Highlights
-
•
Evidence on surgical LAA excision has been limited to retrospective studies and registries.
-
•
Surgical LAA excision is associated with high rates of post-operative atrial fibrillation burden.
-
•
Watchman is equivalent to oral anticoagulants in high-bleeding risk AF patients.
Keywords: Atrial fibrillation, Left atrial appendage occlusion, Left atrial appendage occluder, Watchman device, Thrombosis, Stroke
Abbreviations: ACC, American College of Cardiology; AF, Atrial fibrillation; AHA, American Heart Association; DRT, Device related thrombus; ESC, European Society of Cardiology; FDA, Food and Drug Administation; HR, Hazard ratio; LAA, Left atrial appendage; LAAE, Left atrial appendage exclusion; LGE, Late gadolinium enhancement; OAC, Oral anticoagulation; OR, Odds ratio; TOE, Trans-oesopheageal echocardiogram
Abstract
Atrial fibrillation is one of the most common cardiovascular disorders encountered by clinicians in clinical practice. Patients with atrial fibrillation are at risk of cerebrovascular and systemic embolic events, which may be attenuated by commencement of anticoagulation therapy. Even so, due to extremely high bleeding risk certain patients may not be suitable for long-term anticoagulation therapy. The left atrial appendage is a common site for thrombus formation in patients with atrial fibrillation. Left atrial appendage exclusion, either surgical or percutaneous, has been performed to ostensibly reduce the risk of cerebrovascular events and potentially minimise or omit anticoagulation therapy in select patients. This review summarises the role of the left atrial appendage in cerebrovascular events, current evidence with modification of the left atrial appendage and future trials that may change practice with these procedures.
1. Introduction
Atrial fibrillation (AF) has a prevalence of up to 9% in patients above the age of 80 years [1]. It may lead to significant morbidity including recurrent hospitalisations, heart failure and stroke. Many algorithms have been developed to risk-stratify patients at high risk of stroke to guide treatment with anticoagulation therapy, with the CHA2DS2VASC score being the most commonly used [2]. Current guidelines recommend anticoagulation therapy with CHA2DS2VASC score of 2 in males and 3 in females, with direct oral anticoagulant (OAC) being the most preferred option [3], [4].
Even so, some patients with AF are not commenced anticoagulation due to high bleeding risk such as previous haemorrhagic complications. The HASBLED score calculates the risk of bleeding on OAC, with smaller therapeutic window of anticoagulation therapy in patients with high HASBLED scores due to elevated bleeding risks [5]. This has led to increased uptake of surgical and percutaneous approaches to left atrial appendage exclusion (LAAE), to potentially limit or omit OAC in high bleeding risk patients with AF. This review article evaluates the role of left atrial appendage (LAA) modification in stroke risk reduction with atrial fibrillation and the potential outcomes from these procedures.
2. Left atrial anatomy and function
The LAA is a vestigial muscular extension of the left atrium, located in the anterolateral segment. Its external appearance is that of a flattened tubular structure with its tip commonly directed antero-superiorly with a total average length of 25.9 ± 0.7 mm [6]. The orifice has an oval shape, with a mean maximum and minimum diameter of 17.4 ± 4 mm and 10.9 ± 4.2 mm, respectively [7]. The orifice is separated from the left pulmonary veins by the left lateral ridge whilst smooth muscular walls of the LAA vestibule separates it from the mitral annulus inferiorly [8].
Internally, the cavity of the appendage has complex indentations formed by the pectinate muscles [9], which may be mistaken with LAA thrombi in thicker or prominent muscle bundles. Pectinate muscles cover up to 85% of the LAA, whilst the remaining LAA wall is paper-thin [9]. Patients with permanent AF have larger LAA cavity and lower coverage of pectinate muscle compared to patients in sinus rhythm, suggesting structural remodeling due to long-term arrhythmogenicity [9], [10]. Lobes are visible external protrusions of the LAA body, with majority of the population having two lobes (54%), followed by three lobes (23%), one lobe (20%) and four lobes (3%) [6]. These lobes are common sites of thrombus formation in AF [11].
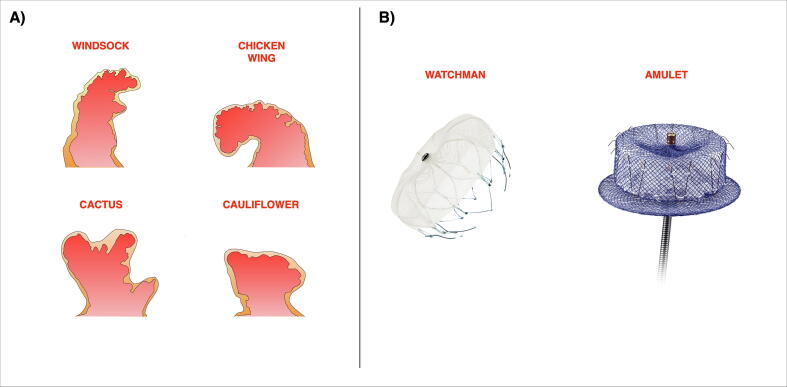
With advanced imaging techniques such as computed tomography and cardiac magnetic resonance, there is better appreciation of the different anatomical morphologies of LAA (Fig. 1A). The most common type is the “chicken wing”, occurring in 48% of patients [12]. It has a dominant lobe with a prominent mid or distal bend, that leads to the distal portion folding back on itself. Next is the “cactus” morphology (30%) with a dominant central lobe and smaller secondary lobes arising from it. The “windsock” occurs in 19%, with a tapering dominant lobe and resembles its nomenclature. The “cauliflower” is rare at 3%, with a shorter length that lacks a dominant lobe but has numerous smaller lobes.
Fig. 1.
A) Different anatomical morphologies of the left atrial appendage. B) The Watchman device is self-expanding nitinol device with a polyethylene terephthalate membrane on the outer surface. The Amulet device is a self-expanding nitinol mesh with a proximal disc and distal lobe.
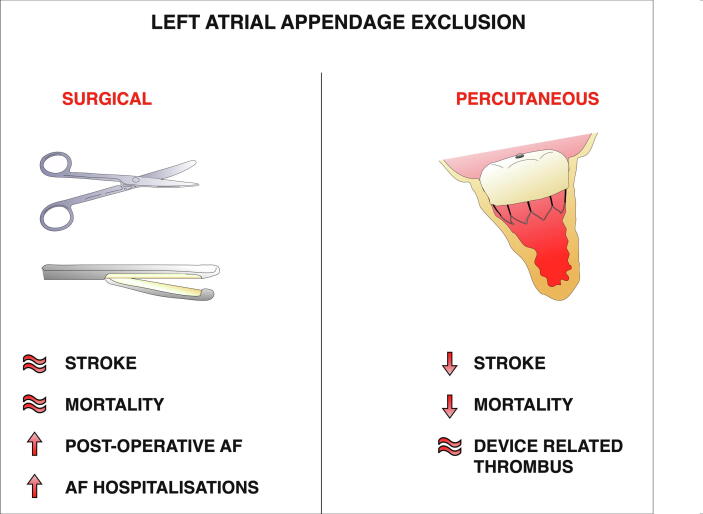
Main Illustration.
. Illustration comparing the potential benefits and risks arising from the surgical and percutaneous left atrial appendage exclusion procedures.
3. Role of left atrial appendage in stroke with atrial fibrillation
The LAA is the most common anatomical site for thromboembolic disease with AF [13]. With AF, there is reduced contractility and hence increased blood stasis in the LAA, which increases the risk of clot formation and thromboembolic phenomenon. Certain LAA anatomy may increase the likelihood of causing cerebrovascular events. Multilobed LAA have reduced emptying velocities and have been shown to increase the risk of stroke [11]. The LAA “cactus” morphology have been shown to be a strong risk for stroke as compared to the “chicken wing” variant, with the latter being a protective factor [12].
However, not all left atrial thrombi are located in the LAA as 10% present as intra-mural thrombus in non-valvular AF [13]. In valvular AF, the prevalence of left atrial thrombi is equal in both LAA and non-LAA sites. Therefore, though LAA modification may ostensibly reduce the risk of stroke in AF, the residual risk is not negligible. Furthermore, novel imaging modalities have demonstrated other plausible mechanisms behind the thromboembolic phenomenon with AF.
Myocardial tissue characterisation may be accurately assessed with magnetic resonance imaging, providing further insight on the role of atrial fibrosis in AF and stroke risk. Late gadolinium enhancement (LGE) of the atria has been shown to predict patients at risk of AF, to suggest the role of atrial fibrosis as an arrhythmogenic and/or cardiomyopathic precursor to clinical AF [14]. Presence of atrial fibrosis (detected with LGE) in patients with and without AF has also been shown to be a strong risk factor of stroke [15], regardless of LAA morphology or appendageal fibrosis. Moreover, greater severity of left atrial fibrosis leads to increased risk of cerebrovascular events [16]. This suggest a complex interplay between atrial myopathy, left atrial fibrosis and LAA anatomical factors behind the thromboembolic risk in AF.
The LAA may also play a protective role in preventing AF. High left atrial filling pressures increases left atrial volume which leads to the development of AF through greater atrial myocardial stretch and stimulation of pulmonary vein electrical activity [17], [18]. The LAA acts as a buffer to offload the increased pressure in the left atrium by enlarging in size due to its increased compliance [19] and preventing long-term left atrial remodeling that lead to AF [20].
4. Surgical left atrial appendage exclusion
In patients at high bleeding risk or contraindications to anticoagulation, LAA modification may be considered to potentially reduce the thromboembolic risk in AF. Interest in the relationship between LAA and thromboembolic events with AF led to the initial development of surgical techniques to modify the LAA. It was first described in a series of surgical LAAE in canines in 1947 [21], which indicated a possible thrombo-prophylactic approach in patients with mitral stenosis. In 1949, the first case series was described in the prevention of arterial thrombosis [22]. Several techniques were described over the next few decades and in the 1980′s, amputation of both atrial appendages was performed as part of the Cox-Maze procedure for AF [23]. Surgical LAAE can also be performed concomitantly with either cardiac bypass or valve surgery.
Various surgical techniques have been developed to exclude the LAA. Surgical excision involves either an epicardial suture encircling the base of LAA or amputation of the LAA at its orifice with suture reinforcement. Stapler excision involves a cutting or non-cutting device that excludes the LAA, that may be reinforced with a bovine pericardial strip [24]. Left atrial ligation devices such as the AtriClip (Atricure, West Chester, OH) allows delivery of a braided polyester at the base of the LAA, effectively excluding the body of the LAA from the left atrium [25]. There is limited data on the comparison of these techniques, which is further hampered by small studies preventing meaningful evaluation [26], [27].
However, the high failure rate of surgical intervention has raised doubts on its potential therapeutic impact in reducing stroke risk. Surgical LAAE failure is defined as either persistent LAA connection or residual stump. A prospective study assessed the success rate of surgical LAAE in 157 patients with trans-oesophageal echocardiography (TOE), noted a failure rate of 60% following surgical excision or stapler excision procedures [27]. Another study described a failure rate of 36% with LAA ligation techniques [28].
Concerningly, incomplete LAAE has been shown to have high rates of spontaneous echo contrast or thrombus [28]. This is likely due to greater blood stasis and reduced ability to empty the LAA from a smaller orifice due to an incomplete LAA closure. This may lead to severe consequences, as an incomplete LAAE has been strongly associated with cerebral and systemic embolisation [29]. Factors that may lead to increased surgical LAAE failure rates include large left atrial size and significant mitral regurgitation [28].
There has not been any large randomised trials with surgical LAAE. The “Left Atrial Appendage Occlusion Study” (LAAOS) II trial [30] was designed as a feasibility study for the upcoming LAAOS III trial. In LAAOS II, only 51 patients were randomised to either LAAE or medical therapy, demonstrating the safety of this procedure. There have been a few registry data that provides further insight on the impact of this procedure on thromboembolic risk. Melduni et al published a propensity-matched analysis of more than 10,000 patients from a high-volume centre, comparing 461 patients with surgical LAAE to matched pairs based on 28 covariates [31]. At a median follow-up of 9.1 years, the authors conclude that LAAE did not reduce the risk of stroke with univariable and multivariable analysis (unadjusted hazard ratio [HR], 1.08; 95% confidence interval [CI], 0.74–1.60). The rates of mortality were also similar in both groups (unadjusted HR, 0.92; 95% CI, 0.76–1.13).
However, another large contemporary registry demonstrated different findings [32]. The authors compared 4374 patients with surgical LAAE to 8590 propensity matched patients from a Medicare registry. The follow-up was significantly shorter, with a mean of 2.1 years. Overall, surgical LAAE patients had lower rates of mortality (3.01 vs 4.30 events per 100 person-years) and stroke (1.14 vs 1.59 events per 100 person-years) when compared to the propensity matched cohort. These findings were only in patients with AF at baseline on post-hoc analysis.
There are a few plausible, possible explanations behind the difference in outcomes between these two studies. Firstly, Yao et al’s registry had a shorter mean follow-up when compared to Melduni et al’s registry (mean 2.1 years vs median 9.1 years). In Meduni et al’s registry, there appears to be a reduction in stroke rates for the first two years with surgical LAAE on visual inspection, though the rates of stroke were similar at a median of 9.1 years. There was no landmark analysis performed at 2 years, so these findings should be taken with caution. Furthermore, Yao et al’s registry also relied on a Medicare database and outcomes from an administrative database (Social Security Death Master File), leading to potential reporting and ascertainment bias. Melduni et al’s registry performed standardised biannual telephone reviews, with combined review of clinical and administrative database.
Another retrospective study [33] demonstrated LAAE was associated with a reduction in thromboembolic events and all-cause mortality. Almost all of the LAAE patients received concurrent surgical AF ablation (94%), thus it is unclear if this finding is driven by either procedures or a synergistic effect. A recently published large meta-analysis [34] demonstrated a reduction in thromboembolic events without a mortality benefit with LAAE. This was only demonstrated in studies that had high proportion of patients with pre-operative AF (greater than 70%). This study was limited by low proportion of randomised trials, lack of routine neurological events and a variety of surgical LAAE used. Nevertheless, there may be a reduction in thromboembolic events with LAAE in patients with prior AF.
Concerningly some studies demonstrate significantly increased AF burden with surgical LAAE (Main Illustration 1) [31], [32]. Even more concerning was that up to half of the patients undergoing surgical LAAE did not have AF prior, potentially introducing AF to these patients from this procedure. Post-operative AF was more common after LAAE (odds ratio [OR], 3.88; 95% CI 2.89–5.20), a trend that continues after discharge as they were also more likely to have hospitalisations (0.14 vs 0.02 hospitalisations per person-year) and outpatient visits (6.68 vs 0.61 outpatient visits per person-year) when compared to non-LAAE patients [31], [32].
For patients without previous AF, this procedure may lead to ineffective utilisation of healthcare, increased bleeding complications from OAC and polypharmacy. Increased AF burden post LAAE could be explained by the potential arrhythmogenic nature of this procedure and the role of LAA in protecting the left atrium from increased filling pressures. From a health economic standpoint, it may not be ideal to perform a procedure funded through healthcare subsidisation that leads to increased utilisation of healthcare in the long-term without any clear improvements in outcome.
5. Percutaneous left atrial appendage exclusion
The PLAATO transcatheter device (Appriva Medical Inc, Sunnyville, CA) provided a proof-of-concept in 2001 and excluded the LAA with a polytetrafluoroethylene membrane and a self-expanding nitinol cage at its orifice. Unfortunately, it had a few drawbacks and difficult implantation technique, leading to its withdrawal in 2006. Since then, the Watchman device (Boston Scientific, Marlborough, MA) has dominated the scene of percutaneous LAAE and is the only device approved by the U.S Food and Drug Administration [35]. It will be the focus of this section.
The Watchman is a self-expanding nitinol device (Fig. 1B) that is deployed at the orifice of the LAA via transseptal puncture, with fixed barbs around its mid-portion to anchor it to the LAA wall. It has a polyethylene terephthalate membrane on the outer surface that occludes the LAA. Several studies have examined its efficacy in non-valvular AF. The PROTECT AF study randomised 707 patients in a 2:1 fashion to either a Watchman device or warfarin therapy alone [36]. At a mean follow-up of 2.3 years, the Watchman device was non-inferior to warfarin with regards to its composite primary end point of stroke, systemic embolism and cardiovascular death, though it met the superiority criteria at an extended follow-up of 3.8 years [37].
However, the PREVAIL trial, which randomised 407 patients with non-valvular AF (2:1 ratio to either Watchman device or warfarin therapy), did not exhibit non-inferiority for a similar composite end point [38]. This was likely due to its low event rate, shorter follow up (18 months) and smaller patient cohort. Even so, it did demonstrate non-inferiority of the Watchman device, with the co-primary composite efficacy end point of stroke and systemic embolism. A combined patient-level meta-analysis of both the PROTECT-AF and PREVAIL trial, involving 1,114 patients, demonstrated that the Watchman device was non-inferior to warfarin therapy with regards to the composite primary end point of stroke, systemic embolism and cardiovascular death at 5-year follow-up [39]. The Watchman device also demonstrated lower rates of disabling/fatal stroke (HR 0.45), haemorrhagic stroke (HR: 0.20), all-cause death (HR 0.73), cardiovascular/unexplained death (HR 0.59) and post-procedural bleeding (HR 0.48) when compared to warfarin therapy. Yet, both the PROTECT-AF and PREVAIL trials required at least six weeks of warfarin therapy to enable endothelisation of the device, which may not be suitable for high bleeding risk patients [36], [38].
Real-world experience has demonstrated the efficacy of the Watchman device in patients with non-valvular AF with high bleeding risk and/or contraindications to OAC. The EWOLUTION trial is a large registry (n = 1025) with a cohort of high stroke risk (CHA2DS2VASC = 4.5 ± 1.6) and high bleeding risk patients (73% of the patients were considered unsuitable for OAC) [40]. Recently, its 2-year outcomes were published, demonstrating lower rates of ischaemic stroke and major bleeding, with a relative risk reduction of 83% and 41%, respectively, with the Watchman device when compared to a historical cohort [41]. The ASAP study included 150 patients with AF ineligible for OAC that were treated with antiplatelets alone following Watchman device [42]. The authors conclude that the Watchman device maybe performed safely without transitioning to warfarin, with low rates of ischaemic (1.7% per year) and haemorrhagic stroke (0.7% per year) at a mean of follow-up of 14 months.
Implant success of Watchman devices have steadily improved over time, from 91% with the PROTECT-AF study (enrolment until 2008), 95.1% with the PREVAIL study (enrolment until 2012) to 98.5% with the EWOLUTION study (enrolment until 2015), suggesting increased proceduralist competency over time [36], [38]. Other real-world registries has been reassuring, as seen with the Post Approval US experience and combined Continuous Access to PROTECT-AF (CAP) and PREVAIL (CAP-2) registry, with success rates of 95.6% and 94%, respectively [43], [44]. Complication rates have gradually reduced as well, with early safety outcomes (defined as a composite of pericardial tamponade, procedural stroke, device embolisation and vascular complications within 7 days) reduced from 8.7% in PROTECT-AF to 4.2% in PREVAIL (p < 0.01) [38]. Real-world registries also support these findings, with reported complication rates of below 3% [43], [44]. Residual device leak, defined as peri-device flow on TOE, has been reported in up to 32% of Watchman devices, with only a third of these cases deemed major (≥3 mm) at one-year follow-up [45]. Another recent study demonstrated a prevalence of device leak of 3.9%, though a cut-off greater or equal to 5 mm of colour flow was used [46]. Reassuringly, residual device leak has not been associated with stroke or systemic embolisation, with the Watchman and other percutaneous LAAE devices [45], [47], [48].
A recent randomised study (PRAGUE-17) comparing the Watchman device with OAC in high risk bleeding and high-risk stroke patients [46]. With regards to the composite end point of stroke, transient ischemic attack, systemic embolism, cardiovascular death, major or nonmajor clinically relevant bleeding, or procedure-/device- related complications, the Watchman device was non-inferior to novel oral anticoagulation, which was primarily apixaban (in 95%). Secondary end point of non-procedure bleeding demonstrated a trend of lower rates with the Watchman device at a median of 20 months of follow-up (hazard ratio 0.53, 95% CI 0.26–1.06, p = 0.07). Over time, this may be in favour of the Watchman device due to the accumulative risk of bleeding from OAC.
Device related thrombus (DRT) may occur following percutaneous LAAE, with a prevalence ranging from 3.7 to 7.2%. It is detected with TOE or cardiac computed tomography post-implantation which may occur at variable time-points; as early as at implantation and later at one year. Dual antiplatelet therapy and OAC has been shown to be a protective factor in the development of DRT, whilst older age and previous history of stroke were strong risk factors [49]. Evidence surrounding DRT with cerebrovascular events were initially inconclusive due to limited numbers and incomplete follow-up. But recent pooled data from the PROTECT-AF, PREVAIL and both its continued access registries have shown DRT were associated with increased ischaemic stroke on multivariate analysis (HR 2.6) [50]. Another French registry of both Amplatzer and Watchman devices demonstrated a correlation between DRT and cerebrovascular events [49].
The Amplatzer Amulet (Abbott Laboratories, Chicago, IL) is another percutaneous LAAO device. It has self-expanding nitinol mesh with a disc in the proximal segment (the appendageal seal) that connects to the distal lobe via an articulated waist (Fig. 1B). The initial version, the Amplatzer Cardiac Plug demonstrated high procedural success (97.3%) with acceptable periprocedural adverse events (4.9%) that driven by cardiac tamponade and major bleeding [51].
The Amulet device is the newer iteration, with larger disc diameters and stiffer stabilising wires. It has an inner wire system that permits device orientation and post-deployment adjustment. This has further reduced the risk of peri-device leak, as demonstrated in the Amulet Observational Study (1.8%) [52]. The longer-term outcomes from this large registry was recently published, demonstrating excellent implant success (99.1%) and acceptable periprocedural adverse events (4.0%) [53]. It was shown to reduce the risk of ischaemic stroke by 67% when compared to predicted risk. Interestingly the rate of DRT with the Amulet device is lower than the Watchman device (1.6% vs 3.7–4.1%), which may allude to the differences in appendageal sealing mechanisms between both devices [53], [54].
6. Current guideline recommendations
The European Society of Cardiology (ESC) provides a weak recommendation (Class IIb, Level C) for concurrent surgical LAAE in patients with AF undergoing cardiac surgery or thorascopic AF surgery [3]. Even so, the guidelines strongly recommend (Class I, Level B) continuing OAC therapy for stroke prevention following surgical LAAE. The American College of Cardiology (ACC) and American Heart Association (AHA) recently published a focused update to their AF guidelines [4]. Whilst maintaining a weak recommendation for LAAE in patients with AF undergoing cardiac surgery (Class IIb), it has increased its level of evidence to Level B in accordance to the ESC guidelines.
Both ESC and ACC/AHA guidelines have similar weak recommendations (Class IIb, Level B) for percutaneous LAAE in patients with AF with high stroke risk and contraindications of long-term OAC due to excessive bleeding risk. Even so, both highlight uncertainties regarding optimal patient selection and periprocedural antithrombotic regimen with percutaneous LAAE, especially with ongoing concerns regarding the clinical sequelae of DRT.
7. Future directions
As highlighted earlier, there has been no large randomised trial involving surgical LAAE. The LAAOS III trial will systematically assess the impact of surgical LAAE in patients with AF on cerebrovascular events and arterial embolism [55]. This multicentre, randomised trial will aim to recruit 4700 patients with AF undergoing cardiac surgery to either LAAE or no LAAE, with the preferred surgical approach as LAA amputation with double layered suture closure. Other surgical methods such as stapler excision and ligation devices, that are Food and Drug Administration (FDA) approved, are permitted. All cerebrovascular events will be assessed with neuroimaging and neurologist assessment. Results are expected to be available within the next few years.
There has been concerns on the high rate of post-operative AF following surgical LAAE, with likely lower events in ligation devices. The “AtriClip Left Atrial Appendage Exclusion Concomitant to Structural Heart Procedures” (ATLAS) trial aims to determine the impact of prophylactic LAA ligation with the AtriClip device by randomising patients undergoing cardiac surgery without previous AF to either AtriClip or no AtriClip treatment arms. The primary outcome is post-operative AF and will have pre-specified analysis on healthcare utilisation from AF such as readmissions, length of stay and financial costs with each treatment arm. This is a multicentre trial in the United States and aims to enroll 2000 patients.
A patient-level meta-analysis has previously demonstrated non-inferiority of Watchman devices when compared to warfarin therapy in the composite end point of stroke, systemic embolism and cardiovascular death at long-term follow-up [39]. However, patients in these trials received at least six weeks of warfarin therapy post procedure which may be a contraindication for extremely high bleeding risk patients. The PRAGUE-17 study highlighted earlier demonstrated the safety of withdrawing OAC following the Watchman device. In high risk bleeding and high-risk stroke patients [46], the Watchman device was non-inferior to direct OAC in preventing major cardiovascular and cerebrovascular events. In this study, the Watchman arm received aspirin and clopidogrel for three months, followed by aspirin long term. Similar studies are under way comparing OAC with the newer-generation Watchman FLX (CHAMPION-AF trial) [NCT04394546] and Amplatzer Amulet devices (CATALYST trial) [NCT04226547]. Another trial examines whether Watchman FLX is a suitable alternative to OAC following catheter ablation (OPTION trial) [NCT03795298].
The “Assessment of the Watchman Device in Patients Unsuitable for Oral Anticoagulation” (ASAP-TOO) trial aims to assess the safety and efficacy of omitting warfarin therapy in high bleeding risk patients following Watchman device implantation [56]. The study aims to recruit 888 patients with AF and randomising them in 2:1 fashion to either Watchman device (with aspirin alone) versus control (aspirin or no therapy, at the physician’s discretion). Patients should be deemed as high bleeding risk by two study physicians. The primary effectiveness end point of this study is the time to first stroke/embolic event whilst the primary safety end point is the composite end point of death, ischaemic stroke, systemic embolism or device/procedural-related event requiring cardiac surgery or endovascular intervention. Follow-up will be at 3, 6 and 12 months, then bi-annual until 60 months post randomisation. This study has stopped recruitment due to low enrolment rates.
The AMULET IDE trial [NCT02879448] will be a non-inferiority trial comparing the safety and efficacy of the Amulet device with the Watchman device. This study has randomised 1878 patients with non-valvular AF to either the Amulet or Watchman device, in a 1:1 fashion. The primary safety outcome is a composite of procedure-related complications, death and major bleeding at 12 months. The primary efficacy outcome is a composite of ischaemic stroke and systemic embolism at 18 months whilst the primary mechanism of action endpoint was device closure defined as residual jet of less than 5 mm (detected on transoesophageal echocardiogram). Future and upcoming trials are summarised in Table 1.
Table 1.
Future trials in both surgical and percutaneous LAAE.
| Study | Cohort | Number of patients | Treatment Arms | Recruitment Details | Primary end points |
|---|---|---|---|---|---|
| AMULET-IDE [NCT02879448] | Non-valvular AF | 1878 | 1) Amulet device | Commenced: Aug 2016 | 1) Safety: Death, procedure-related complications and major bleeding at 12 months |
| 2) Watchman device | Expected full completion: Aug 2024 | 2) Efficacy: Ischaemic stroke and systemic embolism at 18 months | |||
| 3) Device: Device closure (residual jet ≤ 5 mm) at 45 days | |||||
| CHAMPION-AF [NCT04394546] | Non-valvular AF | 3000 | 1) Watchman FLX | Commenced: Oct 2020 | 1) Occurrence of stroke, CV death and systemic embolism at 36 months (non-inferiority) |
| 2) Direct OAC | Expected completion: Dec 2027 | 2) Non-procedural bleeding at 36 months (superiority) | |||
| OPTION [NCT03795298] | Non-valvular AF following catheter ablation | 1600 | 1) Watchman FLX | Commenced: May 2019 | 1) Occurrence of stroke, CV death and systemic embolism at 36 months (non-inferiority) |
| 2) Direct OAC | Expected completion: Nov 2024 | 2) Non-procedural bleeding at 36 months (superiority) | |||
| CATALYST [NCT04226547] | Non-valvular AF | 2650 | 1) Amulet device | Commenced: July 2020 | 1) Occurrence of stroke, CV death and systemic embolism at 24 months (non-inferiority) |
| 2) Direct OAC | Expected completion: Dec 2024 | 2) Major bleeding or non-procedural clinical events at 24 months (superiority) | |||
| 3) Ischaemic stroke or systemic embolism at 36 months (non-inferiority) | |||||
| ASAP-TOO [NCT02928497] | Non-valvular AF not suitable for OAC | 888 | 1) Watchman device | Terminated early due to slow recruitment. Results pending. | 1) Safety: Death, ischaemic stroke, systemic embolism and major complications at 7 days |
| 2) Single or no antiplatelet therapy | 2) Efficacy: Time to first event of ischaemic stroke or embolism up to 5 years | ||||
| PINNACLE FLX [NCT02702271] | Non-valvular AF | 458 | Single arm Watchman FLX device | Commenced: May 2018 | 1) Safety: Death, ischaemic stroke, systemic embolism and major complications at 7 days |
| Expected completion: Feb 2021 | |||||
| 2) Device: LAA closure with any peri-device flow < 5 mm at 12 months | |||||
| LAAOS III [NCT01561651] | AF undergoing cardiac surgery | 4812 | 1) LAA occlusion | Commenced: July 2012 | 1) Stroke or systemic arterial embolism at 4 years |
| 2) No LAAO occlusion | Expected completion: Nov 2022 | ||||
| ATLAS [NCT02701062] | AF undergoing cardiac surgery | 562 | 1) Atriclip device | Commenced: Feb 2016 | 1) Stroke, major bleeding, MI or death within 2 days. |
| 2) Warfarin | Completed recruitment on June 2019 | ||||
AF = atrial fibrillation, CV = cardiovascular, LAA = left atrial appendage, OAC = oral anticoagulation.
8. Conclusion
Clearly there remains a void in evidence as to the role of LAA modification in patients undergoing cardiac surgery, and based on current evidence, the widespread practice is not justified. Given the high cost of these procedures both initially and in the future with potential increases in AF hospitalisations, more clinical data are required before this can be routinely adopted. Moreover, rather than using warfarin as the gold standard, future trials should have direct OAC as the appropriate comparator, as seen in a recent percutaneous LAAO trial. Percutaneous LAAO device have demonstrated good safety and efficacy profile in both observational and randomized studies, which may play a larger role in stroke modification for AF patients with high bleeding risk.
Contributorship Statement
Both HR and JL were involved in the initial write-up, revision and confirming the final version of this manuscript.
Data Sharing Statement
No additional data available.
Disclosure
The authors have nothing to disclose.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sector.
Declaration of Competing Interest
The authors report no relationships that could be construed as a conflict of interest.
References
- 1.Go A.S., Hylek E.M., Phillips K.A. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Friberg L., Rosenqvist M., Lip G.Y. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur. Heart J. 2012;33:1500–1510. doi: 10.1093/eurheartj/ehr488. [DOI] [PubMed] [Google Scholar]
- 3.Hindricks G., Potpara T., Dagres N. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) Eur. Heart J. 2020 [Google Scholar]
- 4.January C.T., Wann L.S., Calkins H. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019;74:104–132. doi: 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Pisters R., Lane D.A., Nieuwlaat R., de Vos C.B., Crijns H.J., Lip G.Y. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 6.Veinot J.P., Harrity P.J., Gentile F. Anatomy of the normal left atrial appendage: a quantitative study of age-related changes in 500 autopsy hearts: implications for echocardiographic examination. Circulation. 1997;96:3112–3115. doi: 10.1161/01.cir.96.9.3112. [DOI] [PubMed] [Google Scholar]
- 7.Su P., McCarthy K.P., Ho S.Y. Occluding the left atrial appendage: anatomical considerations. Heart. 2008;94:1166–1170. doi: 10.1136/hrt.2006.111989. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Minguez J.R., Gonzalez-Fernandez R., Fernandez-Vegas C. Anatomical classification of left atrial appendages in specimens applicable to CT imaging techniques for implantation of amplatzer cardiac plug. J. Cardiovasc. Electrophysiol. 2014;25:976–984. doi: 10.1111/jce.12429. [DOI] [PubMed] [Google Scholar]
- 9.Shirani J., Alaeddini J. Structural remodeling of the left atrial appendage in patients with chronic non-valvular atrial fibrillation: implications for thrombus formation, systemic embolism, and assessment by transesophageal echocardiography. Cardiovasc. Pathol. 2000;9:95–101. doi: 10.1016/s1054-8807(00)00030-2. [DOI] [PubMed] [Google Scholar]
- 10.Ernst G., Stollberger C., Abzieher F. Morphology of the left atrial appendage. Anat. Rec. 1995;242:553–561. doi: 10.1002/ar.1092420411. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto M., Seo Y., Kawamatsu N. Complex left atrial appendage morphology and left atrial appendage thrombus formation in patients with atrial fibrillation. Circ. Cardiovasc. Imaging. 2014;7:337–343. doi: 10.1161/CIRCIMAGING.113.001317. [DOI] [PubMed] [Google Scholar]
- 12.Di Biase L., Santangeli P., Anselmino M. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J. Am. Coll. Cardiol. 2012;60:531–538. doi: 10.1016/j.jacc.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 13.Blackshear J.L., Odell J.A. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann. Thorac. Surg. 1996;61:755–759. doi: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 14.Siebermair J., Suksaranjit P., McGann C.J. Atrial fibrosis in non-atrial fibrillation individuals and prediction of atrial fibrillation by use of late gadolinium enhancement magnetic resonance imaging. J. Cardiovasc. Electrophysiol. 2019;30:550–556. doi: 10.1111/jce.13846. [DOI] [PubMed] [Google Scholar]
- 15.Fonseca A.C., Alves P., Inacio N. Patients with undetermined stroke have increased atrial fibrosis: a cardiac magnetic resonance imaging study. Stroke. 2018;49:734–737. doi: 10.1161/STROKEAHA.117.019641. [DOI] [PubMed] [Google Scholar]
- 16.King J.B., Azadani P.N., Suksaranjit P. Left atrial fibrosis and risk of cerebrovascular and cardiovascular events in patients with atrial fibrillation. J. Am. Coll. Cardiol. 2017;70:1311–1321. doi: 10.1016/j.jacc.2017.07.758. [DOI] [PubMed] [Google Scholar]
- 17.Hof I., Chilukuri K., Arbab-Zadeh A. Does left atrial volume and pulmonary venous anatomy predict the outcome of catheter ablation of atrial fibrillation? J. Cardiovasc. Electrophysiol. 2009;20:1005–1010. doi: 10.1111/j.1540-8167.2009.01504.x. [DOI] [PubMed] [Google Scholar]
- 18.Kalifa J., Jalife J., Zaitsev A.V. Intra-atrial pressure increases rate and organization of waves emanating from the superior pulmonary veins during atrial fibrillation. Circulation. 2003;108:668–671. doi: 10.1161/01.CIR.0000086979.39843.7B. [DOI] [PubMed] [Google Scholar]
- 19.Davis C.A., 3rd, Rembert J.C., Greenfield J.C., Jr. Compliance of left atrium with and without left atrium appendage. Am. J. Physiol. 1990;259:H1006–H1008. doi: 10.1152/ajpheart.1990.259.4.H1006. [DOI] [PubMed] [Google Scholar]
- 20.Hoit B.D., Walsh R.A. Regional atrial distensibility. Am. J. Physiol. 1992;262:H1356–H1360. doi: 10.1152/ajpheart.1992.262.5.H1356. [DOI] [PubMed] [Google Scholar]
- 21.Hellerstein H.K., Sinaiko E., Dolgin M. Amputation of the canine atrial appendages. Proc. Soc. Exp. Biol. Med. 1947;66:337. doi: 10.3181/00379727-66-16084p. [DOI] [PubMed] [Google Scholar]
- 22.Madden J.L. Resection of the left auricular appendix; a prophylaxis for recurrent arterial emboli. J. Am. Med. Assoc. 1949;140:769–772. doi: 10.1001/jama.1949.02900440011003. [DOI] [PubMed] [Google Scholar]
- 23.Cox J.L., Boineau J.P., Schuessler R.B. Successful surgical treatment of atrial fibrillation. Review and clinical update. JAMA. 1991;266:1976–1980. [PubMed] [Google Scholar]
- 24.Gillinov A.M., Pettersson G., Cosgrove D.M. Stapled excision of the left atrial appendage. J. Thorac. Cardiovasc. Surg. 2005;129:679–680. doi: 10.1016/j.jtcvs.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 25.Salzberg S.P., Plass A., Emmert M.Y. Left atrial appendage clip occlusion: early clinical results. J. Thorac. Cardiovasc. Surg. 2010;139:1269–1274. doi: 10.1016/j.jtcvs.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 26.Lee R., Vassallo P., Kruse J. A randomized, prospective pilot comparison of 3 atrial appendage elimination techniques: Internal ligation, stapled excision, and surgical excision. J. Thorac. Cardiovasc. Surg. 2016;152:1075–1080. doi: 10.1016/j.jtcvs.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Kanderian A.S., Gillinov A.M., Pettersson G.B., Blackstone E., Klein A.L. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J. Am. Coll. Cardiol. 2008;52:924–929. doi: 10.1016/j.jacc.2008.03.067. [DOI] [PubMed] [Google Scholar]
- 28.Katz E.S., Tsiamtsiouris T., Applebaum R.M., Schwartzbard A., Tunick P.A., Kronzon I. Surgical left atrial appendage ligation is frequently incomplete: a transesophageal echocardiograhic study. J. Am. Coll. Cardiol. 2000;36:468–471. doi: 10.1016/s0735-1097(00)00765-8. [DOI] [PubMed] [Google Scholar]
- 29.Aryana A., Singh S.K., Singh S.M. Association between incomplete surgical ligation of left atrial appendage and stroke and systemic embolization. Heart Rhythm. 2015;12:1431–1437. doi: 10.1016/j.hrthm.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 30.Whitlock R.P., Vincent J., Blackall M.H. Left Atrial Appendage Occlusion Study II (LAAOS II) Can. J. Cardiol. 2013;29:1443–1447. doi: 10.1016/j.cjca.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Melduni R.M., Schaff H.V., Lee H.C. Impact of left atrial appendage closure during cardiac surgery on the occurrence of early postoperative atrial fibrillation, stroke, and mortality: a propensity score-matched analysis of 10 633 Patients. Circulation. 2017;135:366–378. doi: 10.1161/CIRCULATIONAHA.116.021952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao X., Gersh B.J., Holmes D.R., Jr. Association of surgical left atrial appendage occlusion with subsequent stroke and mortality among patients undergoing cardiac surgery. JAMA. 2018;319:2116–2126. doi: 10.1001/jama.2018.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman D.J., Piccini J.P., Wang T. Association between left atrial appendage occlusion and readmission for thromboembolism among patients with atrial fibrillation undergoing concomitant cardiac surgery. JAMA. 2018;319:365–374. doi: 10.1001/jama.2017.20125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin Gutierrez E., Castano M., Gualis J. Beneficial effect of left atrial appendage closure during cardiac surgery: a meta-analysis of 280 585 patients. Eur. J. Cardiothorac. Surg. 2020;57:252–262. doi: 10.1093/ejcts/ezz289. [DOI] [PubMed] [Google Scholar]
- 35.Waksman R., Pendyala L.K. Overview of the Food and Drug Administration circulatory system devices panel meetings on WATCHMAN left atrial appendage closure therapy. Am. J. Cardiol. 2015;115:378–384. doi: 10.1016/j.amjcard.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Reddy V.Y., Doshi S.K., Sievert H. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-Year Follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) Trial. Circulation. 2013;127:720–729. doi: 10.1161/CIRCULATIONAHA.112.114389. [DOI] [PubMed] [Google Scholar]
- 37.Reddy V.Y., Sievert H., Halperin J. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988–1998. doi: 10.1001/jama.2014.15192. [DOI] [PubMed] [Google Scholar]
- 38.Holmes D.R., Jr., Kar S., Price M.J. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J. Am. Coll. Cardiol. 2014;64:1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 39.Reddy V.Y., Doshi S.K., Kar S. 5-Year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J. Am. Coll. Cardiol. 2017;70:2964–2975. doi: 10.1016/j.jacc.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 40.Boersma L.V., Ince H., Kische S. Efficacy and safety of left atrial appendage closure with WATCHMAN in patients with or without contraindication to oral anticoagulation: 1-Year follow-up outcome data of the EWOLUTION trial. Heart Rhythm. 2017;14:1302–1308. doi: 10.1016/j.hrthm.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 41.Boersma L.V., Ince H., Kische S. Evaluating real-world clinical outcomes in atrial fibrillation patients receiving the WATCHMAN left atrial appendage closure technology. Circ. Arrhythm. Electrophysiol. 2019;12:e006841. doi: 10.1161/CIRCEP.118.006841. [DOI] [PubMed] [Google Scholar]
- 42.Reddy V.Y., Mobius-Winkler S., Miller M.A. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology) J. Am. Coll. Cardiol. 2013;61:2551–2556. doi: 10.1016/j.jacc.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 43.Holmes D.R., Jr., Reddy V.Y., Gordon N.T. Long-term safety and efficacy in continued access left atrial appendage closure registries. J. Am. Coll. Cardiol. 2019;74:2878–2889. doi: 10.1016/j.jacc.2019.09.064. [DOI] [PubMed] [Google Scholar]
- 44.Reddy V.Y., Gibson D.N., Kar S. Post-Approval U.S. Experience with left atrial appendage closure for stroke prevention in atrial fibrillation. J. Am. Coll. Cardiol. 2017;69:253–261. doi: 10.1016/j.jacc.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 45.Viles-Gonzalez J.F., Kar S., Douglas P. The clinical impact of incomplete left atrial appendage closure with the Watchman Device in patients with atrial fibrillation: a PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) substudy. J. Am. Coll. Cardiol. 2012;59:923–929. doi: 10.1016/j.jacc.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 46.Osmancik P., Herman D., Neuzil P. Left Atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J. Am. Coll. Cardiol. 2020;75:3122–3135. doi: 10.1016/j.jacc.2020.04.067. [DOI] [PubMed] [Google Scholar]
- 47.Viles-Gonzalez J.F., Reddy V.Y., Petru J. Incomplete occlusion of the left atrial appendage with the percutaneous left atrial appendage transcatheter occlusion device is not associated with increased risk of stroke. J. Interv. Card Electrophysiol. 2012;33:69–75. doi: 10.1007/s10840-011-9613-x. [DOI] [PubMed] [Google Scholar]
- 48.Freixa X., Tzikas A., Sobrino A., Chan J., Basmadjian A.J., Ibrahim R. Left atrial appendage closure with the Amplatzer Cardiac Plug: impact of shape and device sizing on follow-up leaks. Int. J. Cardiol. 2013;168:1023–1027. doi: 10.1016/j.ijcard.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 49.Fauchier L., Cinaud A., Brigadeau F. Device-related thrombosis after percutaneous left atrial appendage occlusion for atrial fibrillation. J. Am. Coll. Cardiol. 2018;71:1528–1536. doi: 10.1016/j.jacc.2018.01.076. [DOI] [PubMed] [Google Scholar]
- 50.Dukkipati S.R., Kar S., Holmes D.R. Device-related thrombus after left atrial appendage closure. Circulation. 2018;138:874–885. doi: 10.1161/CIRCULATIONAHA.118.035090. [DOI] [PubMed] [Google Scholar]
- 51.Tzikas A., Shakir S., Gafoor S. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the AMPLATZER Cardiac Plug. EuroIntervention. 2016;11:1170–1179. doi: 10.4244/EIJY15M01_06. [DOI] [PubMed] [Google Scholar]
- 52.Landmesser U., Schmidt B., Nielsen-Kudsk J.E. Left atrial appendage occlusion with the AMPLATZER Amulet device: periprocedural and early clinical/echocardiographic data from a global prospective observational study. EuroIntervention. 2017;13:867–876. doi: 10.4244/EIJ-D-17-00493. [DOI] [PubMed] [Google Scholar]
- 53.Hildick-Smith D., Landmesser U., Camm A.J. Left atrial appendage occlusion with the Amplatzer Amulet device: full results of the prospective global observational study. Eur. Heart J. 2020;41:2894–2901. doi: 10.1093/eurheartj/ehaa169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rashid H.N., Layland J. Association between device-related thrombus and the neo-appendage with left-atrial appendage occlusion devices. Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa803. [DOI] [PubMed] [Google Scholar]
- 55.Whitlock R., Healey J., Vincent J. Rationale and design of the Left Atrial Appendage Occlusion Study (LAAOS) III. Ann Cardiothorac Surg. 2014;3:45–54. doi: 10.3978/j.issn.2225-319X.2013.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holmes D.R., Reddy V.Y., Buchbinder M. The Assessment of the Watchman Device in Patients Unsuitable for Oral Anticoagulation (ASAP-TOO) trial. Am. Heart J. 2017;189:68–74. doi: 10.1016/j.ahj.2017.03.007. [DOI] [PubMed] [Google Scholar]