID-19 patients. The median age of the patients was 50 (IQR 25), with a range from 30 to 60 years and most patients were female 54 (65.9%) (Table 1 ). Of all cases, 29 (35.4%) were contacts and 43 (52.4%) were returning travelers, mainly from Iraq (65% of the total returning travelers). Comorbidities were present in nearly half of patients, with G6PD deficiency being the highest (33%), hypertension (27%), and diabetes mellitus (26%). Of the total cases, sickle cell trait was seen in 6 (7.3%) and G6PD deficiency was seen in 27 (32.9%) with no difference between asymptomatic and symptomatic patients.
Table 1.
Characteristics and underlying comorbidities of included COVID-19 patients.
| Characteristics | All patients (N = 82) | Symptomatic patients (N = 37) | Asymptomatic patients (N = 45) | P value |
|---|---|---|---|---|
| Median age (IQR) – yr. | 50 (24) | 44 (25) | 53 (25) | 0.907 |
| Male – no. (%). | 28 (34.1) | 13 (35.1) | 15 (33.3) | 0.524 |
| Female – no. (%). | 54 (65.9) | 24 (64.9) | 30 (66.7) | 0.524 |
| Asthma – no. (%). | 4 (4.9) | 1 (2.7) | 3 (6.7) | 0.623 |
| Diabetes Mellitus – no. (%). | 22 (26.8) | 5 (13.5) | 17 (37.8) | 0.023 |
| Hypertension no. (%). | 22 (26.8) | 10 (27.0) | 12 (26.7) | 1.000 |
| Cerebrovascular disease no. (%). | 2 (2.5) | 0 (0) | 2 (4.4) | 0.248 |
| Malignancy – no. (%). | 3 (3.7) | 1 (2.7) | 2 (4.4) | 1.000 |
| Sickle cell trait – no. (%). | 6 (7.3) | 3 (8.1) | 3 (6.7) | 0.592 |
| G6PD – no. (%). | 27 (32.9) | 12 (32.4) | 15 (33.3) | 0.366 |
| Contact – no. (%). | 29 (35.4) | 16 (43.2) | 13 (28.9) | 0.133 |
| Travel – no. (%). | 43 (52.4) | 11 (29.7) | 32 (71.1) | <0.0001 |
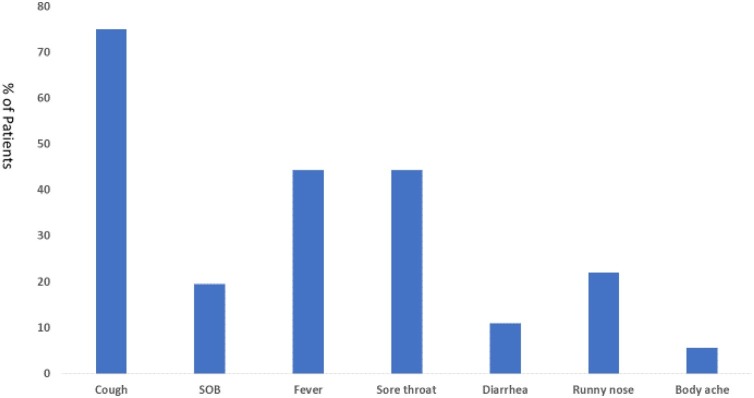
There were 45 (55%) asymptomatic patients and the remaining were symptomatic patients (Table 1). Asymptomatic patients were more likely to have diabetes mellitus (37.8% vs. 13.5% (P = 0.023) and being travelers (71.1% vs. 29.7%; P = <0.0001). Of the patients with symptoms, the most frequent symptoms were cough (75%), fever (44.4%), and sore throat (44.4%) (Fig. 1 ).
Fig. 1.
Percentage of Common Symptoms among Symptomatic COVID-19 patients.
SOB: Shortness of breath.
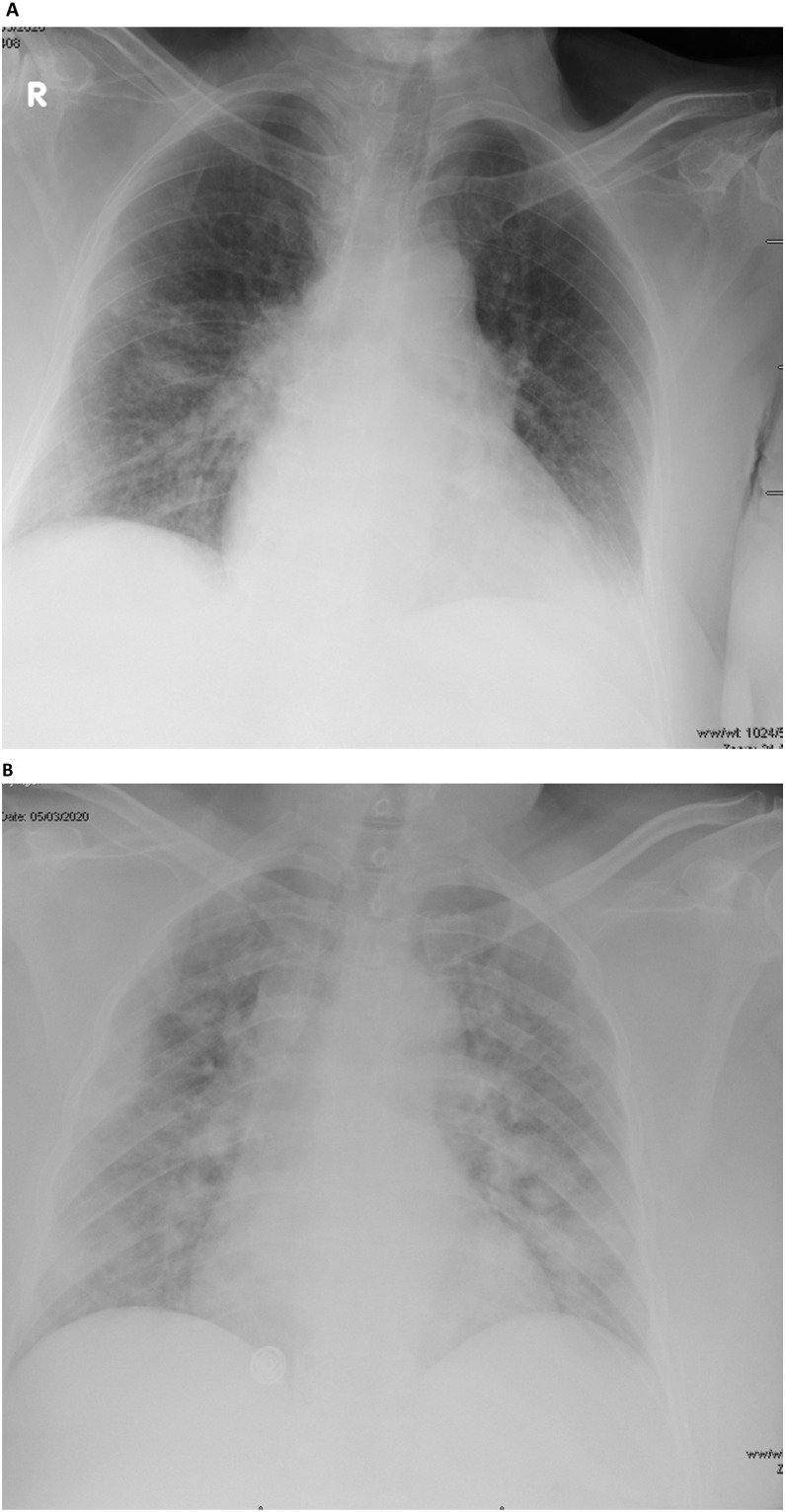
Chest radiographs were abnormal in 46% of the symptomatic and 15.5% of asymptomatic patients (P value = 0.0035) (Fig. 2 ). Unilateral pulmonary infiltration was observed in 8 (21.6%) of symptomatic and 5 (11%) of asymptomatic patients (P value = 0.2337), whereas bilateral infiltrates were seen in 9 (24.3%) and 2 (4.4%) of symptomatic and asymptomatic patients, respectively (P = 0.0191).
Fig. 2.
A portable anterior-posterior chest radiograph showing bilateral infiltrate in an asymptomatic (A) and symptomatic (B) COVID-19 patients.
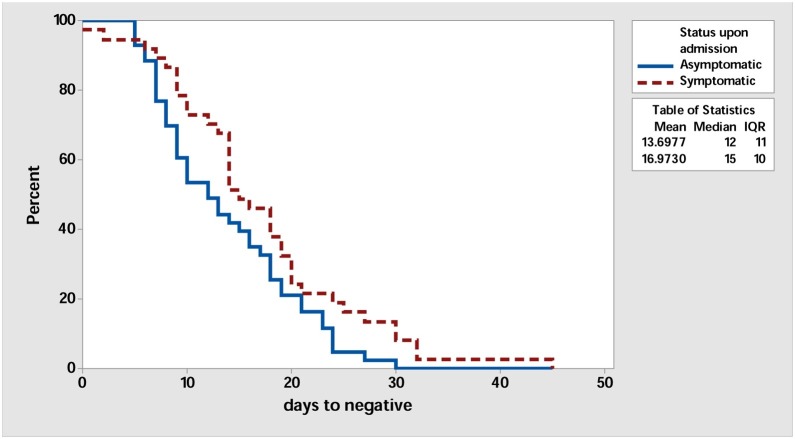
Laboratory findings of the included patients are shown in Table 2 . The majority of the patients had normal WBC, neutrophil counts, and lymphocyte counts. However, there was statistically significant difference in the mean of CRP of 2.19 and 0.44 in symptomatic and asymptomatic patients, respectively (P = 0.004). In addition, LDH was higher in symptomatic (215.57) than asymptomatic (182.79) patients (P = 0.016). The Neutrophils/Lymphocyte Count Ratio (NLR) was 1.77 and 1.30 for the symptomatic and asymptomatic cases, respectively (P = 0.091). There was no significant difference in the time to negative RT-PCR in both groups as shown in Fig. 3 with mean days to negative of 13.6 and 16.9 for asymptomatic and symptomatic group, respectively (P value = 0.42).
Table 2.
Common Laboratory Findings among COVID-19 Patients as a group and in symptomatic and asymptomatic groups.
| Parameter – mean | All patients (N = 82) | Symptomatic patients (N = 37) | Asymptomatic patients (N = 45) | P value |
|---|---|---|---|---|
| WBC x109 cells/L | 5.19 | 4.86 | 5.46 | 0.564 |
| Neutrophils x109 cells/L | 2.91 | 3.77 | 2.21 | 0.179 |
| Lymphocytes x109 cells/L | 2.39 | 3.02 | 1.87 | 0.275 |
| NLR* | 1.51 | 1.77 | 1.30 | 0.091 |
| Platelets x109 cells/L | 256.0 | 247.19 | 263.24 | 0.424 |
| ESR mm/hr | 26.07 | 29.06 | 23.31 | 0.337 |
| CRP mg/L | 1.27 | 2.19 | 0.44 | 0.004 |
| LDH mg/dl | 197.95 | 215.57 | 182.79 | 0.016 |
| D-Dimer | 0.73 | 0.78 | 0.69 | 0.668 |
| Ferritin ng/mL | 339.11 | 398.98 | 288.22 | 0.199 |
| ALT U/L | 23.78 | 27.22 | 20.94 | 0.179 |
| AST U/L | 21.45 | 25.69 | 18.15 | 0.260 |
| Total Bilirubin mg/dl | 8.75 | 10.52 | 7.22 | 0.082 |
| Conjugated Bilirubin mg/dl | 4.31 | 5.16 | 3.61 | 0.195 |
| Protein g/dl | 74.08 | 74.21 | 73.98 | 0.897 |
| Albumin mg/dl | 43.21 | 42.04 | 44.14 | 0.128 |
| ALP IU/L | 73.31 | 72.11 | 74.29 | 0.783 |
| Urea mmol/L | 5.05 | 4.85 | 5.21 | 0.794 |
| Creatinine umol/L | 79.27 | 97.95 | 63.92 | 0.193 |
| PT | 16.77 | 19.58 | 14.45 | 0.381 |
| PTT | 29.52 | 30.20 | 28.95 | 0.305 |
| INR | 1.14 | 1.35 | 0.96 | 0.243 |
| CK U/L | 101.19 | 134.22 | 72.88 | 0.053 |
| CKMB U/L | 22.04 | 18.561 | 25.01 | 0.287 |
NLR is Neutrophils/Lymphocyte Count Ratio.
Fig. 3.
Survival Plot for days to negative comparing asymptomatic (solid line) and asymptomatic (dashed line) groups.
Of all the patients, 23% received antimicrobial therapy, 29.3% had antiviral therapy and 25.6% had hydroxychloroquine. Of all the patients, 4 (4.87%) required intensive care admission as shown in Table 3 .
Table 3.
Characteristics of Critical COVID-19 patients.
| Total patients (n = 4) | Time till transfer* | SOFA score | Mortality estimation | NLR |
|---|---|---|---|---|
| Patient no.1 | 1 | 14 | ≥95.2% | 4.170 |
| Patient no.2 | 1 | 2 | ≤33.3% | 1.848 |
| Patient no.3 | 0 | 5 | 1.882 | |
| Patient no.4 | 3 | 16 | ≥95.2% | 8.960 |
Time in days from the first day of hospital admission to ICU admission; SOFA, Sequential Organ Failure Assessment; NLR, Neutrophil-Lymphocyte Ratio.
Discussion
In this study, we present the clinical, laboratory data and radiographic features of the initial COVID-19 patients that were reported from Saudi Arabia. The majority of these patients were travel-related and were asymptomatic and based on the ministry of health regulation at that time all patients were required to be hospitalized. The Kingdom of Saudi Arabia had taken strict measures to control the COVID-19 pandemic [6,7]. The country had designated COVID-19 hospitals to provide management for COVID-19 patients. Healthcare workers are advised to use droplet and contact precautions when dealing with suspected COVID-19 patients. These precautions are upgraded to airborne infection transmission when performing aerosol generating procedures. In addition, the Kingdom had adopted early utilization of universal masking even in public areas coupled with social distancing and hand hygiene. These precautionary measures are fundamental in the prevention of COVID-19 [16].
The mean age of the patients in the current study was 50 years and was slightly higher than the reported mean age in previous studies from Saudi Arabia that reported 36 years [10] and a median age of 44 years in another study [12]. The clinical picture of COVID-19 cases is variable and ranges from asymptomatic, to symptomatic, and severe disease [17,18]. Only a small number (4 patients; 4.8%) in our sample required ICU admission which was different from other studies reporting ICU admission in 50% of the patients [8] but is similar to the rate of 4.7% reported in a study from Saudi Arabia [10] and another study from China [14]. This difference is easily explained by the fact that we had to admit even asymptomatic patients based on the country’s rules and regulation. However, the number of the critical cases in this study is small to make any generalizable conclusions.
The clinical presentations of symptomatic patients showed that fever was present in less than half of the patients. The presence of fever other studies is variable and ranges from 36% to 98.6% [11,17]. In a large study of 24,410 adults infected by SARS-CoV-2, 78% had fever on presentation [19].
About half of the included patients had comorbidities and this is in contrast to the cited percentage of 32%–93% in different studies [17,20]. There are various pre-existing comorbidities that were associated with COVID-19. We found hypertension (27%) and diabetes mellitus (26%). In a systematic review that included patients till April 7th, 2020, 11% of the included patients had diabetes mellitus [21]. Other studies had also showed high rates of hypertension and diabetes mellitus among COVID-19 patients including studies from Saudi Arabia [10,11], Oman [22] and other parts of the world [2,23,24].
In this study, 54% of the cases were asymptomatic. Asymptomatic COVID-19 is one of the features of this pandemic. The exact percentage of asymptomatic disease is not known [18,25]. The large percentage of asymptomatic patients in this study is likely related to the inclusion of returning travelers. Asymptomatic COVID-19 prevalence is related to the tested groups, and was about 50% in the ship cruise and nursing facilities [[26], [27], [28], [29], [30]]. In a large study of about 500 COVID-19 patients, asymptomatic patients represented 7.9% of the cases [31]. And another study from Saudi Arabia showed that 9.3% were asymptomatic [10].
In the current study, there was no difference in the WBC between symptomatic and asymptomatic patients. In a previous study, the mean WBC was 5.00 (4.03–6.28) in symptomatic and 5.11 (4.15–6.41) in asymptomatic patients [31]. This difference was small but was statistically significant. Most of the cases in the current study did not have lymphopenia. However, previous studies showed lymphopenia in 70% of patients [17]. The Neutrophils/Lymphocyte Count Ratio (NLR) of ≥3.13 was used as a predictor of the need for intensive care unit admission. It was found that elevated NLR and age were significantly associated with illness severity [32]. Thus, it is not surprising that the NLR was not statistically significant in the symptomatic and asymptomatic cases in the current study. However, patients who required ICU admissions had higher NLR which was proportionally related to the calculated mortality rate. In addition, the case fatality rate had varied and likely related to the presence of comorbidities [8,17]. In a recent study, there was a disparity in the death rates where 22.4% of COVID-19 deaths in the USA were among African Americans who constitute 13.4% of the USA population [33]. This is an interesting observation and deserves further evaluation in this part of the world.
CRP is an inflammatory marker and had been used in the early diagnosis of pneumonia. We found that symptomatic patients had higher level of CRP than asymptomatic patients. In a study of 15 asymptomatic patients, 10 (66.7%) had elevated CRP levels [34]. There was a correlation between the level of CRP and severity of COVID-19 cases. In one study, CRP levels increased as the disease progressed and the mean (±SD) was 1.52 ± 1.56 in mild disease compared to 16.76 ± 18.38 in moderate group, 54.15 ± 1.06 in severe disease and 105.00 ± 12.73 in critical group [35]. The progression of COVID-19 was associated with increasing CRP in another study and was a poor prognostic factor [36]. In addition, CRP elevation and eosinopenia had been used as triage markers in fever clinics [37].
There was no difference in the levels of ferritin and D-dimer between asymptomatic and mildly symptomatic patients in the current study. Ferritin is an acute phase reactant and it had been reported to increase as the disease severity increases and thus had been used as a prognostic factors [36]. In one study, ferritin levels were elevated in patients with severe disease COVID-19 or worse disease [38]. Similarly, D-dimer levels statistically significantly higher in severe disease compared to mild and moderate disease [38,39]. Thus, these studies explain the reason that there was no difference in the current study that did not include severe disease. In addition, there are different studies documenting no significant difference in cytokines between asymptomatic and symptomatic patients [40] and other studies documenting such a difference. These differences in the various studies including our study may be related to the population of patients being included, the time of the observations during the spectrum and course of COVID-19 disease.
In Qatif area, Glucose-6-phosphate dehydrogenase deficiency (G6PD) deficiency and sickle cell disease are common hematologic problems with rates of 30.6% and 20–30% of newborns are heterozygous for the sickle gene, respectively [41,42]. The current study showed that sickle cell disease was present in 6 (7.3%) and G6PD deficiency in 27 (32.9%) of patients with no difference between asymptomatic and symptomatic patients. There was no previous study describing the association between G6PD deficiency and COVID-19 [43]. However, it was shown that G6PD deficiency enhances human coronavirus 229E experimental infection [41]. It was assumed that G6PD deficiency may result in increased hemolysis and thus death in patients with COVID-19 [44]. We also found no difference in the occurrence of sickle cell trait between asymptomatic and systematic patients and only one patient had sickle cell disease. In a recent study from Bahrain, 6 of 378 SCD patients who were tested for SARS-CoV-2 were positive [45]. The extent of the effect of hematologic disorders on patients with COVI-19 is not yet established [[46], [47], [48]].
In the current study, chest radiographs were abnormal in 46% of symptomatic and 15.5% of asymptomatic patients. Although, routine chest radiography is not usually done in asymptomatic patients. One study in China showed that 94% of 58 asymptomatic patients had CT-scan findings [8]. Another study showed that 60% of asymptomatic patients had abnormalities confined to one lung and 90% of mildly symptomatic patients had abnormalities in both lungs [9]. Similarly, we found that bilateral radiographic abnormalities were more common in symptomatic patients.
One of the interesting observations in this study is the fact that there was no significant difference in the time to negative RT-PCR in asymptomatic and symptomatic groups. The sensitivity of RT-PCR is usually low in early stages of SARS-CoV-2 infection and ranges between 45 and 60% [49]. SARS-CoV-2 had a modest viral loads in the early stage and peaked approximately 10 days after symptoms onset [50]. Reasons for low sensitivity of PCR may include variations in the accuracies of different tests, low initial viral load or improper clinical sampling [49]. Understanding the viral dynamics profile of SARS-CoV-2 is an important tools to enhance the knowledge of the disease and mode of transmission [51]. In this study, the mean days to negative RT-PCR was 13.6 for asymptomatic and 16.9 for symptomatic patients. Previous studies showed that the median duration of viral shedding was 19 days (range, 6–37 days) from initial viral detection in 23 patients with mild or no symptoms [52]. Another study showed that more than one month lapsed prior to achieving PCR negativity in asymptomatic patients [53]. It is also important to acknowledge the phenomena of intermittent positivity as detected by RT-PCR as reported in previous studies [13,54,55].
In this study, we described differences and similarities between asymptomatic and symptomatic COVID-19 patients. The study is a retrospective and mainly a descriptive study and as such has few limitations. The study did not include sufficient number of severe cases requiring intensive care management, and did not span over a long follow up time to characterize the outcome of all cases. Being a cross-sectional study also limited full description and characterization of the clinical courses and outcomes. In addition, this is a single center study and included mainly returning travelers who had to be hospitalized to comply with the local guidelines. The study also did not address the predictors of progression to symptomatic disease vs. those factors contributing to patients remaining to be asymptomatic. The study included all hospitalized patients but a large percentage of them would not require hospitalization when considered at this time of the pandemic when such patients are cared for at home. Thus, the study results are not generable to all hospitalized patients.
In conclusion, initially reported COVID-19 cases in Saudi Arabia were mainly asymptomatic returning travelers. There was a significant difference in radiographic presentation between asymptomatic and symptomatic patients and the majority of patients had comorbidities. The study compared asymptomatic and symptomatic patients and further studies are needed to further elucidate factors contributing to the development of symptoms in COVID-19 patients.
Funding
No funding sources.
Competing interests
None declared.
Ethical approval
The study was approved by the institutional research ethic committee of Qatif central hospital (QCH), Eastern province, Saudi Arabia (QCH-SREC0195/2020).
References
- 1.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;6736:1–9. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao D., Yao F., Wang L., Zheng L., Gao Y., Ye J. A Comparative Study on the Clinical Features of Coronavirus 2019 (COVID-19) pneumonia with other pneumonias. Clin Infect Dis. 2020;71:756–761. doi: 10.1093/cid/ciaa247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peeri N.C., Shrestha N., Rahman M.S., Zaki R., Tan Z., Bibi S. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int J Epidemiol. 2020;49:717–726. doi: 10.1093/ije/dyaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Tawfiq J.A., Memish Z.A. COVID-19 in the Eastern Mediterranean Region and Saudi Arabia: prevention and therapeutic strategies. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Tawfiq J.A., Sattar A., Al-Khadra H., Al-Qahtani S., Al-Mulhim M., Al-Omoush O. Incidence of COVID-19 among returning travelers in quarantine facilities: a longitudinal study and lessons learned. Travel Med Infect Dis. 2020;38 doi: 10.1016/j.tmaid.2020.101901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Tawfiq J.A., Leonardi R., Fasoli G., Rigamonti D. Prevalence and fatality rates of COVID-19: what are the reasons for the wide variations worldwide? Travel Med Infect Dis. 2020;35 doi: 10.1016/j.tmaid.2020.101711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alsofayan Y.M., Althunayyan S.M., Khan A.A., Hakawi A.M., Assiri A.M. Clinical characteristics of COVID-19 in Saudi Arabia: a national retrospective study. J Infect Public Health. 2020;13:920–925. doi: 10.1016/j.jiph.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Omari A., Alhuqbani W.N., Zaidi A.R.Z., Al-Subaie M.F., AlHindi A.M., Abogosh A.K. Clinical characteristics of non-intensive care unit COVID-19 patients in Saudi Arabia: A descriptive cross-sectional study. J Infect Public Health. 2020;13(11):1639–1644. doi: 10.1016/j.jiph.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barry M., AlMohaya A.E., AlHijji A., Akkielah L., AlRajhi A., Almajid F. Clinical characteristics and outcome of hospitalized COVID-19 patients in a MERS-CoV endemic area. J Epidemiol Glob Health. 2020;10:214–221. doi: 10.2991/jegh.k.200806.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AlJishi J.M., Al-Tawfiq J.A. Intermittent viral shedding in respiratory samples of patients with SARS-CoV-2: observational analysis with infection control implications. J Hosp Infect. 2020;10 doi: 10.1016/j.jhin.2020.09.011. S0195-6701(20)30426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E. Acute respiratory distress syndrome: the Berlin definition. JAMA - J Am Med Assoc. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 16.Tirupathi R., Bharathidasan K., Palabindala V., Salim S.A., Al-Tawfiq J.A. Comprehensive review of mask utility and challenges during the COVID-19 pandemic. Infez Med. 2020;28:57–63. [PubMed] [Google Scholar]
- 17.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. China. JAMA - J Am Med Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Tawfiq J.A. Asymptomatic coronavirus infection: MERS-CoV and SARS-CoV-2 (COVID-19) Travel Med Infect Dis. 2020;35:101608. doi: 10.1016/j.tmaid.2020.101608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant M.C., Geoghegan L., Arbyn M., Mohammed Z., McGuinness L., Clarke E.L. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): a systematic review and meta-analysis of 148 studies from 9 countries. PLoS One. 2020;15 doi: 10.1371/journal.pone.0234765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–-506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baradaran A., Ebrahimzadeh M.H., Baradaran A., Kachooei A.R. Prevalence of comorbidities in COVID-19 patients: a systematic review and meta-analysis. Arch Bone Jt Surg. 2020;8:247–255. doi: 10.22038/abjs.2020.47754.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khamis F., Al-Zakwani I., Al Naamani H., Al Lawati S., Pandak N., Omar M.B. Clinical characteristics and outcomes of the first 63 adult patients hospitalized with COVID-19: an experience from Oman. J Infect Public Health. 2020;13:906–913. doi: 10.1016/j.jiph.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aggarwal S., Garcia-Telles N., Aggarwal G., Lavie C., Lippi G., Henry B.M. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): early report from the United States. Diagnosis. 2020;7:91–96. doi: 10.1515/dx-2020-0046. [DOI] [PubMed] [Google Scholar]
- 24.Feng Y., Ling Y., Bai T., Xie Y., Huang J., Li J. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201:1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gandhi M., Yokoe D.S., Havlir D.V. Asymptomatic transmission, the Achilles’ heel of current strategies to control Covid-19. N Engl J Med. 2020;395(10223):497–506. doi: 10.1056/nejme2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMichael T.M., Currie D.W., Clark S., Pogosjans S., Kay M., Schwartz N.G. Epidemiology of Covid-19 in a Long-Term Care Facility in King County, Washington. N Engl J Med. 2020;382(21):2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimball A., Hatfield K.M., Arons M., James A., Taylor J., Spicer K. Asymptomatic and presymptomatic SARS-COV-2 infections in residents of a long-term care skilled nursing facility - King County, Washington, March 2020. Morb Mortal Wkly Rep. 2020;69:377–381. doi: 10.15585/MMWR.MM6913E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arons M.M., Hatfield K.M., Reddy S.C., Kimball A., James A., Jacobs J.R. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382(22):2081–2090. doi: 10.1056/nejmoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roxby A.C., Greninger A.L., Hatfield K.M., Lynch J.B., Dellit T.H., James A. Detection of SARS-CoV-2 among residents and staff members of an independent and assisted living community for older Adults - Seattle, Washington, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:416–418. doi: 10.15585/mmwr.mm6914e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roxby A.C., Greninger A.L., Hatfield K.M., Lynch J.B., Dellit T.H., James A. Outbreak Investigation of COVID-19 among residents and staff of an independent and assisted living community for older adults in Seattle, Washington. JAMA Intern Med. 2020;180(8):1101–1105. doi: 10.1001/jamainternmed.2020.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mei X., Zhang Y., Zhu H., Ling Y., Zou Y., Zhang Z. Observations about symptomatic and asymptomatic infections of 494 patients with COVID-19 in Shanghai, China. Am J Infect Control. 2020;48:1045–1050. doi: 10.1016/j.ajic.2020.06.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang A.P., ping Liu J., qiang Tao W., ming Li H. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84 doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tirupathi R., Muradova V., Shekhar R., Salim S.A., Al-Tawfiq J.A., Palabindala V. COVID-19 disparity among racial and ethnic minorities in the US: a cross sectional analysis. Travel Med Infect Dis. 2020;38:101904. doi: 10.1016/j.tmaid.2020.101904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu T., Huang R., Zhu L., Wang J., Cheng J., Zhang B. Epidemiological and clinical features of asymptomatic patients with SARS-CoV-2 infection. J Med Virol. 2020;92:1884–1889. doi: 10.1002/jmv.25944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L. C-reactive protein levels in the early stage of COVID-19. Med Mal Infect. 2020;50:332–334. doi: 10.1016/j.medmal.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X., Wang L., Yan S., Yang F., Xiang L., Zhu J. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical center, Wuhan, China. Int J Infect Dis. 2020;94:128–132. doi: 10.1016/j.ijid.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Q., Ding X., Xia G., Chen H.G., Chen F., Geng Z. Eosinopenia and elevated C-reactive protein facilitate triage of COVID-19 patients in fever clinic: a retrospective case-control study. EClinicalMedicine. 2020;23 doi: 10.1016/j.eclinm.2020.100375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y., Dong Y., Wang L., Xie H., Li B., Chang C. Characteristics and prognostic factors of disease severity in patients with COVID-19: the Beijing experience. J Autoimmun. 2020;112:102473. doi: 10.1016/j.jaut.2020.102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu X., Yu MQ Shen Q., Wang L.Z., Di Yan R., Zhang M.Y. Analysis of inflammatory parameters and disease severity for 88 hospitalized covid-19 patients in Wuhan, China. Int J Med Sci. 2020;17:2052–2062. doi: 10.7150/ijms.47935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han H., Xu Z., Cheng X., Zhong Y., Yuan L., Wang F. Descriptive, retrospective study of the clinical characteristics of asymptomatic COVID-19 patients. MSphere. 2020;5 doi: 10.1128/msphere.00922-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Y.-H., Tseng C.-P., Cheng M.-L., Ho H.-Y., Shih S.-R., Chiu D.T.-Y. Glucose-6-Phosphate dehydrogenase deficiency enhances human coronavirus 229E infection. J Infect Dis. 2008;197:812–816. doi: 10.1086/528377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.el Mouzan M.I., al Awamy B.H., al Torki M.T., Niazi G.A. Variability of sickle cell disease in the Eastern Province of Saudi Arabia. J Pediatr. 1989;114(6):973–976. doi: 10.1016/s0022-3476(89)80440-8. [DOI] [PubMed] [Google Scholar]
- 43.Al-Abdi S., Al-Aamri M. G6PD deficiency in the COVID-19 pandemic: ghost within Ghost. Hematol Oncol Stem Cell Ther. 2020 doi: 10.1016/j.hemonc.2020.04.002. S1658-3876(20)30044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamming O.J., Terczyńska-Dyla E., Vieyres G., Dijkman R., Jørgensen S.E., Akhtar H. Interferon lambda 4 signals via the IFNλ receptor to regulate antiviral activity against HCV and coronaviruses. EMBO J. 2013;32:3055–3065. doi: 10.1038/emboj.2013.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.AbdulRahman A., AlAli S., Yaghi O., Shabaan M., Otoom S., Atkin S.L. COVID-19 and sickle cell disease in Bahrain. Int J Infect Dis. 2020;101:14–16. doi: 10.1016/j.ijid.2020.09.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heilbronner C., Berteloot L., Tremolieres P., Dupic L., De Saint Blanquat L., Lesage F. Patients with sickle cell disease and suspected COVID‐19 in a pediatric ICU. Br J Haematol. 2020 doi: 10.1111/bjh.16802. bjh.16802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karimi M., De Sanctis V. Implications of SARSr-CoV 2 infection in thalassemias: do patients fall into the “high clinical risk” category? Acta Biomed. 2020;91:50–56. doi: 10.23750/abm.v91i2.9592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vives Corrons J.-L., De Sanctis V. Rare anaemias, sickle-cell disease and COVID-19. Acta Biomed. 2020;91:216–217. doi: 10.23750/abm.v91i2.9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Tawfiq J.A., Memish Z.A. Diagnosis of SARS-CoV-2 infection based on CT scan vs. RT-PCR: reflecting on experience from MERS-CoV. J Hosp Infect. 2020;105(2):154–155. doi: 10.1016/j.jhin.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao A.T., Tong Y.X., Zhang S. Profile of RT-PCR for SARS-CoV-2: a preliminary study from 56 COVID-19 patients. Clin Infect Dis. 2020;71(16):2249–2251. doi: 10.1093/cid/ciaa460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Tawfiq J.A. Viral loads of SARS-CoV, MERS-CoV and SARS-CoV-2 in respiratory specimens: what have we learned? Travel Med Infect Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyamae Y., Hayashi T., Yonezawa H., Fujihara J., Matsumoto Y., Ito T. Duration of viral shedding in asymptomatic or mild cases of novel coronavirus disease 2019 (COVID-19) from a cruise ship: a single-hospital experience in Tokyo, Japan. Int J Infect Dis. 2020;97:293–295. doi: 10.1016/j.ijid.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiao A.T., Tong Y.X., Zhang S. False-negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: rather than recurrence. J Med Virol. 2020;92(10):1755–1756. doi: 10.1002/jmv.25855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Da Liu W., Chang S.Y., Wang J.T., Tsai M.J., Hung C.C., Hsu C.L. Prolonged virus shedding even after seroconversion in a patient with COVID-19. J Infect. 2020;81(2):318–356. doi: 10.1016/j.jinf.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]