Abstract
Background
Worldwide, seasonal influenza causes significant mortality and severe infections may cause cardiac injury. High-sensitive-troponins (hsTnT) are sensitive and specific markers of myocardial damage. This study investigated the prognostic impact of hsTnT on 30-day mortality in hospitalised influenza patients.
Methods
This retrospective study included influenza patients ≥ 18 years, who had hsTnT performed during admission in two tertiary-hospitals in South Australia. Diagnosis of influenza was confirmed by polymerase–chain-reaction (PCR) test and hsTnT > 14 ng/L with a change of > 20% during admission was considered to be indicative of acute-cardiac injury. Clinical characteristics, complications and 30-day mortality were compared among four groups of patients: hsTnT unavailable, hsTnT negative, chronically elevated hsTnT and acutely elevated hsTnT. Cox-proportional hazard regression determined the hazard of death at 30-days following hospital discharge after adjustment for co-variates.
Results
Between January 2016 -March 2020, 1828 influenza patients, mean age 66.4 years, were hospitalised. Troponin results were available for 617 (47.7%) patients, of whom, 62 (10%) had acute myocardial injury and 232 (37.6%) had chronic hsTnT elevation. Both inpatient and 30-day mortality were significantly higher among patients with acute (P < 0.001) and chronic hsTnT (P < 0.001) when compared to other groups. When compared to patients with negative hsTnT, acute but not chronic hsTnT elevation was significantly associated with 30-day mortality after adjustment for various co-variates (HR 8.30, 1.80–17.84, P value = 0.013).
Conclusions
This is the largest available analysis of cardiac-specific biomarker hsTnT in patients with influenza. An acutely elevated hsTnT was associated with 30-day mortality among hospitalised influenza patients.
Keywords: Influenza, High sensitive troponin, Mortality, Acute coronary syndrome, Acute cardiac injury
1. Introduction
Influenza most often causes a self-limited respiratory illness but, in some instances, may be severe enough to require hospitalisation and may even lead to death. Worldwide, more than 1 million deaths may be associated with an influenza pandemic with some strains of avian influenza having a case fatality rate of as high as 60% [1], [2]. In 2017, there were 29,000 influenza related hospitalisations and 745 deaths were reported due to influenza in Australia [3].
The most common complication associated with influenza is pneumonia, although, influenza may also lead to exacerbation of other underlying chronic medical problems (e.g. congestive heart failure (CHF), atrial fibrillation etc.) [4], [5]. In addition, influenza may lead to cardiac injury which may manifest as myocardial infarction (MI), myocarditis and CHF [6], [7]. Observational studies have suggested that influenza may be associated with an increased cardiovascular morbidity and mortality especially in older people (>85 years) during winter months [8], [9]. Case control studies indicate an increased risk of hospital admissions for acute MI and CHF during influenza season and have also suggested that there is a protective role of influenza vaccination and reduced risk of vascular events with use of antivirals for influenza infection among patients with a history of cardiovascular disease [10], [11], [12].
Although influenza poses a significant burden to the health of the global population, there are still gaps in the understanding of precise magnitude of this burden measured in terms of long-term outcomes, complications and costs, especially in patients with severe infection. Cardiac troponins have emerged as sensitive and specific markers of myocardial injury and elevated levels may indicate myocardial involvement during acute influenza infection [13]. Studies [14], [15] indicate higher immediate mortality among influenza patients who have elevated troponin levels. The impact of troponin levels in predicting long term clinical outcomes among patients with influenza is unknown. Therefore, the aim of the present study was to retrospectively analyse hsTnT serum levels as a marker of 30-day mortality among patients who needed hospitalisation for severe influenza.
2. Methods
We retrospectively analysed data obtained by reviewing the electronic medical records of all patients ≥ 18 years who had an admission with a confirmed influenza infection at two tertiary teaching hospitals (Flinders Medical Centre (FMC) and Royal Adelaide Hospital (RAH)) in Adelaide, between January 2016 to March 2020. Patients were classified as having a confirmed influenza infection in the presence of the International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Australian Modification (ICD-10-AM) [16] diagnosis code of influenza (ICD-10-AM J9 or J10) and a laboratory confirmed influenza infection, which was defined as occurring in a patient in whom influenza virus was detected in a respiratory sample on the basis of a positive reverse-transcription polymerase chain reaction (RT-PCR) test.
Cardiac injury was identified through analysis of electronic laboratory records of patients who had confirmed influenza infection. A case of acute cardiac injury was defined as a rise or fall of hsTnT with at least one value above 99th percentile upper reference limit (URL) [17]. Troponins were measured with the Roche hsTnT assay (Roche Elecsys Troponin T level of > 14 ng/L corresponds to 99th percentile URL) and > 20% rise or fall in hsTnT was regarded as a significant change to indicate an acute cardiac injury [18]. Ethical approval was granted by the Southern Adelaide Human Clinical Research Ethics Committee (SA HREC) and this study was registered with the Australia and New Zealand Clinical Trial Registry (ANZCR) no 12618000451202.
From the EMR, we abstracted the following variables for all patients who had had a hospital admission with laboratory confirmed influenza infection: age, sex, Charlson Comorbidity Index (CCI) [19], smoking status, history of diabetes, ischaemic heart disease (IHD), chronic obstructive pulmonary disease (COPD), bronchial asthma, interstitial lung disease (ILD), chronic kidney disease (CKD), malignancy and immunosuppression. Serum creatinine and c-reactive protein (CRP) levels available during admission were recorded. We also recorded complications during hospital admission: pneumonia, respiratory failure, acute respiratory distress syndrome (ARDS), sepsis and septic shock. Patients were determined to have an acute coronary syndrome (ST-elevation MI (STEMI), non-ST-elevation MI (NSTEMI) or unstable angina) during the index admission based on documentation in the discharge summaries or physician progress notes. The severity of influenza related illness was described by the number of medical emergency response team (MET) calls during admission, proportion of patients having multiple (≥2) MET calls, proportion of patients who went to the intensive care unit (ICU), time spent in hours in the ICU and in hospital mortality. The length of hospital stay (LOS) was adjusted for inpatient mortality. We also recorded mortality and unplanned hospital readmissions within 30 days following discharge.
3. Statistical analyses
Data were analysed for normality using histograms and continuous variables were analysed using one-way analysis of variance (ANOVA) or Kruskal-Wallis H test, while categorical variables were analysed using chi squared or Fisher’s exact test, where appropriate. For this study, patients who had an elevated hsTnT (>99th percentile URL) along with a significant rise or fall (>20%) during admission were determined to have an acute cardiac injury and were classified as acutely elevated hsTnT group, while those with elevated hsTnT with no significant rise or fall during admission were classified as chronically elevated hsTnT group. We compared clinical characteristics, complications and outcomes between four groups: patients where hsTnT results were unavailable, hsTnT negative, chronic hsTnT elevation and acute hsTnT elevation. We used cox proportional hazard model to determine the impact of acutely elevated hsTnT during admission on 30-day mortality after adjustment for the following confounding variables: age, sex, CCI, creatinine levels and severity of illness as determined by C-RP levels, presence of sepsis, number of MET calls during admission and time spent in the ICU. Logistic regression was used with 30-day mortality as an outcome variable and hsTnT as continuous variable after adjustment for the above mentioned co-variates. We computed mortality risk over time by using the Nelson-Aalen cumulative hazard estimate [20] and risk curves were compared among four groups. The log-rank test was used to compare survival difference between patients with or without acute hsTnT elevation. All tests were two-sided and a P value < 0.05 indicated statistical significance. All statistical analyses were performed using STATA vs16.0 (StataCorp., Texas).
4. Results
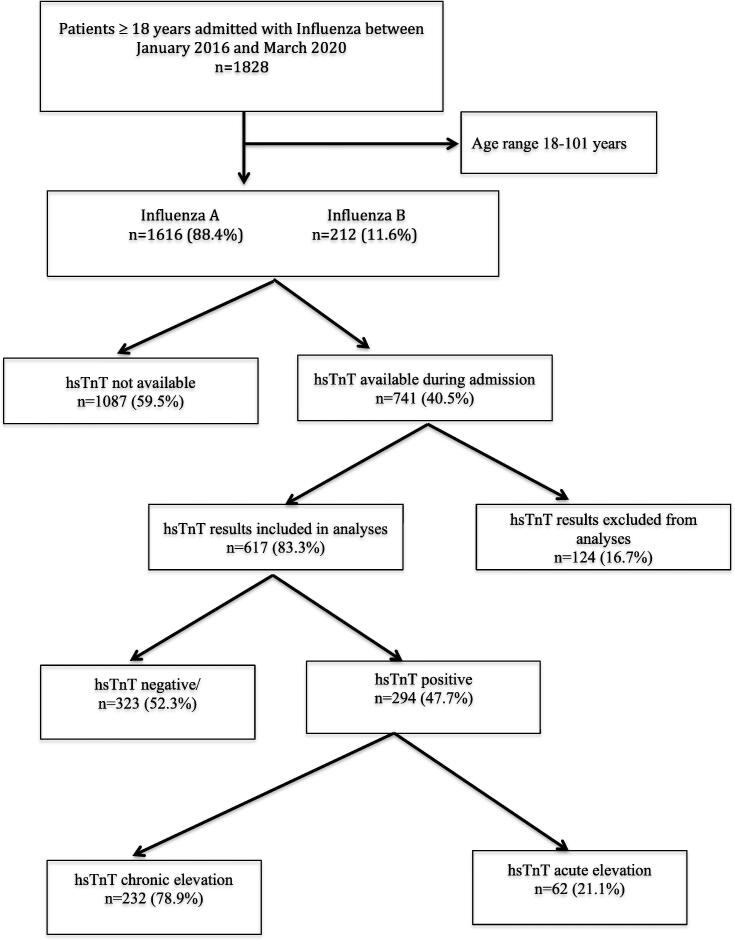
A total of 1828 hospitalised patients ≥ 18 years tested positive for influenza between January 2016 to March 2020. Of these, 1616 (88.4%) patients were identified as having influenza A and 212 (11.6%) as influenza B infection. High sensitive TnT results were available for 741 (40.5%) patients, of whom, 124 (16.7%) patients were excluded because the reported hsTnT assay detection limits were > 20 ng/L, while 1087 (59.5%) did not have hsTnT performed during admission (Fig. 1). Among 617 patients where hsTnT results were available and were included in the analyses, 294 (47.7%) patients had a positive hsTnT while 323 (52.3%) had negative hsTnT. Of 294 patients who had positive hsTnT, 62 (21.1%) patients had an evidence of acute myocardial injury, as reflected by an elevation of troponin levels (>14 ng/L) along with a significant rise or fall during admission, while 232 (78.9%) patients had chronically elevated hsTnT without significant change during admission (Fig. 1). One hundred and fifty five (46.9%) patients had more than two hsTnT results available during admission. The mean (SD) hsTnT levels were 9.4 (3.3) ng/L among patients who had negative hsTnT as compared to 135.2 (621.2) ng/L among patients who were in chronically elevated hsTnT group and 170.5 (265.1) ng/L in those with acutely elevated hsTnT group.
Fig. 1.
Study flow diagram (hsTnT, high sensitive troponin).
The mean age was 66.4 years (SD 20.1; range 18–101) and majority were females (52.3%). More than two-thirds of patients had no previous history of coronary artery disease and less than half were smokers. Diagnosis of ACS was made in 23 (37.7%) patients who were in acutely elevated hsTnT group, of whom, 3 patients (13%) died during admission in comparison to 1(3.8%) death among patients who were in chronically elevated hsTnT group. Among 23 patients who developed ACS, NSTEMI was diagnosed in 22 (96%) patients and 1(4%) patient developed STEMI. Echocardiogram results were available for 41 patients and mean ejection fraction (EF) was not significantly different between the two groups (P < 0.05).
Table 1 shows differences in the baseline characteristics of influenza patients among four groups of influenza patients according to hsTnT results: hsTnT unavailable, hsTnT negative, chronic hsTnT elevation and acute hsTnT elevation. Influenza patients who had an acute or chronic hsTnT elevation were more likely to be older with a history of IHD, diabetes, COPD, asthma, CKD and had a significantly higher CCI when compared to other groups. Similarly, when compared to other groups patients with acute or chronic hsTnT elevation were more likely to have a higher creatinine and C-RP levels. However, no differences were observed in regard to the degree of immunosuppression or influenza sub-types between various groups (Table 1).
Table 1.
Baseline characteristics of patients.
| Variable | hsTnT not available (n = 1087) | hsTnT negative (n = 323) | Chronically elevated hsTnT (n = 232) | Acutely elevated hsTnT (n = 62) | P value |
|---|---|---|---|---|---|
| Age mean (SD) | 62.7 (21.1) | 66.4 (18.5) | 79.7 (12.1) | 78.3 (13.7) | <0.001 |
| Age group n (%) | |||||
| < 40 | 214 (84.9) | 35 (13.9) | 1(0.4) | 2 (0.8) | <0.001 |
| 40–59 | 202 (69.9) | 67 (23.2) | 17 (5.9) | 3 (1.0) | |
| 60–79 | 384 (63.3) | 133 (21.9) | 69 (11.4) | 21 (3.4) | |
| > 80 | 287 (51.6) | 88 (15.8) | 145 (26.1) | 36 (6.5) | |
| Sex male n (%) | 473 (46.4) | 132 (42.7) | 125 (54.4) | 31 (50.8) | 0.050 |
| Influenza type n (%) | 0.252 | ||||
| Influenza A | 958 (88.1) | 281 (87.0) | 213 (91.8) | 57 (91.9) | |
| Influenza B | 129 (11.9) | 42 (13.0) | 19 (8.2) | 5 (8.1) | |
| CCI mean (SD) | 1.1 (1.5) | 0.9 (1.3) | 2.2 (2.1) | 2.9 (1.8) | <0.001 |
| Smoking history n (%) | 481 (44.3) | 162 (50.2) | 124 (53.5) | 30 (48.4) | 0.057 |
| IHD n (%) | 155 (14.3) | 49 (15.2) | 74 (31.9) | 24 (38.7) | <0.001 |
| Diabetes n (%) | 237 (21.8) | 63 (19.5) | 86 (37.1)) | 26 (41.9) | <0.001 |
| COPD n (%) | 241 (22.2) | 82 (25.4) | 94 (40.5) | 23 (37.1) | <0.001 |
| Asthma n (%) | 79 (7.3) | 31 (9.6) | 7 (3.0) | 0 | 0.003 |
| ILD n (%) | 13 (1.2) | 0 | 5 (2.1) | 2 (3.2) | 0.047 |
| CKD n (%) | 8 (0.7) | 2 (0.6) | 5 (2.1) | 3 (4.8) | 0.005 |
| Malignancy n (%) | 124 (11.4) | 33 (10.2) | 32 (13.8) | 10 (16.1) | 0.401 |
| Immunosuppressed n (%) | 6 (0.5) | 1 (0.3) | 2 (0.8) | 1 (1.6) | 0.600 |
| hsTnT mean (SD) (ng/L) | 135.2 (621.2)) | 170.5 (265.1) | <0.001 | ||
| EF mean (SD) | 52.4 (13.2) | 48.8(11.2) | 0.354 | ||
| Creatinine mean (SD) (μmol/L) | 106.4 (111.4) | 82.7 (31.5) | 143.2 (143.9) | 149.3 (126.3) | <0.001 |
| CRP mean (SD) (mg/L) | 55.7 (67.5) | 59.5 (76.4) | 68.0 (73.5) | 65.8 (65.5) | 0.032 |
hsTnT, (high sensitive troponin); SD, (standard deviation); CCI, (charlson comorbidity index); IHD, (ischaemic heart disease); COPD, (chronic obstructive lung disease), ILD, (interstitial lung disease), CKD, (chronic kidney disease); EF, (ejection fraction).
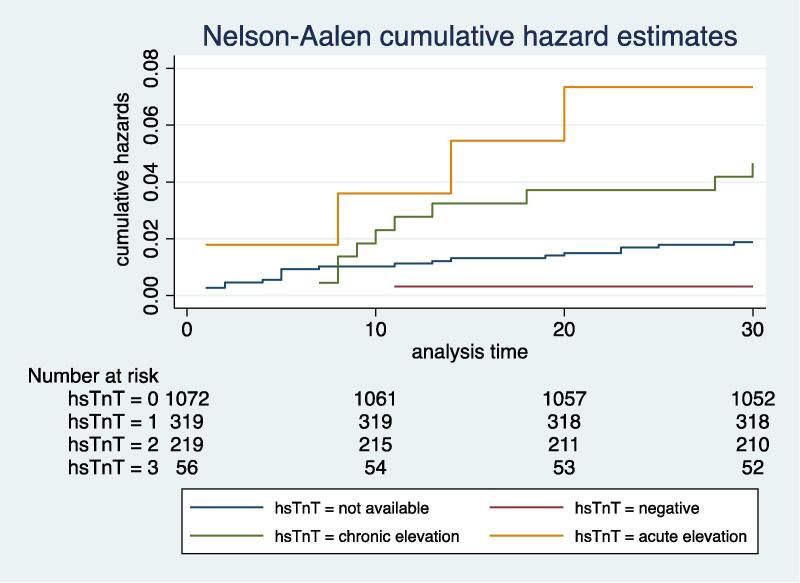
Influenza patients who had an acute or chronic hsTnT elevation had a higher incidence of sepsis, septic shock, and respiratory failure, however, other complications such as pneumonia, ARDS and myocarditis were not significantly different when compared to those who had negative hsTnT or where hsTnT results were unavailable (Table 2). When compared to patients with negative hsTnT, patients in the acutely elevated or chronically elevated hsTnT group had a greater severity of illness as reflected by the higher number of MET calls, a higher proportion of patients who had multiple (>2 MET calls), proportion of patients who needed ICU admission and time spent in the ICU (Table 2). Median length of hospital stay was significantly longer (P < 0.001) and inpatient mortality was significantly higher (9.7% vs 5.6% vs 1.6% vs 1.3%, P value < 0.001) in patients with acute or chronic hsTnT elevation when compared to those where hsTnT was negative or unavailable. HsTnT levels had a significant but weak positive correlation with LOS (r = 0.14, P < 0.05) and time spent in the ICU (r = 0.20, P < 0.05) but had no correlation with the number of MET calls. At 30 days following hospital discharge, 10 (16.1%) patients who had an acute hsTnT elevation during admission died as compared to 23 (9.9%) deaths among patients who had chronic hsTnT elevation and 5 (1.6%) deaths in the group who had negative hsTnT (P < 0.001), but there was no difference in the number of unplanned hospital readmissions between different groups (Table 2). The hazard of death at 30 days following hospital discharge among patients who had acute hsTnT elevation was 8.3 times higher when compared to those who had negative hsTnT, even after adjustment for factors such as age, sex, creatinine, CCI and the severity of illness as assessed by factors such as CRP levels, presence of sepsis, MET calls and need for an ICU admission (HR 8.30, 95% CI 1.80–17.84, P value = 0.013) (Table 3). Nelson-Aalen curve showed significant survival difference between two groups (Fig. 2) and log rank test was statistically significant (χ2 27.10, P < 0.001). Logistic regression showed that hsTnT levels during admission were significantly associated with 30-day mortality after adjustment for various co-variates (coefficient 0.003, 95% CI 0.0010–0.0057, P = 0.005). However, the hazard of death at 30-days following hospital discharge was not significantly different for patients who had chronic hsTnT elevation when compared to those with negative hsTnT group (HR 5.2, 95% 0.61–45.51, P = 0.131) Table 3.
Table 2.
Complications and outcomes.
| Variable | hsTnT not available (n = 1087) | hsTnT negative (n = 323) | Chronically elevated hsTnT (n = 232) | Acutely elevated hsTnT (n = 62) | P value |
|---|---|---|---|---|---|
| In hospital mortality n (%) | 15 (1.3) | 4 (1.2) | 13 (5.6) | 6 (9.7) | < 0.001 |
| 30-day mortality n (%) | 35 (3.2) | 5 (1.6) | 23 (9.9) | 10 (16.1) | < 0.001 |
| 30-day readmissions n (%) | 139 (12.8) | 37 (11.5) | 45 (19.4) | 6 (9.9) | 0.046 |
| LOS median (IQR) | 3 (5) | 4 (5) | 6 (9) | 8 (17) | < 0.001 |
| MET calls n (%) | 27 (4.8) | 16 (6.3) | 29 (16.4) | 15 (36.6) | < 0.001 |
| Number of MET calls mean (SD) | 0.1 (0.4) | 0.1 (0.3) | 0.3 (0.8) | 0.8 (1.1) | < 0.001 |
| Patients with multiple MET calls n (%) |
7 (1.3) | 2 (0.8) | 13 (7.3) | 9 (21.9) | < 0.001 |
| ICU admission n (%) | 50 (4.6) | 32 (9.9) | 31 (13.4) | 20 (32.3) | < 0.001 |
| ICU hours mean (SD) | 6.8 (56.1) | 13.6 (93.8) | 22.2 (87.9) | 91.7 (234.6) | <0.001 |
| Pneumonia mean (SD) | 67 (6.6) | 21 (6.8) | 22 (9.6) | 10 (16.4) | 0.020 |
| Sepsis n (%) | 39 (3.8) | 10 (3.2) | 22 (9.6) | 11 (18.0) | <0.001 |
| Septic shock n (%) | 4 (0.3) | 2 (0.6) | 7 (3.0) | 1 (1.6) | <0.001 |
| Respiratory failure n (%) | 73 (7.2) | 34 (11.0) | 33 (14.4) | 10 (16.4) | 0.001 |
| ARDS n (%) | 6 (0.6) | 3 (1.0) | 3 (1.3) | 1 (1.6) | 0.581 |
| Myocarditis n (%) | 0 | 0 | 1 (0.4) | 1 (1.6) | 0.282 |
| Pericarditis n (%) | 0 | 0 | 0 | 1 (1.6) | 0.035 |
*Multiple MET calls ≥ 2.
hsTnT, (high sensitive troponin); LOS, (length of hospital stay); MET, (medical emergency response team); SD, standard deviation; ICU (intensive care unit), ARDS, (adult respiratory distress syndrome).
Table 3.
Multivariable cox proportional hazard model comparing acute hsTnT and chronic hsTnT with negative hsTnT as baseline.
| Variable | Hazards ratio | CI | P value |
|---|---|---|---|
| Acute hsTnT elevation | 8.30 | 1.80–17.84 | 0.013 |
| Chronic hsTnT elevation | 5.2 | 0.61–45.59 | 0.131 |
| Age | 1.11 | 1.04–1.18 | <0.001 |
| Male sex | 0.56 | 0.20–1.53 | 0.262 |
| CCI | 1.41 | 1.15–1.71 | 0.001 |
| Creatinine | 0.99 | 0.98–0.99 | 0.043 |
| CRP | 1.00 | 0.99–1.01 | 0.630 |
| Sepsis | 4.00 | 0.98–16.33 | 0.053 |
| ICU admission | 1.10 | 0.03–2.14 | 0.428 |
| MET calls | 1.04 | 0.27–3.93 | 0.953 |
CI, confidence interval; hsTnT, high sensitive troponins; CCI, charlson comorbidity index; CRP, C-reactive protein; ICU, intensive care admission; MET, medical emergency response team.
Fig. 2.
Nelson Aalen hazard curve.
5. Discussion
This study suggests that influenza patients who have an evidence of acute cardiac injury as reflected by elevated and significant change in cardiac biomarkers have significantly higher number of complications during hospital admission with a consequent longer LOS and higher inpatient mortality. Mortality remained significantly higher even after 1 month following hospital discharge only in patients who had an acute and significant troponin elevation during admission even after adjustment for various confounders including comorbidities and severity of illness during index admission.
A previous large study [21] among U.S. veterans, which included over 4000 influenza patients over a period of 2 years, of which, cardiac biomarkers were available for 14% patients, found that 24% of influenza patients had elevated cardiac biomarkers and 25% of these patients developed ACS. In comparison, in our study a higher number of patients (39.7%) were found to have elevated cardiac biomarkers with slightly higher (37.7%) rates of ACS. These differences could be related to the higher sensitivity of the troponin assay used in our population [22]. Furthermore, higher rates of ACS in our study are probably related to the differences in cardio vascular disease (CVD) risk factor profile because patients in our study were older (median age 82 years vs. 77 years in the US study). In addition, these differences in rates of ACS may be related to the virulence of circulating influenza strains in respective epidemiological season [23]. Another study [15], which included 264 influenza patients admitted over a period of 5 years where hsTnT results were available, found that 31.8% of patients had elevated cardiac biomarkers and 7.6% of these patients developed ACS, however, patients in this study were much younger (68.7 vs. 82 years) as compared to our population.
Several studies [13], [14], [15], [24] have previously evaluated the impact of influenza viral infection on cardiac function and immediate mortality but only limited studies have determined the long term clinical consequences of severe influenza infection who require hospitalisation. These studies have indicated an increased incidence of arrhythmias, presence of regional wall motion abnormalities and a reduction in ejection fraction along with increased mortality during acute phase of viral illness. A recent study [25] suggests six-fold increased incidence of acute myocardial infarction within one week of laboratory confirmed influenza infection. Of 62 patients with significantly elevated troponins, 23 (37.1%) patients in our study were diagnosed with an ACS and 3 (13%) died during their hospital stay. Interestingly, 14 (60.9%) of our patients who developed ACS had no previous history of CAD. These findings are in contrast to a study by Harris et al, [14] who found that among 33 patients (mean age of 71.2 years) who had elevated troponins, only 33.3% had no previous history of coronary artery disease. This difference might be related to fewer risk factors for CAD in our population because less than half of the patients in our study were smokers or had a history of diabetes.
The present study suggests that influenza patients are at a significantly higher risk of dying during (9.7%) and after one month of hospital discharge (16.1%) and this risk was especially higher among patients who had an acute elevation and significant change in cardiac biomarkers during their admission. These results are similar to a study by Ludwig et al [21] who found that 13% patients with elevated cardiac biomarkers died within 30 days of laboratory-confirmed influenza infection and 61% received diagnosis of NSTEMI or probable NSTEMI. A systematic review [26] suggests that 0.4% to 13% patients hospitalised with influenza may have an associated myocarditis and are more likely to develop CHF. In this review, among 44 patients who developed pericardial effusions 23 patients required advanced cardiac life support with mortality of 22%. The prevalence of influenza associated myocarditis was only 0.12% in our study and thus does not explain higher mortality. However, patients in our study were at a higher risk of death because they were significantly older and had a higher number of comorbidities than those included in this review. Another aspect of cardiac involvement by influenza viral infection may be through exacerbation of pre-existing CAD [4], [27]. Systemic inflammation has been recognised as an important driver of atherosclerosis. Influenza infection promotes inflammation and is associated with a pro-coagulant state, which can destabilise atherosclerotic plaques and can cause vascular occlusion [28], [29]. Recent studies have described an increased risk of myocardial infarction with laboratory confirmed influenza and to a lesser extent with other viral infections [26], [30].
Another possible reason for a higher late mortality among influenza patients could be related to an increased risk of bacterial superinfections, such as pneumonia [31]. In recent decades there has been a high incidence of staphylococcus aureus superinfections, especially during influenza A pandemics [32]. This occurs because influenza viral replication leads to cytopathic effects in the cells and dysregulates cytokine production [31]. This in turn alters the host’s immune response to clear bacteria and achieve an appropriate modulation of the inflammatory cascade to fight infection [33]. A previous study [34] suggests that factors associated with a high 60-day mortality was Asian race, admission to ICU and immunosuppression (patients with human immunodeficiency virus (HIV) or immune dysfunction). In our study, 30-day mortality was significantly higher among patients who had spent longer time in the ICU but was not increased in patients who were immunosuppressed. It is quite possible that elevated cardiac biomarkers in patients with influenza are just a marker of severe viral illness and, as a consequence of immune dysfunction, patients becomes susceptible to new infections with a resultant higher mortality.
A major limitation of this study is that due to the retrospective design, there could have been a selection bias among patients who got evaluated for cardiac injury at the time of admission, as such, the findings of this study cannot be used to triage patients for admission according to the troponin levels. We were unable to get data on antiviral treatment received during admission, as previous studies [34], [35] suggest mortality benefits among patients who receive early antiviral treatment. Vaccination status of the participants was not captured. Evidence [25] suggests that influenza vaccination reduces cardiovascular events and mortality and it is possible that vaccination may have impacted mortality in this study. Another limitation of this study is that there is a possibility that some of the outcome events were not captured by our central computer database, as the data were only available for patients who presented to the hospitals specified and there were no data available for patients who had sought treatment elsewhere e.g. in private hospitals.
6. Conclusions
An acute and significant change in hsTnT among patients with influenza is not only associated with an immediate increased mortality but is also associated with an increased 30-day mortality after discharge. Troponins were elevated in many who lacked a documented history of ischaemic heart disease. As such a finding of acute cardiac troponin elevation among patients with influenza patients should prompt a search for underlying predisposing conditions or risk factors for CAD and these should be meticulously managed according to the current guidelines to improve clinical outcomes.
Grants
This is an unfunded study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Cheng P.Y., Palekar R., Azziz-Baumgartner E. Burden of influenza-associated deaths in the Americas, 2002–2008. Influenza Other Respir Viruses. 2015;9(Suppl 1):13–21. doi: 10.1111/irv.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwong J.C., Li P., Redelmeier D.A. Influenza morbidity and mortality in elderly patients receiving statins: a cohort study. PLoS ONE. 2009;4 doi: 10.1371/journal.pone.0008087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Australian Government Department of Health Canberra. Australian influenza surveillance report No. 12 (reporting period 14 October to 27 October 2017). https://www1.health.gov.au/internet/main/publishing.nsf/Content/ozflu-surveil-no12-17.htm, 2017 (assessed 1 June 2020).
- 4.Estabragh Z.R., Mamas M.A. The cardiovascular manifestations of influenza: a systematic review. Int. J. Cardiol. 2013;167:2397–2403. doi: 10.1016/j.ijcard.2013.01.274. [DOI] [PubMed] [Google Scholar]
- 5.Mamas M.A., Fraser D., Neyses L. Cardiovascular manifestations associated with influenza virus infection. Int. J. Cardiol. 2008;130:304–309. doi: 10.1016/j.ijcard.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 6.Caussin C., Escolano S., Mustafic H. Short-term exposure to environmental parameters and onset of ST elevation myocardial infarction. The CARDIO-ARSIF registry. Int. J. Cardiol. 2015;183:17–23. doi: 10.1016/j.ijcard.2015.01.078. [DOI] [PubMed] [Google Scholar]
- 7.Guan X., Yang W., Sun X. Association of influenza virus infection and inflammatory cytokines with acute myocardial infarction. Inflamm. Res. 2012;61:591–598. doi: 10.1007/s00011-012-0449-3. [DOI] [PubMed] [Google Scholar]
- 8.Fleming D.M. The contribution of influenza to combined acute respiratory infections, hospital admissions, and deaths in winter. Commun. Dis. Public Health. 2000;3:32–38. [PubMed] [Google Scholar]
- 9.Reichert T.A., Simonsen L., Sharma A., Pardo S.A., Fedson D.S., Miller M.A. Influenza and the winter increase in mortality in the United States, 1959–1999. Am. J. Epidemiol. 2004;160:492–502. doi: 10.1093/aje/kwh227. [DOI] [PubMed] [Google Scholar]
- 10.Smeeth L., Thomas S.L., Hall A.J., Hubbard R., Farrington P., Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N. Engl. J. Med. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 11.Siriwardena A.N., Gwini S.M., Coupland C.A. Influenza vaccination, pneumococcal vaccination and risk of acute myocardial infarction: matched case-control study. CMAJ. 2010;182:1617–1623. doi: 10.1503/cmaj.091891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casscells S.W., Granger E., Kress A.M., Linton A. The association between oseltamivir use and adverse neuropsychiatric outcomes among TRICARE beneficiaries, ages 1 through 21 years diagnosed with influenza. Int. J. Adolesc. Med. Health. 2009;21:79–89. doi: 10.1515/ijamh.2009.21.1.79. [DOI] [PubMed] [Google Scholar]
- 13.Eggers K.M., Lindahl B. Application of Cardiac Troponin in Cardiovascular Diseases Other Than Acute Coronary Syndrome. Clin. Chem. 2017;63:223–235. doi: 10.1373/clinchem.2016.261495. [DOI] [PubMed] [Google Scholar]
- 14.Harris J.E., Shah P.J., Korimilli V., Win H. Frequency of troponin elevations in patients with influenza infection during the 2017–2018 influenza season. Int. J. Cardiol. Heart Vasc. 2019;22:145–147. doi: 10.1016/j.ijcha.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pizzini A., Burkert F., Theurl I., Weiss G., Bellmann-Weiler R. Prognostic impact of high sensitive Troponin T in patients with influenza virus infection: A retrospective analysis. Heart Lung. 2020;49:105–109. doi: 10.1016/j.hrtlng.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Roberts R.F., Innes K.C., Walker S.M. Introducing ICD-10-AM in Australian hospitals. Med. J. Aust. 1998;169:S32–S35. doi: 10.5694/j.1326-5377.1998.tb123473.x. [DOI] [PubMed] [Google Scholar]
- 17.Thygesen K., Alpert J.S., Jaffe A.S. Fourth Universal Definition of Myocardial Infarction. J. Am. Coll. Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 18.Thygesen K., Mair J., Giannitsis E. How to use high-sensitivity cardiac troponins in acute cardiac care. Eur. Heart J. 2012;18:2252–2257. doi: 10.1093/eurheartj/ehs154. [DOI] [PubMed] [Google Scholar]
- 19.Murray S.B., Bates D.W., Ngo L., Ufberg J.W., Shapiro N.I. Charlson Index is associated with one-year mortality in emergency department patients with suspected infection. Acad. Emerg Med. 2006;13:530–536. doi: 10.1197/j.aem.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z. Statistical description for survival data. Ann. Transl. Med. 2016;4:401. doi: 10.21037/atm.2016.07.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.A. Ludwig, C. Lucero-Obusan, P. Schirmer, C. Winston, M. Holodniy, Acute cardiac injury events ≤30 days after laboratory-confirmed influenza virus infection among U.S. veterans, 2010-2012, BMC Cardiovasc. Disord. 15 (2015) 109. https://doi.org/10.1186/s12872-015-0095-0. [DOI] [PMC free article] [PubMed]
- 22.Vasile V.C., Jaffe A.S. High-Sensitivity Cardiac Troponin for the Diagnosis of Patients with Acute Coronary Syndromes. Curr. Cardiol. Rep. 2017;19:92. doi: 10.1007/s11886-017-0904-4. [DOI] [PubMed] [Google Scholar]
- 23.Lytras T., Andreopoulou A., Gkolfinopoulou K., Mouratidou E., Tsiodras S. Association between type-specific influenza circulation and incidence of severe laboratory-confirmed cases; which subtype is the most virulent? Clin. Microbiol. Infect. 2019 doi: 10.1016/j.cmi.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 24.Ito T., Akamatsu K., Ukimura A. The Prevalence and Findings of Subclinical Influenza-associated Cardiac Abnormalities among Japanese Patients. Intern. Med. 2018;57:1819–1826. doi: 10.2169/internalmedicine.0316-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwong J.C., Schwartz K.L., Campitelli M.A. Acute Myocardial Infarction after Laboratory-Confirmed Influenza Infection. N. Engl. J. Med. 2018;378:345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 26.Sellers S.A., Hagan R.S., Hayden F.G., Fischer W.A., 2nd The hidden burden of influenza: A review of the extra-pulmonary complications of influenza infection. Influenza Other Respir. Viruses. 2017;11:372–393. doi: 10.1111/irv.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warren-Gash C., Smeeth L., Hayward A.C. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect. Dis. 2009;9:601–610. doi: 10.1016/s1473-3099(09)70233-6. [DOI] [PubMed] [Google Scholar]
- 28.Naghavi M., Wyde P., Litovsky S. Influenza infection exerts prominent inflammatory and thrombotic effects on the atherosclerotic plaques of apolipoprotein E-deficient mice. Circulation. 2003;107:762–768. doi: 10.1161/01.cir.0000048190.68071.2b. [DOI] [PubMed] [Google Scholar]
- 29.Vejpongsa P., Kitkungvan D., Madjid M. Outcomes of Acute Myocardial Infarction in Patients with Influenza and Other Viral Respiratory Infections. Am. J. Med. 2019;132:1173–1181. doi: 10.1016/j.amjmed.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Joseph C., Togawa Y., Shindo N. Bacterial and viral infections associated with influenza. Influenza Other Respir. Viruses. 2013;7(Suppl 2):105–113. doi: 10.1111/irv.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shepardson K.M., Larson K., Johns L.L. IFNAR2 Is Required for Anti-influenza Immunity and Alters Susceptibility to Post-influenza Bacterial Superinfections. Front. Immunol. 2018;9:2589. doi: 10.3389/fimmu.2018.02589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rice T.W., Rubinson L., Uyeki T.M. Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit. Care Med. 2009;40(2012):1487–1498. doi: 10.1097/CCM.0b013e3182416f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynfield R., Davey R., Dwyer D.E. Outcomes of influenza A(H1N1)pdm09 virus infection: results from two international cohort studies. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0101785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reacher M., Warne B., Reeve L. Influenza-associated mortality in hospital care: a retrospective cohort study of risk factors and impact of oseltamivir in an English teaching hospital, 2016 to 2017. Euro Surveill. 2019;24 doi: 10.2807/1560-7917.Es.2019.24.44.1900087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanshaoworakul W., Simmerman J.M., Narueponjirakul U. Severe human influenza infections in Thailand: oseltamivir treatment and risk factors for fatal outcome. PLoS ONE. 2009;4 doi: 10.1371/journal.pone.0006051. [DOI] [PMC free article] [PubMed] [Google Scholar]