Abstract
An imbalance in angiogenic growth factors and poor utero-placental perfusion are strongly associated with preeclampsia. The reduced utero-placental perfusion (RUPP) model that mimics insufficient placental perfusion is used to study preeclampsia. The aim of this study was to develop a refined RUPP model in C57Bl/6 J mice to test the efficacy of MZe786 as a potential inhibitor of soluble Flt-1 for preeclampsia therapy. Murine RUPP (mRUPP) was induced through bilateral ligation of the ovarian arteries at E11.5 that resulted in typical preeclampsia symptoms including increase in mean arterial pressure (MAP), kidney injury and elevated soluble Flt-1 (sFlt-1) levels in the maternal plasma and amniotic fluid. The murine RUPP kidneys showed tubular and glomerular damage along with increased oxidative stress characterised by increased nitrotyrosine staining. The mRUPP displayed abnormal placental vascular histology, reduced expression of placental cystathionine γ-lyase (CSE), the hydrogen sulfide (H2S) producing enzyme, and resulted in adverse fetal outcomes (FGR). Importantly, oral administration of hydrogen sulfide (H2S)-releasing compound MZe786 from E11.5 to E17.5 successfully prevented the development of preeclampsia. Specifically, MZe786 treatment reduced maternal MAP and kidney nitrotyrosine staining and improved fetal outcome. The circulation levels of sFlt-1 were dramatically decreased in MZe786 treated animals implying that H2S released from MZe786 offered protection by inhibiting sFlt-1 levels. MZe786 prevent preeclampsia and warrant a rapid move to randomised control clinical trial.
Keywords: Preeclampsia, Soluble Flt-1, Hydrogen sulfide, Nitrosative stress, Mouse model
Graphical abstract

Highlights
-
•
Refined mouse reduced uterine perfusion pressure (mRUPP) model exhibits preeclampsia symptoms.
-
•
Mouse RUPP induces maternal hypertension, kidney injury, elevates circulating sFlt-1 levels and promotes nitrosative stress.
-
•
Mouse RUPP reduces expression of the protective enzyme, placental cystathionine γ-lyase and causes poor fetal outcome.
-
•
H2S releasing aspirin, MZe786, acts as an inhibitor of sFlt-1 to successfully prevent preeclampsia and improve fetal outcome.
-
•
MZe786 is a novel drug with therapeutic potential to prevent preeclampsia.
1. Introduction
Preeclampsia is characterised by new hypertension along with complications affecting maternal organs and the feto-placental systems [1,2]. It contributes significantly to poor health outcomes in later life for both the mother and the baby [[3], [4], [5]]. Ten to 30 years after preeclampsia, women are several folds higher risk of developing high blood pressure, heart disease, chronic heart failure and stroke [6]. Despite a better understanding of the disease mechanism [[7], [8], [9], [10], [11], [12]], effective pharmacological therapeutics has yet to be identified. Unfortunately, the only option available today is the early delivery of the baby with all the negative consequences associated with it.
Circulating levels of soluble fms-like tyrosine kinase-1 (sFlt-1), the natural inhibitor of vascular endothelial growth factor (VEGF), is elevated before the clinical onset of preeclampsia [12]. Although this disease is almost unique to humans and higher primates [13], several animal models have been developed to mimic symptoms of preeclampsia. The reduced utero-placental perfusion (RUPP) procedure was first performed in female baboons and guinea-pigs and resulted in hypertension and proteinuria during pregnancy [14,15]. Granger and colleagues modified the RUPP technique in rats with reduction of blood flow in abdominal aorta and uterine arteries [[16], [17], [18]].
The first direct evidence that a dysfunctional cystathionine γ-lyase (CSE)/hydrogen sulfide (H2S) pathway contributes to the pathogenesis of preeclampsia [7] was demonstrated using CSE inhibition, which induced preeclampsia-like features in pregnant mice owing to the inhibition of H2S production. Intraperitoneal injection of a slow-releasing H2S-generating compound GYY4137 restored fetal growth and reduced sFlt-1 levels in pregnant animals [7].
Here we report a refined mouse RUPP (mRUPP) model in a common inbred strain of C57Bl/6 J mice, which caused an increase in sFlt-1 levels, induced hypertension, kidney damage, altered placental morphology and adverse fetal outcome. Importantly, we demonstrate the successful use of this model to test the efficacy of an orally active H2S-releasing MZe786 to inhibit sFlt-1 and reversed the clinical characteristics of preeclampsia.
2. Materials and methods
For detailed descriptions of the materials and methods used, please see Supplementary file.
2.1. Materials
The H2S-releasing molecule, MZe786 (2-acetyloxybenzoic acid 4-(3-thioxo-3H-1,2-dithiol-5-yl)phenyl ester) with a defined pharmacological profile, was used as previously described [[19], [20], [21]].
2.2. Methods
2.2.1. Refined murine reduced uterine perfusion pressure (mRUPP) model
Twelve to twenty-week-old C57Bl/6 J males were mated with similar aged C57Bl/6 J female mice. The first day of pregnancy (E0.5) was defined by the presence of vaginal plug the following morning. At E11.5, pregnant mice were anesthetised with 2% isoflurane. Abdominal cavity was opened by an incision through the linea alba. Using magnetic retractors, the skin and muscle was gently pulled for a better visual of the organs and pups of the mice. The ovarian arteries on either side were identified and ligated above the ovaries using 7-0 silk sutures (Fig. 1B). The incisions were closed using Vicryl 6.0 sutures. Sham control group underwent comparative manipulations but without ovarian artery ligation. All surgeries were carried out under aseptic conditions and buprenorphine (0.05 mg/kg) i. p. was administered for 48 h post-surgery. mRUPP and Sham were either treated with drug carrier (0.5% carboxymethyl cellulose in PBS) or 25 mg/kg or 50 mg/kg of MZe786 from E11.5 to E17.5 via gavage. On E16.5, mice were placed in metabolic cages to collect urine for 24 h. On day E17.5 the mice were sacrificed, and the tissues were harvested.
Fig. 1.
Refined RUPP model in C57Bl6/J mice. (A) Diagram illustrates the experimental protocol for mouse RUPP model. Female C57B16/J were time mated with similar aged males and on E11.5 of pregnancy RUPP/Sham surgery was performed. The mice were then divided into groups to receive 50 mg/kg of MZe786 orally daily from E11.5 to E17.5. Control mice received drug career (0.5% carboxymethyl cellulose in PBS). All mice were harvested on E17.5 (B) Schematic representation of mouse uterus showing placement of ligation in the ovarian arteries. (C) Blood flow rate in the ovarian artery at base line (pre-mRUPP) and after RUPP surgery (post-mRUPP). The blood flow rate was significantly decreased post ligation. Data expressed as mean (±SEM) and analysed by non-parametric t-test, n = 7.
2.2.2. Acute blood flow assessment
The blood flow in the ovarian artery was assessed using laser Doppler flowmetry (LDF) on mRUPP pre- and post-ligation as previously described [22].
2.2.3. Blood pressure analysis
Blood pressure was measured on E17.5 as described previously [7].
2.2.4. Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay kits for murine sFlt-1 and KIM-1 were obtained from R&D Systems, UK and assays performed according to the manufacturer's specifications.
2.2.5. Immunohistochemistry
Murine kidney and placental tissue sections were prepared for immunohistochemistry as previously described [23].
2.2.6. Histological analysis
Murine kidney and placental tissues were embedded with paraffin and stained for Hematoxylin and eosin (H&E). H&E stained kidney and placenta tissues were imaged using NanoZoomer (Hamamatsu, Japan). Area of the labyrinth zone was measured and analysed using ImageJ. Kidney analysis was done in a semi-quantitative manner as described previously [24,25]. Two researchers performed blindly and independently, the analysis of placenta and kidney (see Table 1).
2.2.7. Statistical analysis
Data are presented as either mean and SEM or median and range as appropriate. Comparison between two groups was performed using Mann-Whitney U test (non-parametric). Comparisons among three or more groups were performed using Two-way ANOVA. Statistical significance was set at p < 0.05.
3. Results
3.1. Refined mRUPP model in C57Bl/6 J mice
We used C57Bl/6 J mice to develop a refined mRUPP model that could successfully mimic appropriate clinical and biochemical signs of preeclampsia. In contrast to the traditional method of ligating abdominal aorta and ovarian artery, we performed surgical occlusion of ovarian arteries on either side (Fig. 1B). Importantly the surgery was performed much earlier into pregnancy (E11.5) compared to the traditional method (E14.5), providing a longer ‘therapeutic window’ (Fig. 1A). Laser Doppler blood flow measurements showed that the ligation of ovarian arteries proximal to ovaries significantly reduced arterial flow post-ligation (Fig. 1C). The flow rate in the abdominal aorta remained unchanged (Supplementary Fig. 1C) by this procedure.
3.2. MZe786 improved maternal outcome in mRUPP
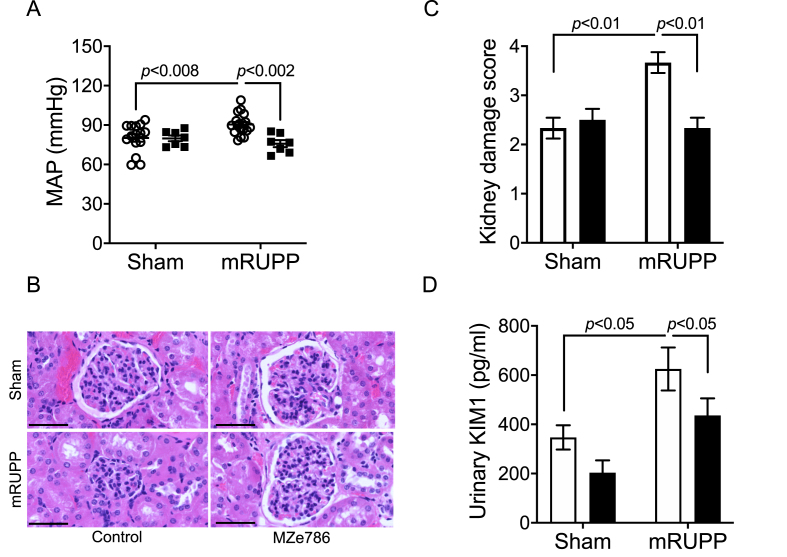
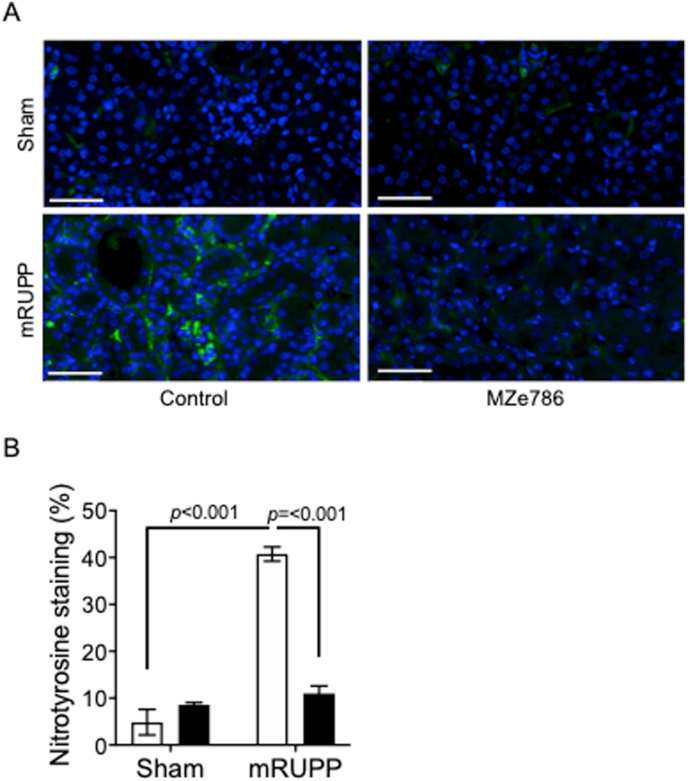
Mice that underwent mRUPP surgery showed typical preeclamptic features such as high mean arterial blood pressure compared to Sham operated mice (Fig. 2A). In humans, preeclampsia affects kidney function during pregnancy with a four-fold increased risk of developing end-stage renal disease within 10 years after pregnancy [26]. Semi-quantitative, blinded evaluation of kidney histology revealed histopathological alterations in the mRUPP kidneys such as glomerular degeneration, narrowing or disappearance of Bowman's space, tubular atrophy, fibrosis and vascular congestion (Fig. 2B and C). The size of glomeruli in mRUPP group appeared smaller compared to Sham and did not reach statistical significance (Supplementary Fig. 2A). Likewise, the renal artery wall appeared to be thicker in mRUPPs compared to Sham and again was not statistically significant (Supplementary Fig. 2B). The levels of Kidney Injury Marker (KIM-1) in urine were significantly increased in mRUPP mice confirming the renal injury in this model (Fig. 2D). We have previously shown evidence that preeclampsia is associated with reduced circulating levels of H2S [7]. Here we show that oral administration of H2S releasing molecule, MZe786 significantly improve maternal outcome in the mRUPP model. Specifically, the mean arterial blood pressure was significantly reduced in MZe786 treated mRUPPs (Fig. 2A). Two doses of MZe786 were tested, 25 mg/kg and 50 mg/kg. The dose of 25 mg/kg of MZe786 did not reduce blood pressure in mRUPPs (Supplementary Fig. 3). Hence, 50 mg/kg dose was selected for future studies. The mRUPP/Sham mice that received drug carrier (orally) were used as controls. MZe786 also significantly reduced the renal injury induced by mRUPP as evidenced by reduced kidney damage score (Fig. 2B and C) and decrease in KIM-1 levels in urine (Fig. 2D). The mRUPP kidneys showed higher oxidative stress as evidenced by increased nitrotyrosine staining compared to Sham (Fig. 3A and B). Importantly, MZe786 effectively suppressed oxidative stress as evidenced from marked reduction in nitrotyrosine staining in the treated group (Fig. 3A and B).
Fig. 2.
H2S-releasing molecule, MZe786 rescued preeclamptic phenotype in mRUPP model. (A) Mean arterial blood pressure (MAP) recorded at day 17.5 of gestation of Sham or mRUPP mice treated with MZe786 or control. MAP was significantly upregulated in mRUPP mice (n = 15) compared to Sham (n = 15). MZe786 (n = 7) effectively reduced the increase in MAP induced by mRUPP. (B) Representative H&E images of kidney from different groups (scale bar represents 50 μm). Sham kidney section showed normal renal cortex and glomerular tufts. mRUPP group showed marked kidney damage including glomerular degeneration with absence of bowman's capsular space, disorganised tubules, fibrosis and vascular congestion. MZe786 treated Sham and mRUPP showed architecture similar to Sham, n = 6. (C) The graph shows the scoring of the pathological changes, n = 6. (D) Urine levels of KIM-1 in Sham or mRUPP mice treated with MZe786 or control. mRUPP induced significantly high levels of KIM-1. MZe786 treated mice showed significant reduction in KIM-1 levels, n = 6. Data expressed as mean (±SEM) and analysed by 2-way ANOVA. Clear circles represent group that received drug career and black squares represent group that received MZe786. White bars represent group that received drug career and black bars represent group that received MZe786.
Fig. 3.
H2S-releasing molecule, MZe786 reduced renal oxidative stress in mRUPP model. (A) Nitrotyrosine levels, a footprint of peroxynitrite production, in kidneys of different groups are shown (scale bar represents 50 μm). The nitrotyrosine levels was upregulated in mRUPP compared to Sham. The levels of nitrotyrosine remain unchanged in Sham treated with MZe786. MZe786 treatment significantly decreased nitrotyrosine production in mRUPP kidneys. (B) Graph showing quantification of nitrotyrosine staining. Data expressed as mean (±SEM) and analysed by 2-way ANOVA, n = 4. White bars represent group that received drug career and black bars represent group that received MZe786.
3.3. MZe786 rescue fetal outcome in mRUPP
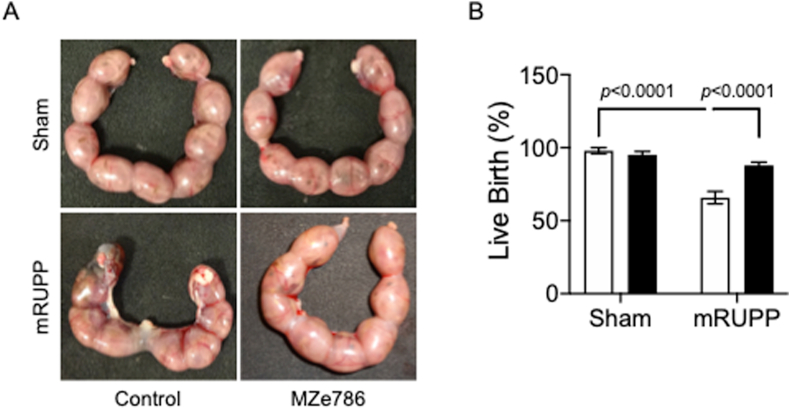
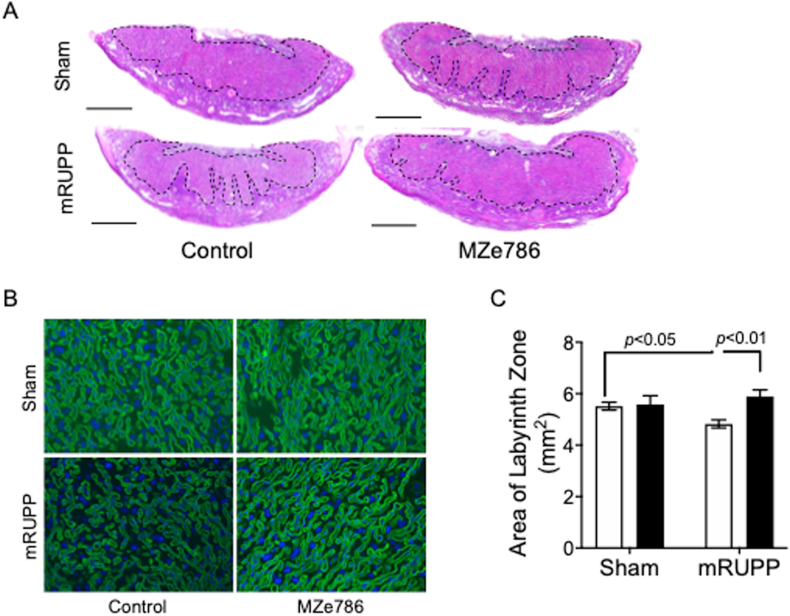
The percentage of viable fetuses was significantly lower in mRUPP compared to Sham (Fig. 4A and B). H2S releasing molecule, MZe786, significantly increased the live birth in mRUPP (Fig. 4A and B). The mouse placenta is comprised of three distinct zones; the maternally derived decidua, and the conceptus-derived junctional and labyrinth zones. The labyrinth zone consist of two layers of multinucleated syncytiotrophoblast (SynT) cells resulting in a large surface area for nutrient and gas exchange between mother and fetus [27]. Blinded histological analysis of placental sections showed that the area of labyrinth zone was significantly reduced in mRUPP compared to Sham (Fig. 5A and C). This reduction was reversed successfully by oral administration of MZe786 (Fig. 5A and C). In mature placenta, fetal-derived endothelial cells line the maternal blood spaces [28]. Using isolectin B4 to highlight the fetal endothelial cells [7], we compared the anatomic features of labyrinth zone in Sham, mRUPP and MZe786 treatment groups. In control mice, the labyrinth showed organised fetal vessels with well-developed branching morphogenesis (Fig. 5B). The mRUPP placentas showed irregular branching. More importantly, MZe786 treatment group showed organised fetal vessels similar to Sham (Fig. 5B).
Fig. 4.
Fetal outcome was rescued by MZe786 in mRUPP (A) Representative images of uterine horn (E17.5) showing increased fetal loss in mRUPP and the rescue effect of MZe786 in mRUPP. (B) Live birth rate expressed as percentage in Sham and mRUPP treated with MZe786 or control. The live births in mRUPP (n = 9) was significantly lower compared to Sham (n = 6). Importantly MZe786 (n = 8) rescued mRUPP induced miscarriages, significantly increasing live births. No adverse effect was seen in Sham treated with MZe786 (n = 6). Data expressed as mean (±SEM) and analysed by 2-way ANOVA. White bars represent group that received drug career and black bars represent group that received MZe786.
Fig. 5.
MZe786 reversed altered placental morphology in mRUPP model. (A) Gross histological assessment of placental morphology showed marked reduction of placental labyrinth zone (indicated by dashed lines) in mRUPP compared to Sham (scale bar represents 1 mm). Oral administration of MZe786 significantly reduced this structural alteration induced by mRUPP. Placenta of Sham mice treated with MZe786 remain unchanged (n = 4). (B) Isolectin B4 staining of labyrinth zone showing altered vasculature in the mRUPP mice and rescue when treated with MZe786. (C) Graph showing the measurement of area of labyrinth zone using Image J software. Results are representative or expressed as mean (±SEM) and analysed by 2-way ANOVA, n = 7. White bars represent group that received drug career and black bars represent group that received MZe786.
3.4. MZe786 rescue preeclampsia phenotype by suppressing sFlt-1 in mother and fetus
Soluble Flt-1 is the anti-angiogenic factor with the highest strength of association with preeclampsia; elevated levels of sFlt-1 are reported in plasma [29], placenta [12] and amniotic fluid [30] of preeclamptic women. Previous study from Ahmed's laboratory demonstrated that endothelial knockdown of CSE resulted in increased release of sFlt-1 [7]. We investigated whether similar changes in levels of sFlt-1 occur in the refined mRUPP model in which we also observed decreased placental expression of CSE following RUPP surgery (Fig. 6A). Expression levels of sFlt-1 were significantly increased in placental tissue (Fig. 6B) and amniotic fluid (Fig. 6C) in mRUPP. The maternal sFlt-1 levels were 3-fold higher in mRUPP compared to Sham controls (Fig. 6D). In contrast, circulating VEGF levels remained unchanged (Supplementary Figure 4). To determine whether MZe786 inhibits sFlt-1 release, we assessed sFlt-1 levels in pregnant mice treated with MZe786. In mRUPP, MZe786 effectively reduced the sFlt-1 levels to Sham control levels (Fig. 6D). The levels of sFlt-1 in amniotic fluid (Fig. 6C) and placenta expression of sFlt-1 mRNA were (Fig. 6B) also significantly decreased compared to mice that received drug carrier as control.
Fig. 6.
MZe786 rescue PE phenotype in mRUPP by downregulating sFlt-1. qPCR data showing decreased CSE mRNA expression in mRUPPs compared to Sham (A). mRNA expression levels of sFlt-1 in placenta (B). ELISA data showing protein levels of sFlt-1 in amniotic fluid (C), and plasma (D). CSE mRNA expression was significantly downregulated in mRUPPs and the sFlt-1 levels were correspondingly upregulated in mRUPPs compared to Sham. MZe786 effectively lowered sFlt-1 expression in mRUPP mice. Data expressed as mean (±SEM) and analysed by 2-way ANOVA, n = 6. White bars represent group that received drug career and black bars represent group that received MZe786.
4. Discussion
We recently reported that MZe786 rescues mitochondrial activity by stimulating cardiac mitochondrial biogenesis and antioxidant defence in heme oxygenase-1 deficient mice under high sFlt-1 environment [19]. With a good safety profile, MZe786 was a good H2S releasing candidate for testing in an animal model of preeclampsia [20]. The beneficial role of MZe786 has been reported in atherosclerosis [31] and is shown to have strong antithrombotic effect [32]. Here, we report the development of a refined RUPP model in mice and demonstrate the clear beneficial effect of MZe786 in rescuing preeclampsia symptoms and suppressing maternal and placental sFlt-1 levels. Acute kidney injury is commonly associated with preeclampsia and has high rates of association with maternal and perinatal mortality [33]. Consistent with the high KIM-1 levels in the urine of mRUPP pregnancy, the histology of the kidneys showed damage including tubular and glomerular degeneration, fibrosis and vascular congestion. MZe786 suppressed KIM-1 and improved renal function.
A common complication of hindlimb ischemia is complete paraplegia due to restriction of abdominal aorta in the rat RUPP model [34] indicating that aortic restriction occludes blood flow to the entire hindquarters of the animal. Hence, the symptoms of preeclampsia observed in these animals cannot be classified as specific to insufficient uteroplacental perfusion. Attempts to improve the model by restricting the blood flow specifically to the uteroplacental units in rats resulted in increased blood pressure. However, many of the prominent features of preeclampsia including elevated sFlt-1 levels and renal damage were absent [35,36]. An earlier report of RUPP in C57Bl/6 J mice used restriction of abdominal aorta and ovarian arteries making the preeclamptic symptoms unspecific to uteroplacental perfusion [18]. The study showed increased blood pressure and serum sFlt-1 levels, but placental sFlt-1 expression remain unchanged and kidney damage was absent [18]. In the present study, by ligating the ovarian arteries only, we avoided interfering with the abdominal blood flow (Supplementary Fig. 1). Fushima et al. in an earlier study had shown that the positional effect of ligation of the vascular arcade [37]. They bilaterally ligated ovarian vessels distal to ovarian branches, uterine vessels, or both in an outbred strain of mice at E14.5. The study showed increased blood pressure, glomerular endotheliosis and reduction in pup weights post-surgery [37]. However, the important feature of increased maternal plasma sFlt-1 levels and placental expression of sFlt-1 were absent, which is a major component of preeclampsia. Importantly, we have performed the surgery earlier at E11.5 in C57Bl/6 J, which corresponds to the beginning of the second trimester of mouse pregnancy and approximately similar in gestation period when preeclampsia develops in women. This has allowed us to increase the therapeutic window for testing drugs. All other RUPP models reported so far have performed surgery at a later day of E14.5, when placentation is fully established.
E11.5 is a crucial time when placentation develops. This study reports marked pathophysiological changes in the placenta of RUPP pregnancy. There was significant reduction in placental labyrinth zone in mRUPP pregnancy, which is a crucial site for nutrient and gas exchange between mother and fetus. The labyrinth zone also showed marked anatomical changes with disorganised fetal vessels. Typical features such as blood pressure, sFlt-1 levels were significantly higher in mRUPPs compared to Sham controls.
Performing the surgery at E11.5 also gives a good ‘therapeutic window’. The protective effect of H2S has been widely reported in cardiovascular [[38], [39], [40], [41]], neurodegenerative diseases [42,43] and in multiple cancer pathophysiology [[44], [45], [46]]. In this study, we used MZe786, which is a H2S releasing aspirin as a source of H2S. Chronic use of aspirin is frequently associated with gastropathy, partly due to, redox imbalances [47]. MZe786 has been shown to counter the redox imbalance processes through increased H2S/glutathione production, heme oxygenase-1 promoter activity and by suppression of 8-isoprostane-prostaglandin F2α (8-isoprostane) formation [20]. MZe786 has been shown to effectively reverse pathological symptoms in a rat model of metabolic syndrome [48]. The study compared the effect of MZe786 with its parent compound aspirin and confirmed the clear beneficial effect of MZe786 over aspirin [21,48]. Since the clear beneficial role of MZe786 over aspirin was already established, we chose not to include an aspirin-only group to minimise animal usage, as our objective was to see if MZe786 reduced sFlt-1 levels. Aspirin was reported not to inhibit sFlt-1 [49]. Primarily, the mode of action of MZe786 involves H2S release. The H2S release profile of MZe786 shows a significant early increase in circulating H2S and the administration of MZe786 also increases Cysteine and GSH levels in the kidneys [20,50]. H2S is reported to act as a relaxing factor for blood vessels by altering K+ channel activity and cGMP levels in vascular smooth muscle cells [51,52]. In agreement, we saw reduced blood pressure in mRUPP mice treated with MZe786. MZe786 successfully reduced KIM-1 levels and prevented kidney damage. The mRUPP kidneys showed increased nitrotyrosine staining indicating the presence of excess peroxynitrate, which is known to alter mitochondrial function to generate free radicals. Endothelial nitric oxide is regulated by VEGF [53]. In preeclampsia, the abnormally high sFlt-1 levels is responsible for reduced VEGF activity [54]. This may explain the dysregulation of intracellular NO and superoxide and the resultant production of excess peroxynitrate [55]. Importantly, MZe786 significantly reduced nitrotyrosine staining in kidneys.
Notably, MZe786 administration was found to ameliorate placental structural alterations as indicated by increased labyrinth zone compared to mRUPPs. Increase in labyrinth zone area has been associated with a positive adaptation of placenta for better nourishment of fetus in mice [56]. Importantly, MZe786 improved fetal outcome in mRUPPs as evidenced by increase in viable fetuses and more organised fetal vasculature.
Angiogenic imbalance caused by increased circulation of sFlt-1 is highlighted as a prime culprit in preeclampsia, contributing to most of the preeclamptic symptoms. Hence any intervention that would reduce the elevated levels of sFlt-1 may improve pregnancy outcome and protect the mother from permanent vascular damage. We have previously shown that dysregulation of H2S by inhibition of CSE increase sFlt-1 release [7]. Surgically induced preeclampsia consistently showed increased maternal and fetal expression levels of sFlt-1 and marked reduction in the placental expression of CSE. Our recent studies have shown that CSE/H2S pathway sustain endothelial mitochondrial bioenergetics and loss of CSE increased the production of mitochondrial-specific superoxide [19]. Mitochondria are the main source of reactive oxygen species during ischemia in renal tissue [57]. A key regulator of reactive oxygen species detoxification and oxidative phosphorylation is the nuclear transcriptional co-activator peroxisome proliferator-activated receptor co-activator (PGC)-1α [58]. Previous reports show reduced expression of PGC-1α in renal tissue from RUPP rats [59]. PGC-1α is also a master regulator of mitochondrial biogenesis signals [60]. We recently demonstrated the ability of MZe786 to rescue mitochondrial biogenesis and anti-oxidant defence in heme oxygenase-1 deficient mice exposed to a high sFlt-1 environment [19]. Here, we show that MZe786 successfully reduced maternal and fetal sFlt-1 levels and reduced oxidative stress in mRUPP kidneys as evidenced by decreased nitrotyrosine staining. The administration H2S-releasing molecules have previously shown to protect against oxidative stress in models of unilateral ureteric obstruction induced renal injury [61] and bilateral renal ischemia in rats [62]. H2S treatment protects the mitochondrial health by reducing apoptosis, mitochondrial swelling and loss of mitochondrial integrity induced by renal ischemia/reperfusion injury in rats [63]. MZe786 suppressed the increase sFlt-1 that was a likely result of decreased CSE expression in the mRUPP model. As observed in our earlier publications [19], this was probably due, in part, to the increased expression of the antioxidant genes and improved mitochondrial biogenesis that suppressed oxidative stress.
In conclusion, the development of this refined surgically induced model of preeclampsia that captures major preeclampsia features including up-regulation of sFlt-1 and down-regulation of protective enzymes offers a unique opportunity to test novel therapeutics for the prevention of preeclampsia. MZe786 significantly limited the development of preeclampsia in the mRUPP model by suppressing sFlt-1 and nitrosative stress as well as improving antioxidant genes [21] and cellular mitochondrial function [64,65]. This compound warrants urgent investigation in clinical settings.
5. Statement of author contributions
AA conceived the original idea and SA and KW supervised the project. JS and HR carried out the vast majority of the experiments and analysed the data. KL, SA carried out acute blood flow measurements. AS was involved in designing and manufacture of MZe786. FAA contributed to data analysis and fund raising for the project with AA. JS, HR and AA wrote the manuscript. All authors were involved in the discussion of the results and reviewing the manuscript and gave their final approval of the submitted manuscript.
Conflict(s) of Interest/Disclosure(s)
JS, SA, HR, KL, AS and FAA declare they have no conflict of interest. AA is the Chairman and the majority shareholder in MirZyme Therapeutics. AA and KW are inventors for the use of hydrogen sulfide compounds in the treatment of preeclampsia.
Acknowledgements
This work was supported in part by grants from the British Heart Foundation (FS/15/72/31676) and Medical Research Council (G0700288) to AA and grants (FP-51-42 and IFPHI-058-130-2020) from the Deanship of Scientific Affairs, King Abdulaziz University, Jeddah to FAA and AA.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101814.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Fondjo L.A., Boamah V.E., Fierti A., Gyesi D., Owiredu E.W. Knowledge of preeclampsia and its associated factors among pregnant women: a possible link to reduce related adverse outcomes. BMC Pregnancy Childbirth. 2019;19(1):456. doi: 10.1186/s12884-019-2623-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young B.C., Karumanchi S.A. Toward a better diagnosis for preeclampsia. Clin. Chem. 2016;62(7):913–915. doi: 10.1373/clinchem.2016.254920. [DOI] [PubMed] [Google Scholar]
- 3.Amaral L.M., Cunningham M.W., Jr., Cornelius D.C., LaMarca B. Preeclampsia: long-term consequences for vascular health. Vasc. Health Risk Manag. 2015;11:403–415. doi: 10.2147/VHRM.S64798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams D. Long-term complications of preeclampsia. Semin. Nephrol. 2011;31(1):111–122. doi: 10.1016/j.semnephrol.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Staff A.C. Long-term cardiovascular health after stopping pre-eclampsia. Lancet. 2019;394(10204):1120–1121. doi: 10.1016/S0140-6736(19)31993-2. [DOI] [PubMed] [Google Scholar]
- 6.Breetveld N.M., Ghossein-Doha C., van Kuijk S., van Dijk A.P., van der Vlugt M.J., Heidema W.M. Cardiovascular disease risk is only elevated in hypertensive, formerly preeclamptic women. BJOG. 2015;122(8):1092–1100. doi: 10.1111/1471-0528.13057. [DOI] [PubMed] [Google Scholar]
- 7.Wang K., Ahmad S., Cai M., Rennie J., Fujisawa T., Crispi F. Dysregulation of hydrogen sulfide producing enzyme cystathionine gamma-lyase contributes to maternal hypertension and placental abnormalities in preeclampsia. Circulation. 2013;127(25):2514–2522. doi: 10.1161/CIRCULATIONAHA.113.001631. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed A., Ramma W. Unravelling the theories of pre-eclampsia: are the protective pathways the new paradigm? Br. J. Pharmacol. 2015;172(6):1574–1586. doi: 10.1111/bph.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed A., Rezai H., Broadway-Stringer S. Evidence-based revised view of the pathophysiology of preeclampsia. Adv. Exp. Med. Biol. 2017;956:355–374. doi: 10.1007/5584_2016_168. [DOI] [PubMed] [Google Scholar]
- 10.Cudmore M., Ahmad S., Al-Ani B., Fujisawa T., Coxall H., Chudasama K. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115(13):1789–1797. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 11.Egbor M., Ansari T., Morris N., Green C.J., Sibbons P.D. Morphometric placental villous and vascular abnormalities in early- and late-onset pre-eclampsia with and without fetal growth restriction. BJOG. 2006;113(5):580–589. doi: 10.1111/j.1471-0528.2006.00882.x. [DOI] [PubMed] [Google Scholar]
- 12.Maynard S.E., Min J.Y., Merchan J., Lim K.H., Li J., Mondal S. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 2003;111(5):649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crosley E.J., Elliot M.G., Christians J.K., Crespi B.J. Placental invasion, preeclampsia risk and adaptive molecular evolution at the origin of the great apes: evidence from genome-wide analyses. Placenta. 2013;34(2):127–132. doi: 10.1016/j.placenta.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Cavanagh D., Rao P.S., Tung K.S., Gaston L. Eclamptogenic toxemia: the development of an experimental model in the subhuman primate. Am. J. Obstet. Gynecol. 1974;120(2):183–196. doi: 10.1016/0002-9378(74)90360-3. [DOI] [PubMed] [Google Scholar]
- 15.Golden J.G., Hughes H.C., Lang C.M. Experimental toxemia in the pregnant Guinea pig (Cavia porcellus) Lab. Anim. Sci. 1980;30(2 Pt 1):174–179. [PubMed] [Google Scholar]
- 16.Alexander B.T., Kassab S.E., Miller M.T., Abram S.R., Reckelhoff J.F., Bennett W.A. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37(4):1191–1195. doi: 10.1161/01.hyp.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 17.Granger J.P., LaMarca B.B., Cockrell K., Sedeek M., Balzi C., Chandler D. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol. Med. 2006;122:383–392. doi: 10.1385/1-59259-989-3:381. [DOI] [PubMed] [Google Scholar]
- 18.Intapad S., Warrington J.P., Spradley F.T., Palei A.C., Drummond H.A., Ryan M.J. Reduced uterine perfusion pressure induces hypertension in the pregnant mouse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;307(11):R1353–R1357. doi: 10.1152/ajpregu.00268.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez-Aranguren LCR H., Ahmad S., Alzahrani F.A., Sparatore A., Wang K., Ahmed A. MZe786 rescues cardiac mitochondrial activity in high sFlt-1 and low HO-1 environment. Antioxidants. 2020;9:598. doi: 10.3390/antiox9070598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sparatore A., Perrino E., Tazzari V., Giustarini D., Rossi R., Rossoni G. Pharmacological profile of a novel H(2)S-releasing aspirin. Free Radic. Biol. Med. 2009;46(5):586–592. doi: 10.1016/j.freeradbiomed.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Rezai H., Ahmad S., Alzahrani F.A., Sanchez-Aranguren L., Dias I.H., Agrawal S. MZe786, a hydrogen sulfide-releasing aspirin prevents preeclampsia in heme oxygenase-1 haplodeficient pregnancy under high soluble flt-1 environment. Redox Biol. 2020;38:101768. doi: 10.1016/j.redox.2020.101768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson R. Technology drives healthcare innovation. EE-Evaluation Engineering. 2017;56(3):10–14. [Google Scholar]
- 23.Ahmad S., Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ. Res. 2004;95(9):884–891. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- 24.Rangan G.K., Tesch G.H. Quantification of renal pathology by image analysis. Nephrology. 2007;12(6):553–558. doi: 10.1111/j.1440-1797.2007.00855.x. [DOI] [PubMed] [Google Scholar]
- 25.Abuyassin B., Badran M., Ayas N.T., Laher I. Intermittent hypoxia causes histological kidney damage and increases growth factor expression in a mouse model of obstructive sleep apnea. PloS One. 2018;13(2) doi: 10.1371/journal.pone.0192084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopes van Balen V.A., Spaan J.J., Cornelis T., Spaanderman M.E.A. Prevalence of chronic kidney disease after preeclampsia. J. Nephrol. 2017;30(3):403–409. doi: 10.1007/s40620-016-0342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eaton M., Davies A.H., Devine J., Zhao X., Simmons D.G., Mariusdottir E. Complex patterns of cell growth in the placenta in normal pregnancy and as adaptations to maternal diet restriction. PloS One. 2020;15(1) doi: 10.1371/journal.pone.0226735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adamson S.L., Lu Y., Whiteley K.J., Holmyard D., Hemberger M., Pfarrer C. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev. Biol. 2002;250(2):358–373. doi: 10.1016/s0012-1606(02)90773-6. [DOI] [PubMed] [Google Scholar]
- 29.Levine R.J., Maynard S.E., Qian C., Lim K.H., England L.J., Yu K.F. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004;350(7):672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 30.Vuorela P., Helske S., Hornig C., Alitalo K., Weich H., Halmesmaki E. Amniotic fluid--soluble vascular endothelial growth factor receptor-1 in preeclampsia. Obstet. Gynecol. 2000;95(3):353–357. doi: 10.1016/s0029-7844(99)00565-7. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H., Guo C., Zhang A., Fan Y., Gu T., Wu D. Effect of S-aspirin, a novel hydrogen-sulfide-releasing aspirin (ACS14), on atherosclerosis in apoE-deficient mice. Eur. J. Pharmacol. 2012;697(1–3):106–116. doi: 10.1016/j.ejphar.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Pircher J., Fochler F., Czermak T., Mannell H., Kraemer B.F., Wornle M. Hydrogen sulfide-releasing aspirin derivative ACS14 exerts strong antithrombotic effects in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 2012;32(12):2884–2891. doi: 10.1161/ATVBAHA.112.300627. [DOI] [PubMed] [Google Scholar]
- 33.Conti-Ramsden F.I., Nathan H.L., De Greeff A., Hall D.R., Seed P.T., Chappell L.C. Pregnancy-Related acute kidney injury in preeclampsia: risk factors and renal outcomes. Hypertension. 2019;74(5):1144–1151. doi: 10.1161/HYPERTENSIONAHA.119.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brennan L., Morton J.S., Quon A., Davidge S.T. Postpartum vascular dysfunction in the reduced uteroplacental perfusion model of preeclampsia. PloS One. 2016;11(9) doi: 10.1371/journal.pone.0162487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schenone M.H., Mari G., Schlabritz-Loutsevitch N., Ahokas R. Effects of selective reduced uterine perfusion pressure in pregnant rats. Placenta. 2015;36(12):1450–1454. doi: 10.1016/j.placenta.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Morton J.S., Levasseur J., Ganguly E., Quon A., Kirschenman R., Dyck J.R.B. Characterisation of the selective reduced uteroplacental perfusion (sRUPP) model of preeclampsia. Sci. Rep. 2019;9(1):9565. doi: 10.1038/s41598-019-45959-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fushima T., Sekimoto A., Minato T., Ito T., Oe Y., Kisu K. Reduced uterine perfusion pressure (RUPP) model of preeclampsia in mice. PloS One. 2016;11(5) doi: 10.1371/journal.pone.0155426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo W., Gui D.D., Yan B.J., Ren Z., Peng L.J., Wei D.H. Hydrogen sulfide switch phenomenon regulating autophagy in cardiovascular diseases. Cardiovasc. Drugs Ther. 2020;34(1):113–121. doi: 10.1007/s10557-019-06927-4. [DOI] [PubMed] [Google Scholar]
- 39.Tran B.H., Yu Y., Chang L., Tan B., Jia W., Xiong Y. A novel liposomal S-Propargyl-Cysteine: a sustained release of hydrogen sulfide reducing myocardial fibrosis via TGF-beta1/smad pathway. Int. J. Nanomed. 2019;14:10061–10077. doi: 10.2147/IJN.S216667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kar S., Shahshahan H.R., Hackfort B.T., Yadav S.K., Yadav R., Kambis T.N. Exercise training promotes cardiac hydrogen sulfide biosynthesis and mitigates pyroptosis to prevent high-fat diet-induced diabetic cardiomyopathy. Antioxidants. 2019;8(12) doi: 10.3390/antiox8120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peter E.A., Shen X., Shah S.H., Pardue S., Glawe J.D., Zhang W.W. Plasma free H2S levels are elevated in patients with cardiovascular disease. J Am Heart Assoc. 2013;2(5) doi: 10.1161/JAHA.113.000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang X., Li C., Xie X., Zhan K.B., Yang S.Q., Tang Y.Y. Hydrogen sulfide inhibits homocysteine-induced neuronal senescence by up-regulation of SIRT1. Int. J. Med. Sci. 2020;17(3):310–319. doi: 10.7150/ijms.38602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song Y.J., Shi Y., Cui M.M., Li M., Wen X.R., Zhou X.Y. H2S attenuates injury after ischemic stroke by diminishing the assembly of CaMKII with ASK1-MKK3-p38 signaling module. Behav. Brain Res. 2020;384:112520. doi: 10.1016/j.bbr.2020.112520. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L., Qi Q., Yang J., Sun D., Li C., Xue Y. An anticancer role of hydrogen sulfide in human gastric cancer cells. Oxid Med Cell Longev. 2015;2015:636410. doi: 10.1155/2015/636410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cai F., Xu H., Cao N., Zhang X., Liu J., Lu Y. ADT-OH, a hydrogen sulfide-releasing donor, induces apoptosis and inhibits the development of melanoma in vivo by upregulating FADD. Cell Death Dis. 2020;11(1):33. doi: 10.1038/s41419-020-2222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu S., Pan J., Cheng X., Zheng J., Wang X., Guan H. Diallyl trisulfide, a H2 S donor, inhibits cell growth of human papillary thyroid carcinoma KTC-1 cells through a positive feedback loop between H2 S and cystathionine-gamma-lyase. Phytother Res. 2020;34(5):1154–1165. doi: 10.1002/ptr.6586. [DOI] [PubMed] [Google Scholar]
- 47.Becker J.C., Domschke W., Pohle T. Current approaches to prevent NSAID-induced gastropathy--COX selectivity and beyond. Br. J. Clin. Pharmacol. 2004;58(6):587–600. doi: 10.1111/j.1365-2125.2004.02198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rossoni G., Manfredi B., Tazzari V., Sparatore A., Trivulzio S., Del Soldato P. Activity of a new hydrogen sulfide-releasing aspirin (ACS14) on pathological cardiovascular alterations induced by glutathione depletion in rats. Eur. J. Pharmacol. 2010;648(1–3):139–145. doi: 10.1016/j.ejphar.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 49.Xu B., Shanmugalingam R., Chau K., Pears S., Hennessy A., Makris A. The effect of acetyl salicylic acid (Aspirin) on trophoblast-endothelial interaction in vitro. J. Reprod. Immunol. 2017;124:54–61. doi: 10.1016/j.jri.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 50.Giustarini D., Del Soldato P., Sparatore A., Rossi R. Modulation of thiol homeostasis induced by H2S-releasing aspirin. Free Radic. Biol. Med. 2010;48(9):1263–1272. doi: 10.1016/j.freeradbiomed.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 51.Bucci M., Papapetropoulos A., Vellecco V., Zhou Z., Pyriochou A., Roussos C. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler. Thromb. Vasc. Biol. 2010;30(10):1998–2004. doi: 10.1161/ATVBAHA.110.209783. [DOI] [PubMed] [Google Scholar]
- 52.Li L., Whiteman M., Guan Y.Y., Neo K.L., Cheng Y., Lee S.W. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117(18):2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 53.Ahmed A., Dunk C., Kniss D., Wilkes M. Role of VEGF receptor-1 (Flt-1) in mediating calcium-dependent nitric oxide release and limiting DNA synthesis in human trophoblast cells. Lab. Invest. 1997;76(6):779–791. [PubMed] [Google Scholar]
- 54.Ahmed A. Heparin-binding angiogenic growth factors in pregnancy: a review. Placenta. 1997;18:215–258. [Google Scholar]
- 55.Craici I.M., Wagner S.J., Weissgerber T.L., Grande J.P., Garovic V.D. Advances in the pathophysiology of pre-eclampsia and related podocyte injury. Kidney Int. 2014;86(2):275–285. doi: 10.1038/ki.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coan P.M., Angiolini E., Sandovici I., Burton G.J., Constancia M., Fowden A.L. Adaptations in placental nutrient transfer capacity to meet fetal growth demands depend on placental size in mice. J. Physiol. 2008;586(18):4567–4576. doi: 10.1113/jphysiol.2008.156133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Plotnikov E.Y., Kazachenko A.V., Vyssokikh M.Y., Vasileva A.K., Tcvirkun D.V., Isaev N.K. The role of mitochondria in oxidative and nitrosative stress during ischemia/reperfusion in the rat kidney. Kidney Int. 2007;72(12):1493–1502. doi: 10.1038/sj.ki.5002568. [DOI] [PubMed] [Google Scholar]
- 58.Mastropasqua F., Girolimetti G., Shoshan M. PGC1alpha: friend or foe in cancer? Genes. 2018;9(1) doi: 10.3390/genes9010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williamson R.D., McCarthy F.P., Manna S., Groarke E., Kell D.B., Kenny L.C. L-(+)-Ergothioneine significantly improves the clinical characteristics of preeclampsia in the reduced uterine perfusion pressure rat model. Hypertension. 2020;75(2):561–568. doi: 10.1161/HYPERTENSIONAHA.119.13929. [DOI] [PubMed] [Google Scholar]
- 60.Fernandez-Marcos P.J., Auwerx J. Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 2011;93(4) doi: 10.3945/ajcn.110.001917. 884S-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang D., Zhang Y., Yang M., Wang S., Jiang Z., Li Z. Exogenous hydrogen sulfide prevents kidney damage following unilateral ureteral obstruction. Neurourol. Urodyn. 2014;33(5):538–543. doi: 10.1002/nau.22450. [DOI] [PubMed] [Google Scholar]
- 62.Ahmad A., Olah G., Szczesny B., Wood M.E., Whiteman M., Szabo C. AP39, A mitochondrially targeted hydrogen sulfide donor, exerts protective effects in renal epithelial cells subjected to oxidative stress in vitro and in acute renal injury in vivo. Shock. 2016;45(1):88–97. doi: 10.1097/SHK.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bos E.M., Leuvenink H.G., Snijder P.M., Kloosterhuis N.J., Hillebrands J.L., Leemans J.C. Hydrogen sulfide-induced hypometabolism prevents renal ischemia/reperfusion injury. J. Am. Soc. Nephrol. 2009;20(9):1901–1905. doi: 10.1681/ASN.2008121269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sanchez-Aranguren L.C., Ahmad S., Dias I.H.K., Alzahrani F.A., Rezai H., Wang K. Bioenergetic effects of hydrogen sulfide suppress soluble Flt-1 and soluble endoglin in cystathionine gamma-lyase compromised endothelial cells. Sci. Rep. 2020;10(1):15810. doi: 10.1038/s41598-020-72371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanchez-Aranguren L.C., Rezai H., Ahmad S., Alzahrani F.A., Sparatore A., Wang K. MZe786 rescues cardiac mitochondrial activity in high sFlt-1 and low HO-1 environment. Antioxidants. 2020;9(7) doi: 10.3390/antiox9070598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.